Patents
Literature
2849results about "Level indications" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
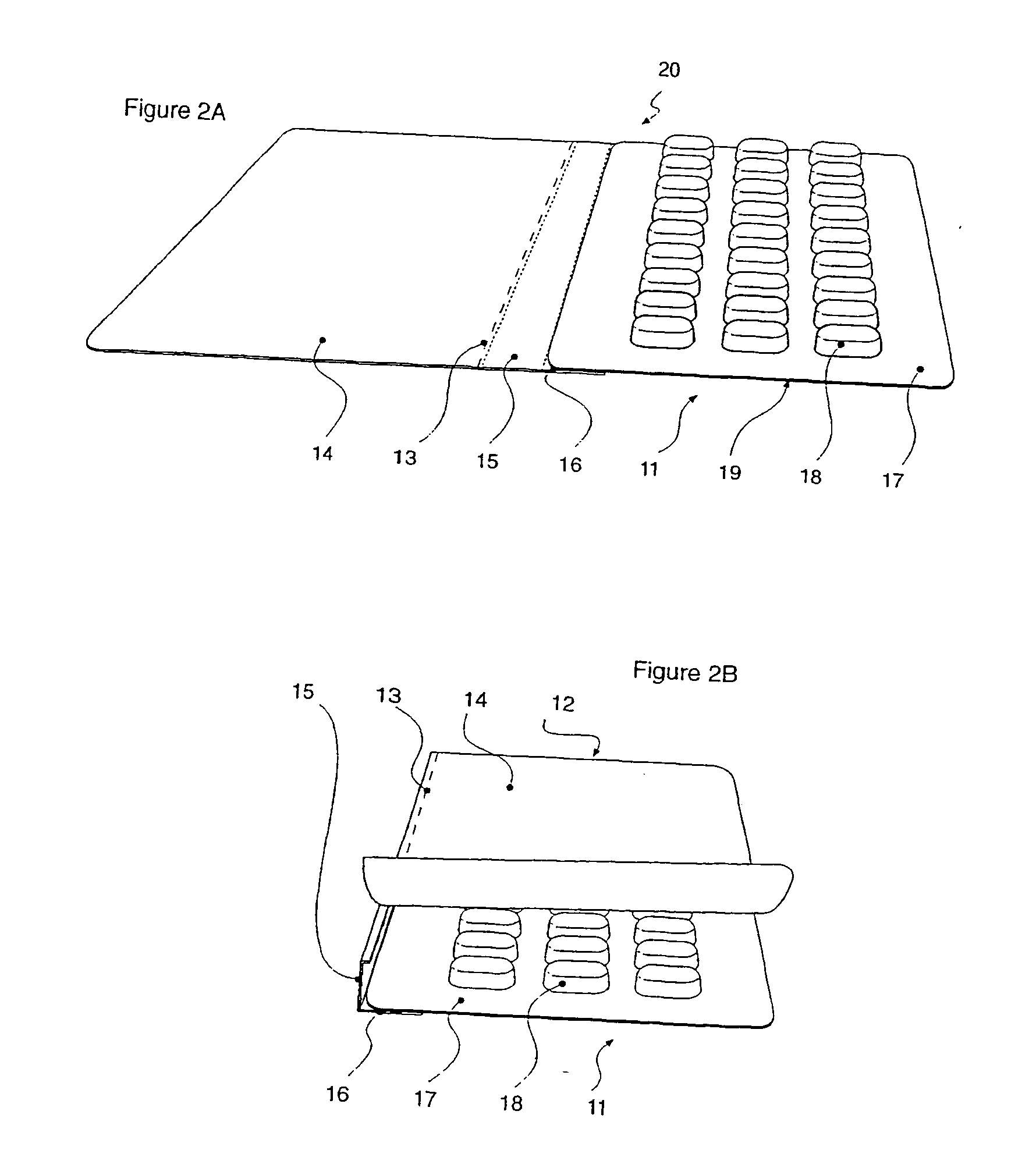
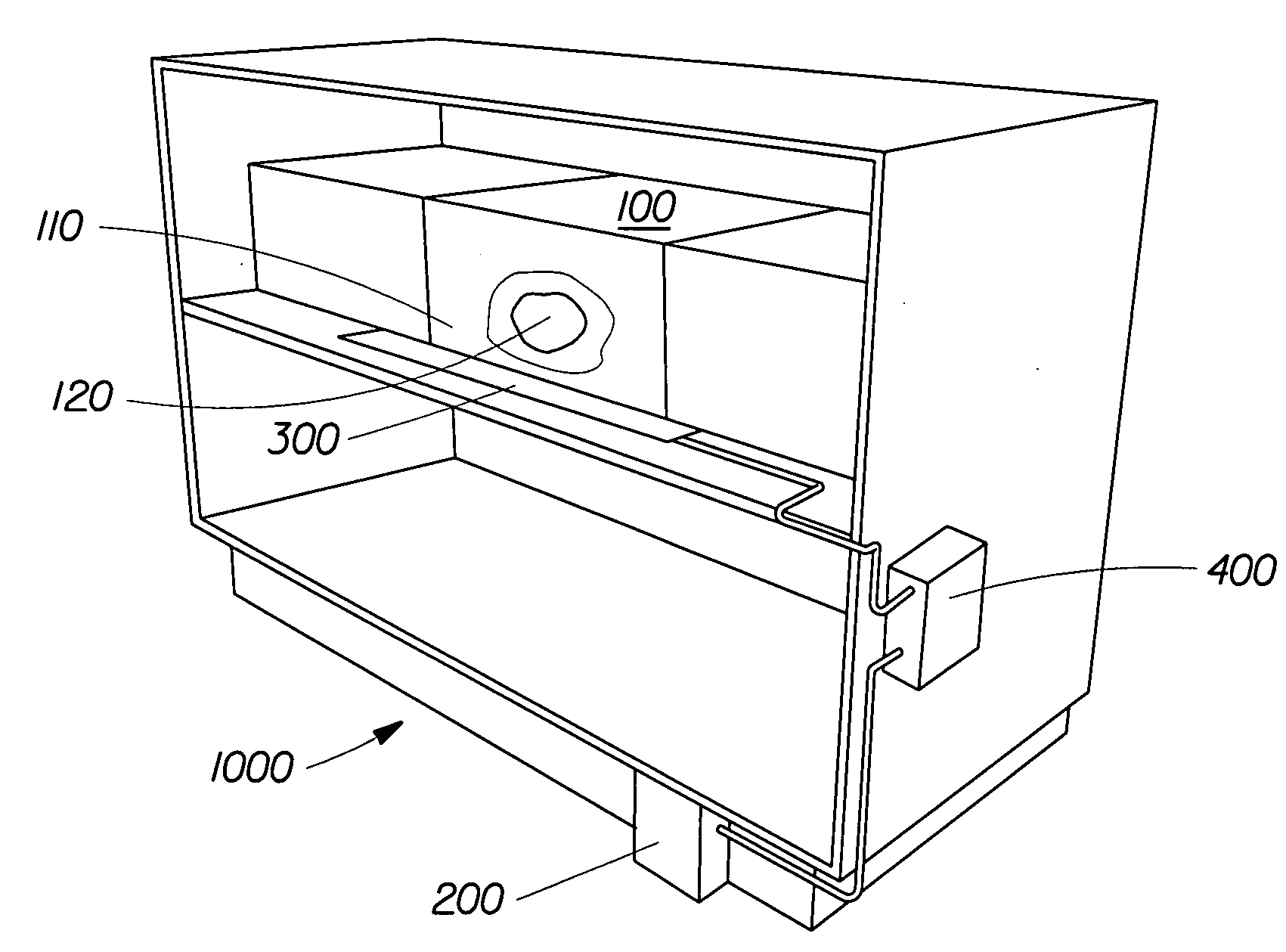
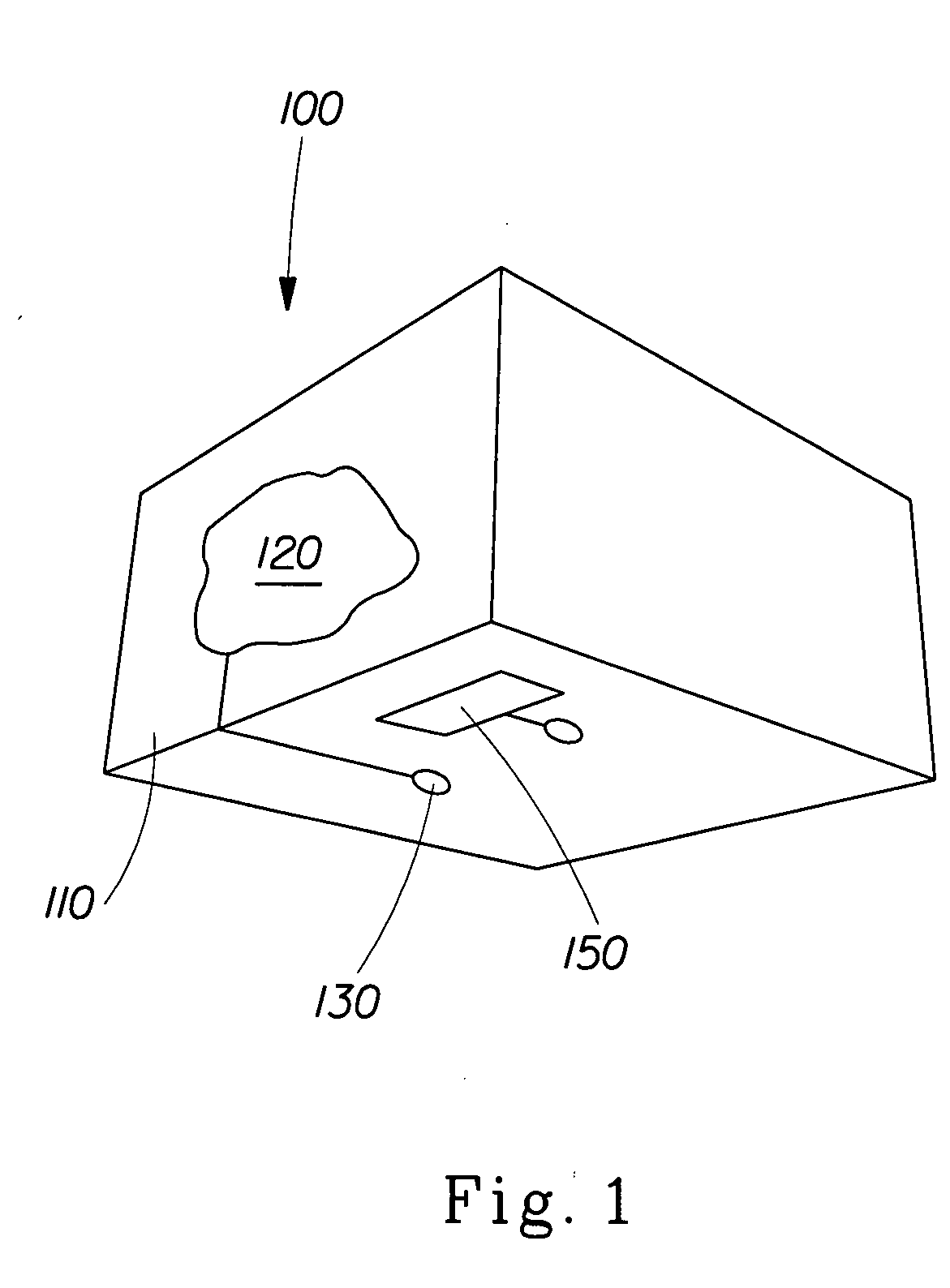
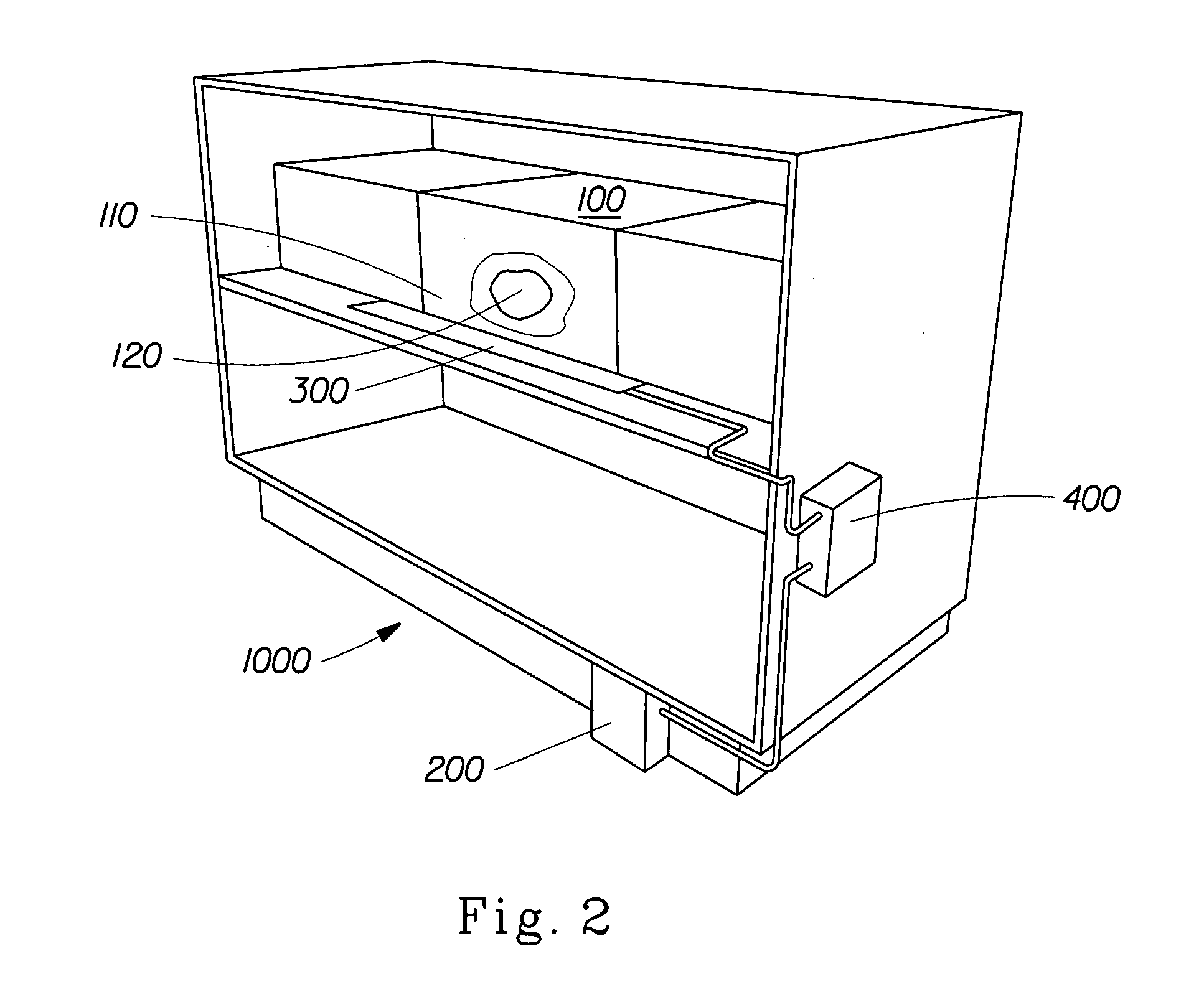
Perfume delivery systems for consumer goods
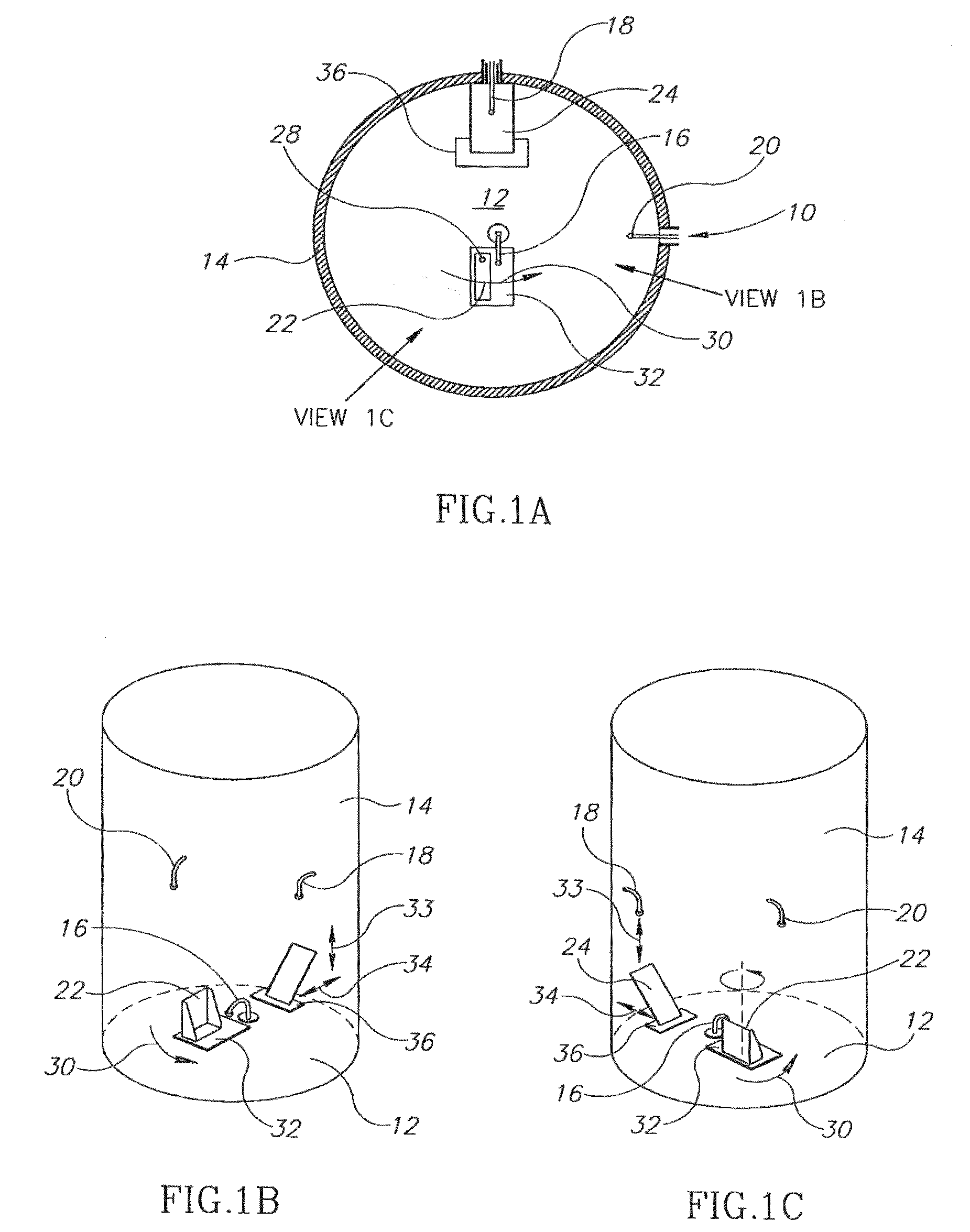
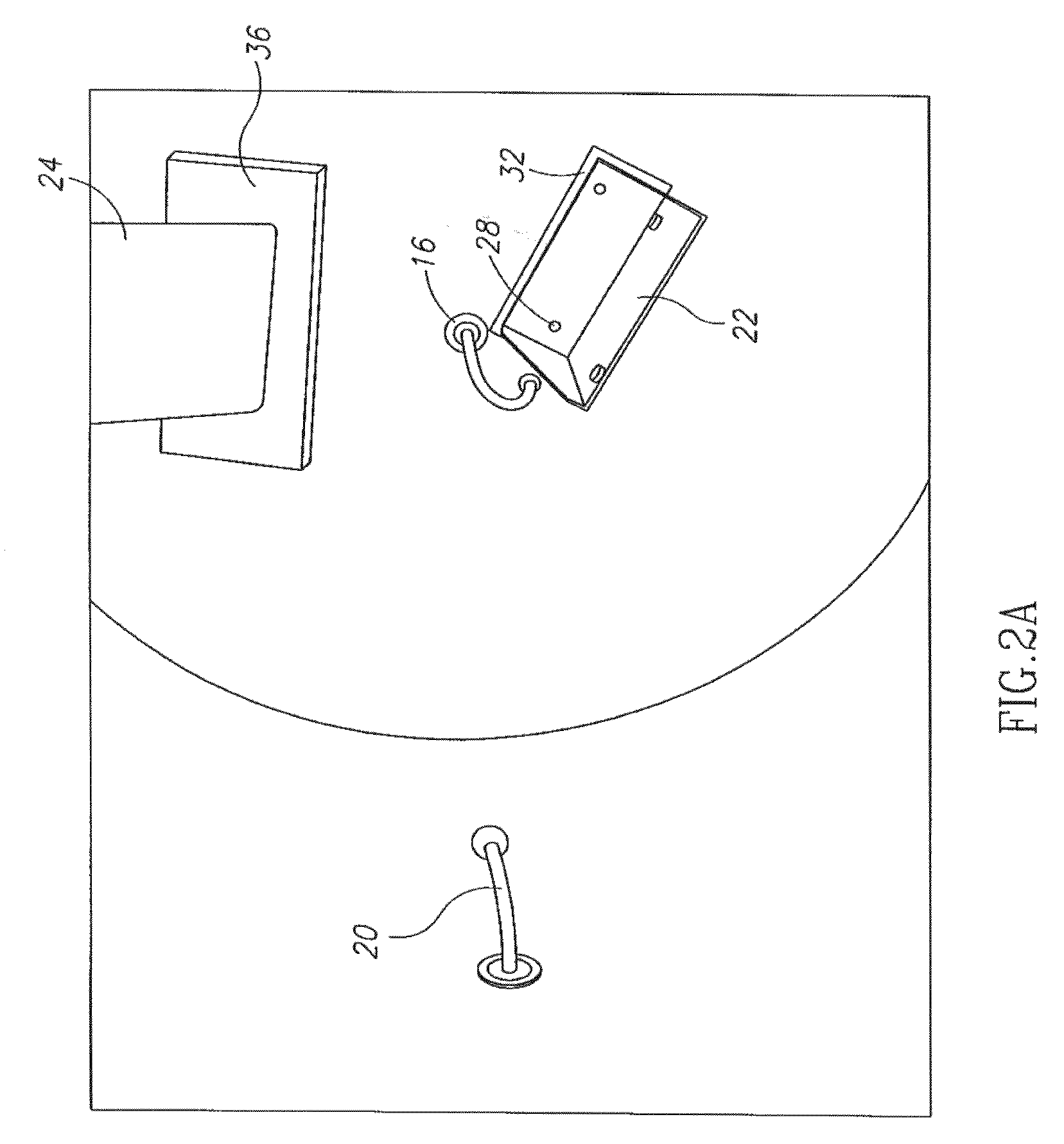
