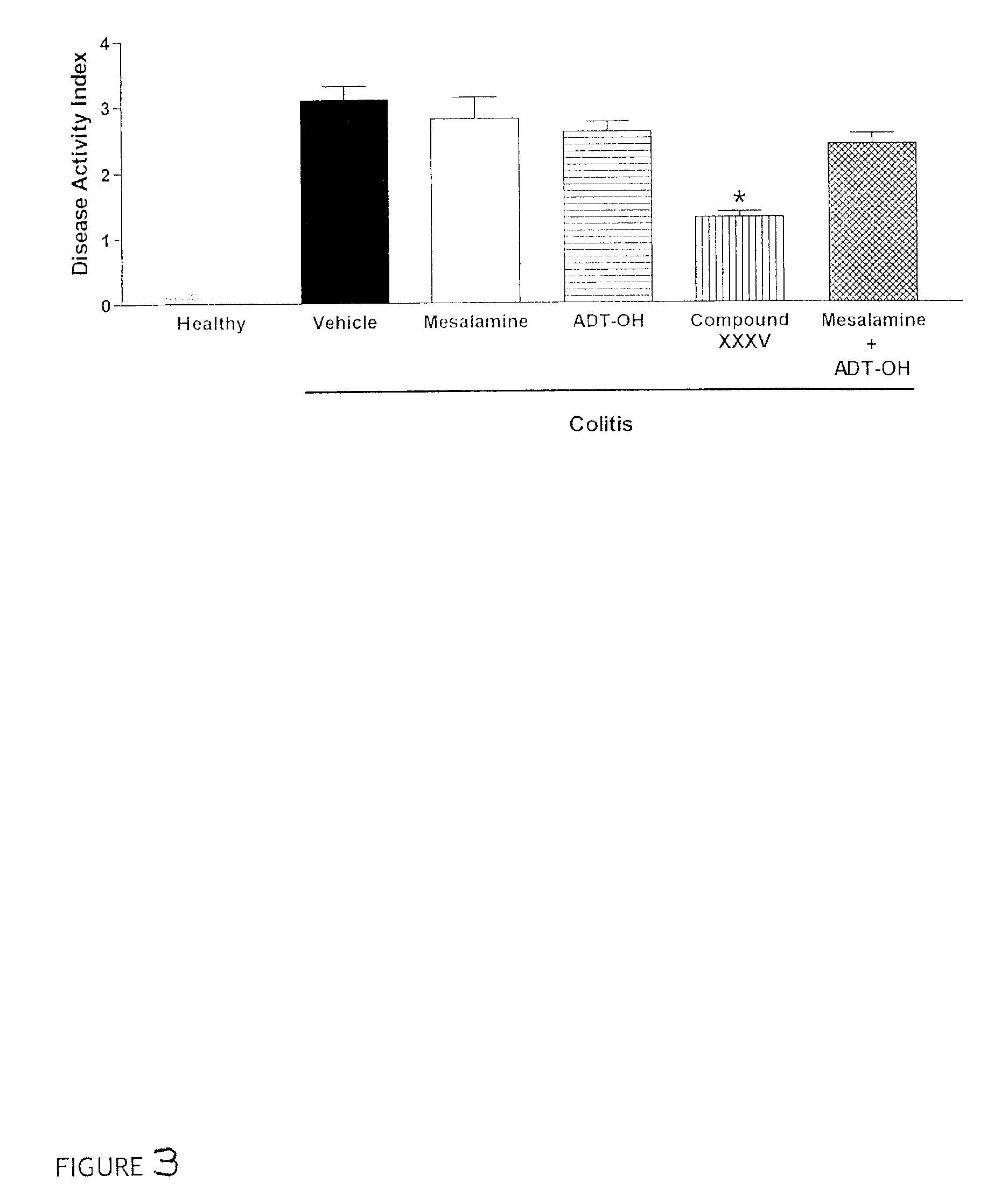
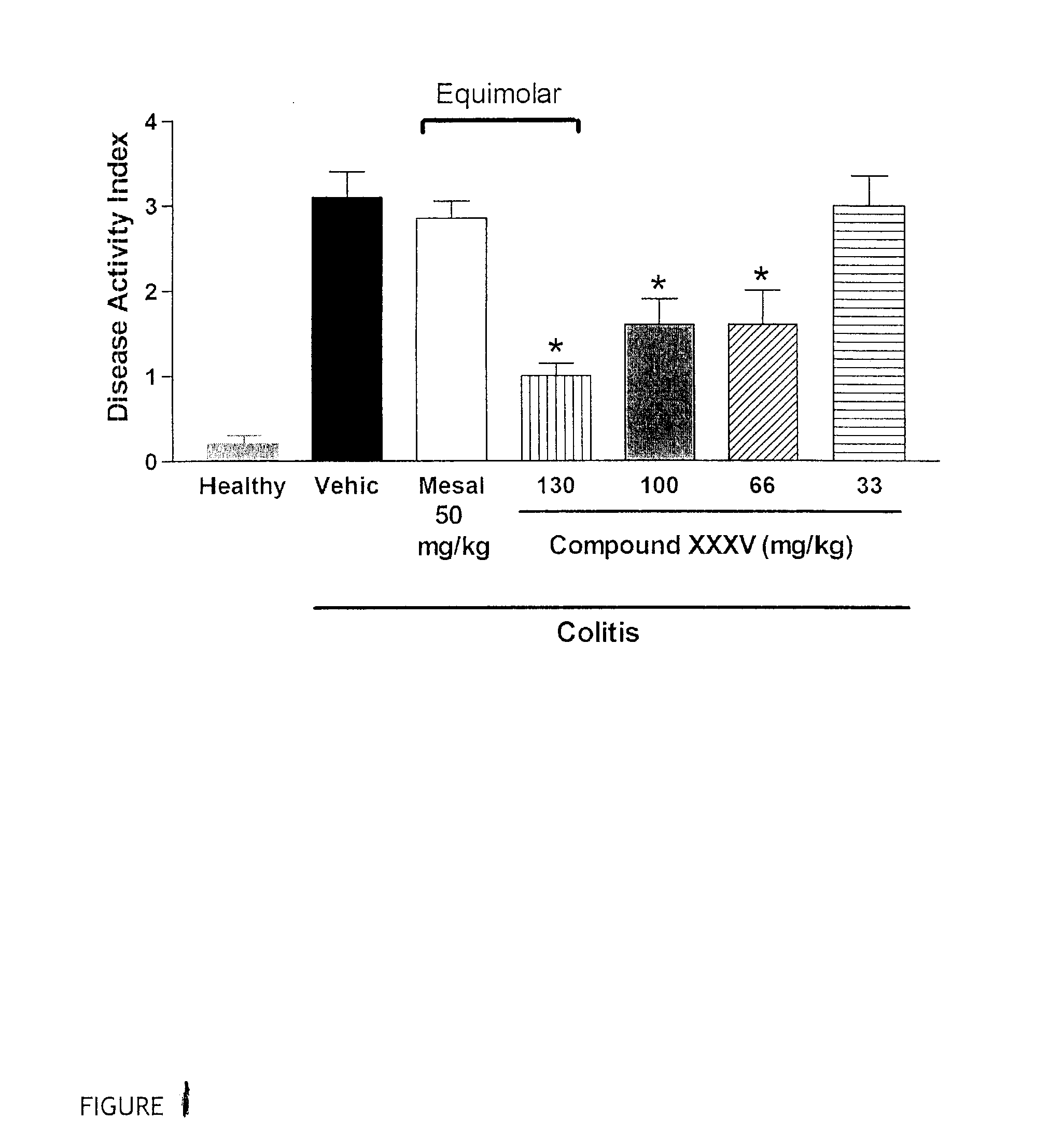
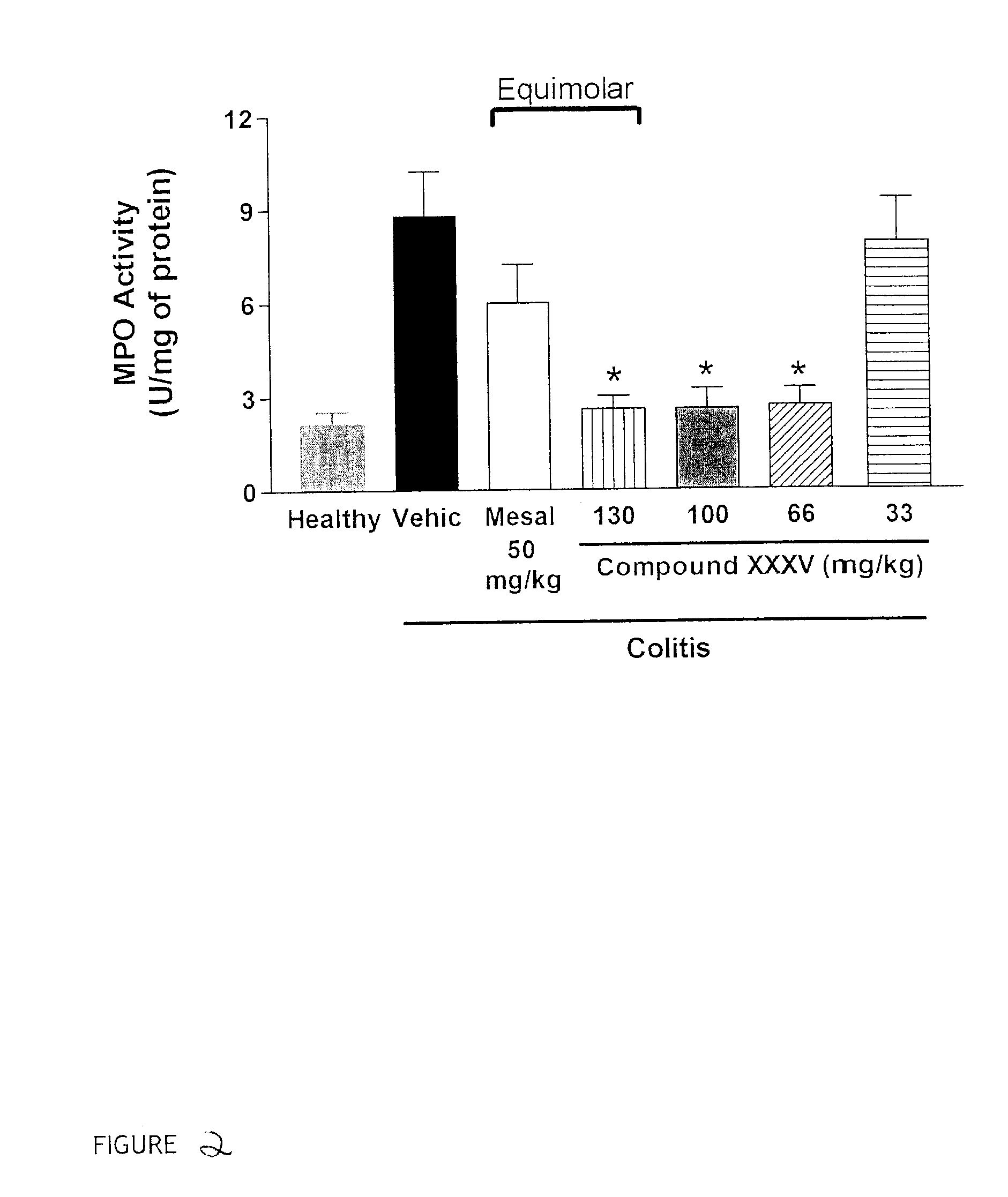
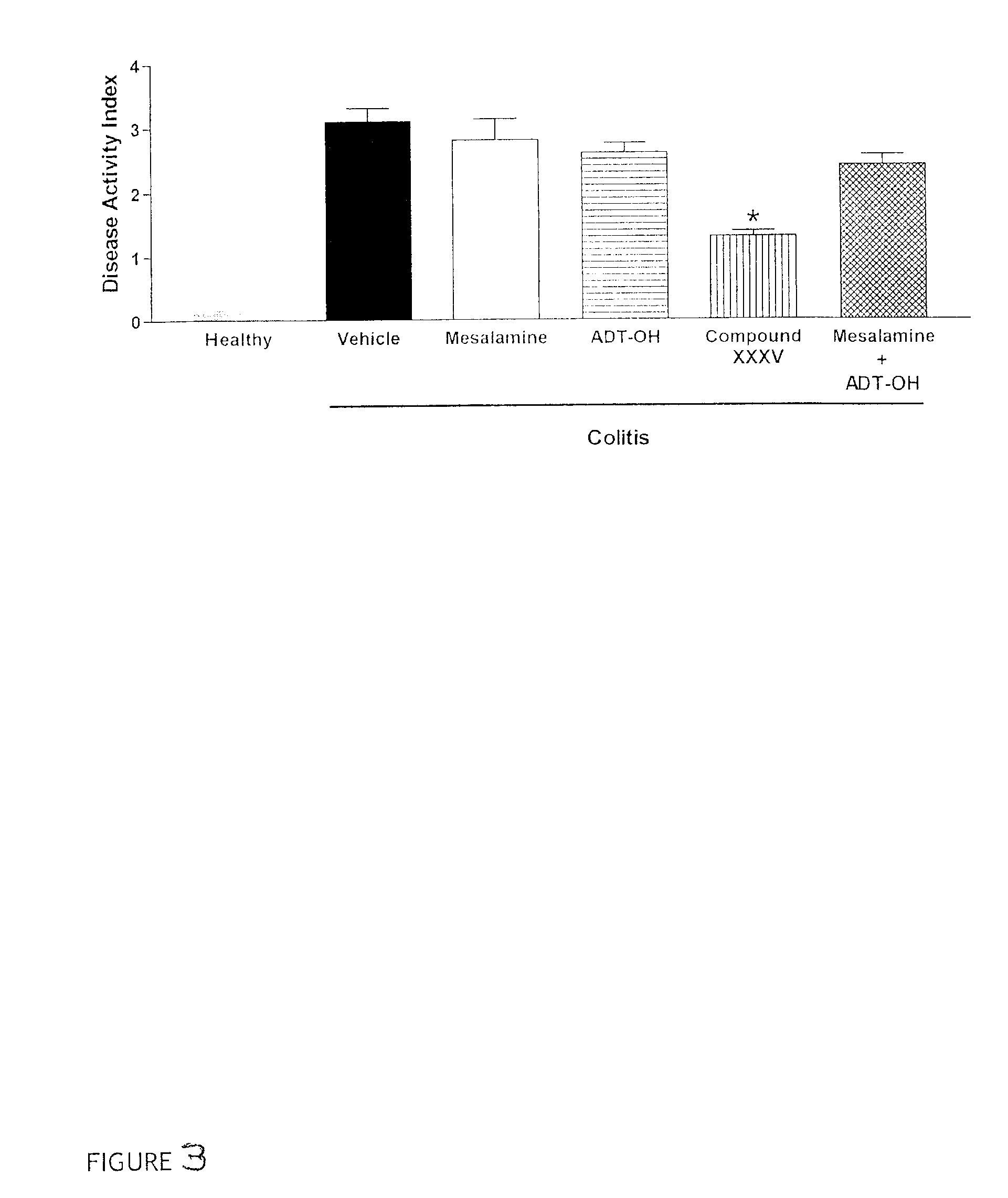
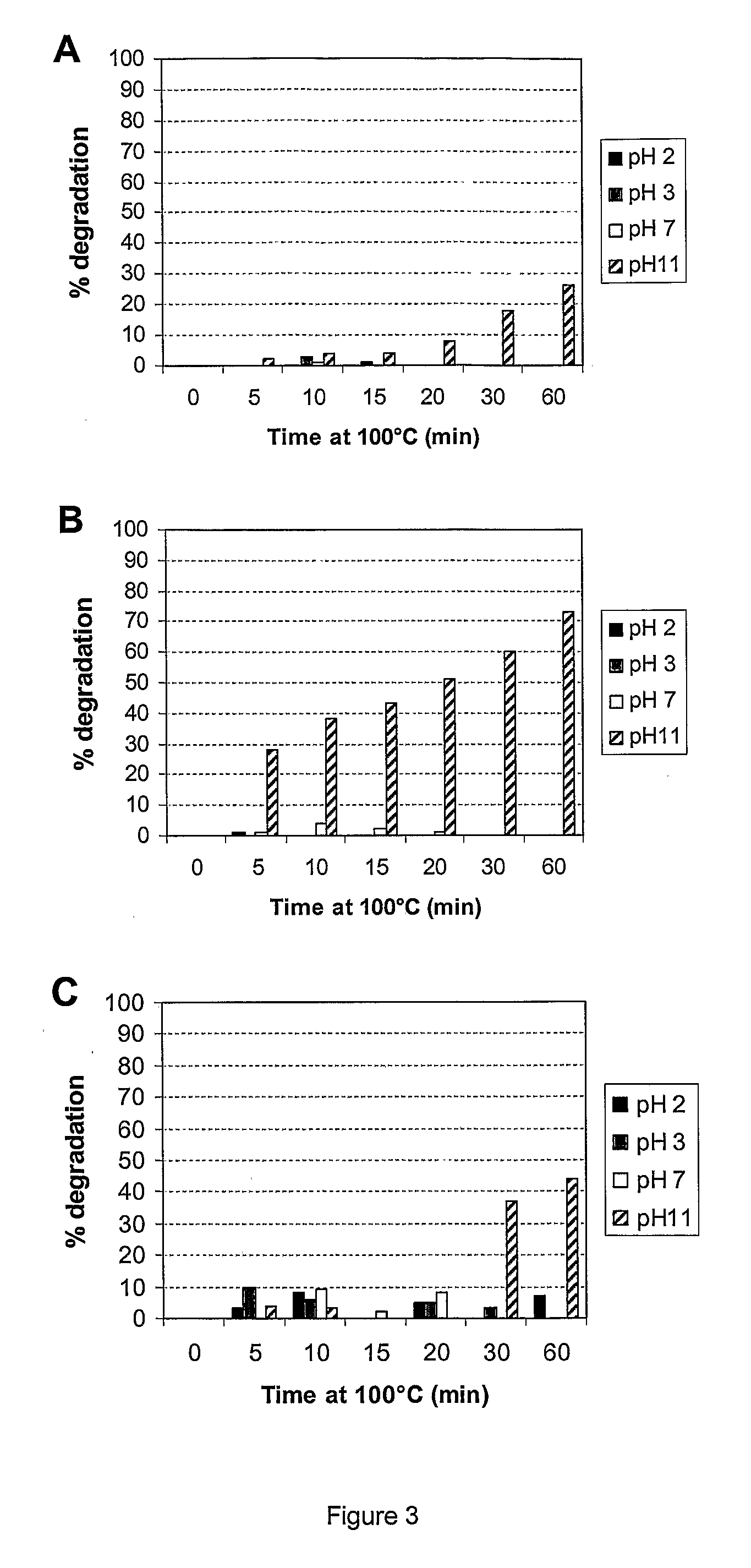
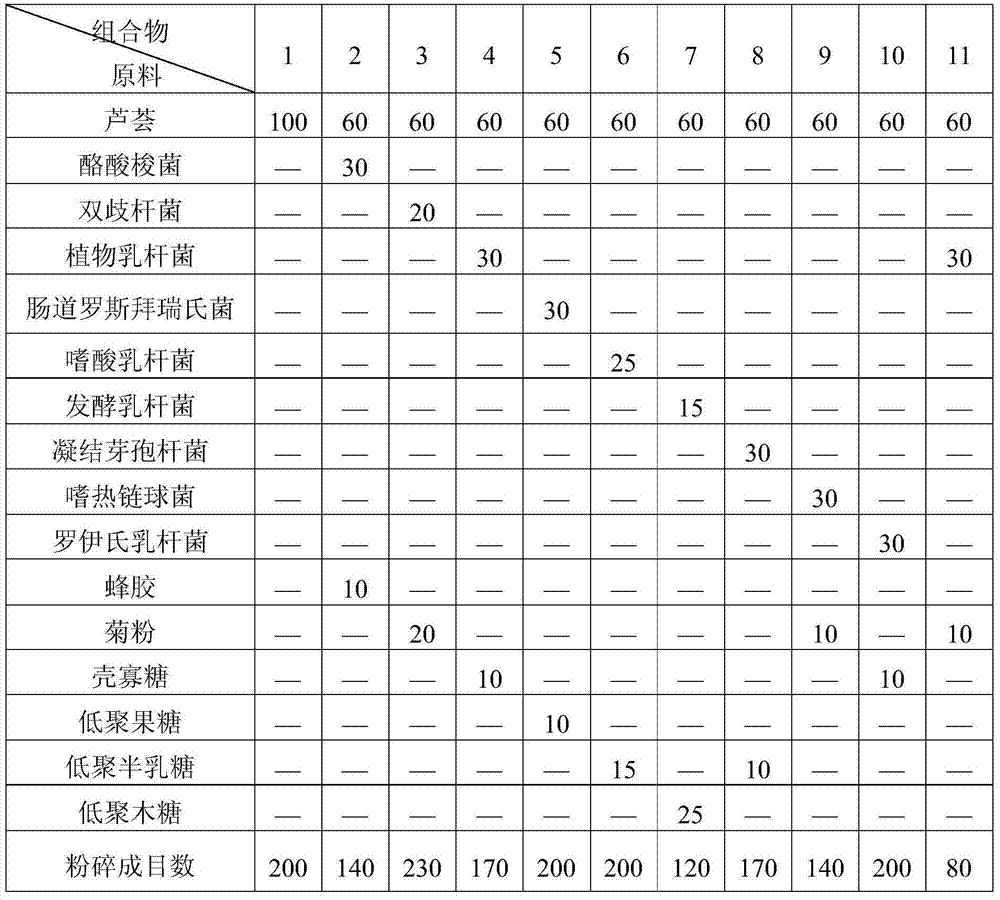
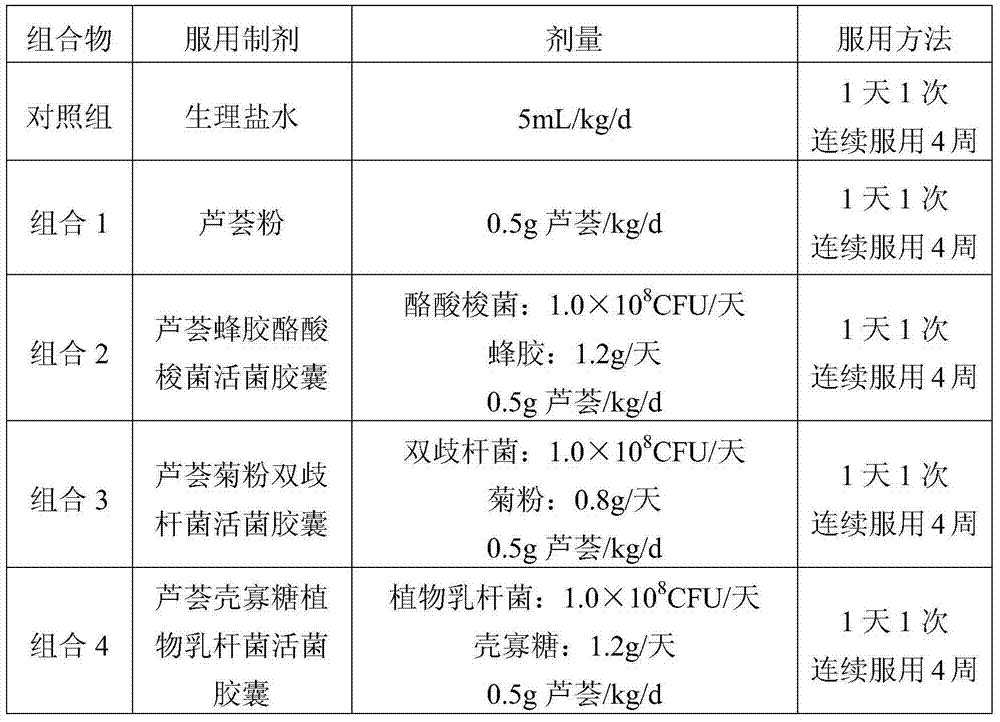
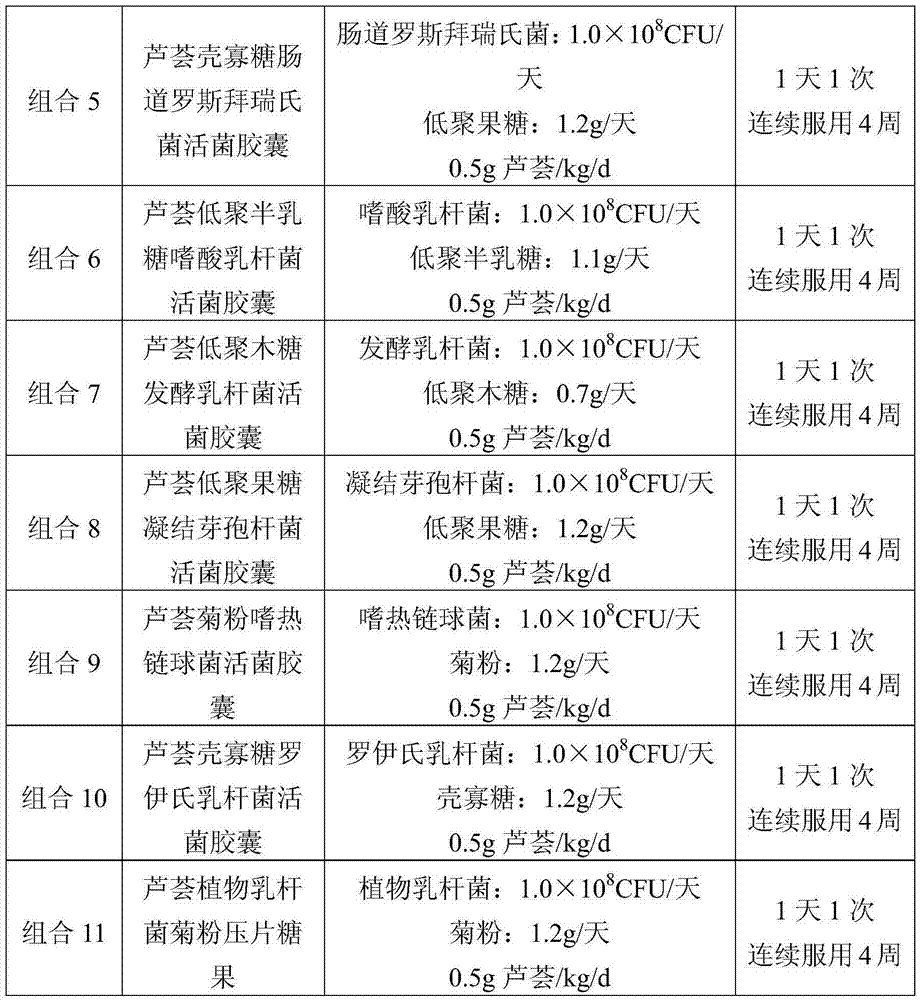
Patents
Literature
4767 results about "Intestino-intestinal" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
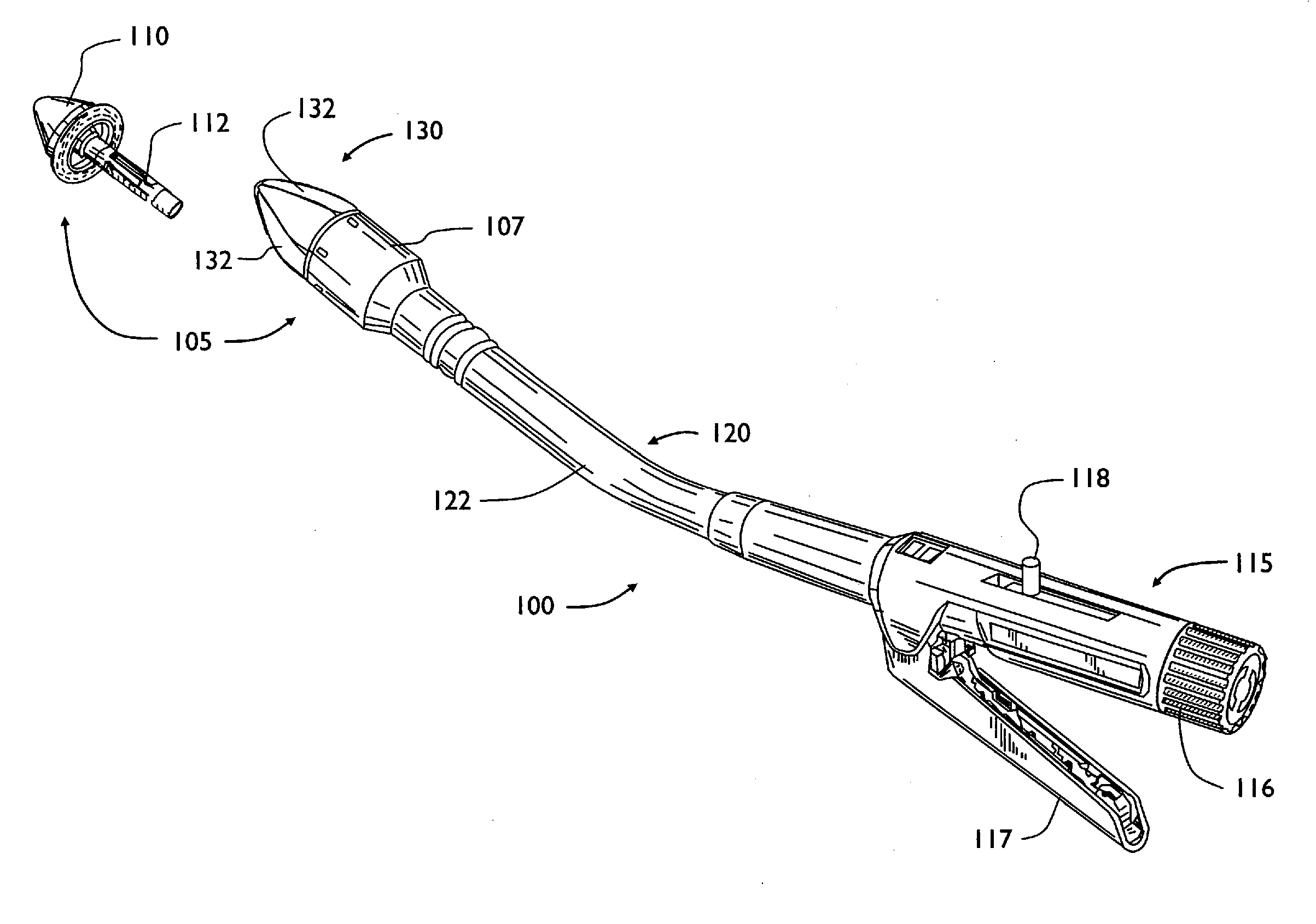
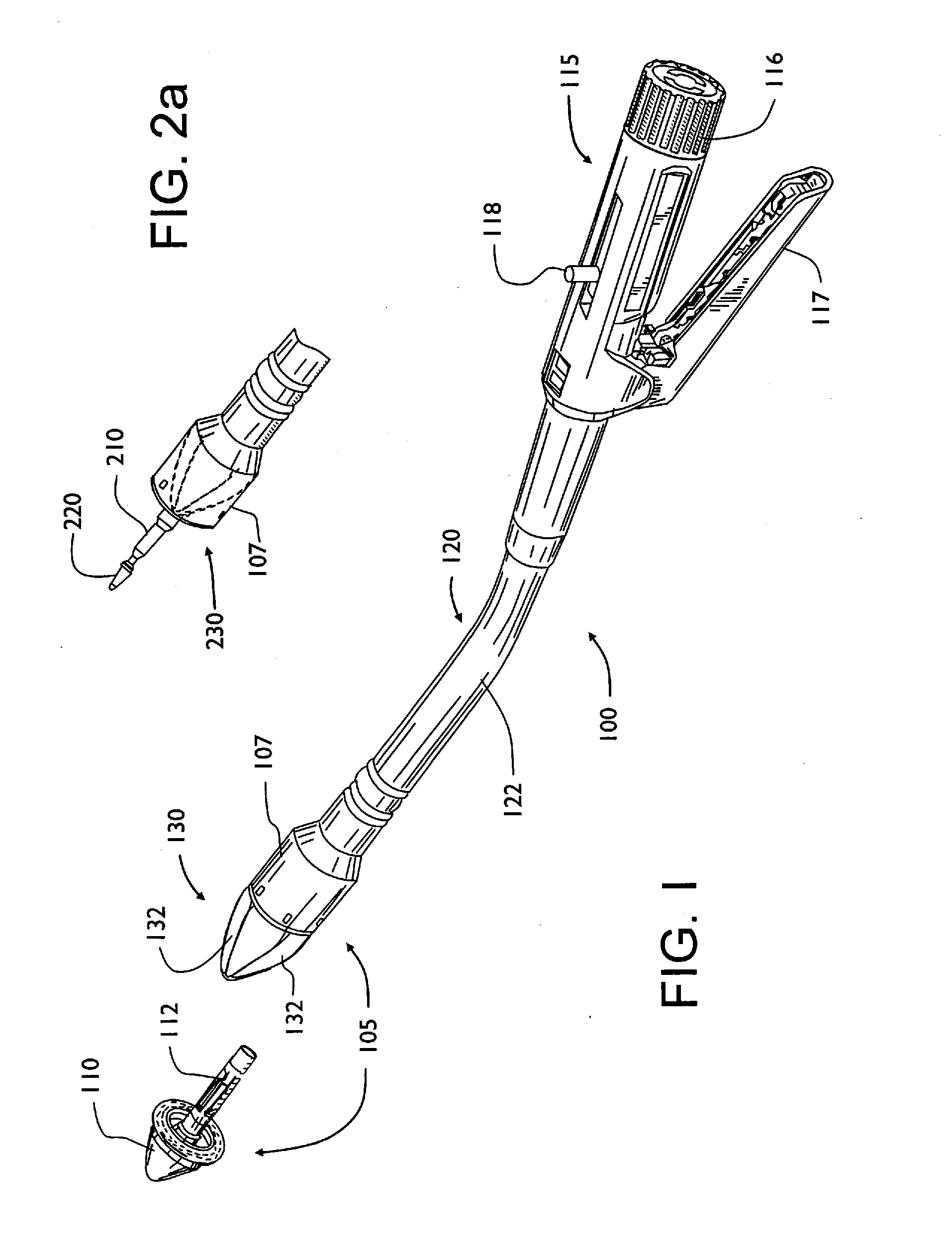
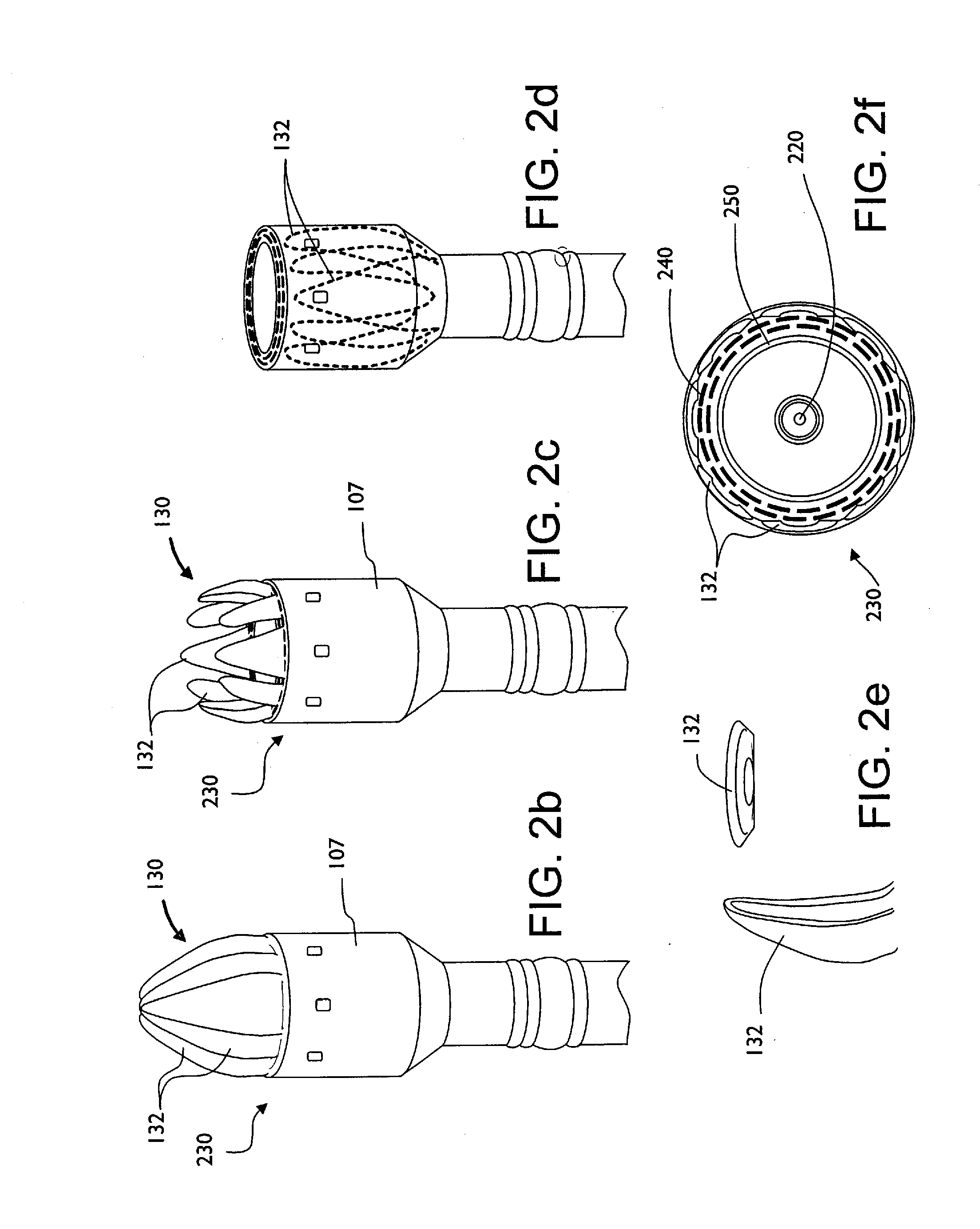
Shield for surgical stapler and method of use
InactiveUS20100163598A1Reduce traumaEasy to browseSuture equipmentsStapling toolsEngineeringSurgical department
A surgical stapler that may have a head assembly, a stapling assembly, a shield, a base head, a handle assembly, and a shaft assembly. A head assembly may have an anvil and anvil shaft, as well as a stapling assembly. A stapling assembly may have a trocar that can be removeably detachable with the anvil, a cannula extension, and a plurality of staples. A shield may be configured to retract from a first extended position where the shield generally covers the stapling assembly or the head assembly, to a second retracted position where the shield generally exposes the stapling assembly or the head assembly. The shield may be integral with the stapler, or provided after-market as an add-on. A surgical stapler may additionally or alternatively include an air or gas pump assembly that can be used to insufflate the rectum and intestinal tract during insertion and advancement of the stapler.
