Periplaneta americana extract enteric-coated preparation and preparation method thereof
An enteric-coated preparation, the technology of Periplaneta americana, applied in the field of enteric-coated preparation of Periplaneta americana extract and its preparation, can solve problems such as easy burst release or delayed release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
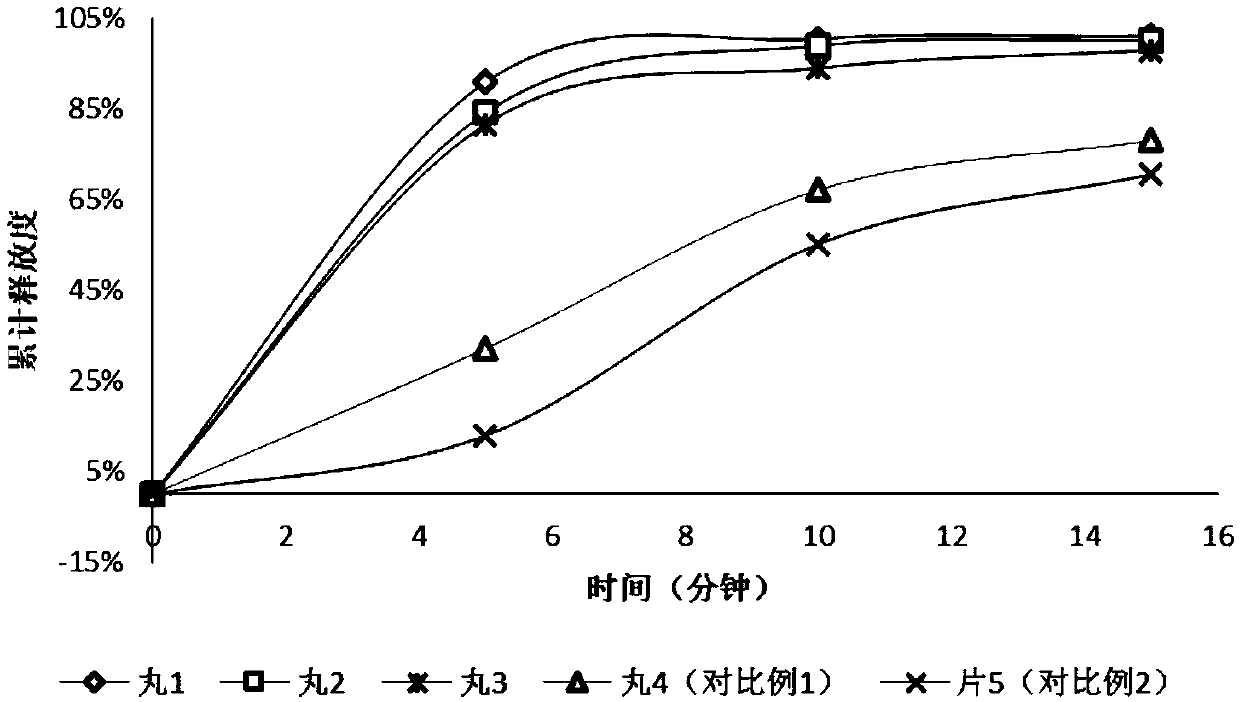
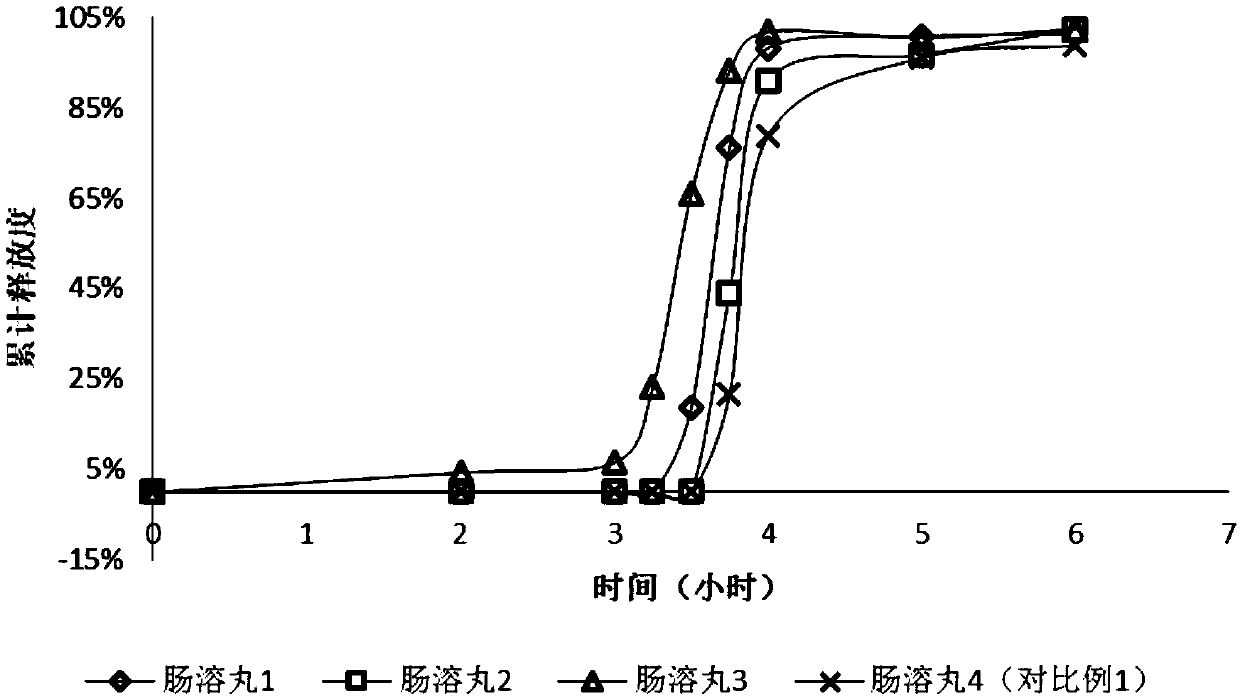
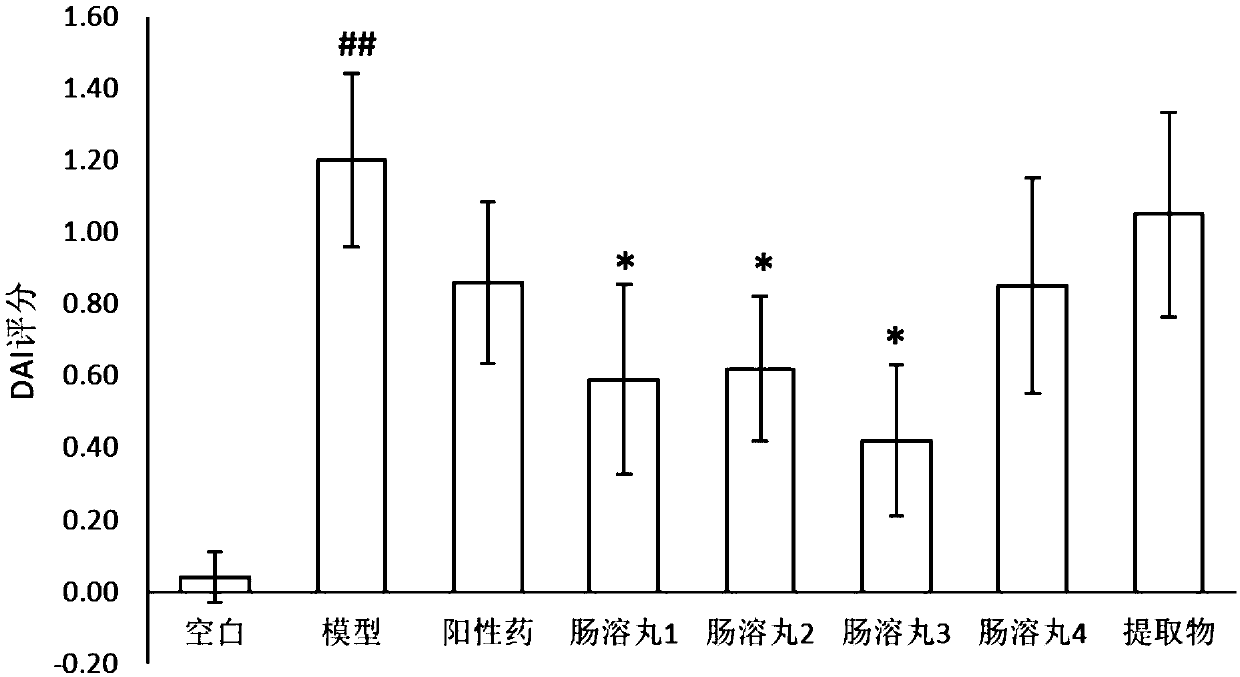
Image
Examples
Embodiment 1
[0061] Example 1: Preparation of Kangfuxin enteric-coated pellets and capsules
[0062] (1) Preparation of blank core
[0063] Microcrystalline Cellulose 400g
[0064] 10% ethanol aqueous solution 400g
[0065] Weigh microcrystalline cellulose and 10% ethanol according to the prescription amount, fully knead and mix the prescription amount of microcrystalline cellulose, add 10% ethanol and continue mixing to obtain a soft material, and slowly put the soft material into the extruder In the funnel, adjust the extrusion speed to be 35rpm to extrude to obtain a strip-shaped soft material. Put the strip-shaped soft material in the spheronizer, adjust the spheronization speed to 800rpm, and spheronize for 10 minutes. The obtained pellet cores are dried in an oven at 40°C for 2 hours to obtain blank pellet cores. The blank pellet cores are obtained by extrusion spheronizer Screening is carried out, and pellets with a particle size of 0.4-1.2 mm are selected for drug-loading layer ...
Embodiment 2