InactiveUS20070275866A1Low vapor pressureRealized benefitsCosmetic preparationsContainer decorationsEngineeringDelivery system
Owner:THE PROCTER & GAMBLE COMPANY
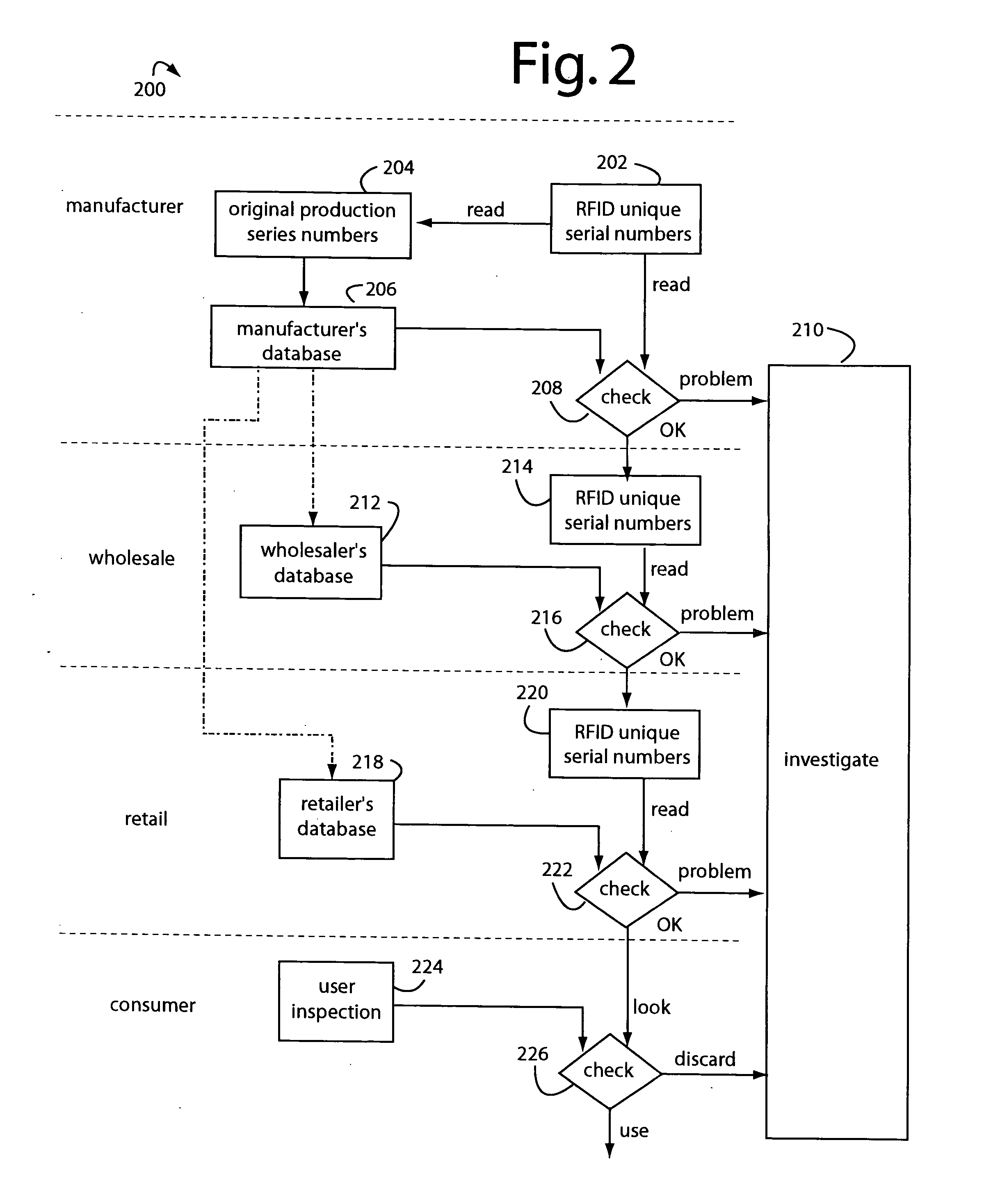
Method and system for preventing counterfeiting of high price wholesale and retail items
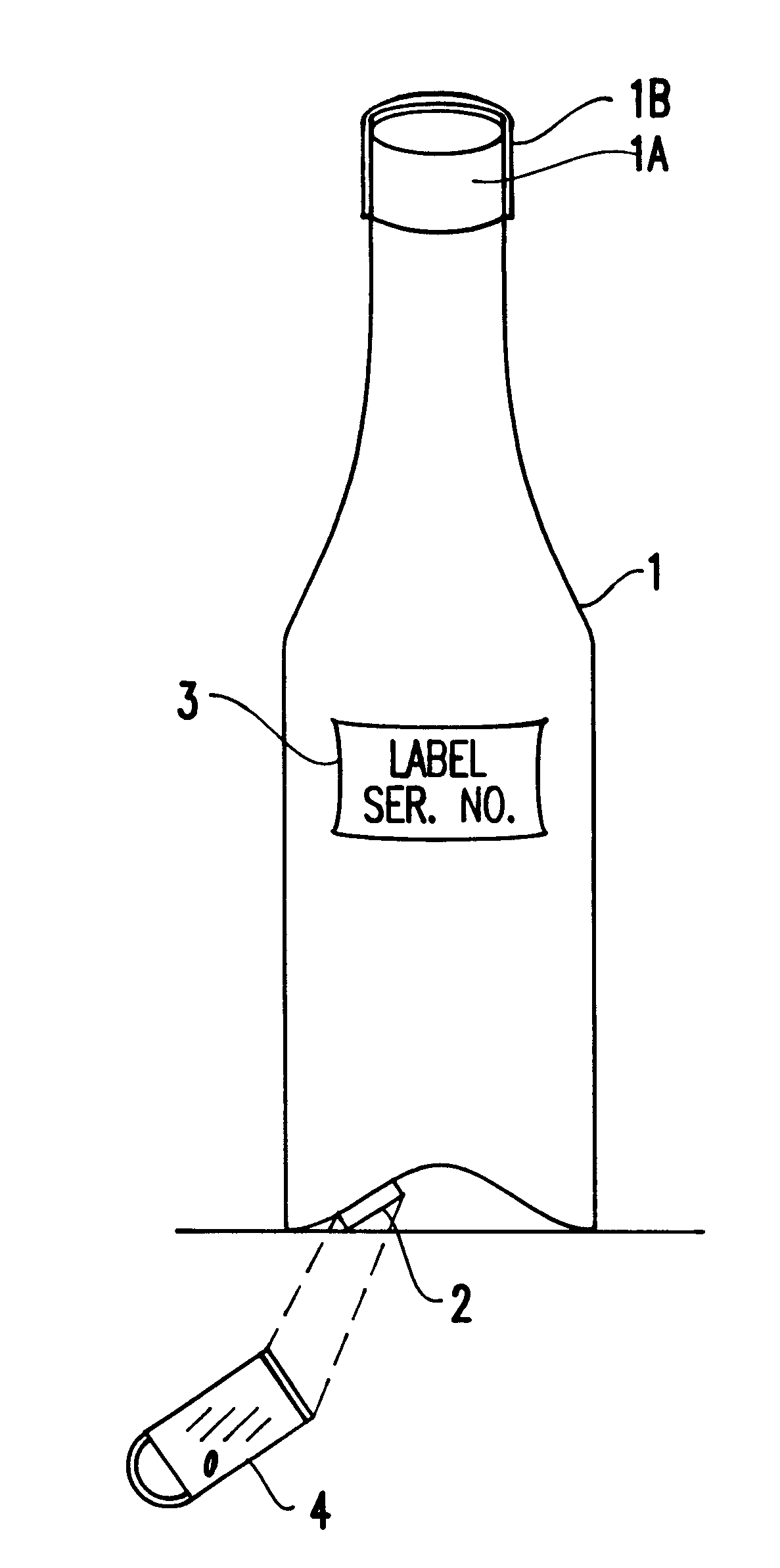
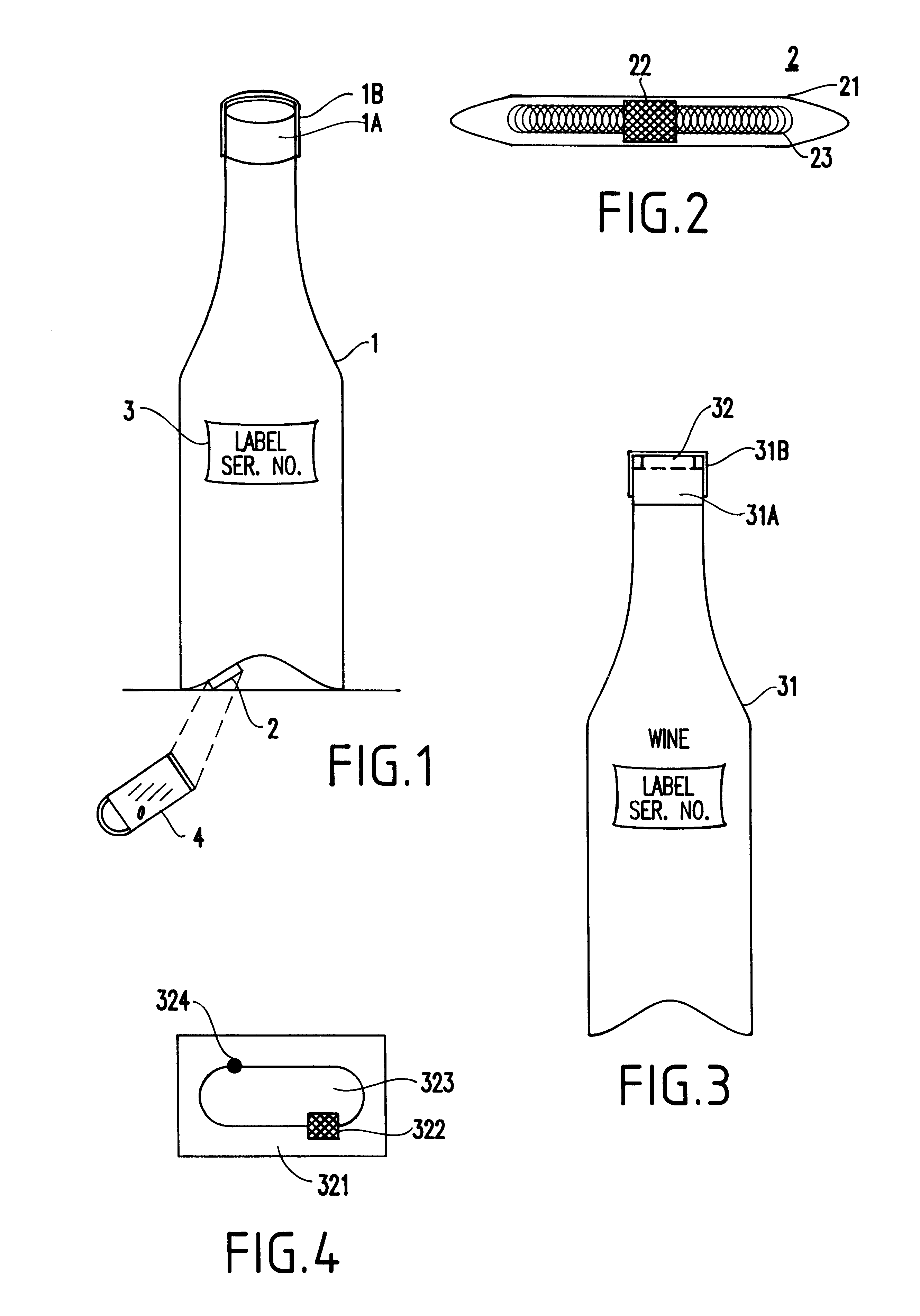
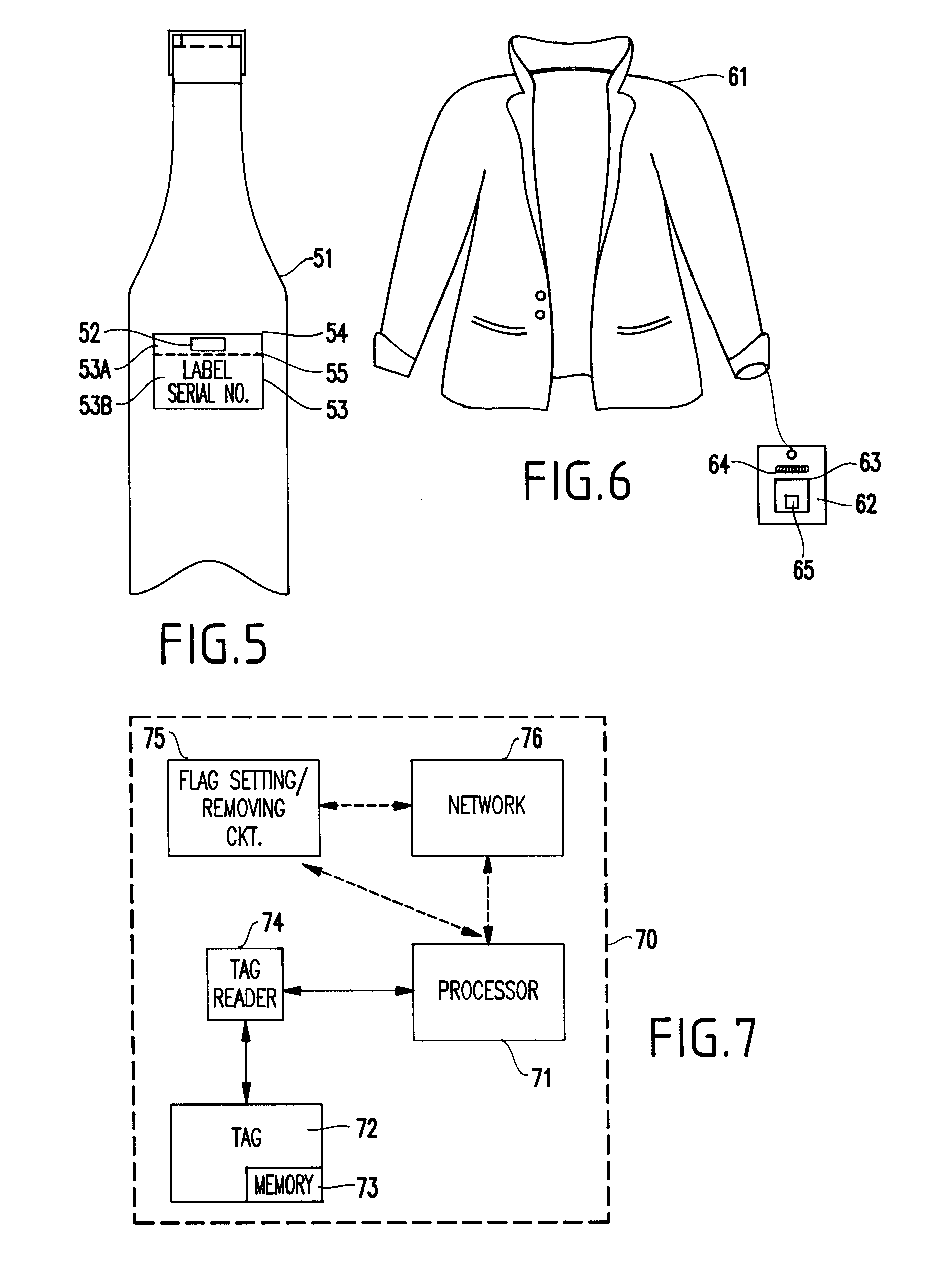
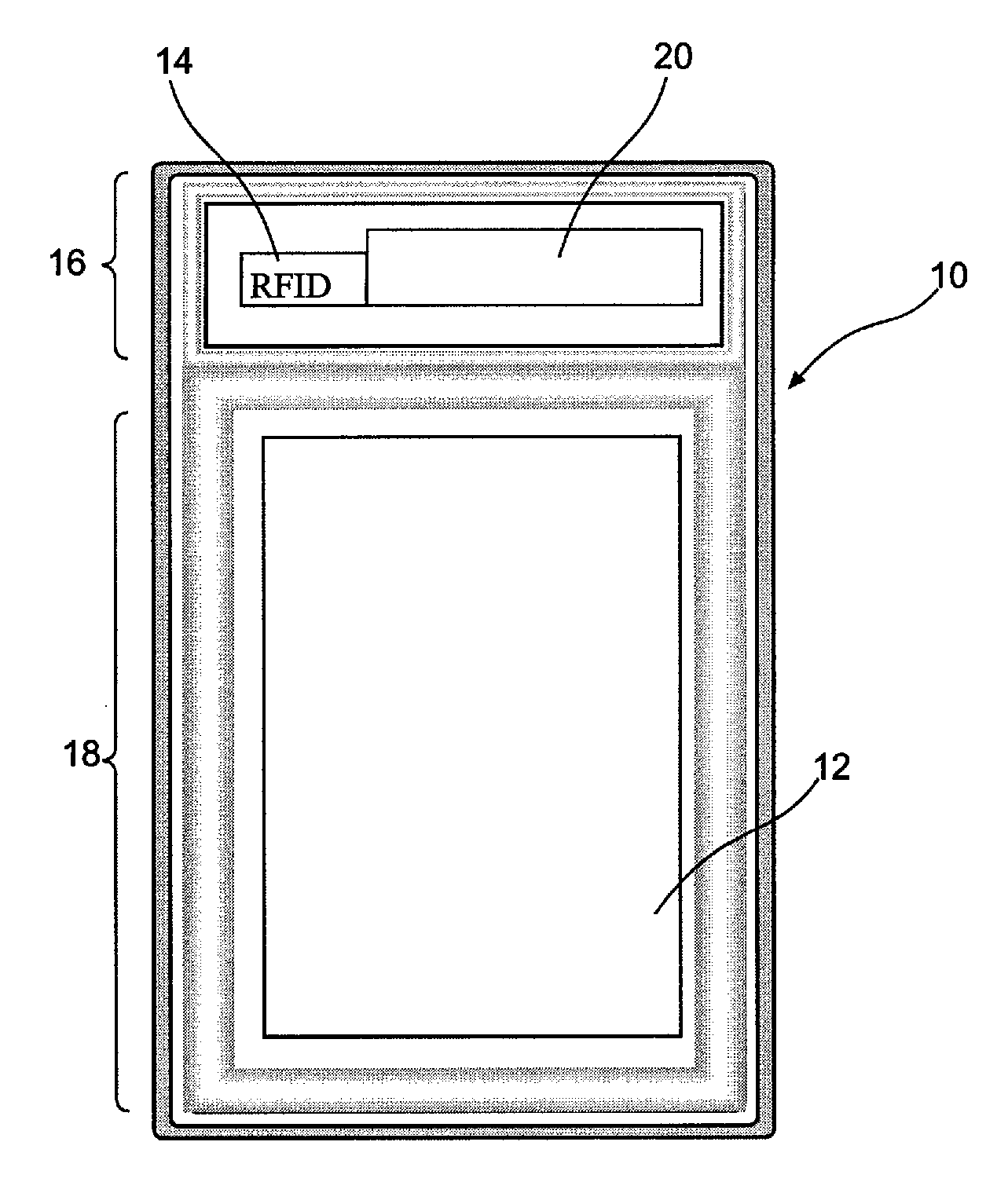