Owner:BELZER GEORGE E
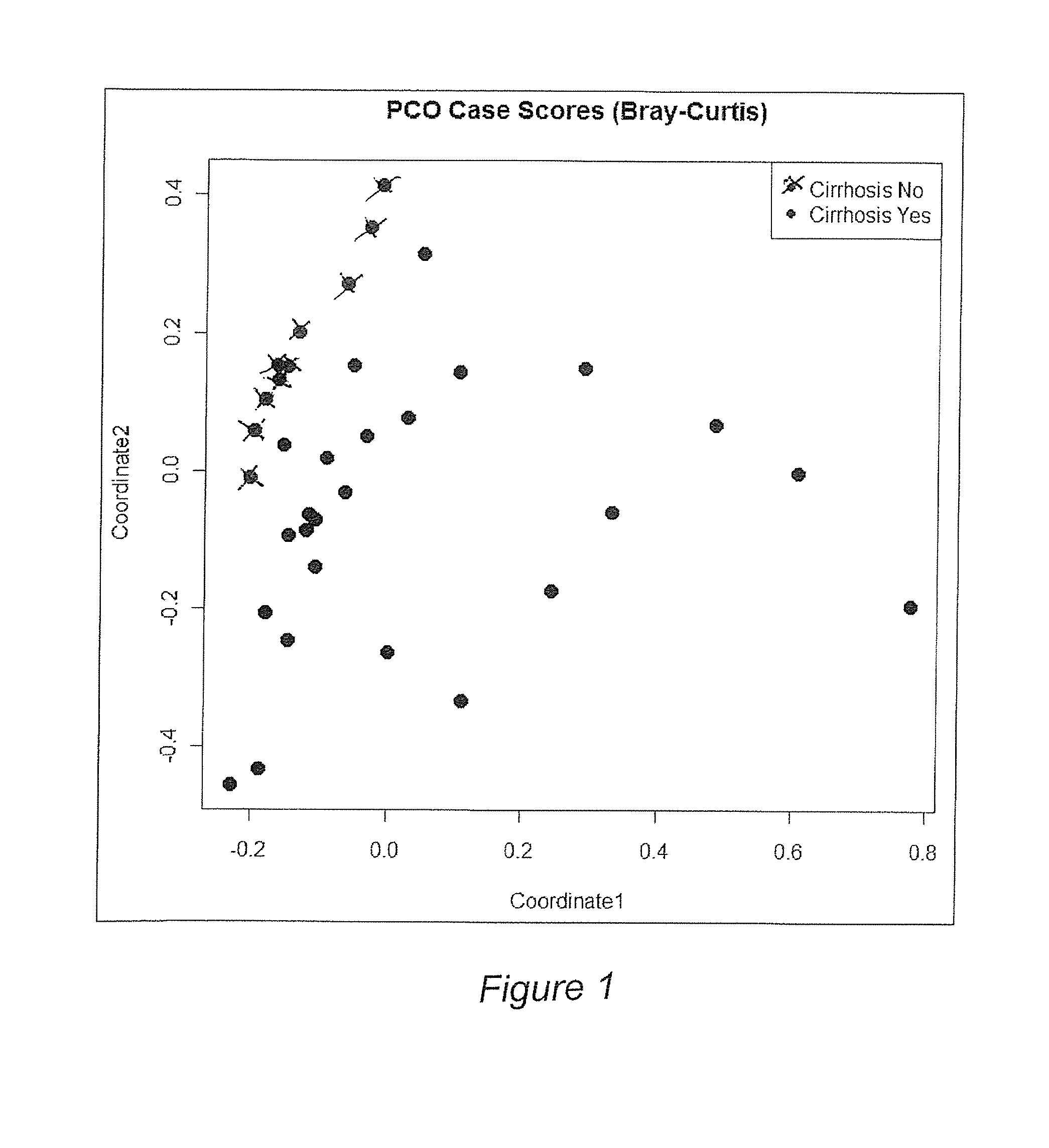
Gut microflora as biomarkers for the prognosis of cirrhosis and brain dysfunction
InactiveUS20140179726A1Efficacy of treatmentReduce the amount requiredBiocideMicrobiological testing/measurementDiseaseTreatment targets
A systems biology approach is used to characterize and relate the intestinal (gut) microbiome of a host organism (e.g. a human) to physiological processes within the host. Information regarding the types and relative amounts of gut microflora is correlated with physiological processes indicative of e.g., a patient's risk of developing a disease or condition, likelihood of responding to a particular treatment, for adjusting treatment protocols, etc. The information is also used to identify novel suitable therapeutic targets and / or to develop and monitor the outcome of therapeutic treatments. An exemplary disease / condition is the development of hepatic encephalopathy (HE), particularly in patients with liver cirrhosis.
Owner:VIRGINIA COMMONWEALTH UNIV +1
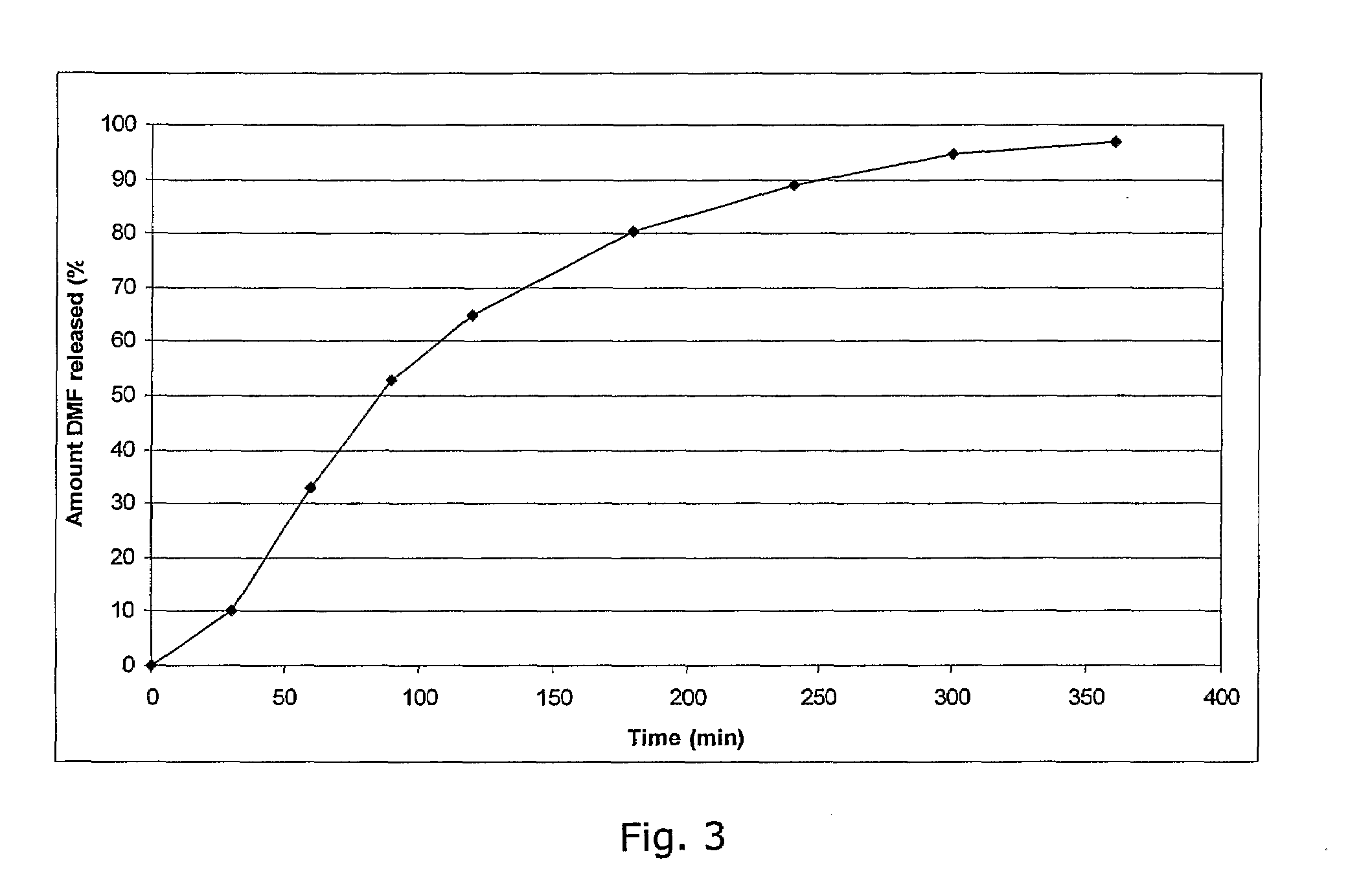
Controlled release pharmaceutical compositions comprising a fumaric acid ester
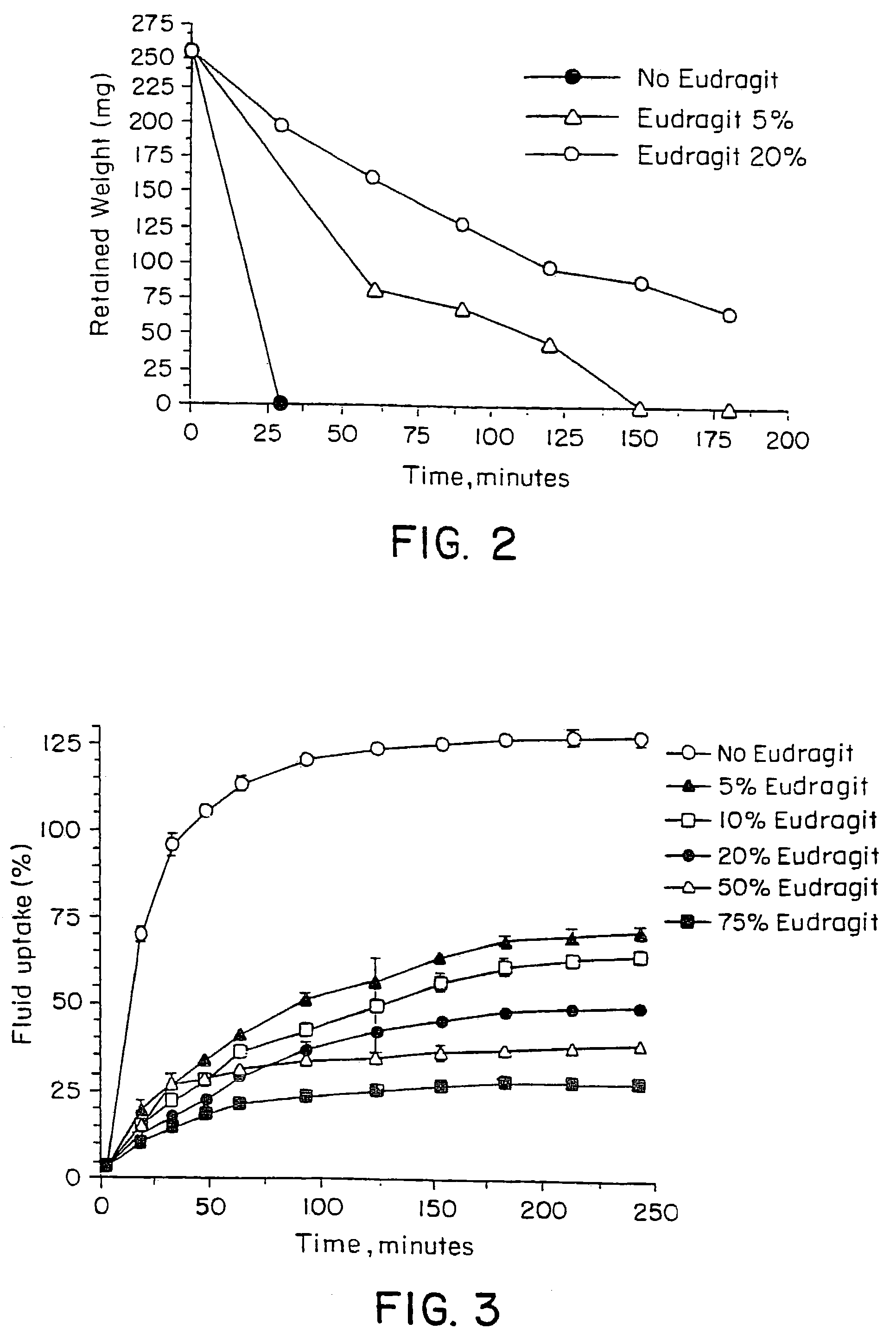
The present invention relates to controlled release pharmaceutical compositions comprising fumaric acid ester(s) as active substance(s). The compositions are suitable for use in the treatment of e.g. psoriasis or other hyperproliferative, inflammatory or autoimmune disorders and are designated to release the fumaric acid ester in a controlled manner so that local high concentrations of the active substance within the gastrointestinal tract upon oral administration can be avoided and, thereby, enabling a reduction in gastro-intestinal related side-effects.
Owner:BIOGEN SWISS MFG GMBH
Probiotic lactic acid bacterium to treat bacterial infections associated with SIDS
Compositions including a non-pathogenic lactic acid-producing bacteria, such as a Bacillus species, spores or an extracellular product of B. coagulans, formulated for oral administration to the intestinal tract for inhibiting bacterial gastrointestinal infections are described. Methods and systems using the compositions for treating gastrointestinal infections, particularly sudden infant death syndrome (SIDS) are also disclosed.
Owner:GANEDEN BIOTECH
Treatment of inflammatory conditions of the intestine
Owner:CSL BEHRING AG
Lactobacillus micro-capsule as well as preparation method and use
ActiveCN101496555AReduce harmHigh viable countBacteria material medical ingredientsAnimal feeding stuffEcological environmentFreeze-drying
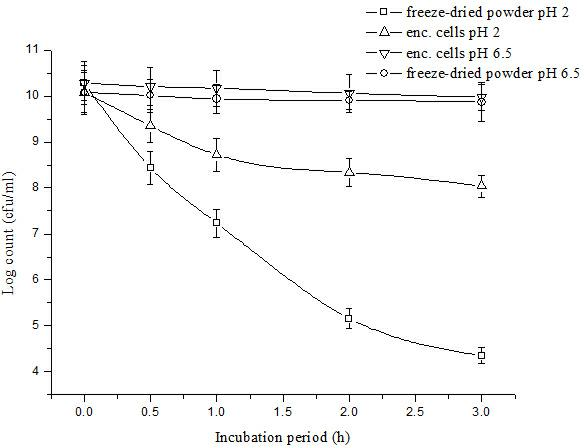
The invention relates to a lactobacillus microcapsule, a method for preparing the same, and the use of the same. The lactobacillus microcapsule consists of an outer layer of wall material, a freeze dried protective agent and lactobacilli. The invention also provides the method for preparing the lactobacillus microcapsule and the use of the lactobacillus microcapsule as a feed additive. The lactobacillus microcapsule can effectively protect the lactobacilli in a core material, prolong the survival time of the lactobacilli at room temperature and improve the tolerance of the lactobacilli to the metal ions in the feed; in addition, the lactobacillus microcapsule has good gastric acid resistance and can disintegrate in the intestinal canal quickly to release the lactobacilli, thereby improving the utilization rate of the lactobacilli, balancing the micro-ecological environment in the intestinal canal, suppressing the growth of pathogenic bacteria, protecting the health of the intestinal canals of animals, reducing the incidence rate of the intestinal canals of the animals and the like.
