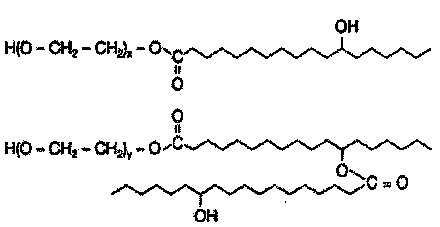
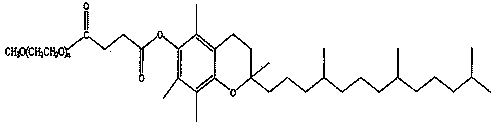
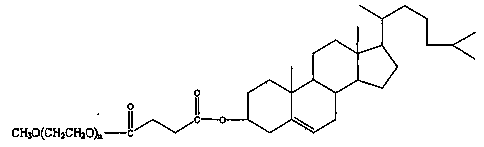
3-n-butylphthalide injection and preparation method thereof
A technology of butylphthalide and injection, which is applied in the field of medicine and can solve the problems of unstable quality, complicated formulation of butylphthalide, and inability to meet clinical drug requirements well.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0109] Example 1 Butylphthalide injection (water injection): contains butylphthalide, soybean lecithin, and water for injection. The prescription dosage of the three is shown in the following table:
[0110] In 100mL:
[0111] Material name Prescription amount % by weight Butylphthalide 0.25g 0.25 Soy Lecithin 4g 4 water for injection Replenish water to 100ml
[0112] Preparation process: Dissolve soybean lecithin in about 90ml of water for injection at 40~60°C, slowly add butylphthalide dropwise, stir well, break up with a high-pressure homogenizer, control the particle size to be less than 50 nanometers, obtain a clear liquid, replenish water To 100ml, add 0.1% activated carbon and stir for 30 minutes, filter to remove carbon, sterilize, pack in 10ml each, and sterilize.
Embodiment 2
[0113] Example 2 Butylphthalide injection (water injection): contains butylphthalide, polyethylene glycol-12-hydroxystearate and water for injection. The prescription dosage of the three is shown in the following table:
[0114] Material name Prescription amount % by weight Butylphthalide 0.25g 0.25 Polyethylene glycol-12-hydroxystearate 1g 1 water for injection Replenish water to 100ml
[0115] Preparation process: Dissolve polyethylene glycol-12-hydroxystearate in about 90ml of water for injection at 20~30°C, slowly add butylphthalide dropwise, stir well, no oil droplets appear on the liquid surface, and a clear liquid is obtained. Replenish water to 100ml, add 0.2% activated carbon and stir for 30 minutes, filter to remove carbon, sterilize, pack in aliquots, 10ml each, and sterilize.
Embodiment 3
[0116] Example 3 Butylphthalide injection (freeze-dried powder injection): contains butylphthalide, polyethylene glycol-12-hydroxystearate and water for injection. The prescription dosage of the three is shown in the following table:
[0117] Material name Prescription amount % by weight Butylphthalide 0.5g 0.5 Polyethylene glycol-12-hydroxystearate 2g 2 Mannitol 6g 6 glucose 1.5g 1.5 water for injection Replenish water to 100ml
[0118] Preparation process: Dissolve polyethylene glycol-12-hydroxystearate in about 90ml of water for injection at 20~30°C, slowly add butylphthalide dropwise, stir well, until no oil drops appear on the liquid surface, add mannitol and glucose Dissolve to obtain a clear liquid, add water to 100ml, add 0.1% activated carbon and stir for 30 minutes, filter to remove carbon, sterilize, subpackage, 5ml each, and freeze-dry.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com