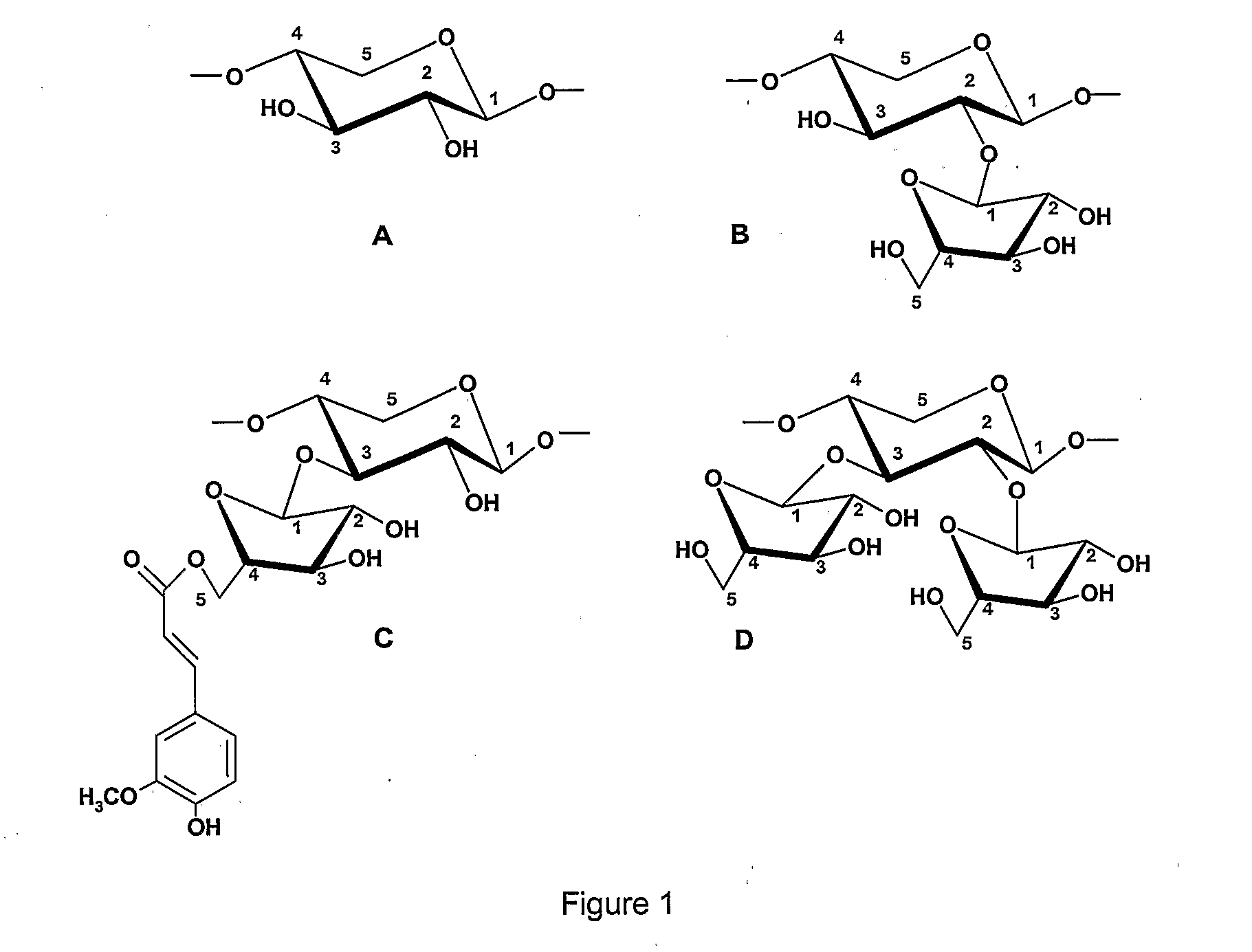
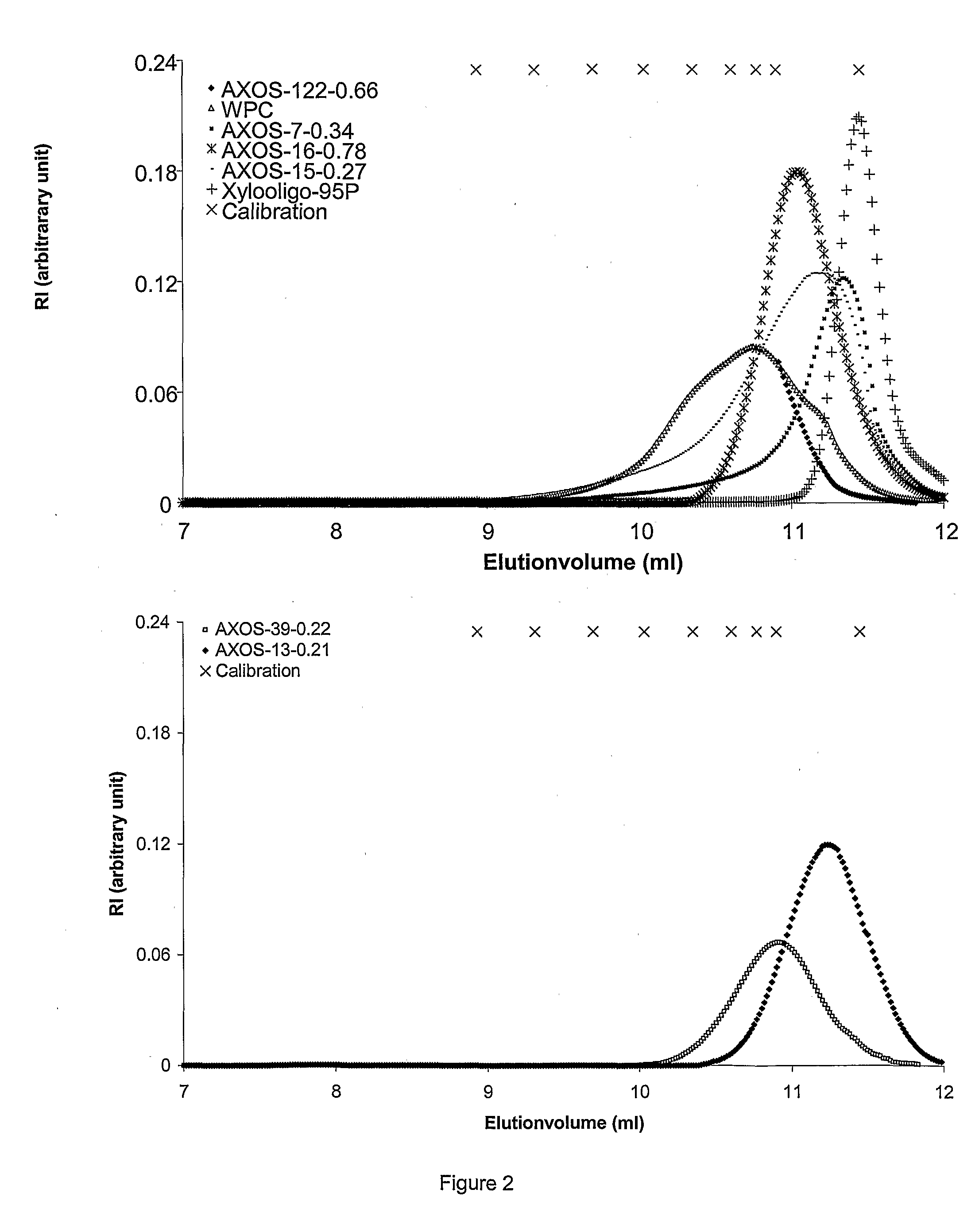
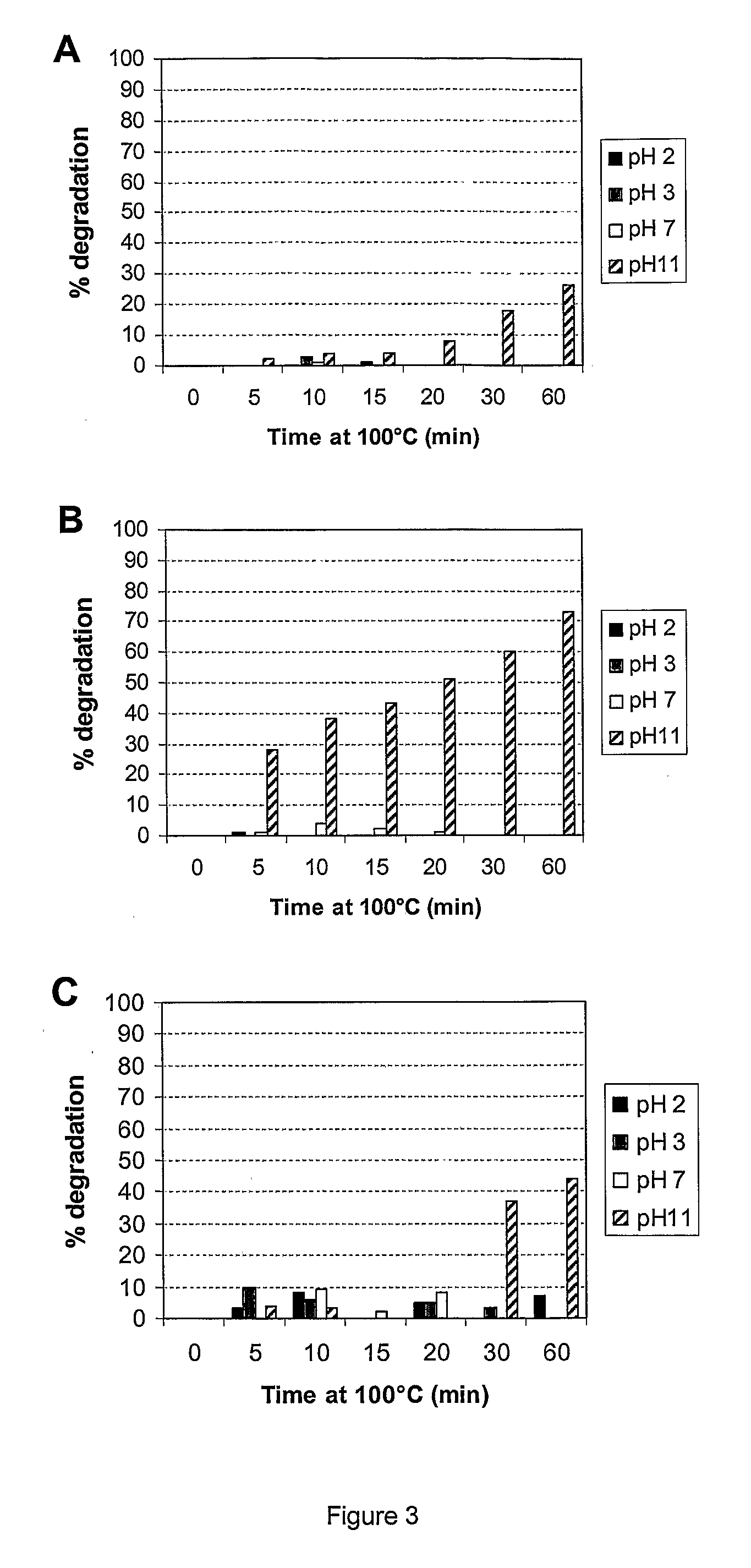
Prebiotic Preparation
a technology of xylan or arabinoxylan, applied in the field of arabinoxylan preparations, can solve the problems of high price level, unfavorable environmental protection, and low cost effect of current manufacturing processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of XOS / AXOS with Average DP>4
Materials and Methods
[0052]Xylooligo-95P. The commercial xylo-oligosaccharide preparation Xylooligo-95P, consisting predominantly of xylobiose, xylotriose, and xylotetraose, was obtained from Suntory Ltd. (Tokyo, Japan).
[0053]Preparation of water-unextractable arabinoxylan from wheat bran. For the preparation of bran water-unextractable arabinoxylan (WU-AX), commercial wheat bran (Meneba Meel BV, Rotterdam, The Netherlands) was used as starting material. Non-arabinoxylan material was partially removed from the bran by enzymic treatment according to the method described in Maes et al. (2004). A suspension of wheat bran in water (1:7 w / v) was first treated with a thermostable a-amylase (Termamyl 120 