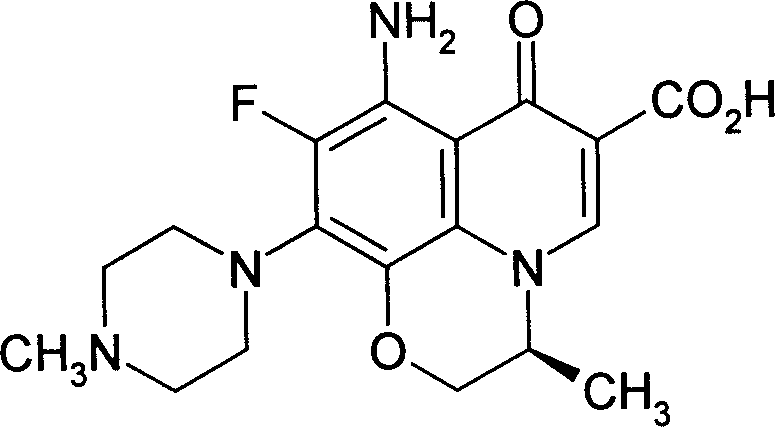
Carbostyrile genus antibiotic preparation took orally
A technology for oral preparations of quinolones, which is applied in the field of oral preparations of quinolone antibiotics, can solve problems such as application limitations, and achieve the effects of low toxic and side effects, strong antibacterial effect, and broad antibacterial spectrum
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Make 1000 oral ordinary tablets with the raw and auxiliary materials in the following weight ratio, with a tablet weight of about 385mg:
[0022] Quinolones Antibiotics 200g
[0023] Pregelatinized starch 50g
[0024] Microcrystalline Cellulose 100g
[0025] 3% HPMC 40% ethanol solution appropriate amount
[0026] Sodium carboxymethyl starch 30g
[0028] Preparation Process:
[0029] 1. Pulverize the quinolone antibiotic raw material and auxiliary materials respectively, and pass through a 100-mesh sieve.
[0030] 2. The main ingredient is mixed with pregelatinized starch and microcrystalline cellulose in proportion by weight, and soft materials are made with 3% HPMC40% ethanol solution, granulated, and granulated after drying.
[0031] 3. Add sodium carboxymethyl starch and magnesium stearate, mix well, and press into tablets.
Embodiment 2
[0033] 1. Prepare 6% ethanol solution of Opadry enteric coating solution
[0034] 2. Take the plain tablet obtained in Example 1 and pour it into a coating pan for coating to obtain an enteric-coated tablet. Coating powder relative to tablet core weight gain: 4.0-5.0%.
Embodiment 3
[0036] Make 1000 oral ordinary tablets with the raw and auxiliary materials in the following weight ratio, with a tablet weight of about 220mg:
[0037] Quinolones Antibiotics 100g
[0038] 20g pregelatinized starch
[0039] Microcrystalline Cellulose 80g
[0040] 3% HPMC 40% ethanol solution appropriate amount
[0041] Sodium carboxymethyl starch 15g
[0043] Preparation Process:
[0044] 1. Pulverize the quinolone antibiotic raw material and auxiliary materials respectively, and pass through a 100-mesh sieve.
[0045] 2. The main ingredient is mixed with pregelatinized starch and microcrystalline cellulose in proportion by weight, and soft materials are made with 3% HPMC40% ethanol solution, granulated, and granulated after drying.
[0046] 3. Add sodium carboxymethyl starch and magnesium stearate, mix well, and press into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com