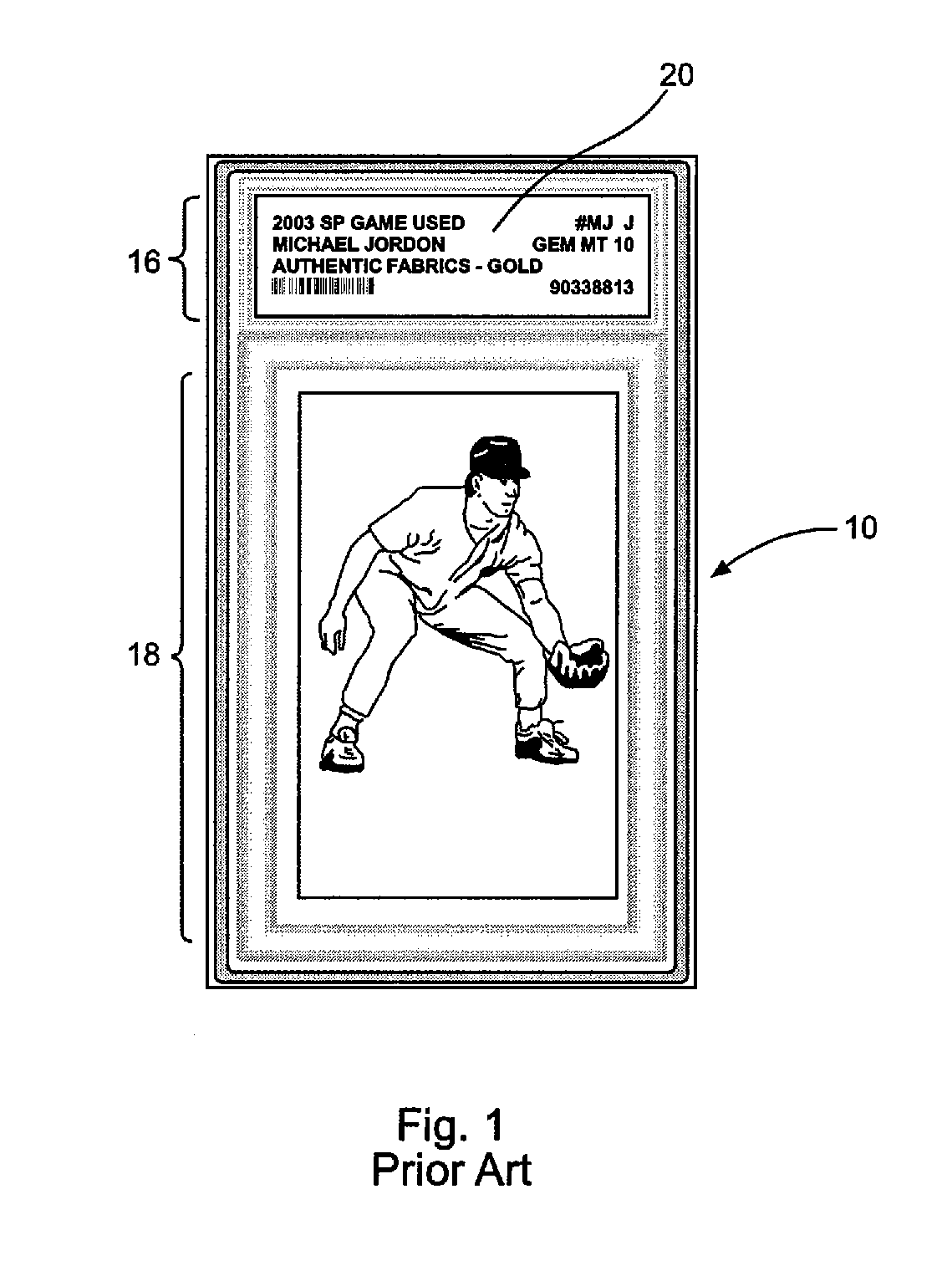
InactiveUS6226619B1Convenient verificationPrevent counterfeitingContainer decorationsLevel indicationsWorld Wide Web
A method and system for preventing counterfeiting of an item, include an interrogatable tag attached to the item. The item includes visible indicia for comparison with secret, non-duplicable information stored in the tag designating authenticity.