[0074] Example 2: Preparation of Kangfuxin enteric-coated pellets and capsules
[0075] Example 2 On the basis of Example 1, the use of the disintegrant cross-linked povidone XL was omitted. In addition, the enteric coating layer was prepared, the amount of Eudragit S100 was 125g, and the coating weight of the enteric coating was increased 25%. Other raw material formulas and preparation steps, process conditions are all with embodiment 1.
Embodiment 3
[0076] Example 3: Preparation of Kangfuxin enteric-coated pellets and capsules
[0077] (1) Preparation of drug-loaded pellet core
[0078] Commercially available fast-collapsing micro-pill core 300g (purchased from Hangzhou Gaocheng Bio-Nutrition Technology Co., Ltd., model: fast-collapsing MCC pellets)
[0079] Hypromellose E5 90g
[0080] American cockroach extract (by dry weight) 180g
[0081] Micronized silica gel 30g
[0082] Weigh 90g of hypromellose E5, add 810ml of purified water, heat to 80°C, stir until completely dissolved, and cool to room temperature. Weigh 180 g of Periplaneta americana extract (by dry weight), add the above solution, stir evenly, and finally add 30 g of micropowder silica gel to obtain the drug-containing coating solution. Weigh the core of the prescribed amount, and use a fluidized bed to coat the drug-loaded layer. The coating amount of the drug-containing coating is 100% of the prescribed amount. During the coating process, the agitator ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| The inside diameter of | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com