Owner:BEIJING DABEINONG TECH GRP CO LTD +1
Method for controlling flow of intestinal contents in a patient's intestines
ActiveUS20090240100A1Eliminate the problemAnti-incontinence devicesSurgical needlesSurgeryBlood circulating
There is provided a method for controlling a flow of intestinal contents in the intestinal passageway of a patient's intestines. The method comprises gently constricting (i.e., without substantially hampering the blood circulation in the intestinal tissue wall) at least one portion of the intestinal tissue wall to influence the flow in the intestinal passageway, and stimulating the constricted wall portion to cause contraction of the wall portion to further influence the flow in the intestinal passageway. The method can be used for restricting or stopping the flow in the intestinal passageway, or for actively moving the fluid in the intestinal passageway, with a low risk of injuring the intestines.
Owner:FORSELL PETER
Suckling piglet feed with low acid-binding capacity and preparation method thereof
InactiveCN101999560AReduce acidityGuaranteed healthy growthFood processingAnimal feeding stuffBiotechnologyAnimal science
The invention discloses preparation of a suckling piglet feed with low acid-binding capacity and a production method thereof. The feed is prepared from corn, bean pulp, fermented bean pulp, whey powder, vitamin, microelements and the like which serve as raw materials by special processing modes such as puffing, crushing, low-temperature granulation and the like. Through the feed and the preparation method, the defects of the conventional suckling piglet feed with high acid-binding capacity are overcome, the condition of low digestibility of the suckling piglet is reduced, the palatability of the feed is improved, the health of the intestinal canal of the piglet is improved and the productivity of the suckling piglets is improved.
Owner:TONGWEI
Enterococcus faecium ANSE228 and application thereof
ActiveCN102031235AIncrease production capacityReduce the death rateAntibacterial agentsBacteriaEscherichia coliStaphylococcus cohnii
The invention provides an Enterococcus faecium ANSE228 of which the collection number is CGMCC No.4082. The invention also provides application of the Enterococcus faecium ANSE228 to inhibition of salmonella pullorum and / or Escherichia coli and / or Staphylococcus aureus. The Enterococcus faecium ANSE228 is obtained by processes of repeated separation, purification, rejuvenation and the like, and has high biological activity, obvious probiotic property, high adversity resistance and the like. The invention also provides a microecological agent which contains the Enterococcus faecium ANSE228. When the microecological agent is added into drinking water and / or feeds for breeding animals, the Enterococcus faecium ANSE228 can be quickly activated and reproduced and a dominant beneficial flora can be formed after the Enterococcus faecium ANSE228 is fed into intestinal canals of the animals, and the Enterococcus faecium ANSE228 has the effects of reducing a harmful flora in the intestinal canals, adjusting microecological balance of the intestinal canals, substituting for medicaments such as antibiotic and the like, and improving weight increment of the animals and the utilization rate of the feeds.
Owner:科润生科技发展有限公司
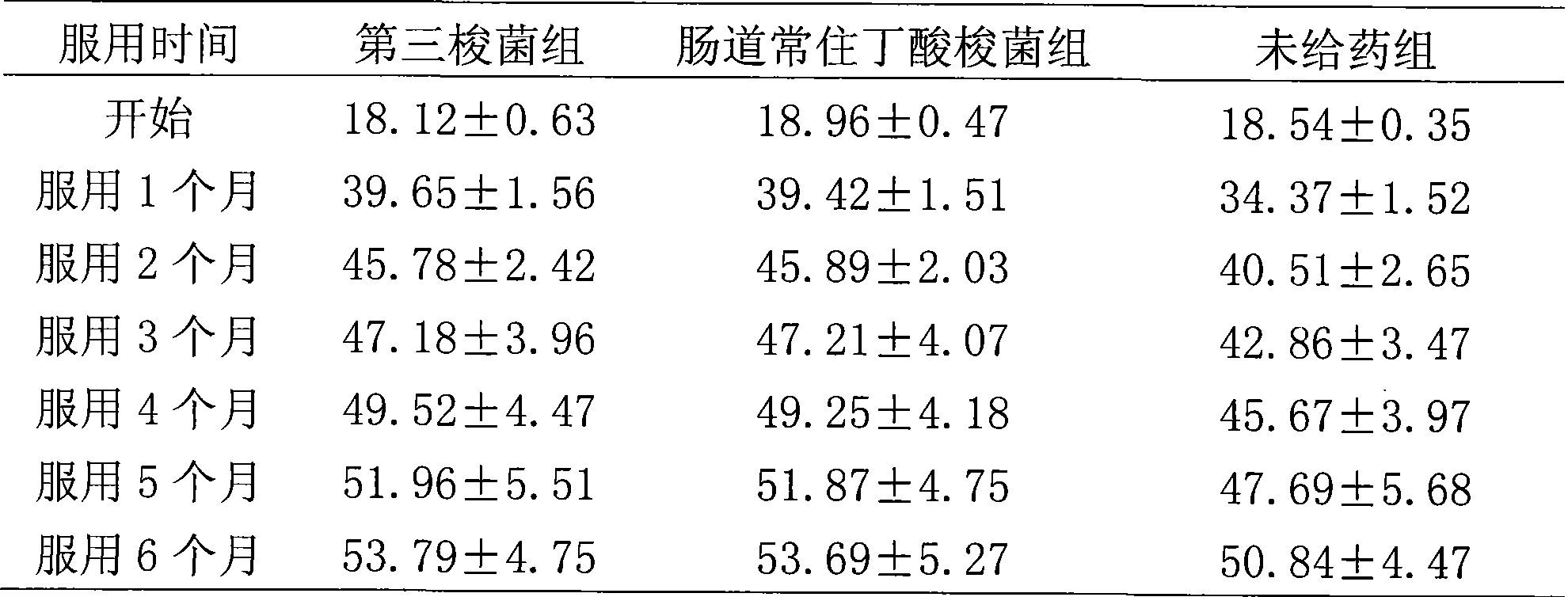
Eubacterium, Clostridium preparation and use thereof
The invention relates to a eubacterium and clostridium preparation and application of the same, in particular to a microecological preparation which is prepared for replenishing butyric acid bacteria and butyric acid produced by intestinal tract by taking single eubacterium, single clostridium or a eubacterium and clostridium composition as a main active composition, and application of the same in treating related diseases through butyric acid production, and belongs to the field of biological medicine.
Owner:QINGDAO EASTSEA PHARMA +1
Swallowable drug delivery device and methods of drug delivery
ActiveCN102905753AImprove pharmacokineticsGood curative effectMetabolism disorderSurgeryIntestinal structureIntestinal walls
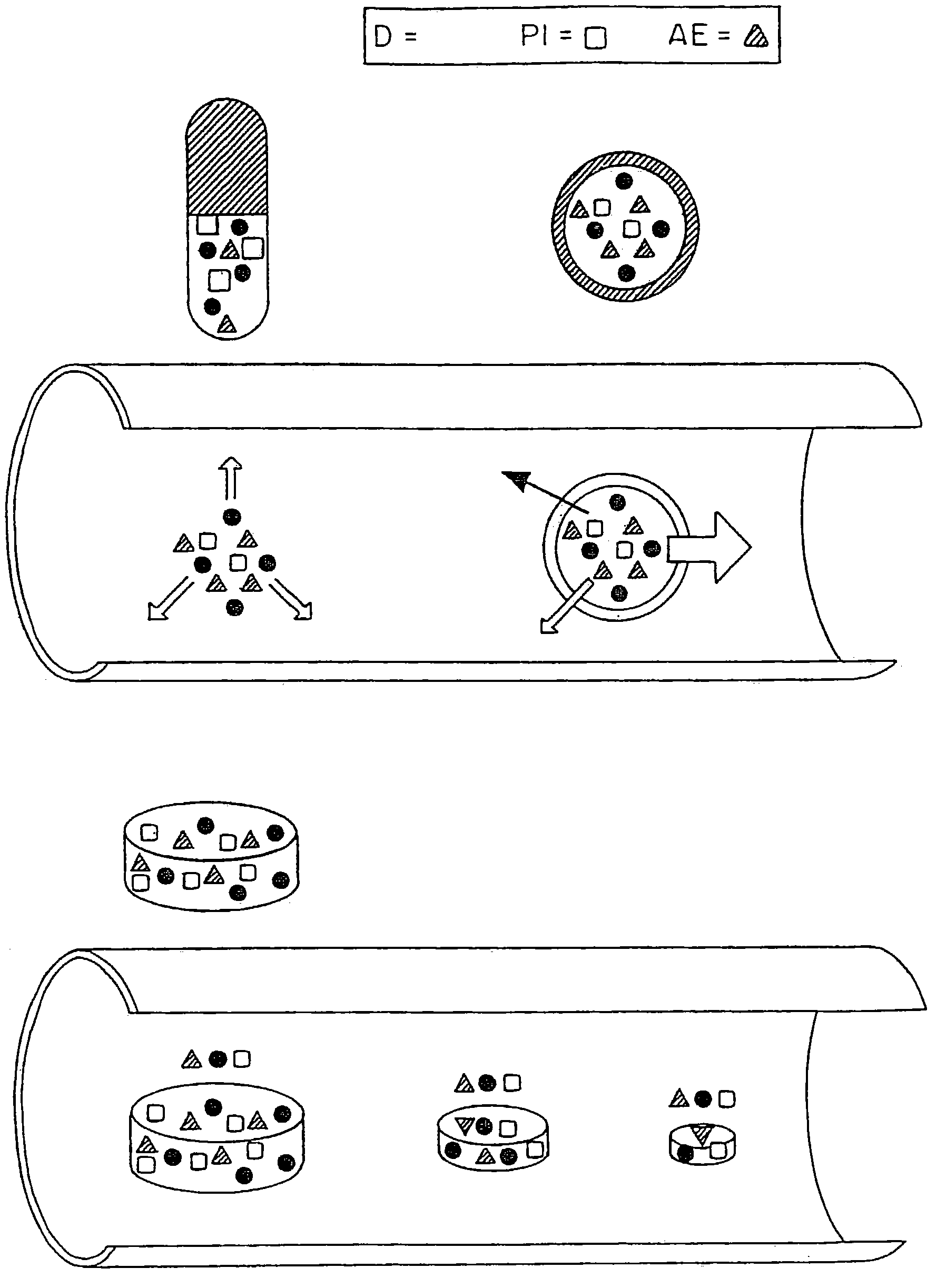
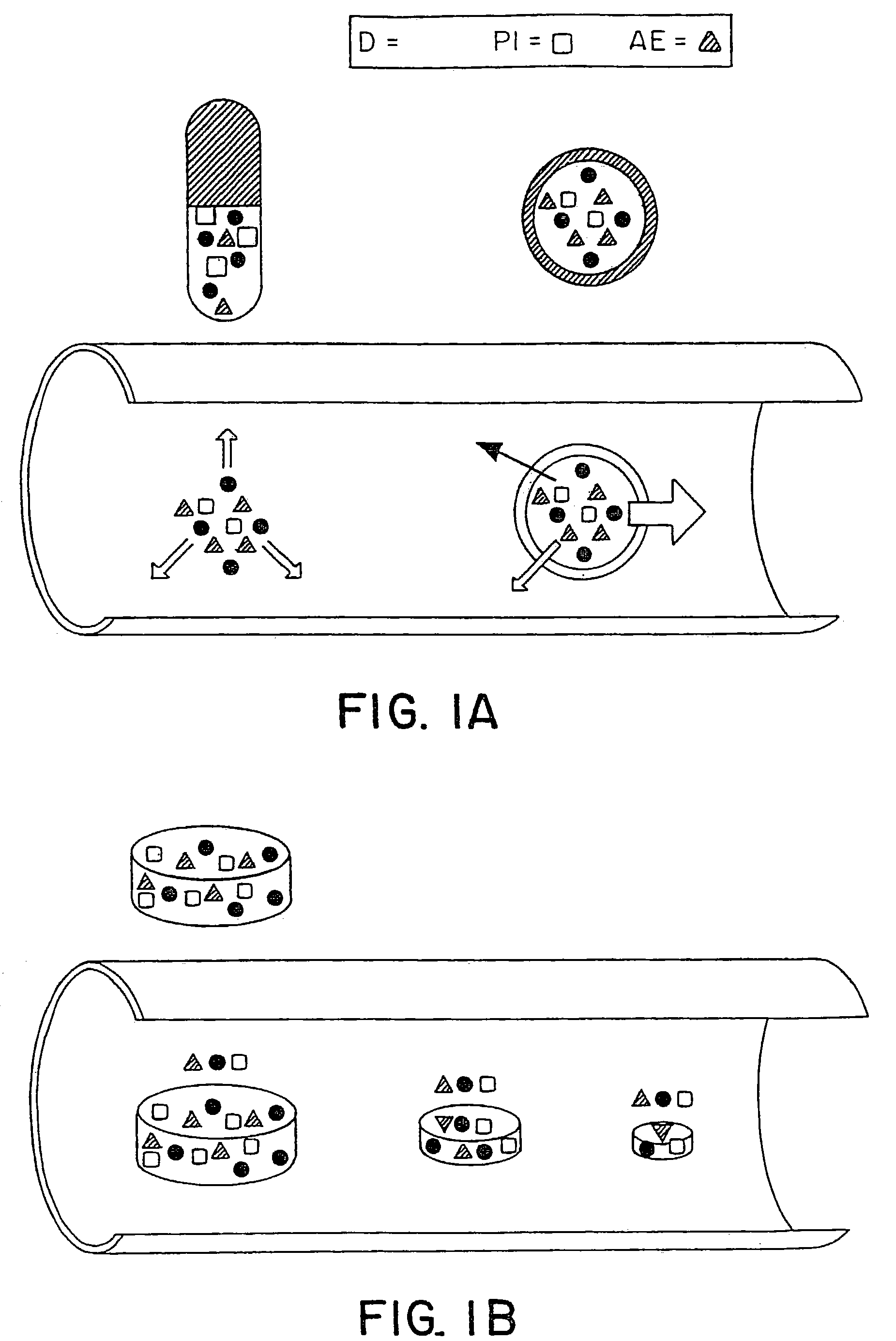
Embodiments of the invention provide swallowable devices, preparations and methods for delivering drugs and other therapeutic agents within the GI tract. Some embodiments provide a swallowable device such as a capsule for delivering drugs into the intestinal wall or other GI lumen. The device comprises a capsule sized to be swallowed and pass through the intestinal tract. The capsule can include at least one guide tube, one or more tissue penetrating members positioned in the guide tube, a delivery member, an actuating mechanism and a release element. The release element degrades upon exposure to various conditions in the intestine so as to release and actuate the actuating mechanism. Embodiments of the invention are particularly useful for the delivery of drugs which are poorly absorbed, tolerated and / or degraded within the GI tract.
Owner:RANI THERAPEUTICS
Fruit and vegetable probiotic tablet and preparation method thereof
ActiveCN104489646AHigh survival rate of live bacteriaImprove stabilityNatural extract food ingredientsFood ingredient functionsCelluloseDrug biological activity
The present invention discloses a fruit and vegetable probiotic tablet and a preparation method thereof. The fruit and vegetable probiotic tablet uses probiotic powders such as lactobacillus plantarum powder as the main raw material, and the preparation method scientifically mixes modified dietary fibers, fruit and vegetable powder, oligosaccharides, plant extracts, protein powder, tea leaf extracts and traditional Chinese medicine extracts and etc., thus improves the content of soluble celluloses which are of real significance for probiotic flora, enhances the physiological activity of celluloses, thereby increases the species of intestinal probiotic flora as well as significantly enhances the colonization ability and time of endogenous and exogenous probiotics in the human intestinal tracts, effectively inhibits the growth and reproduction of harmful intestinal bacteria, especially gram-negative bacteria, and fully regulates the composition of the intestinal probiotic flora. The prepared fruit and vegetable probiotic tablet has a high biological activity, a long human intestinal colonization time, and a significant weight loss effect, and is suitable for a wide range of people.
Owner:南京旭优食品技术有限公司
Targeted gastrointestinal tract delivery of probiotic organisms and/or therapeutic agents
ActiveUS20160022592A1Improve imbalanceAntibacterial agentsBiocideAntibiotic-associated diarrhoeaClostridium difficile infections
The present invention relates to the development of a targeted delivery system for the oral delivery of probiotics or therapeutic agent for various indications, including and not limited to active and prophylaxis treatment of Clostridium difficile infection, antibiotic associated diarrhea, irritable bowel syndrome, Crohn's disease, intestinal flora replacement, supplemental flora treatments for patients taking antibiotics, and for restoration of balance and signaling between the intestinal microbiome and the intestinal cells in patients under treatment of metabolic syndrome manifestations, specifically diabetes, insulin resistance, obesity, hyperlipidemia and hypertension.
Owner:THERABIOME
Therapeutic agent preparations for delivery into a lumen of the intestinal tract using a swallowable drug delivery device
ActiveCN103025319AImprove pharmacokineticsGood curative effectAntibacterial agentsPeptide/protein ingredientsTolerabilityIntestinal walls
Embodiments of the invention provide swallowable devices, preparations and methods for delivering drugs and other therapeutic agents within the GI tract. Many embodiments provide a swallowable device for delivering the agents. Particular embodiments provide a swallowable device such as a capsule for delivering drugs into the intestinal wall or other GI lumen. Embodiments also provide various drug preparations that are configured to be contained within the capsule, advanced from the capsule into the intestinal wall and degrade within the wall to release the drug to produce a therapeutic effect. The preparation can be coupled to an actuator having a first configuration where the preparation is contained in the capsule and a second configuration where the preparation is advanced out of the capsule into the intestinal wall. Embodiments of the invention are particularly useful for the delivery of drugs which are poorly absorbed, tolerated and / or degraded within the GI tract.