Owner:TOSHIBA GLOBAL COMMERCE SOLUTIONS HLDG
Passive tamper-resistant seal and applications therefor
A radio frequency identification seal comprises an antenna including a main antenna portion and at least one break-away portion and an RFID tag coupled and tuned to the antenna. The RFID tag outputs a signature in response to a scanning signal when tuned to the antenna.
Owner:COVELEY SOLBYUNG
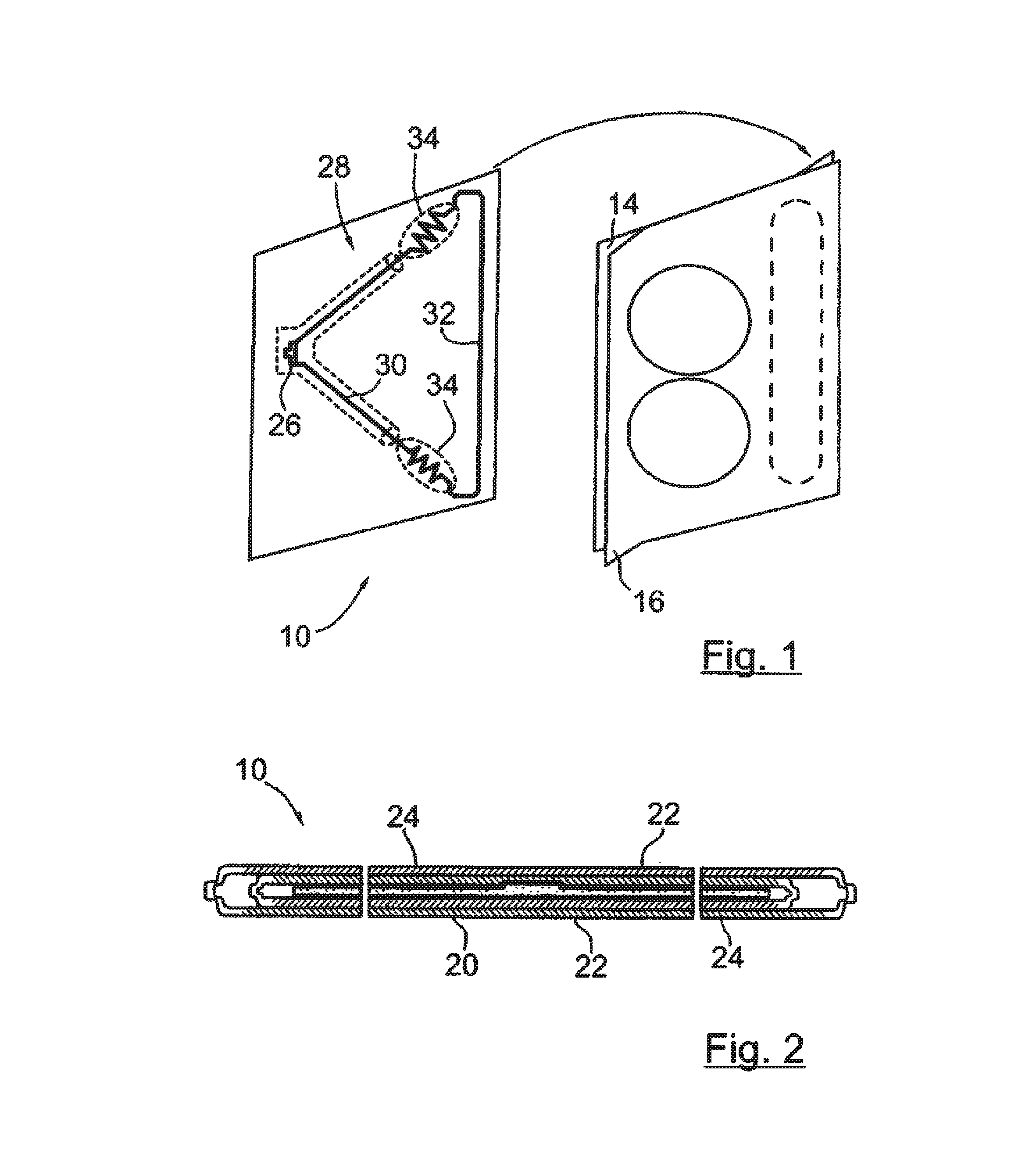
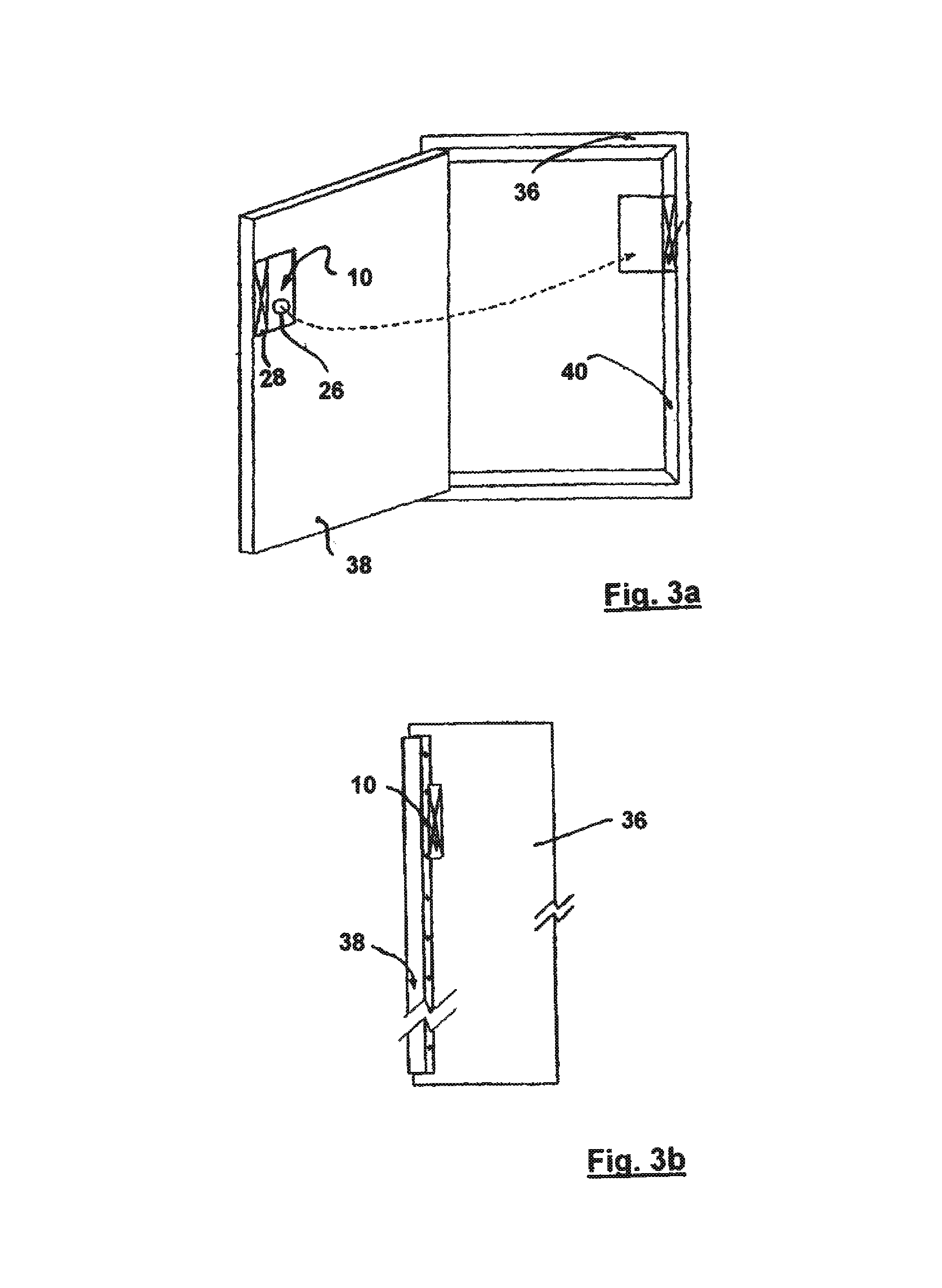
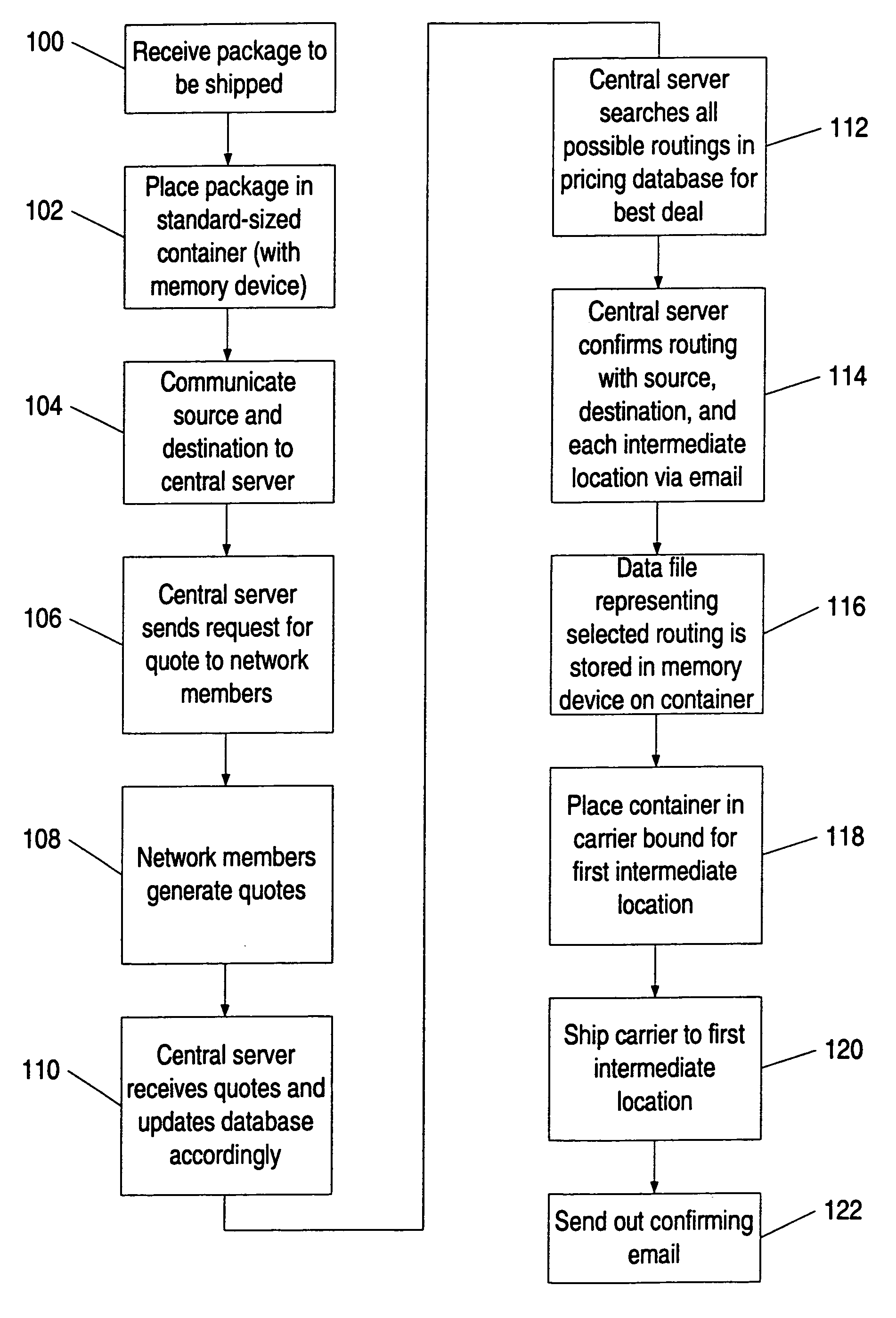
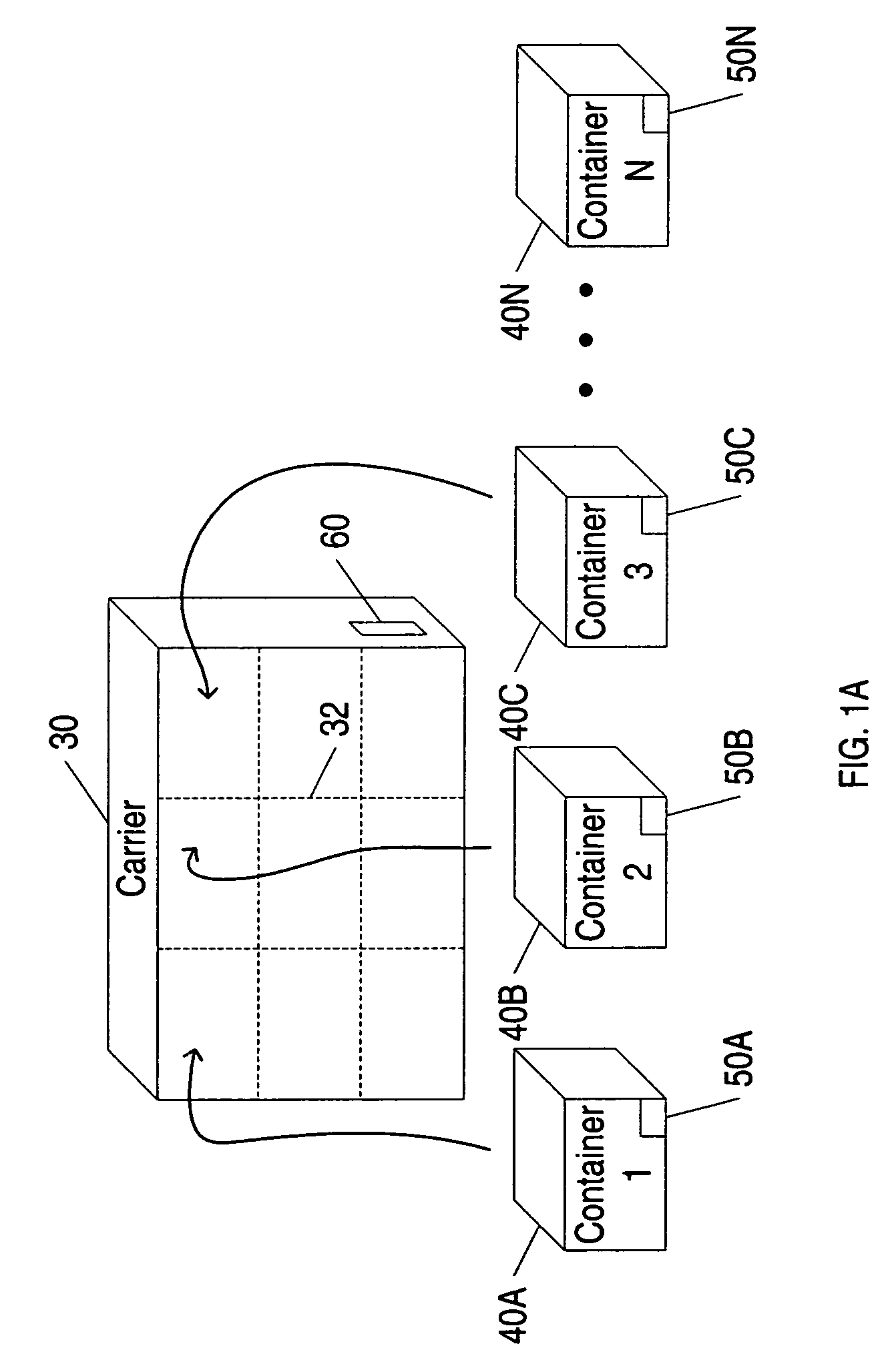
System and method for tracking and routing shipped items
InactiveUS7035856B1Simplify the shipping processSimple processContainer decorationsLevel indicationsData fileFile storage
A method and system for shipping items. A server is configured to send out requests for quotes to a number of regional shipping companies using a network. The server receives responses from the network and selects a route based on the responses. The route may include shipping the item to one or more intermediate destinations before the item arrives at the final destination. The server may create a data file reflecting the selected route. The items are packed in one or more containers, wherein each container has a memory device. At least part of the data file is then stored into the memory device. The memory device may be accessed as needed during shipping to determine where the item is going and when the item needs to arrive. Additional information may also be stored in the memory device, and the device may be updated at intermediate destinations. Each container may take a different routing, and the server may actively search for better routings as the item proceeds along the selected route. The containers may be configured to be placed within carriers that hold multiple containers, and the carriers may also be configured with memory devices.