LS, Novozymes, Bagsvaerd, Denmark; 1 μl / g wheat bran) for 90 min at 90° C. to hydrolyse the starch. After cooling to 50° C., the pH of the suspension was adjusted to 6.0 using concentrated HCl and the suspension was incubated with a protease (Neutra...
example 2
Physicochemical and Sensory Properties of XOS / AXOS Preparations
Materials and Methods
[0070]Oligosaccharide preparations. Xylooligo-95P, AXOS-15-0.27, AXOS-39-0.22, and AXOS-13-0.21 were obtained as described in the Materials and Methods of example 1. The Fructo-oligosaccharide (FOS) preparation was the commercial product Raftilose (Orafti, Tienen, Belgium). The AXOS-15-0.27 used for sensory analysis was first dissolved in water (1:25 w / v) and treated with active carbon to remove possible off-flavours resulting from the production process. The suspension of AXOS-15-0.27 and active carbon (0.75 g / g AXOS-15-0.27) was stirred for 1 h at 18° C., and after decantation, the active carbon was removed by centrifugation (10000 g, 30 min, 18° C.).
[0071]Stability measurements. Stability measurements were carried out on the water-extractable part of oligosaccharide preparations, which was obtained by suspension of the preparations in deionised water (1:10 w / v) followed by shaking (2 h, 18° C.), c...
example 3
Fractionation of AXOS Preparations by Ultrafiltration
Materials and Methods
[0079]Preparation of AXOS. Squeegee WU-AX was prepared as described in the Materials and Methods of example 1. Squeegee WU-AX was suspended in sodium acetate buffer (25 mM, pH 4.7) at 3 g / l and incubated with XAA, an endoxylanase from Aspergillus aculeatus (Shearzyme 500 L, Novozymes, Bagsvaerd, Denmark), at 18.4 U per g squeegee WU-AX for 4 h at 30° C. After inactivation of the enzymes by boiling for 30 min and subsequent filtration of the suspension, AXOS was recovered in the filtrate.
[0080]Separation of AXOS by ultrafiltration. AXOS were fractionated in a dead-end ‘HP4750 stirred cell’ ultrafiltration device (Sterlitech Corporation, Kent, USA). The ultrafiltration membranes used had a molecular mass cut off (MMCO) of either 5 kDa (P005F, Celgard, Wiesbaden, Germany), 10 kDa (PES-10, Synder Filtration, Vacaville, Calif., USA), or 30 kDa (PES-030H, Celgard). Concentration polarization at the membrane surface ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com