Owner:RANI THERAPEUTICS
Controlled release oral drug delivery system
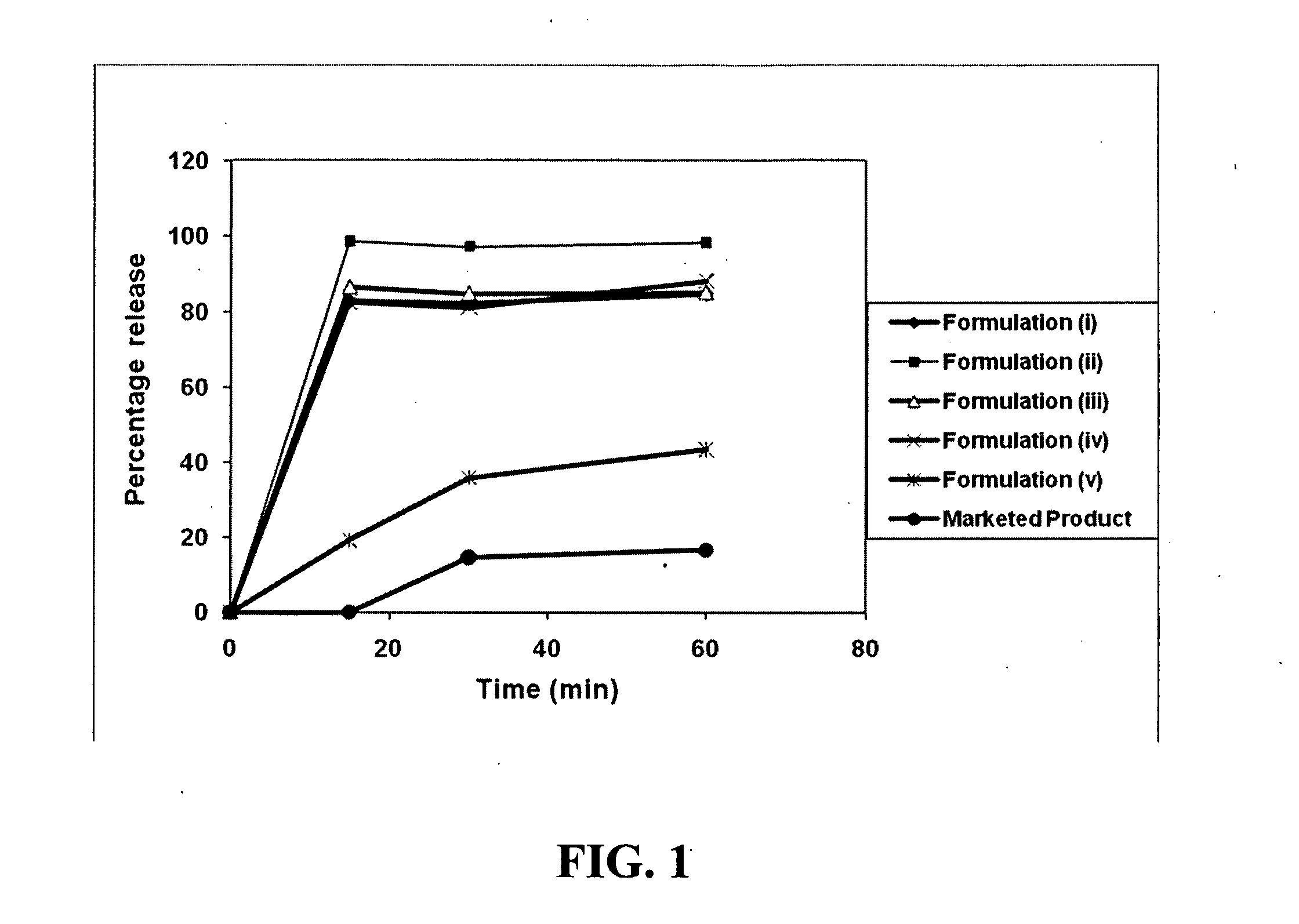
The invention relates to synchronous drug delivery composition comprising a polymeric matrix which comprises hydrogel blended with a hydrophobic polymer, so as to form an erodible matrix, a drug, and, optionally, an agent which enhances intestinal drug absorption and / or an agent which inhibits intestinal drug degradation,wherein erosion of the erodible matrix, permits synchronous release of the drug, the hydrogel and the intestinal drug absorption agent and / or the agent which inhibits intestinal drug degradation.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD
Oral Dosage Form Of Tetrahydrocannabinol And A Method Of Avoiding And/Or Suppressing Hepatic First Pass Metabolism Via Targeted Chylomicron/Lipoprotein Delivery
ActiveUS20110092583A1Easy to transportPromote lymphatic transportBiocideSenses disorderChylomicronCytochrome P450
Self-emulsifying drug delivery systems are provided to improve dissolution, stability, and bioavailability of drug compounds of dronabinol or other cannabinoids. The drug compound(s) are dissolved in an oily medium (e.g. triglycerides and / or mixed glycerides and / or free fatty acids containing medium and / or long chain saturated, mono-unsaturated, and / or poly-unsaturated free fatty acids) together with at least one surfactant. The surfactant promotes self-emulsification, thereby promoting targeted chylomicron / lipoprotein delivery and optimal bioavailability through the mammalian intestinal tract. A dosage form can optionally include co-solvents, anti-oxidants, viscosity modifying agents, cytochrome P450 metabolic inhibitors, P-GP efflux inhibitors, and amphiphilic / non-amphiphilic solutes to induce semi-solid formation for targeted release rates.
Owner:MURTY RAM B +1
In vivo use of water absorbent polymers
InactiveUS20050036983A1Improving fluid absorbing performanceLess interferenceMetabolism disorderDigestive systemMedicineRemove blood
The subject invention is a method and material for removing fluid from the intestinal tract of a host and may be useful in treating animals or human patients suffering from fluid overload states. In one embodiment, the subject method involves ingesting an enterically coated non-systemic, non-toxic, non-digestible, water absorbing polymer which absorbs fluid while passing through the intestinal tract. The polymer is excreted in the feces wherein the polymer and absorbed fluid is removed from the body. Preferred polymers include super absorbent acrylic acid polymers, preferably provided in bead form. The polymers may include functional groups for selectively removing blood borne waste products, e.g. urea, from the G.I. tract.
Owner:SORBENT THERAPEUTICS
Derivatives of 4- or 5-aminosalicylic acid
InactiveUS7910568B2Easily reach colonPromote absorptionBiocideAntipyreticSalicylic acidBULK ACTIVE INGREDIENT
Owner:ANTIBE THERAPEUTICS INC
Derivaitves of 4-Or 5-Aminosalicylic Acid
InactiveUS20080207564A1Less readily absorbedEasily reach colonBiocideAntipyreticThioester synthesisSalicylic acid
The present invention provides new derivatives of 4- or 5-aminosalicylic acid, and a pharmaceutical composition containing these derivatives of 4- or 5-aminosalicylic acid as active ingredients, useful for the treatment of intestinal diseases such as inflammatory bowel disease (IBD) and irritable bowel syndrome (IBS) and for the prevention / treatment of colon cancer. More particularly, these derivatives comprise a hydrogen sulfide releasing moiety linked via an azo, an ester, an anhydride, a thioester or an amide linkage to a molecule of 4- or 5-aminosalicylic acid. Furthermore, the present invention provides a process for preparing these compounds and their use for treating IBD and IBS and the prevention / treatment of colon cancer.
Owner:ANTIBE THERAPEUTICS INC
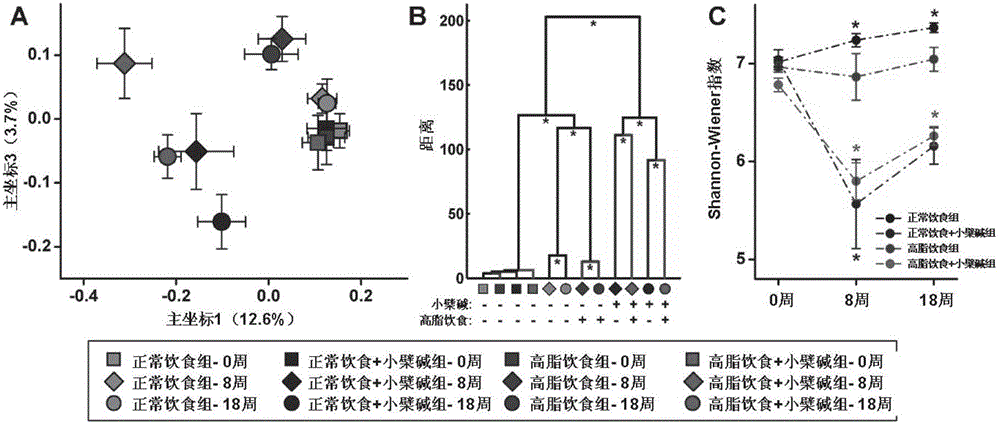
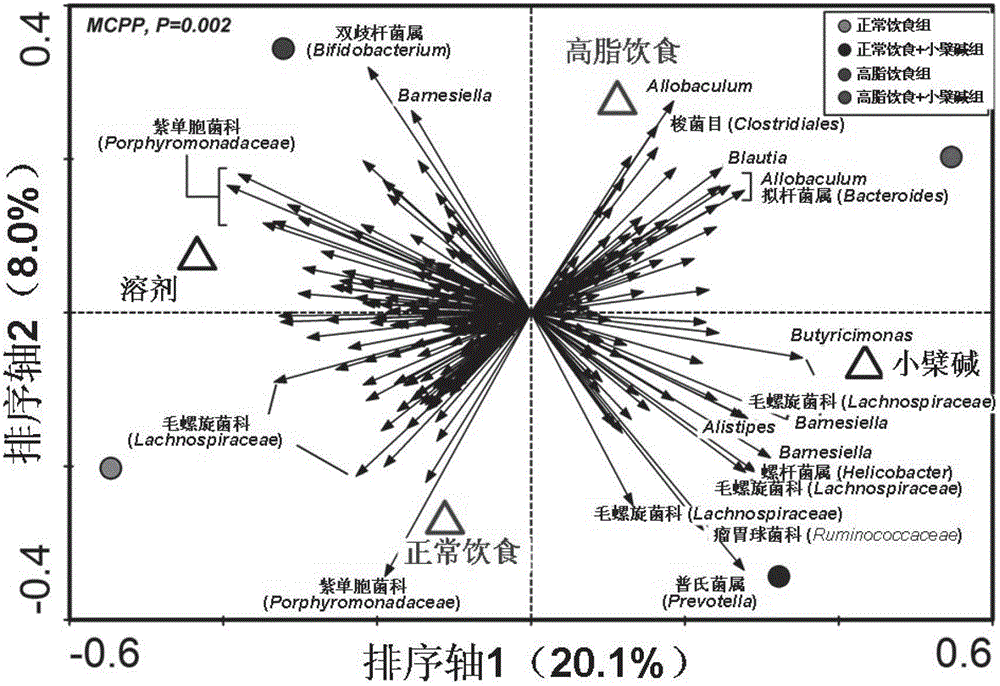
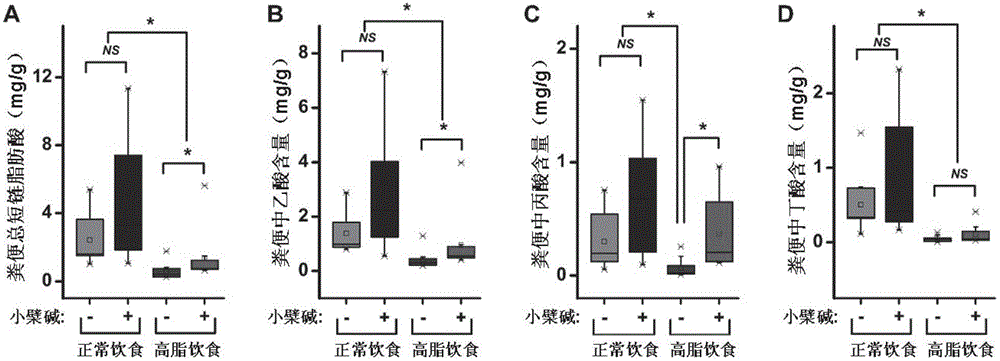
Method for improving intestinal colony structure and application
The invention provides a method for improving an intestinal colony structure and an application in the preparation of medicines, nourishment, health products, foods and beverages used for adjusting the intestinal colony. A high-flux sequence test technology and a multivariate statistical method are employed to find the intestinal colony groups which are closely related with host metabolism. According to the invention, berberine is taken as a sample to establish a novel method for improving the intestinal colony structure, the method comprises the steps of selectively increasing a part of bacteria, such as short chain aliphatic acid producing bacteria and the like, and simultaneously inhibiting a part of strain, such as bacteria capable of producing endotoxin and the like, thereby the disorders of metabolism or an immunization system due to enhancement of system inflammatory diseases induced by improper diet, obesity, insulin resistance and the like can be prevented or treated. The invention also provides a method for screening medicines, compounds and foods having the analogous effects, and a composition for improving the intestinal colony structure. The application of the method can be used for exploiting the medicines, nourishment, health products, foods, beverages and the like which take the intestinal colony as a target.
Owner:SHANGHAI JIAO TONG UNIV +1
Lactobacillus reuteri and its application
ActiveCN107523526AImprove the level ofImprove triglyceridesMilk preparationNervous disorderDiseaseGut flora
The invention relates to the technical field of microorganism, and discloses a Lactobacillus reuteri and its application. The preservation number of the Lactobacillus reuteri CCFM8631 is CGMCC No.14394; the level of mice peripheral neurotransmitter 5-hydroxytryptamine can be significantlyimproved; the rise of the mice peripheral blood testonsterone level and the abundant abnormity of Blautia, Turicibacter, Oscillospira and Bifidobacterium in the intestinal flora by high glucose and high fatty diets are recovered; the tolerated simulative gastrointestinal fluid is rapidly planted in an intestinal tract, so as to significantly improve the pathological injury of metabolic syndrome mice liver and duodenum and rise of triglyceride and total cholesterol content in the serum by high glucose and high fatty diets are significantly improved; the Lactobacillus reuteri can be used for preventing, delaying or treating metabolic disorder such as metabolic syndrome, irritable bowel syndrome, and anxiety, depression and other metal diseases related to irritable bowel syndrome.
Owner:INFINITUS (CHINA) CO LTD
Enterococcus faecium EF08 as well as feed additive and feed containing enterococcus faecium EF08
ActiveCN104195075AReduce excessive consumptionHigh feed conversionBacteriaMicroorganism based processesBiotechnologyAnimal product
The invention discloses an enterococcus faecium EF08 as well as a feed additive and a feed containing the enterococcus faecium EF08. The enterococcus faecium EF08 is collected with the number of CGMCC (China General Microbiological Culture Collection Center) No.5549 in CGMCC. The feed additive disclosed by the invention can be used for reducing the feed excessively consumed by harmful bacteria in intestines by virtue of an equalizing effect of bacteria for equalizing the intestinal environment, furthermore, the conversion rate of the feed for economic animals is increased, the feed for the economic animals can be effectively converted into a primary animal product with an economic value, and then, the effect of increasing the economic values of the economic animals can be improved.