Owner:NIHON DOT COM
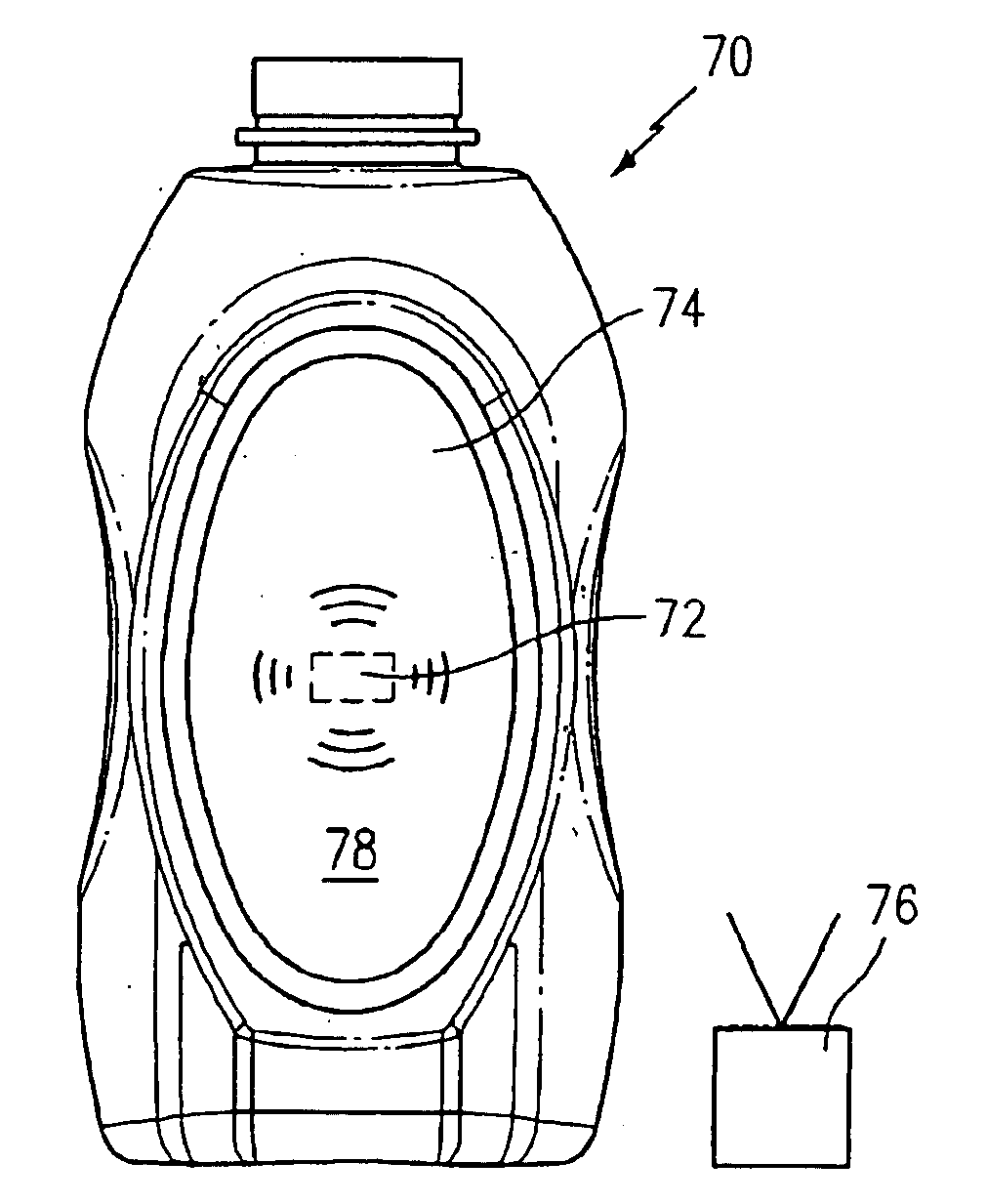
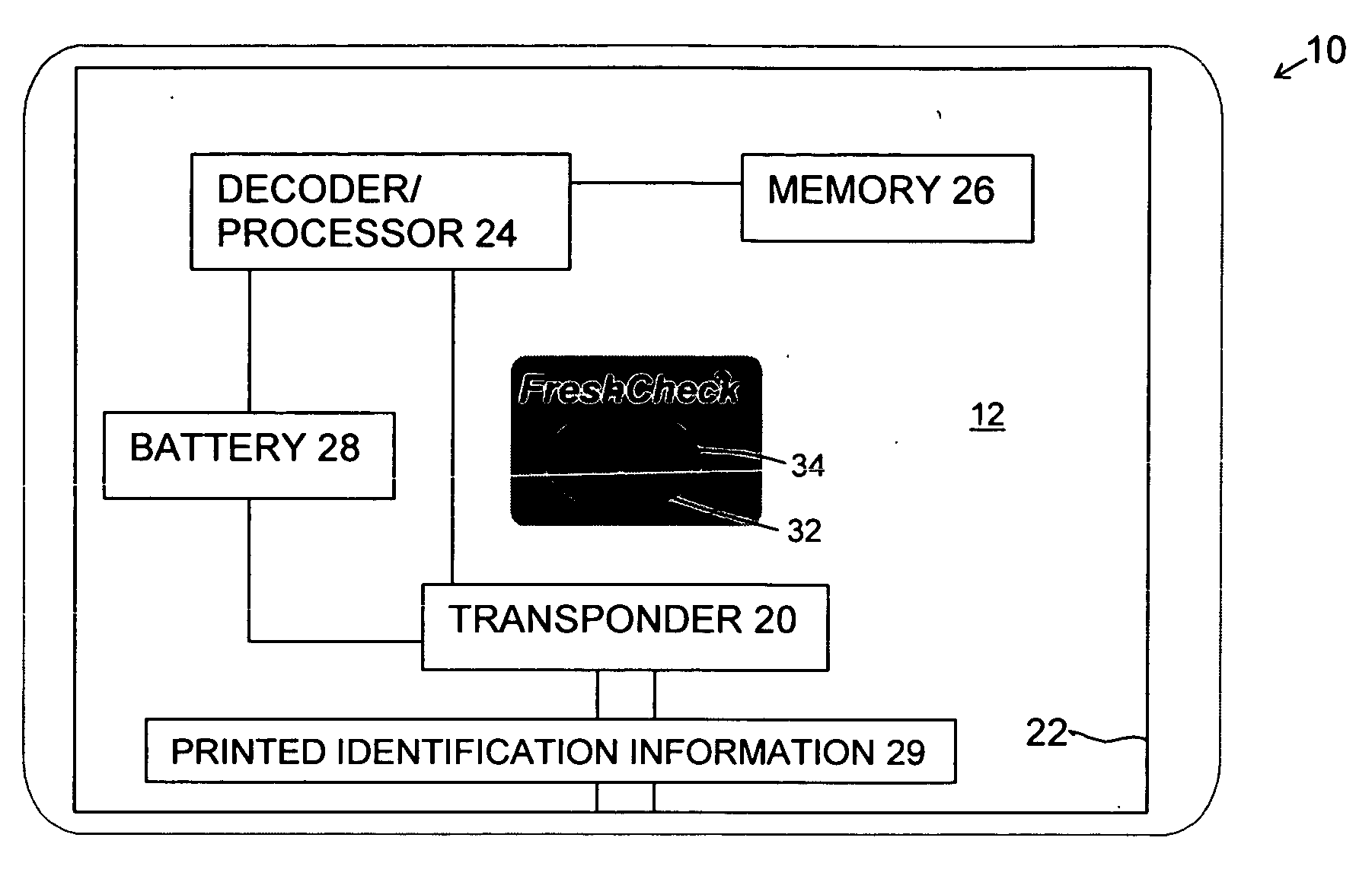
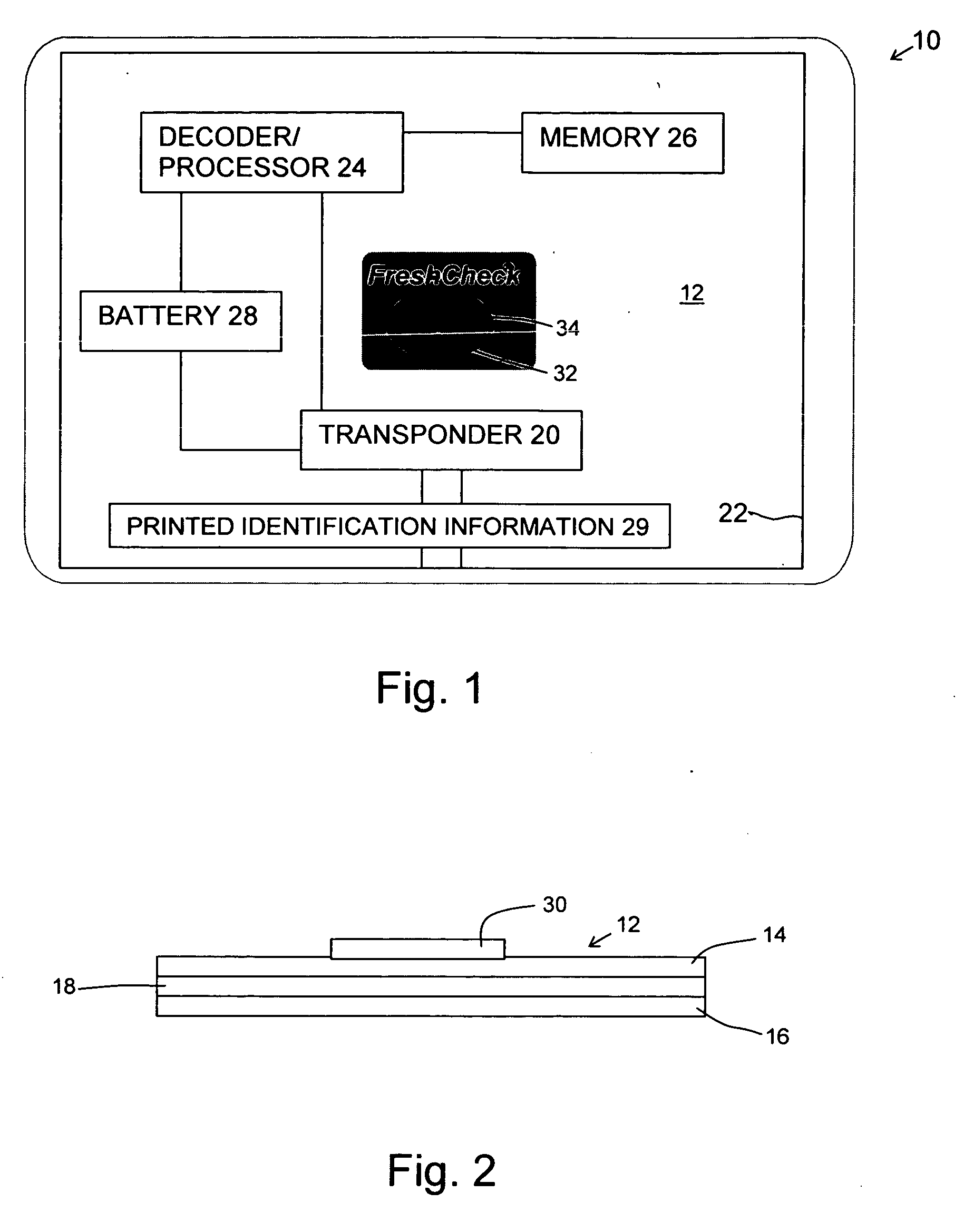
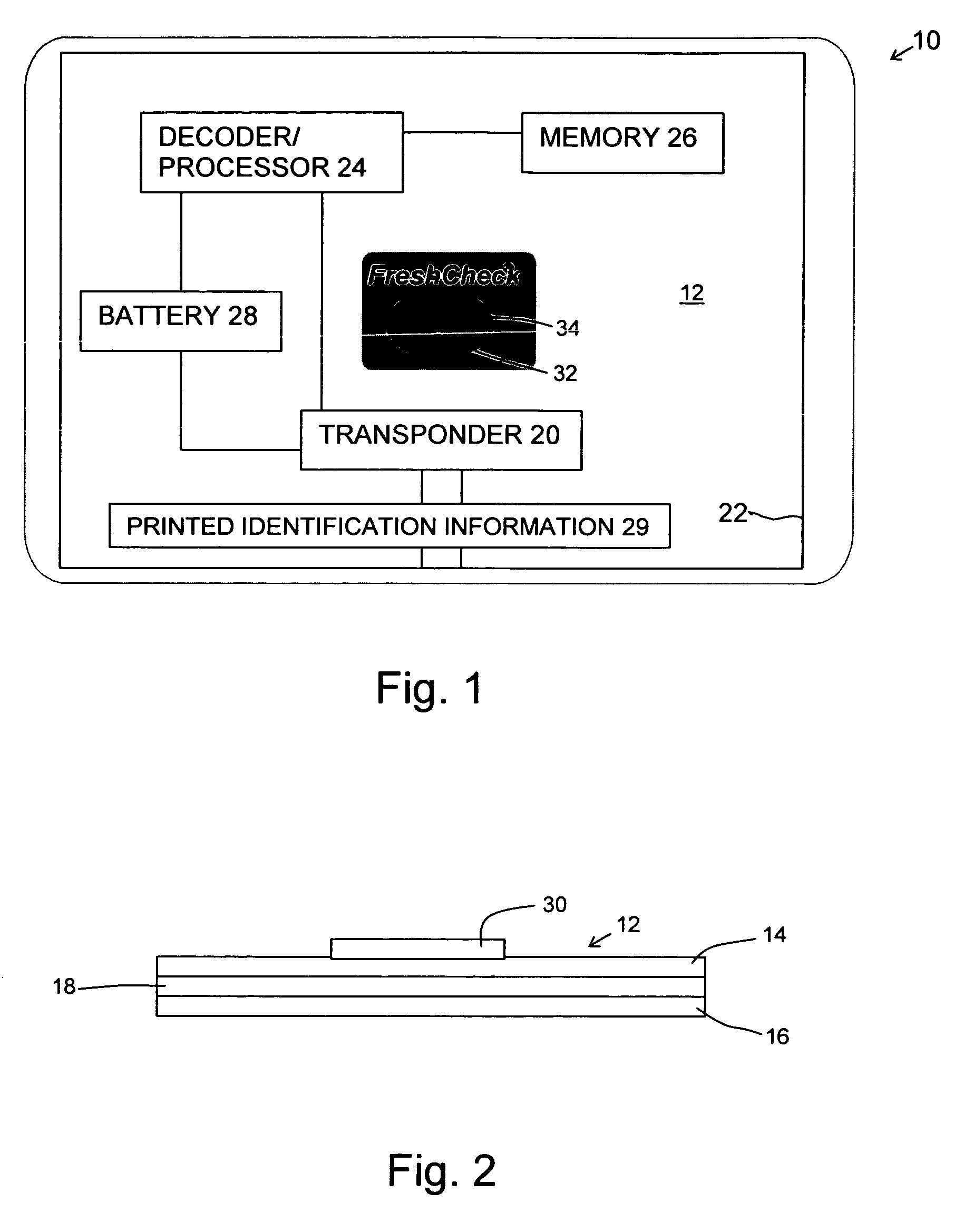
Product dispensing controlled by RFID tags
ActiveUS7009519B2Provide informationContainer decorationsLevel indicationsTimerManufacturing engineering
RFID tags attached to a product contain information regarding the product and communicate this information to a reader. This information can include the age of the product, the amount of product left and preparation or care instructions for the product. To record the age of a product, a timer is initiated in the RFID tag. Thereafter, the elapsed time on the timer can be read and displayed by the reader. For other products, the RFID tag can have contacts monitoring the level of remaining product. Once the contacts sense that the amount of product is low, this information can be transmitted and communicated by the reader. Preparation and care instructions are prerecorded on the tag. Instructions can also be communicated between a product and a base, such as a heater.