Owner:SYNBIO TECH
Bifidobacterium longum and application thereof
ActiveCN108220206AGood ability to tolerate simulated gastrointestinal fluidNo side effectsMilk preparationBacteriaHuman bodyBiotechnology
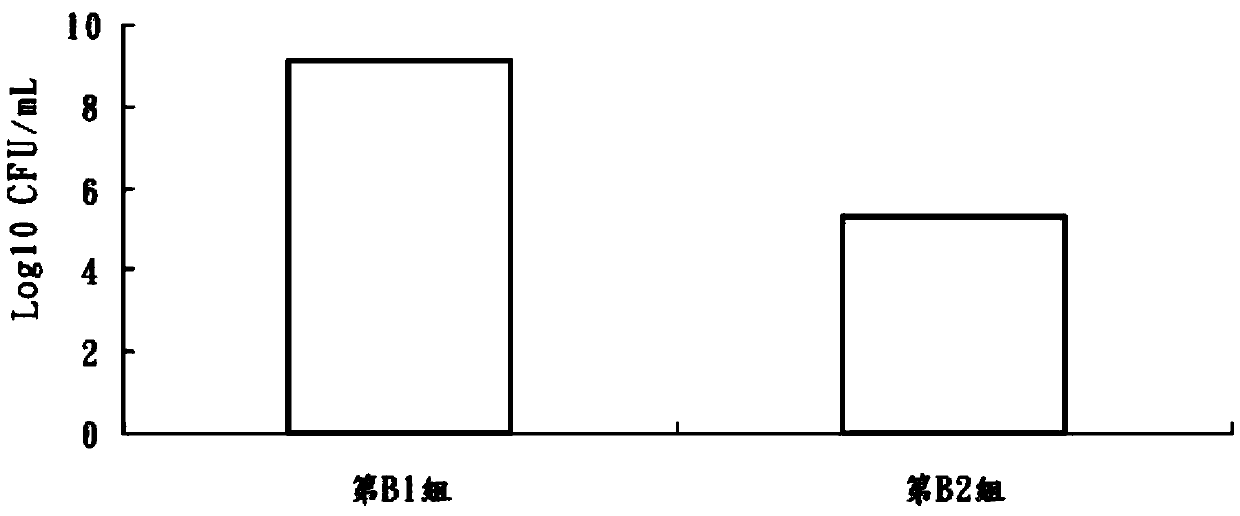
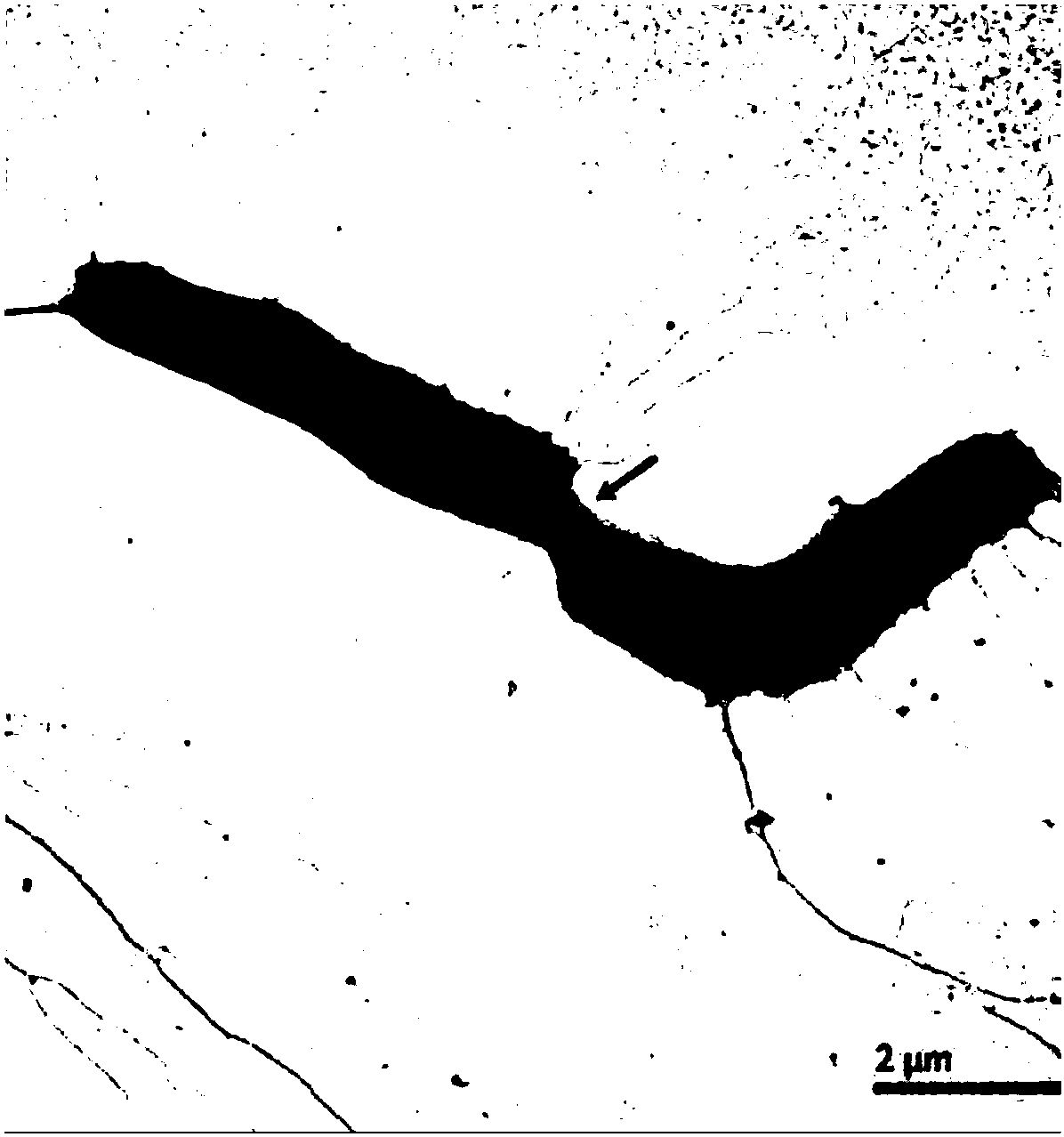
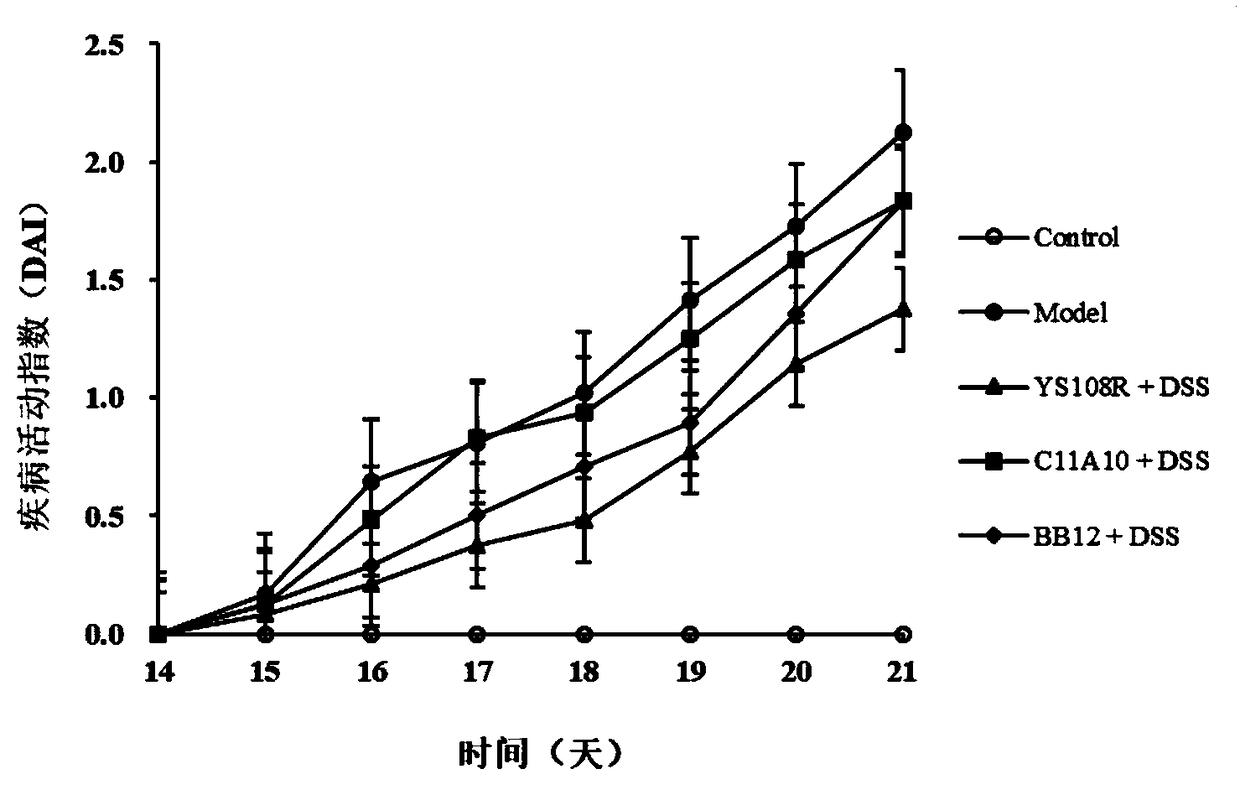
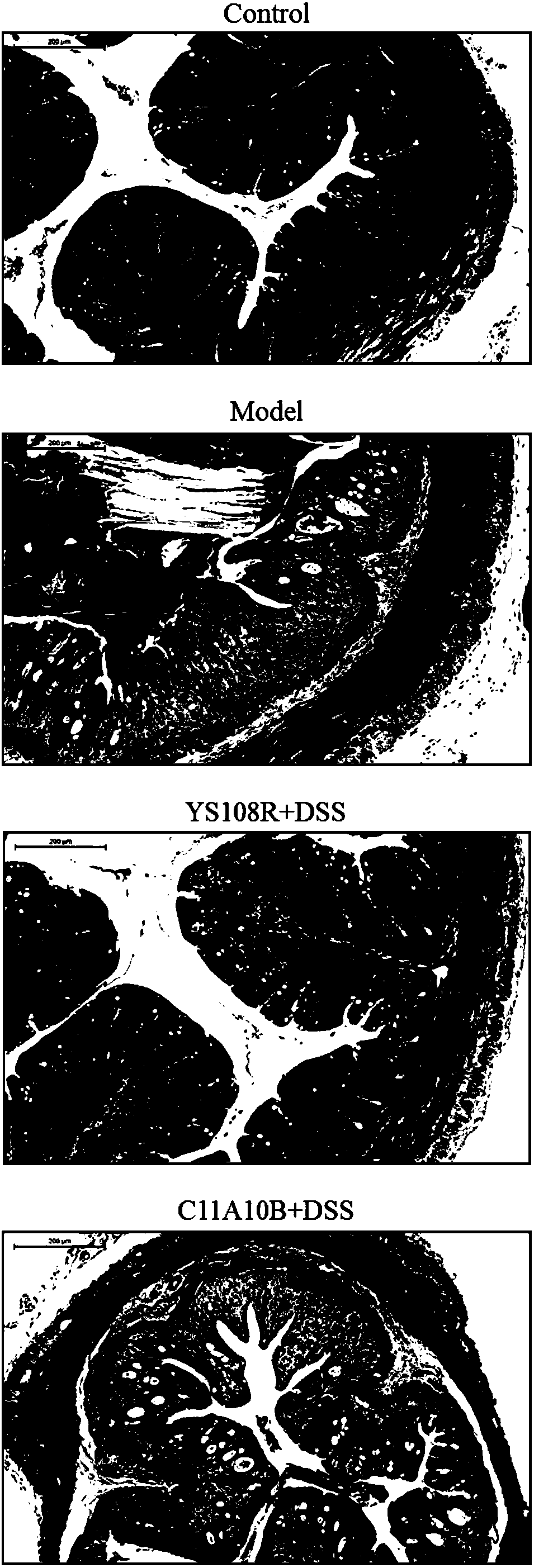
The invention discloses bifidobacterium longum and the application thereof and belongs to the technical field of biologics. The invention provides a bifidobacterium longum YS108R strain which has thecharacteristic of generating viscous exopolysaccharide, and has a remarkable improvement function on DSS (Dextran Sulfate Sodium) induced mouse colitis models. The strain has a relatively good capability of enduring simulated gastrointestinal fluids, is capable of remarkably reducing disease activity indexes of mice in the DSS induction period, and has an effective protection function on colon tissue. In addition, the bifidobacterium longum YS108R disclosed by the invention is separated from intestinal florae of healthy people, is free of toxic and / or side effects on human bodies, and has certain advantages when being compared with conventional medicine treatment. The strain can be used for preparing probiotic powder, fermented milk, and the like, and has wide market prospects.
Owner:无锡特殊食品与营养健康研究院有限公司
Bifidobacterium microcapsule and preparing method thereof
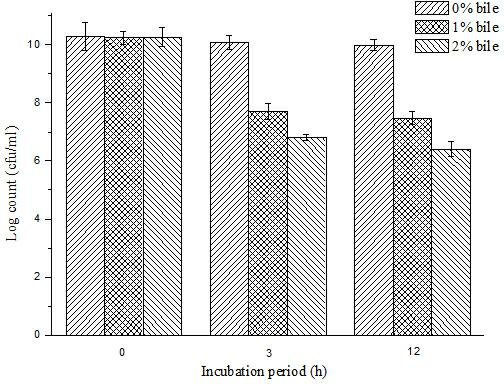
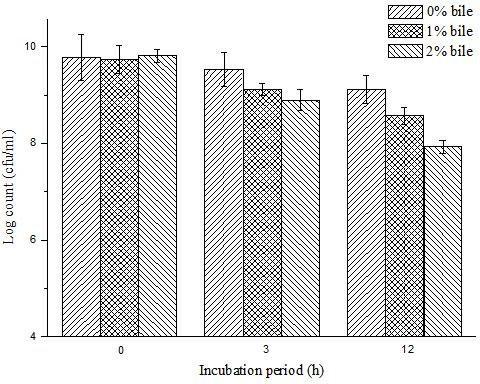
ActiveCN102210659AResistant to gastric acidBile salt resistantMetabolism disorderBacteria material medical ingredientsFreeze-dryingHigh survival rate
The invention specifically relates to a bifidobacterium microcapsule and a preparing method thereof. The bifidobacterium is obligatorily anaerobic and very sensitive to oxygen, PH, temperature, humidity, and other adverse external environment, thereby being very hard to remain activity during production, storage and transport; besides, if taken orally in a form of dry bifidobacterium powder, the bifidobacterium cannot tolerate low-pH value gastric acid, bile salt, and other environments, so a purpose that massive survived bifidobacteria arrive at an intestinal tract and colonise on the intestinal mucosa is hard to be guaranteed. The preparing method disclosed by the invention comprises the following steps of: adding freeze-dried bifidobacterium powder into a sodium alginate solution and then mixing in soybean oil, emulsifying the mixed solution and standing; centrifugally collecting micro-capsules; and drying the micro-capsules by using a vacuum freeze-drying technology. The bifidobacterium microcapsule disclosed by the invention has the advantages of gastric acid resistance, bile salt resistance and entericsolubility, high survival rate of bifidobacterium, simple preparation method, strong practicality, convenience for industrial production, and excellent storage stability and solves the problem of short storage period of probiotics preparations.
Owner:SHAANXI GIANT BIOTECHNOLOGY CO LTD
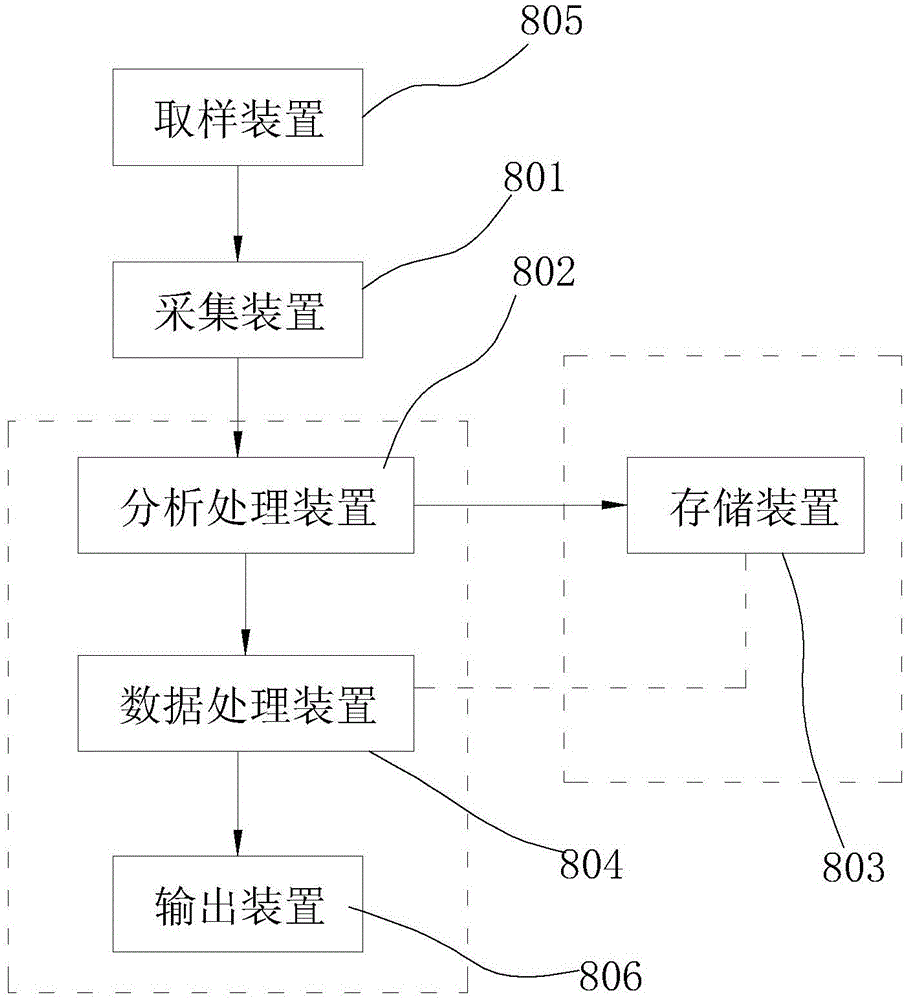
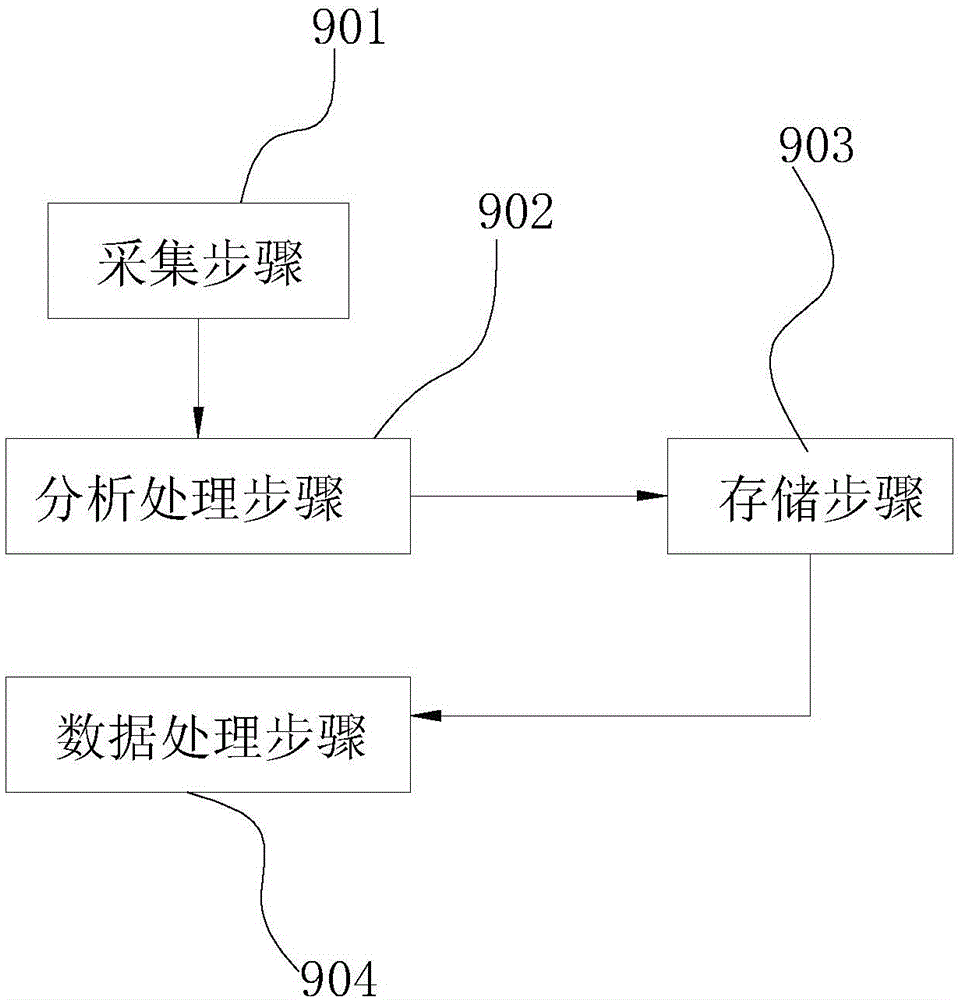
Detection system and method for intestinal flora and dynamic database
ActiveCN105046094AImprove the situationIncrease capacityMicrobiological testing/measurementSpecial data processing applicationsPhysical well beingFlora
The invention discloses a detection system and method for intestinal flora and a dynamic database. The detection system for the intestinal flora comprises: an acquisition device, an analysis and processing device, a storage device and a data processing device, wherein the acquisition device is used for acquiring information of the intestinal flora; the analysis and processing device is used for carrying out amplification processing on the acquired information of intestinal flora to obtain data of intestinal flora at every time of individual detection; the storage device is used for storing the data of intestinal flora; and the data processing device is used for comparing the data of intestinal flora of individuals at each time with the total quantity of the intestinal flora in the storage device. According to the detection system, an examinee can know self intestinal health condition composition through detection of the intestinal flora, and the examinee can know a difference value between oneself and healthy crowd, and then regulates aspects of diet, exercise, living habits and the like, thereby improving the condition of the intestinal flora and the body health condition. According to the dynamic database, the capacity of the database can be expanded in real time to obtain detection parameters of excrement of different types of people at the newest period.