Owner:SC JOHNSON & SON INC
Food preparation
InactiveUS20090236334A1Improve efficiencyIncrease net powerContainer decorationsLevel indicationsEngineeringIngested food
Owner:GOJI LTD
Thermoformed platform
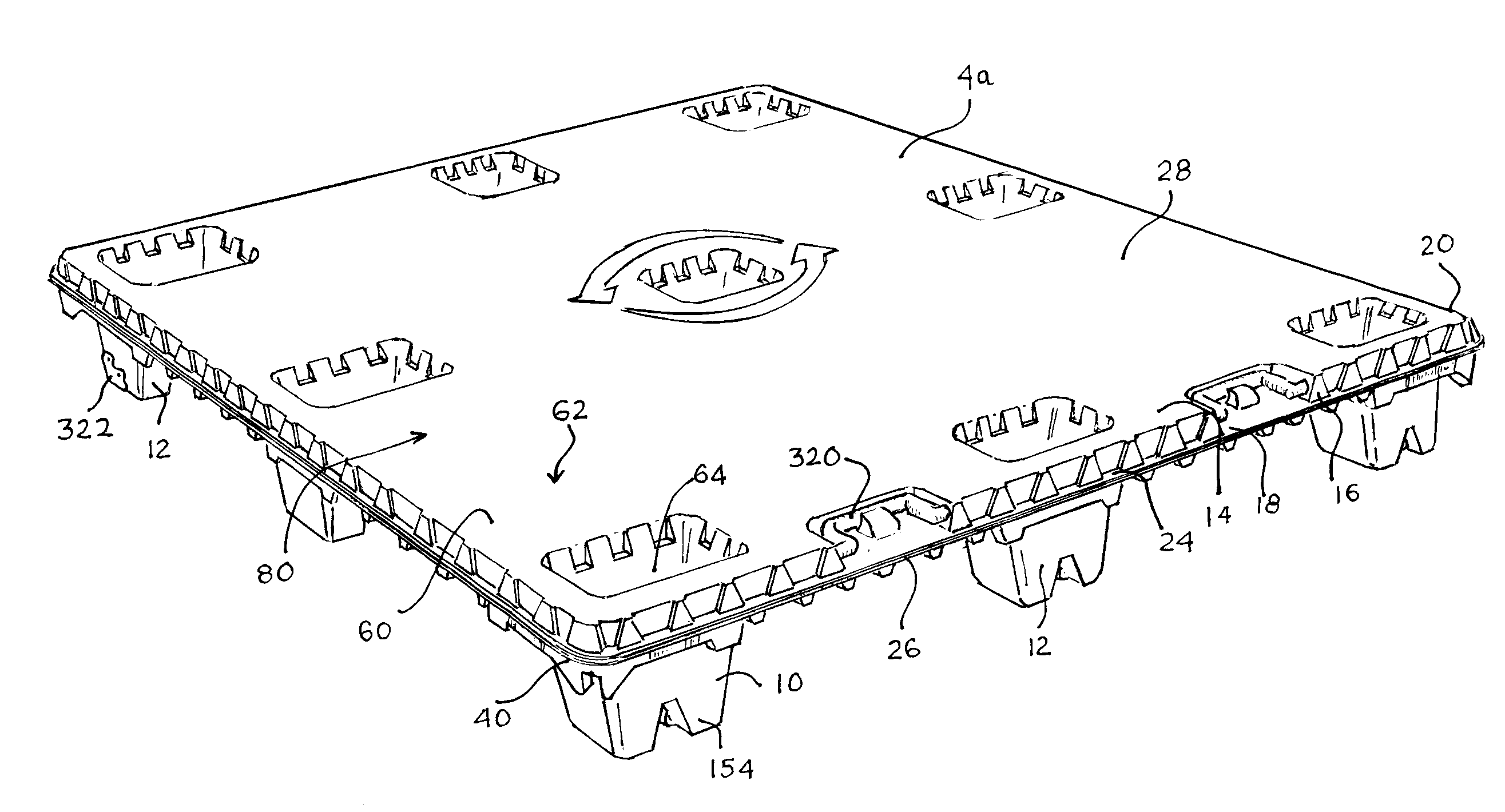
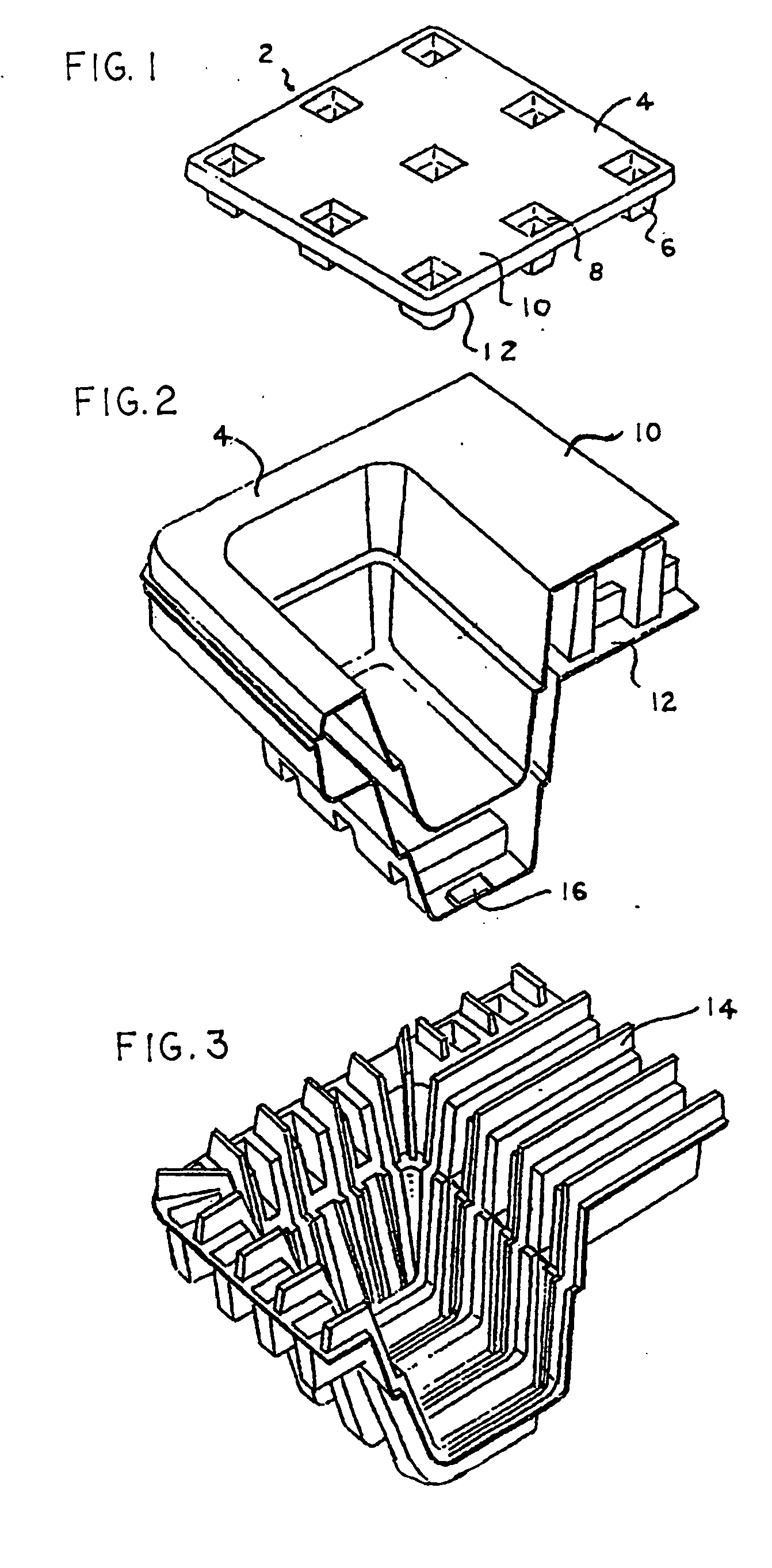
Articles constructed of a plurality of scuffed sheets have improved sheet-to-sheet bond strength and surfaces with high coefficients of friction. Articles constructed out of three scuffed sheets include exterior intumescent polymeric surfaces resisting the spread of combustion flames and insulating the interior surfaces from the high temperature of fire. Articles include electronic apparatus sending an emergency 911 call to a remote monitoring station. Articles are advantageously reinforced with optional rigidifying structures without article modification. Members are joined with snap together features providing an assembled article. Articles include handles for ergonomic manipulation by workers. Articles include elements amenably receiving unitization accessories. The article improvements are demonstrated in the form of industrial platforms, particularly material handling pallets.
Owner:NEXTREME
Blister package with electronic content monitoring system
InactiveUS7113101B2Universal applicabilityEfficient operabilityContainer decorationsLevel indicationsMonitoring systemBlister pack
A replicate can be secured to a blister package intended to contain articles, such as pills, and is used to record the removal of individual articles from the blisters. To remove an article from a blister one will usually press against the blister to push the article through a frangible closure seal, breaking the seal in the process. The replicate includes a backing sheet which carries a plurality of traces alignable with corresponding blisters so that when the article is removed from the blister it will not only break the seal but it will also break the corresponding trace. All of the traces are connected to an integrated circuit which may also be formed or provided on the backing sheet, as is a power source for the integrated circuit. The breaking of the trace is an event that is recorded in the integrated circuit for later accessability. The replicate may be secured to the blister package after the package has been produced by conventional form-fill-seal equipment. The individual traces can be formed into a grid of closely spaced traces so that alignment of the traces with the individual blisters is less critical. The replicates may be formed by printing or other conventional methods on a roll of lidstock. After forming the individual replicates are severed from the roll of lidstock for securement to a blister package.
Owner:INTELLIGENT DEVICES SEZC
Food preparation
ActiveUS20090236335A1Improve efficiencyIncrease net powerContainer decorationsLevel indicationsEngineeringIngested food
Owner:JOLIET 2010 LTD
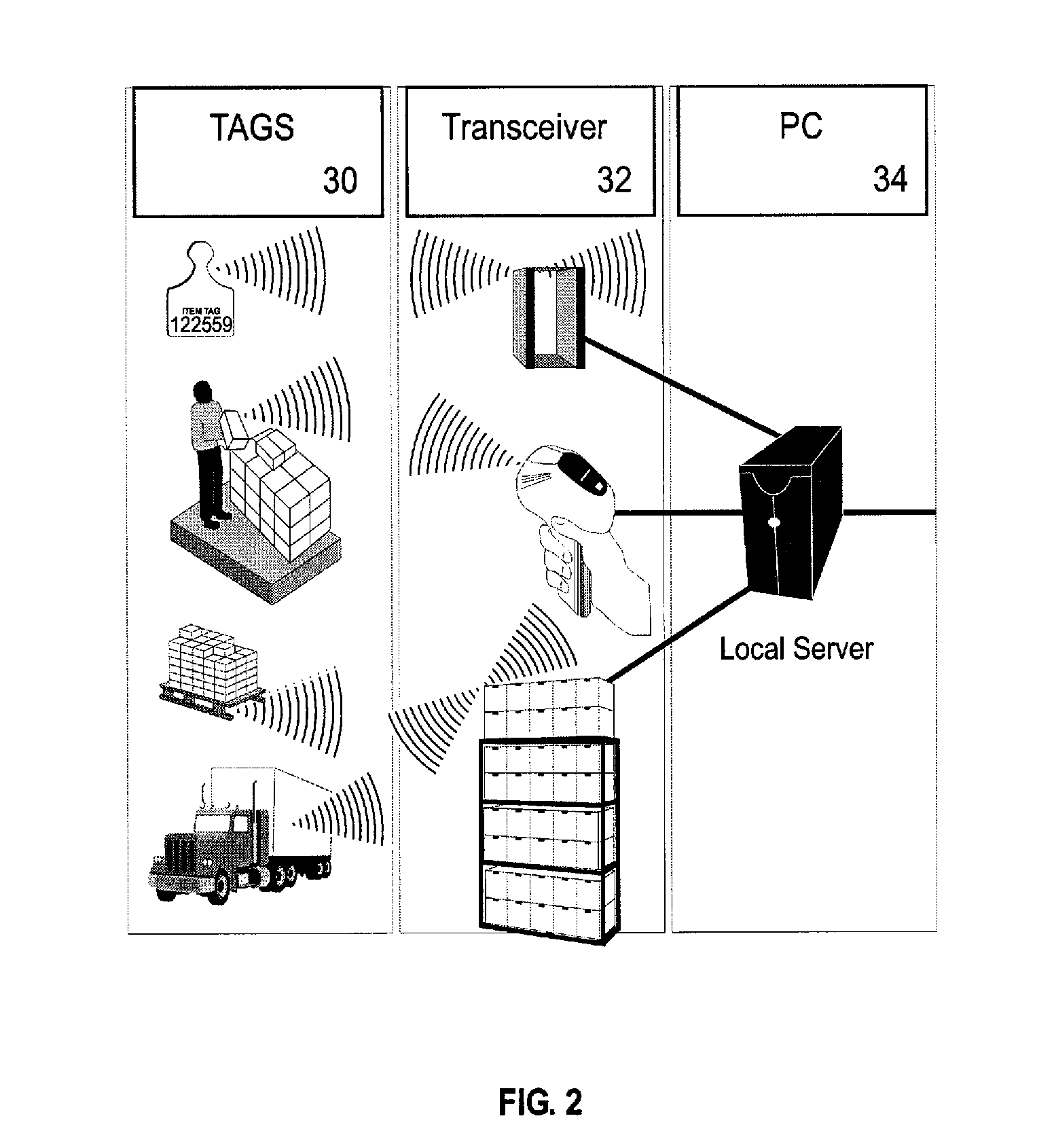
Method, apparatus, and system for tracking unique items
InactiveUS20070187266A1Improve protectionEasy authenticationContainer decorationsLevel indicationsInternet privacyRadio frequency
A method, apparatus, and system for double-sided scanning, tracking, registering, identification storing and further handling by trading or collecting unique items. Specifically, the invention relates to a method, apparatus, and system for locating and tracking unique objects utilizing a computer controlled radio frequency system and radio frequency tags that are associated with unique objects in a manner that facilitates both protection, up-loading to a unique and secure web-based service site allowing secure registration of identified, verified ownership and authentication of the unique objects.
Owner:HH TECH
Thermoformed platform having a communications device
InactiveUS20050241548A1Improve protectionImprove reliability and durabilityContainer decorationsLevel indicationsThermoformingEngineering
An apparatus has a communications device associated therewith. In another aspect of the present invention, a pallet is made from thermoformed polymeric sheets with an attached communications device. A further aspect of the present invention provides a radio frequency identification device attached to an apparatus. In still another aspect of the present invention, a communications device is incorporated into one or more sheets of a pallet or other container prior to forming. Methods of making and using a thermoformed pallet and container, having a communications device, are also provided.