Owner:深圳谱元科技有限公司
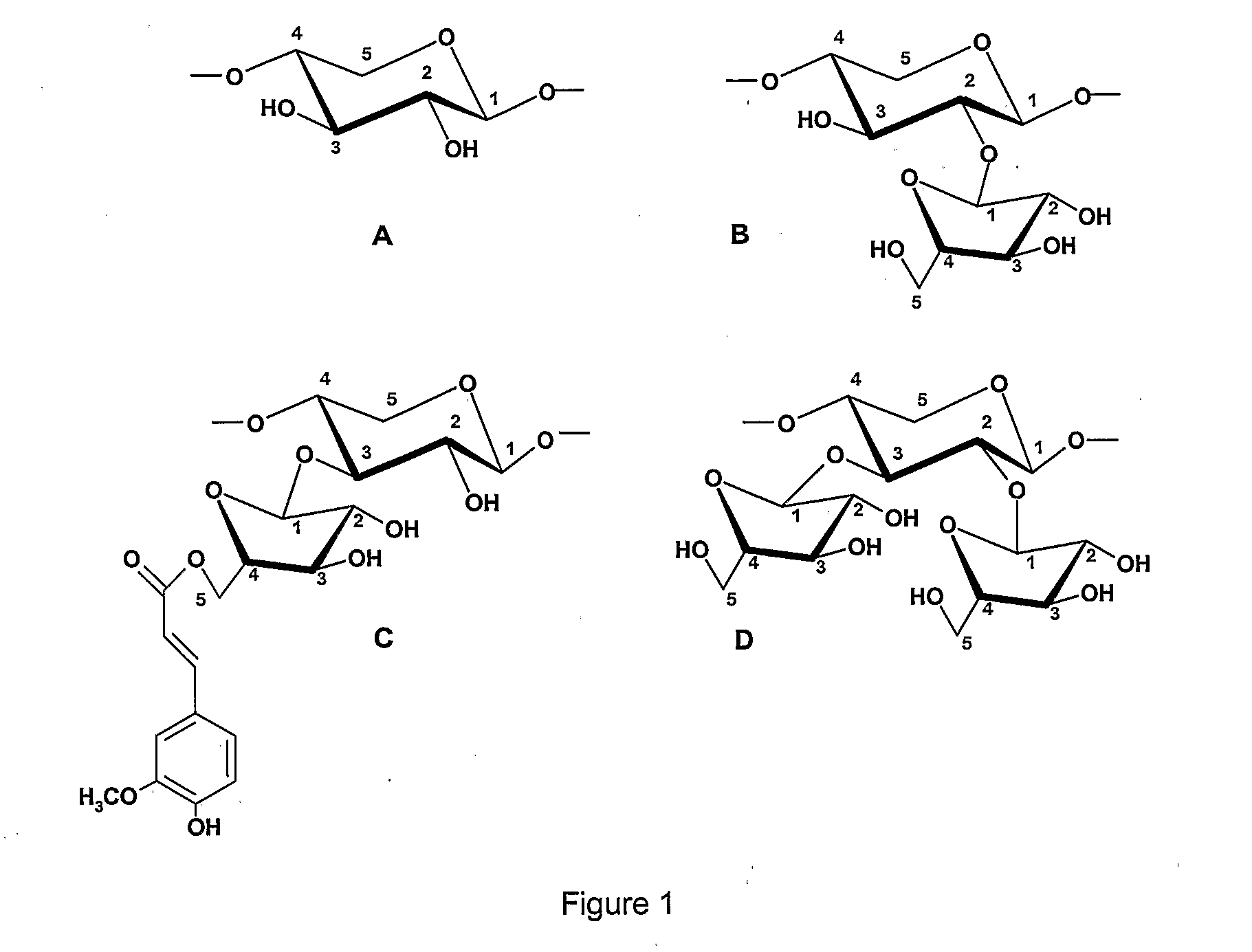
Prebiotic Preparation
InactiveUS20080102162A1Extraordinary viscosity forming potentialSignificant positive effectOrganic active ingredientsDough treatmentArabinoxylanFood science
The present invention relates to a nutritional additive comprising arabinoxylans, which beneficially modulates the human intestinal flora. Furthermore, several food and beverage products comprising the additive are provided as well as methods to prepare the said additive.
Owner:K U LEUVEN RES & DEV
Composition containing aloe, probiotics and prebiotics and application of composition
InactiveCN104740138AIncrease the number ofThe effect of significantly modulating the intestinal flora of animalsOrganic active ingredientsMetabolism disorderBifidobacteriumIntestinal structure
The invention discloses a composition and an application thereof in regulating intestinal flora of animals. The composition contains aloe, probiotics and prebiotics, wherein the mass ratio of the aloe to the probiotics to the prebiotics is (6 to 3 to 1)-(6 to 1 to 3). The inventor discovers that the composition disclosed by the invention can effectively promote the proliferation of useful bacteria such as bifidobacterium and lactobacilli in animal intestines, so as to effectively regulate the intestinal flora of animals; therefore, the composition can be used for preventing or treating diabetes on the basis of the effect of the intestinal flora.
Owner:深圳华大基因农业控股有限公司 +1
Site-specific intestinal delivery of adsorbents, alone or in combination with degrading molecules
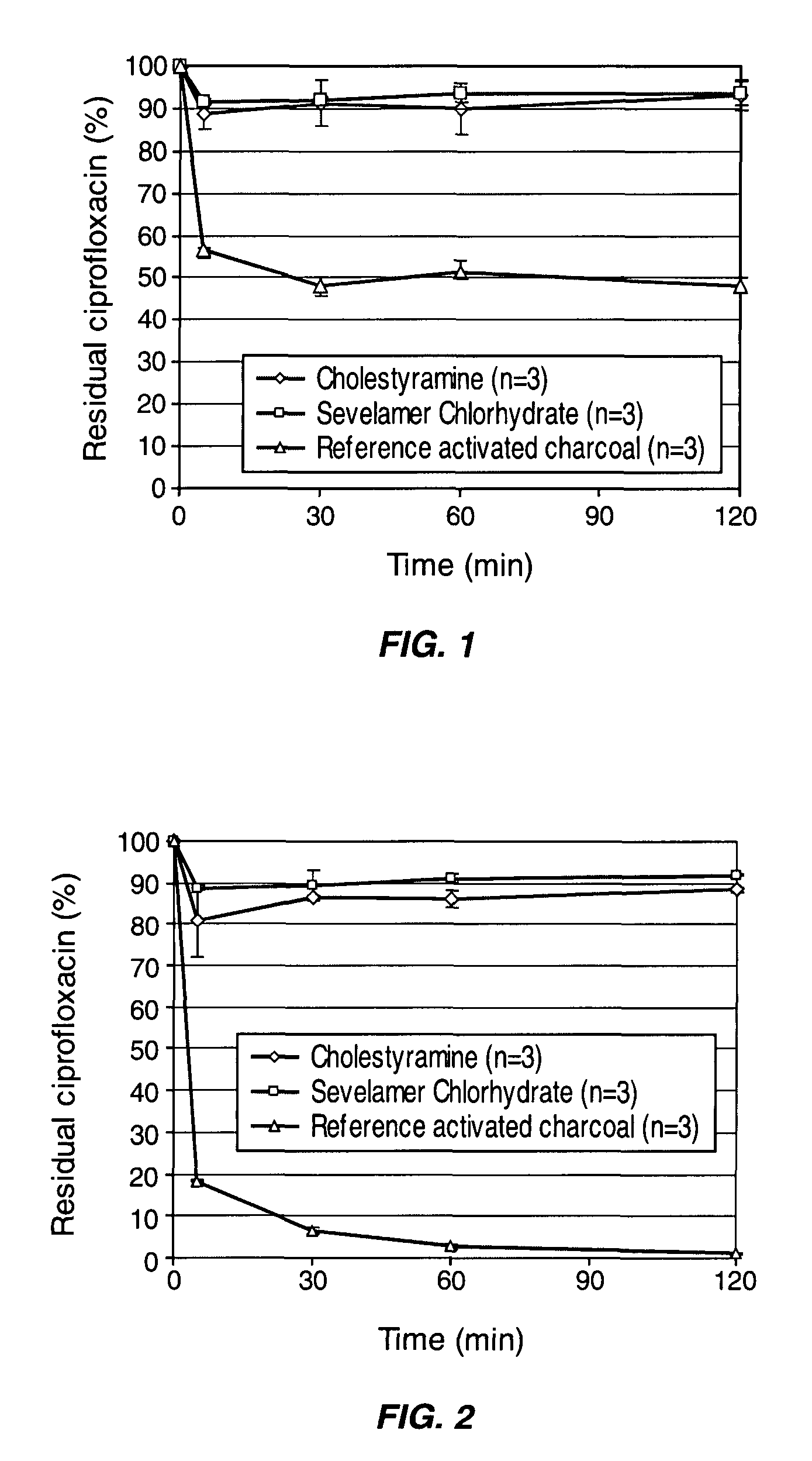
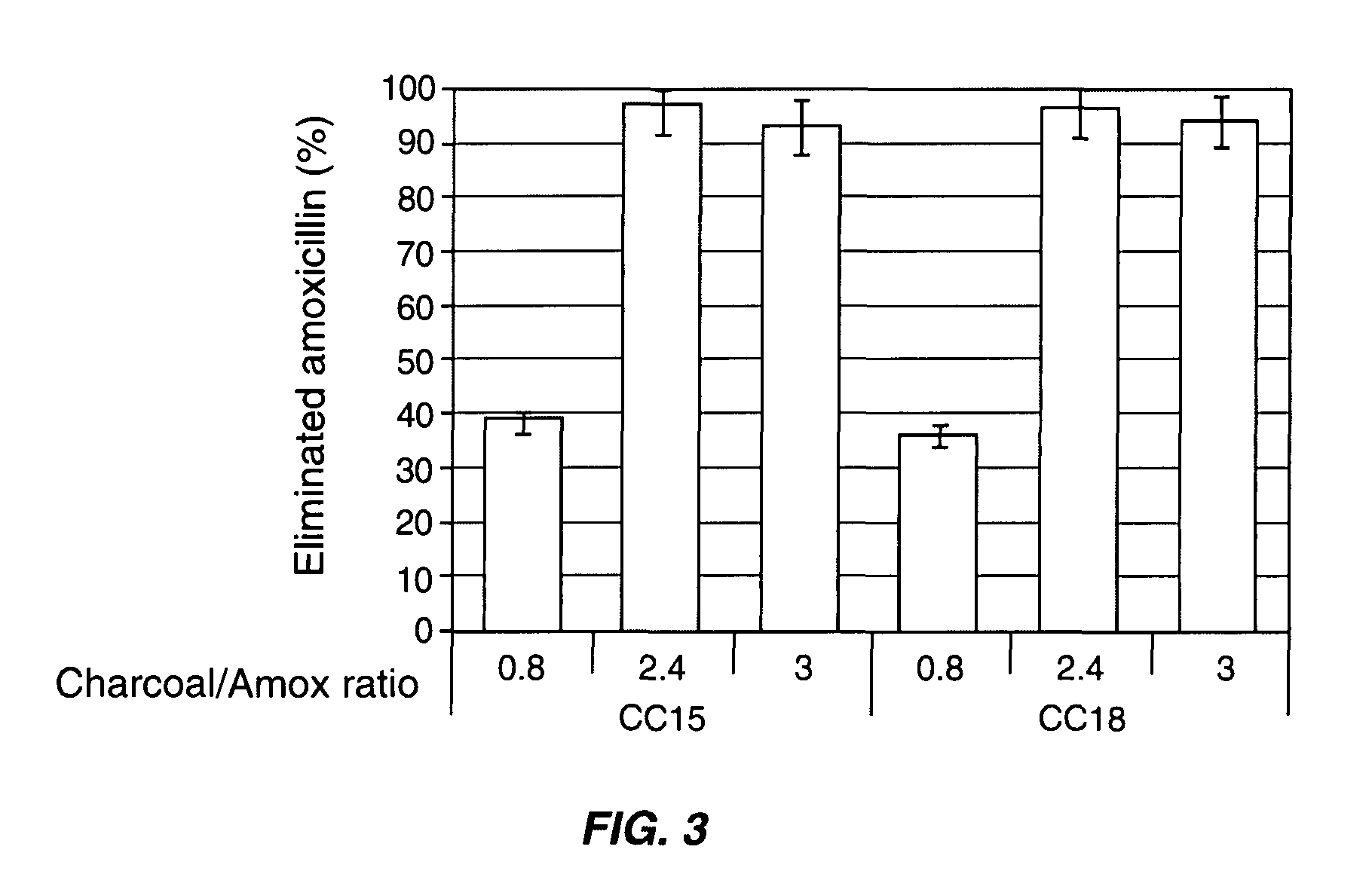
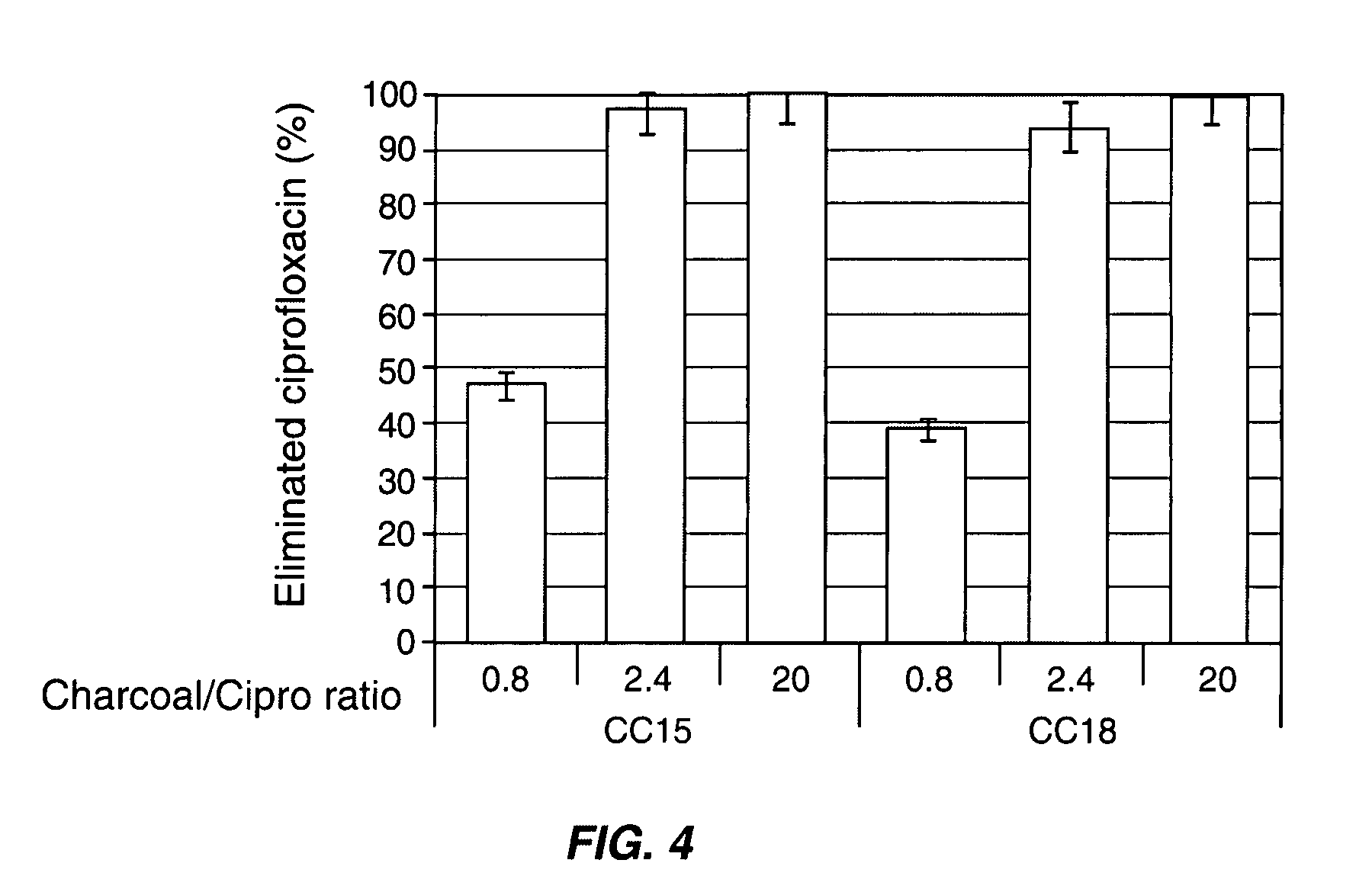
InactiveUS8048413B2Reduce concentrationImprove side effectsAntibacterial agentsBiocideDrug adsorptionMetabolite
Compositions which deliver adsorbents, alone or in combination with active drug “degrading molecules,” in a site-specific manner to the intestine, and which eliminate or at least lower the concentration of residual unwanted material within the intestine, are disclosed. Methods of treatment using the compositions are also disclosed. The material to be eliminated can include residual active antibiotics, metabolites, bacterial or other toxins, and drugs which cause side effects in the gastrointestinal tract. The adsorbents can be formulated in capsules, tablets or any acceptable pharmaceutical composition, and are ideally designed to specifically release the adsorbents in a programmed manner at a specific site of the intestinal tract. The programmed delivery prevents adsorbents from interfering with the normal absorption process of a given molecule after oral absorption, until it reaches the lower part of the small intestine. The compositions can be used to adsorb, and therefore remove, any residual drug, metabolite thereof, or bacterial toxin after oral or parenteral administration which would otherwise cause adverse effects in the lower intestine and / or colon.
Owner:CENT NAT DE LA RECHERCHE SCI +3
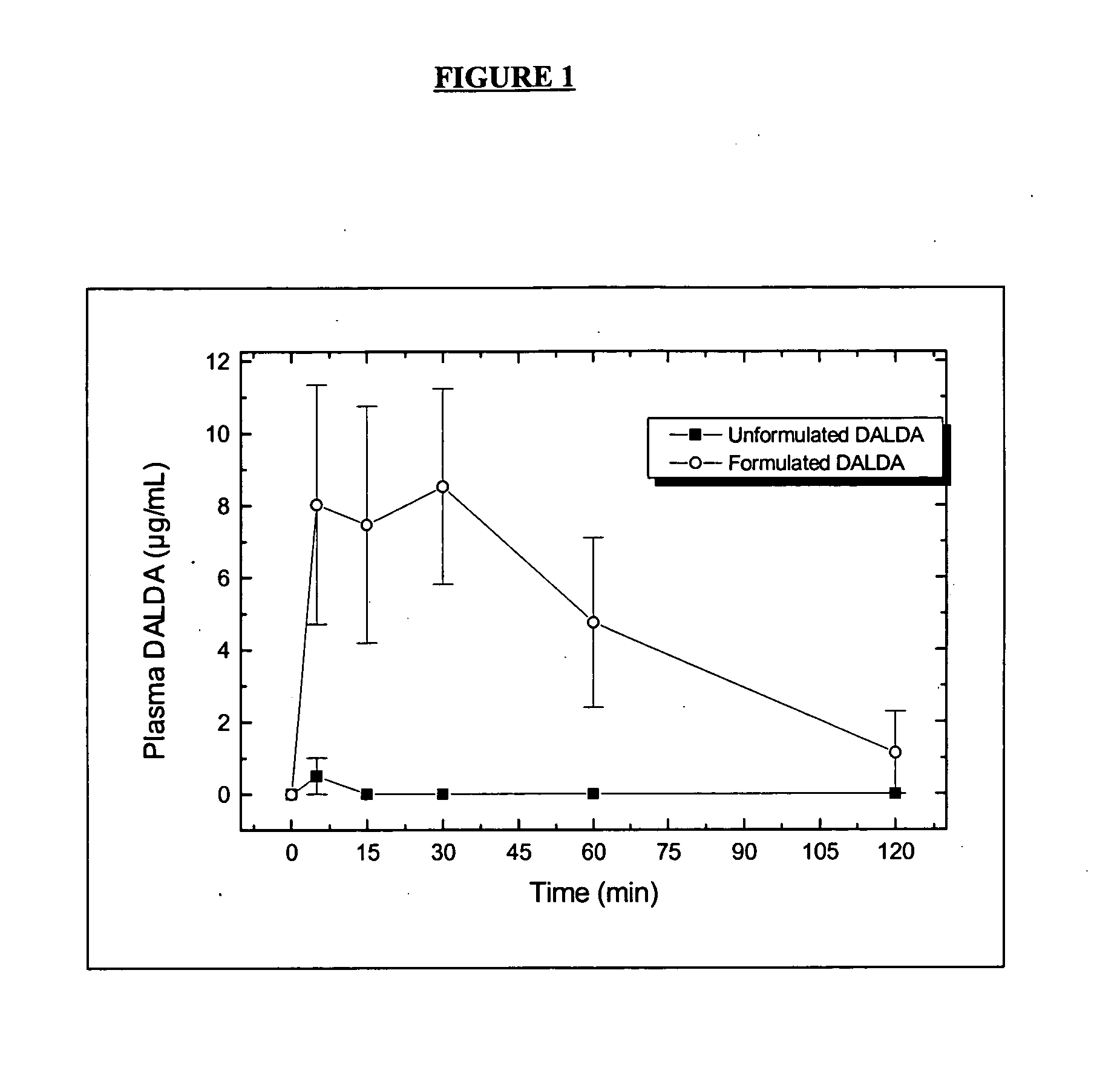
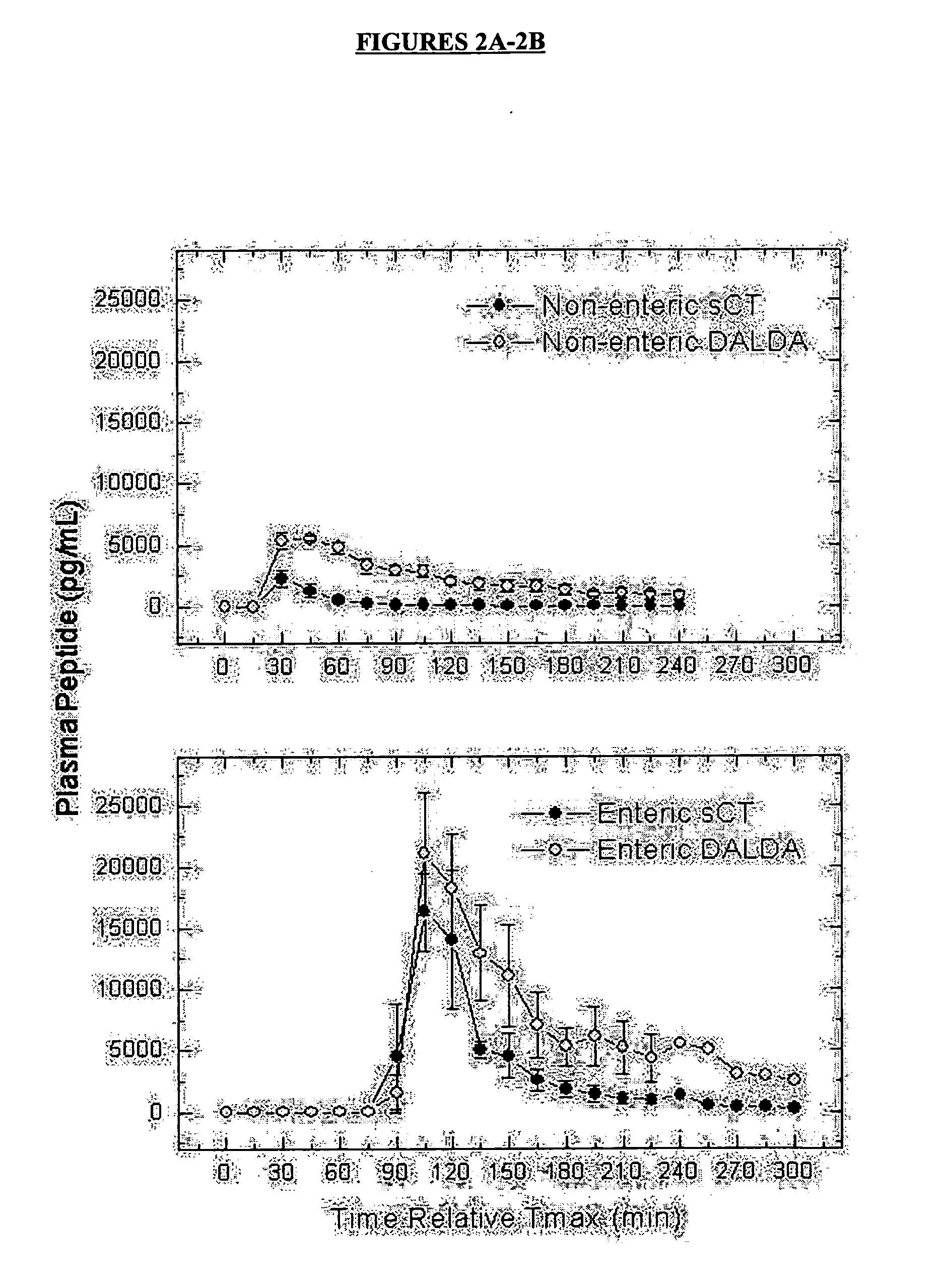
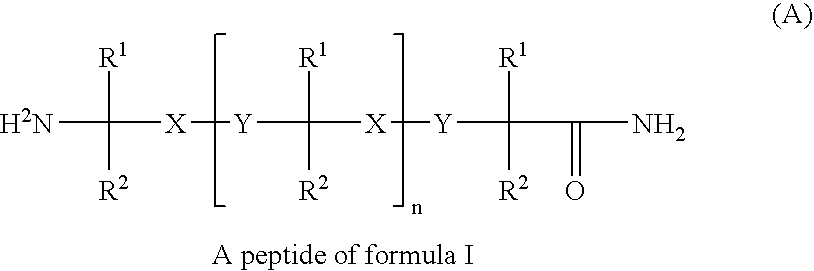
Oral delivery of peptide pharmaceutical compositions
InactiveUS20050282756A1Reliable deliveryNervous disorderDispersion deliveryIntestinal structureOral medication
Bioavailability of peptide active agents to be administered orally is enhanced by a pharmaceutical composition providing targeted release of the peptide to the intestine by combining the composition with an absorption enhancer. Bioavailability is further significantly increased by administering the composition in an acid-resistant protective vehicle which transports components of the invention through the stomach. The composition may optionally further include a sufficient amount of a pH-lowering agent to lower local intestinal pH. All components are released together into the intestine with the peptide.
Owner:UNIGENE LABORATORIES
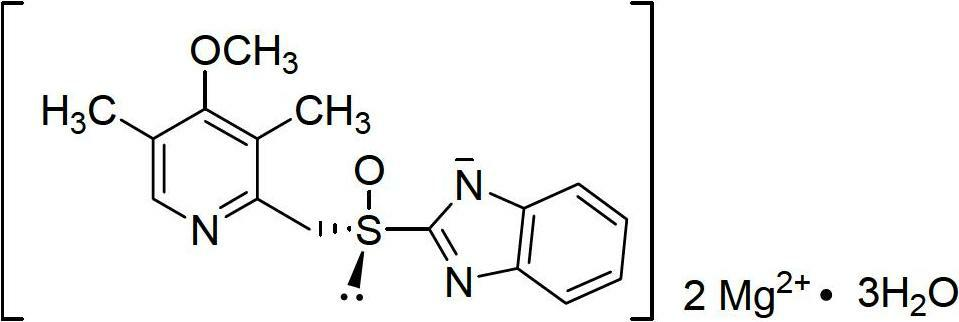
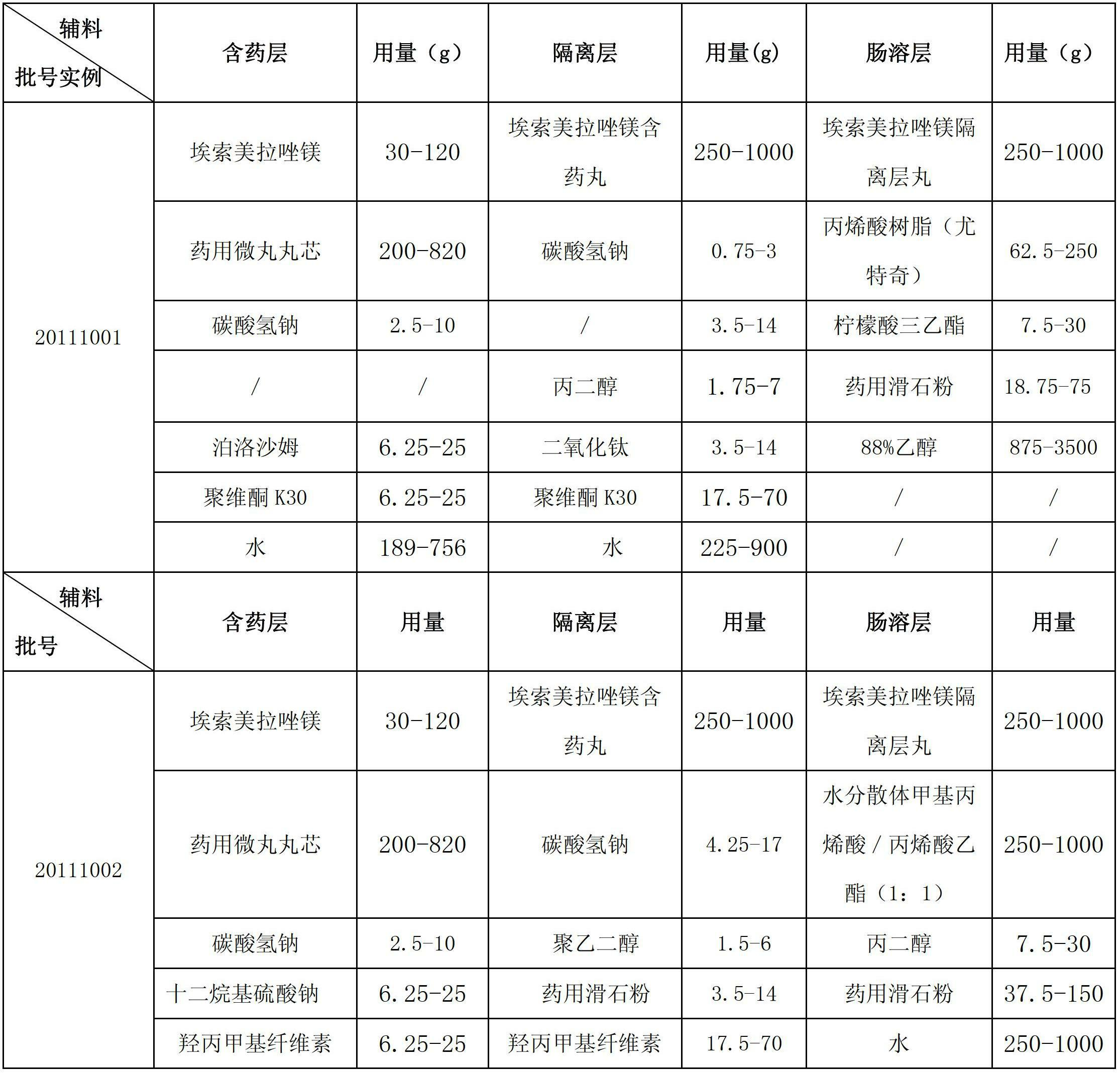
Esomeprazole magnesium enteric-coated pellet and preparation method thereof
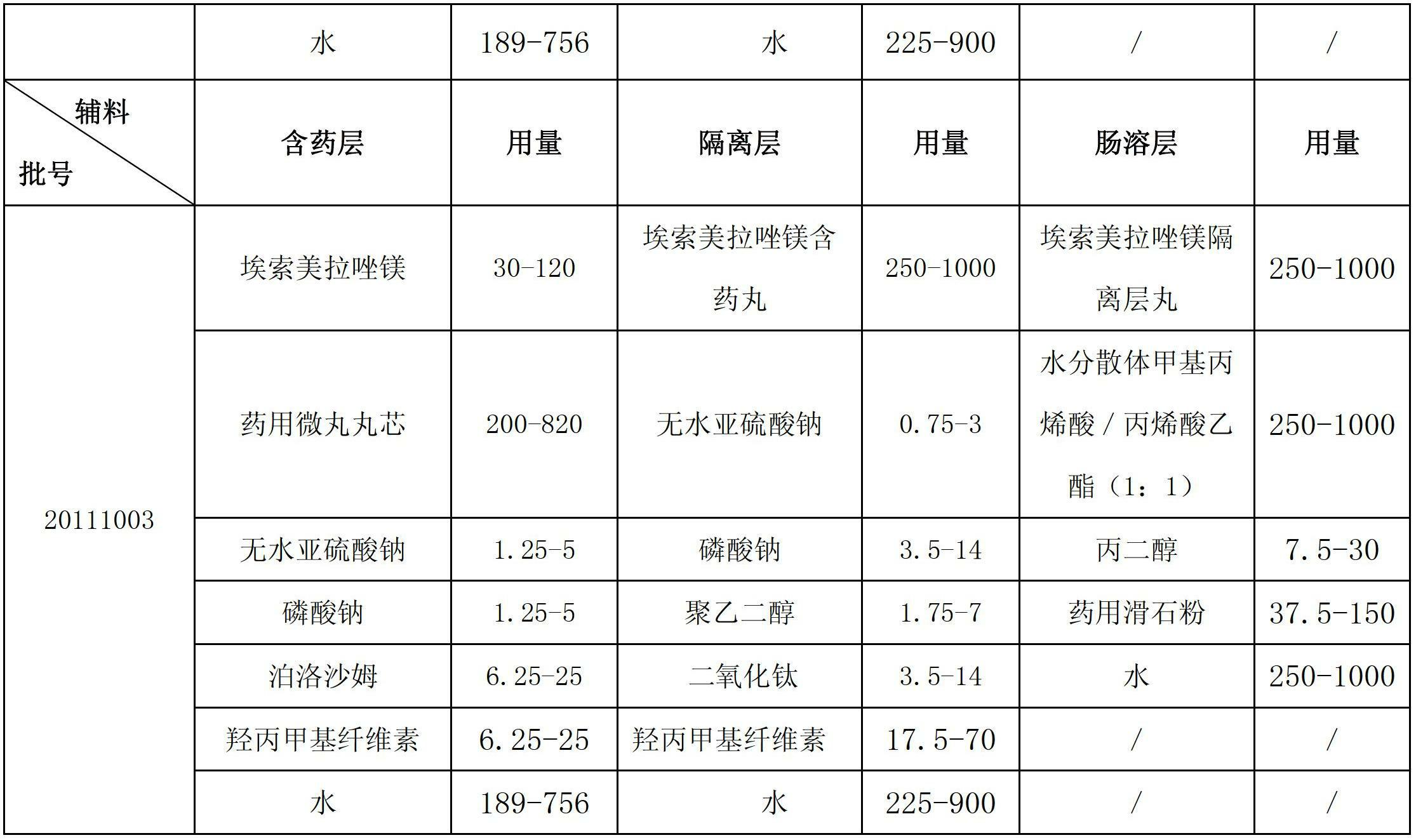
ActiveCN102670521AProtect active ingredientsFix stability issuesOrganic active ingredientsDigestive systemMedicineIsolation layer
The invention belongs to the field of pharmacy and relates to a medicinal preparation taking esomeprazole magnesium as an active ingredient, in particular to an esomeprazole magnesium enteric-coated pellet and a preparation method thereof. According to the esomeprazole magnesium enteric-coated pellet, medicines can be rapidly released in intestinal tracts. The esomeprazole magnesium enteric-coated pellet comprises the following structural layers in sequence from inside to outside: a medicine-contained layer, an isolation layer and an enteric-coated layer.
Owner:珠海润都制药股份有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com