Owner:NEXTREME
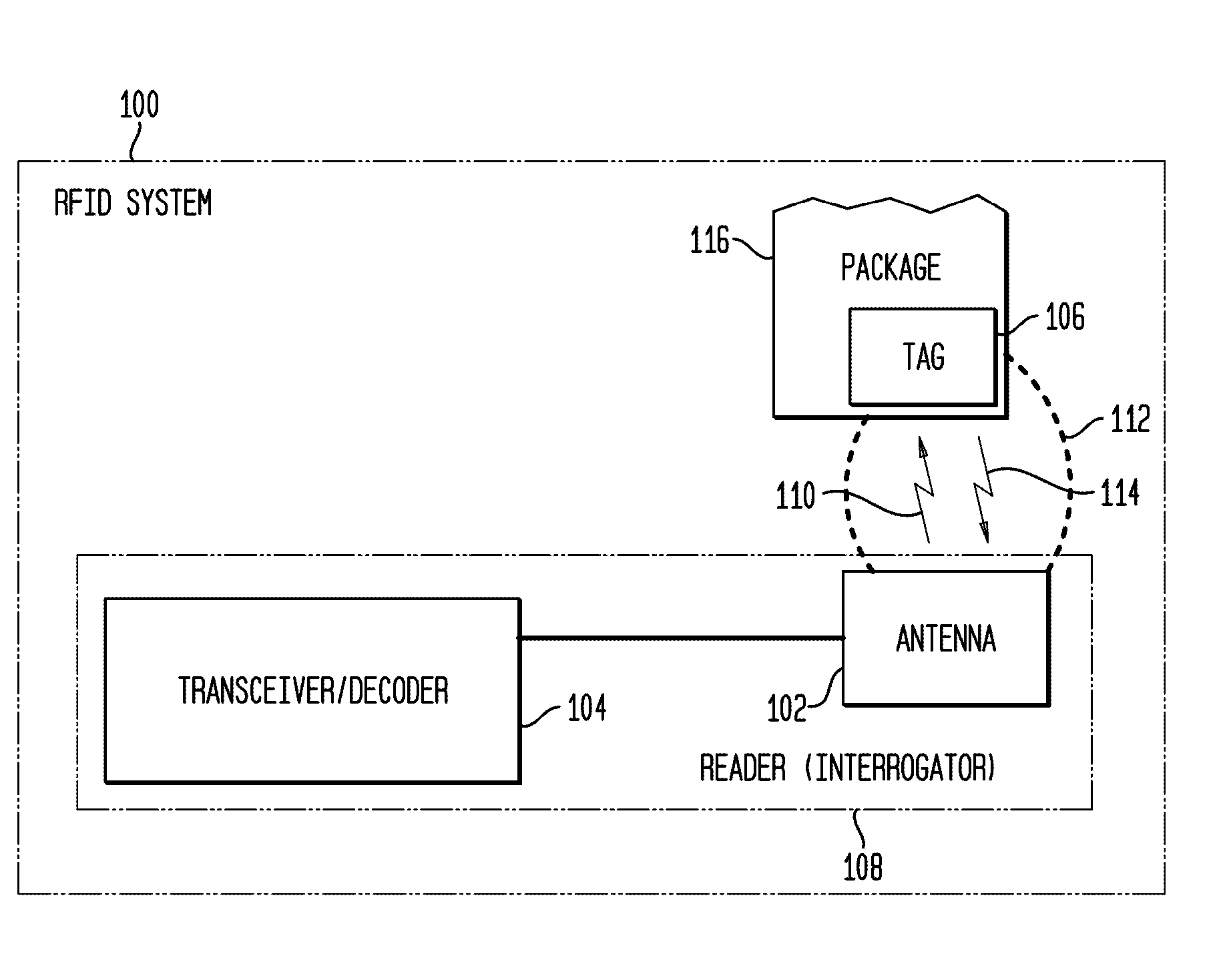
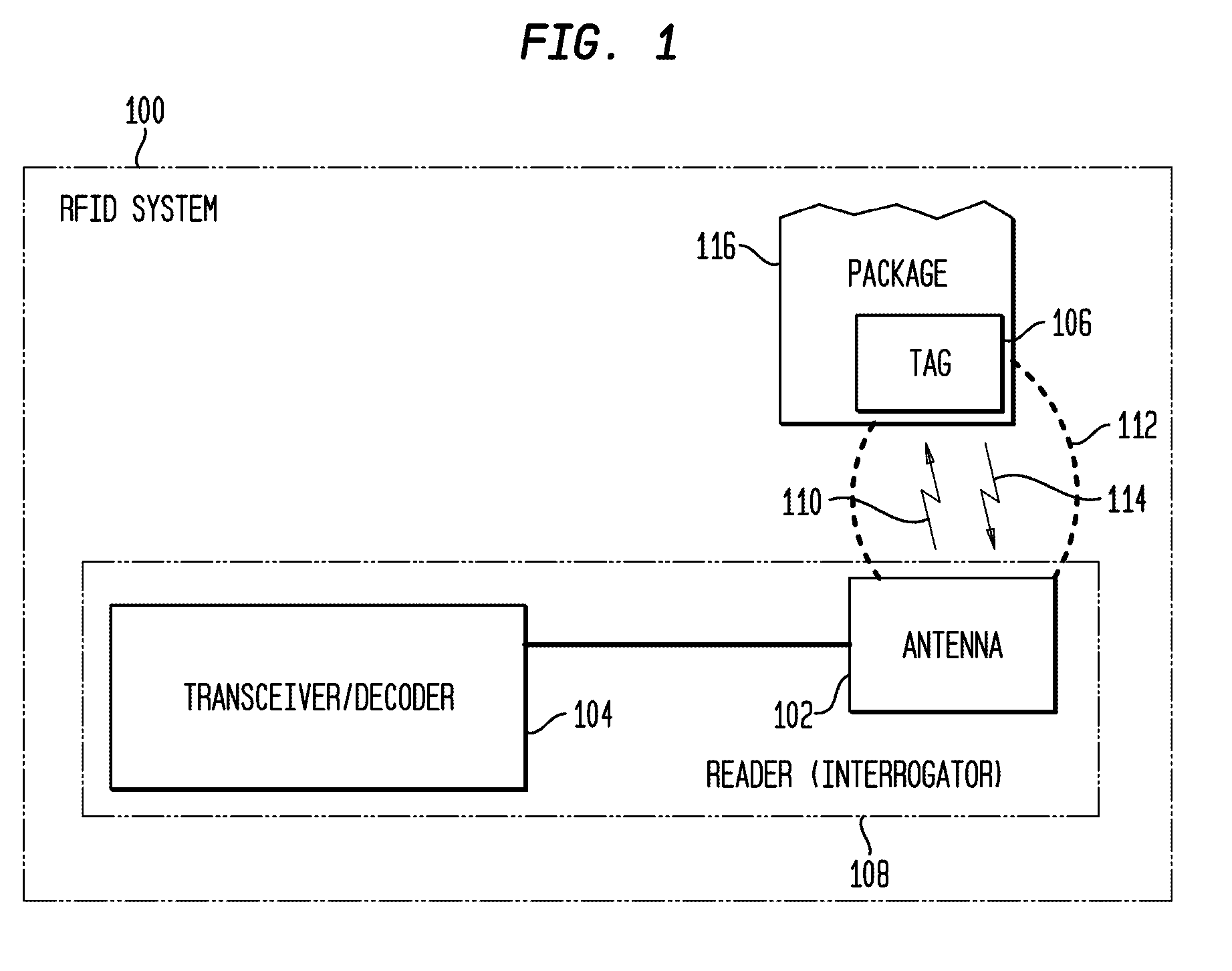
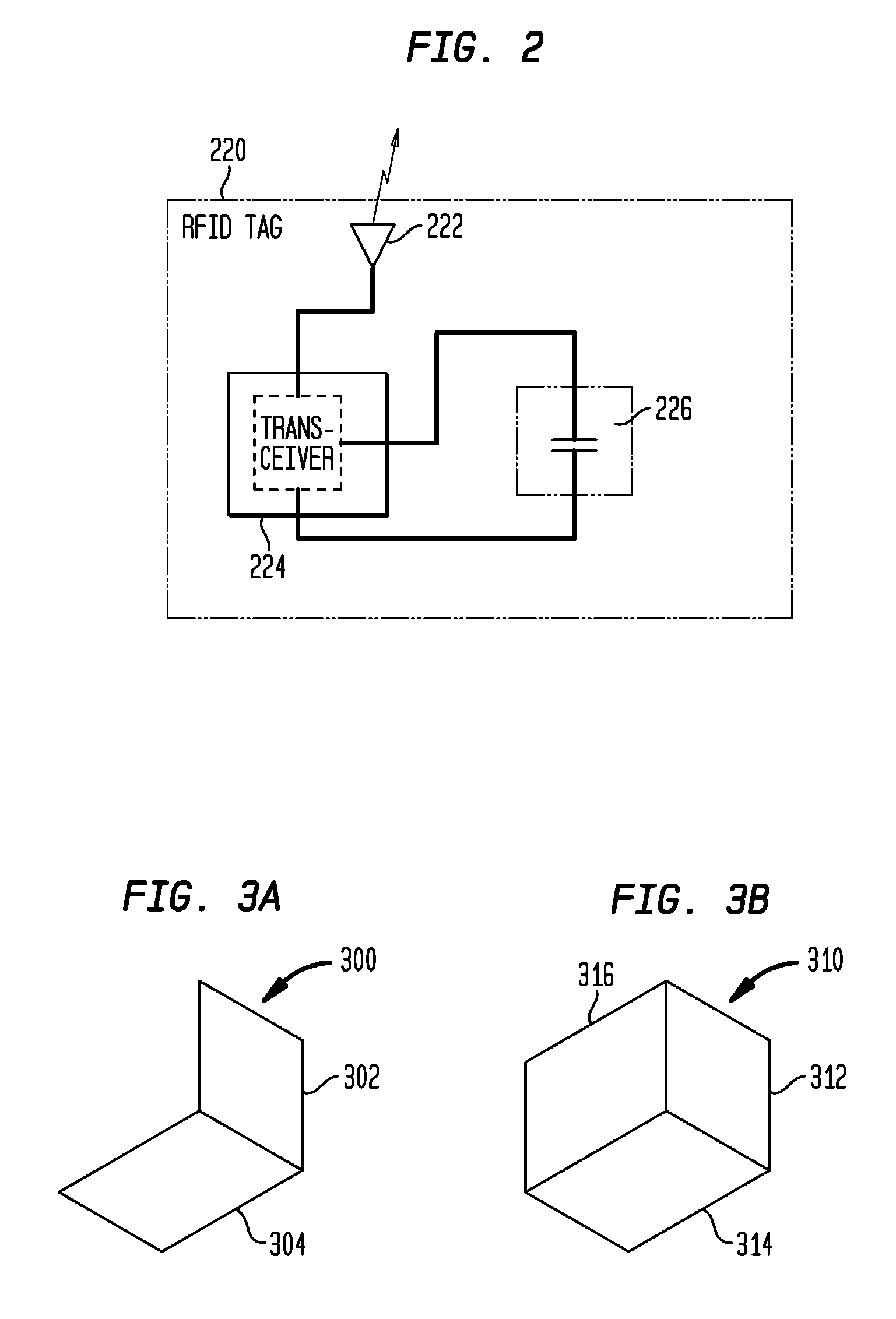
Tamper Evident Radio Frequency Identification System And Package
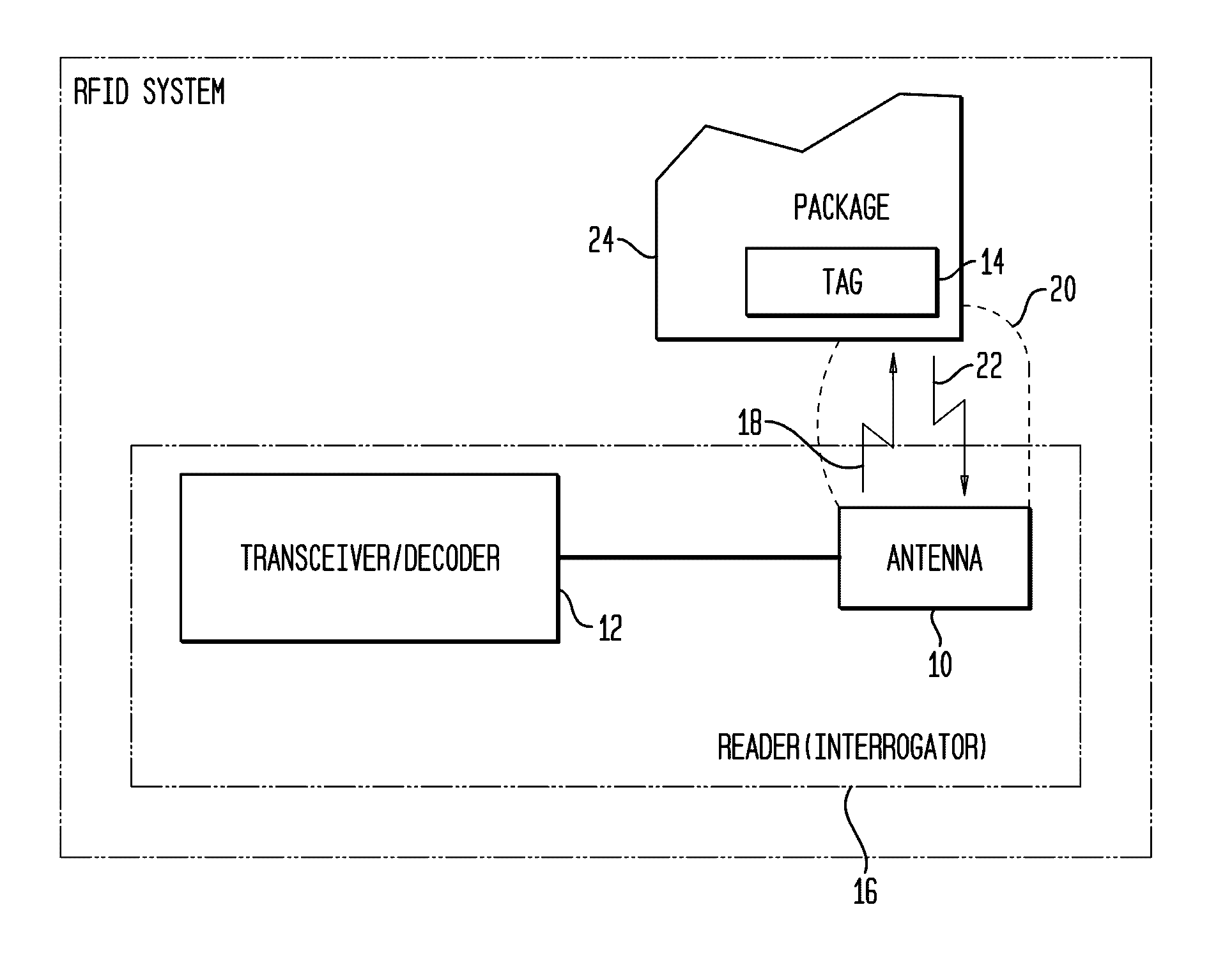
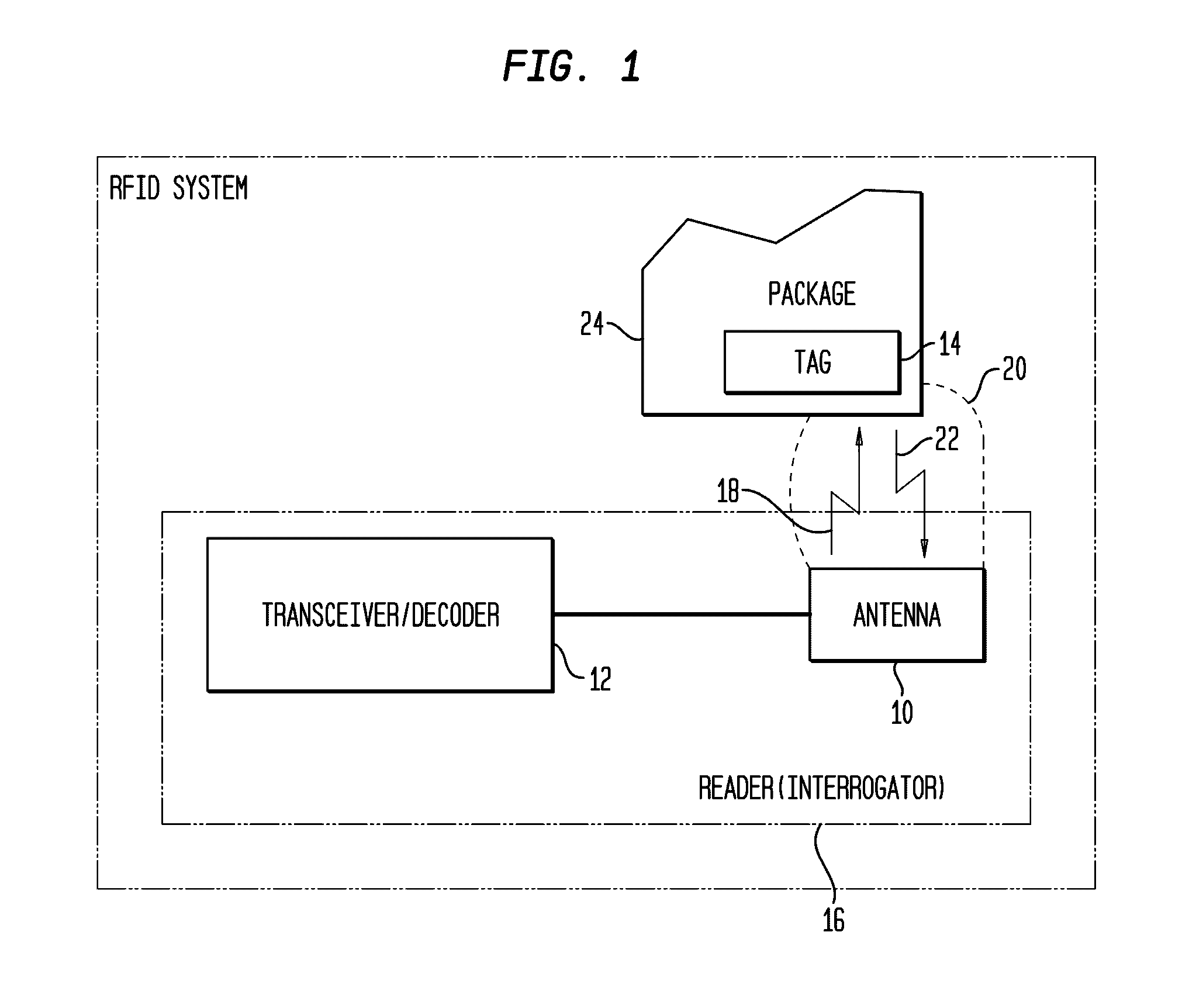
InactiveUS20020067264A1Function is disabledEffective monitoringBurglar alarm with fastening tamperingContainer decorationsEngineeringRadio frequency
<heading lvl="0">Abstract of Disclosure< / heading> A tamper evident package and system where the package has a radio frequency identification tag affixed to or imprinted onto the covering. An interrogator emits an electromagnetic signal to the identification tag which responds with a predetermined signal if the covering of the package is intact and has not been breached or the identification tag may be powered and emit the predetermined signal intermittently or continuously by itself. An attempt to enter the package disables the identification tag and will cause the identification tag, thereafter interrogated, to fail to send a signal or will send a signal that is different from the predetermined signal. The interrogator recognizes the lack of or the different signal as an indication that the integrity of the package has been breached.
Owner:INT PAPER CO
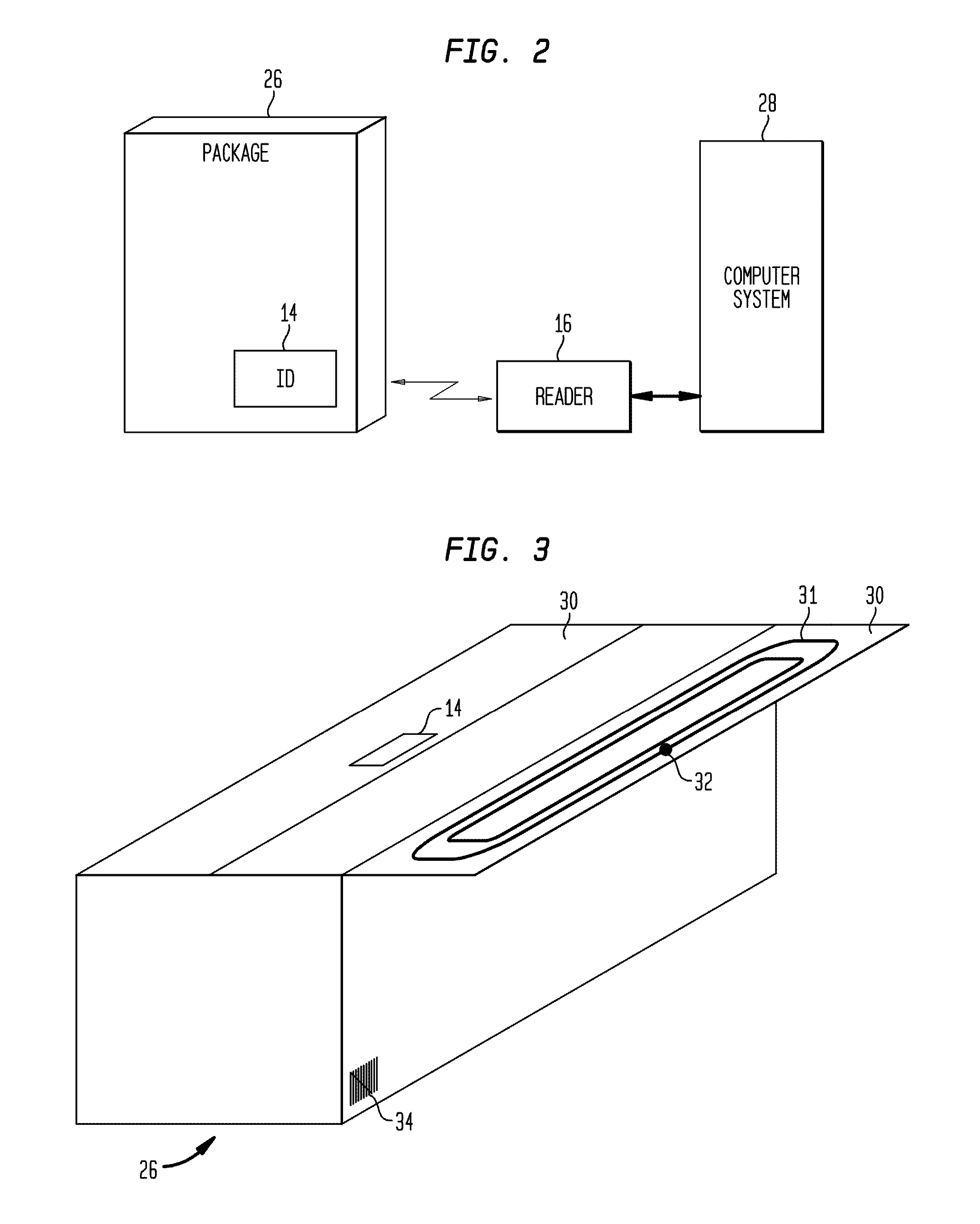
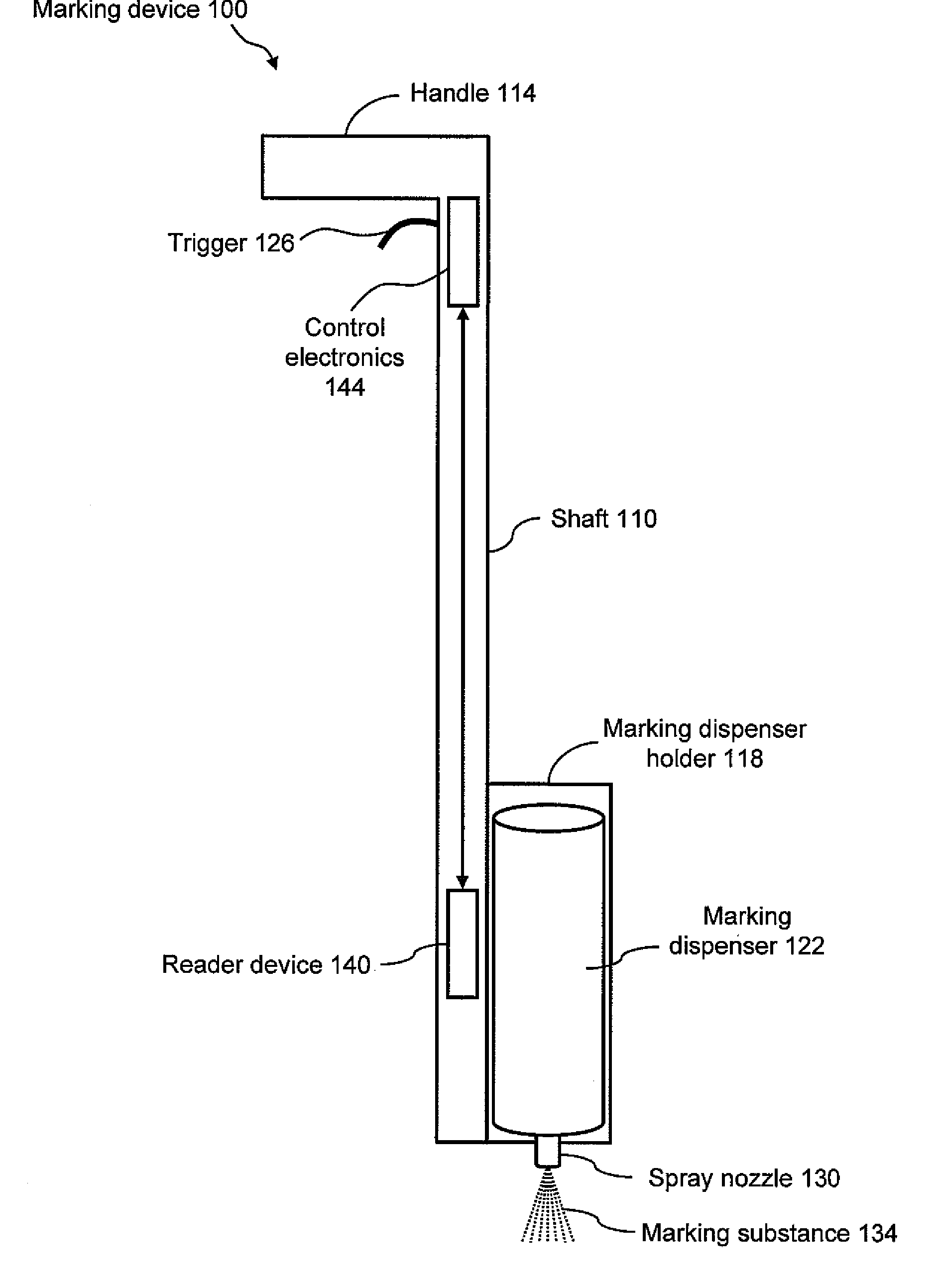
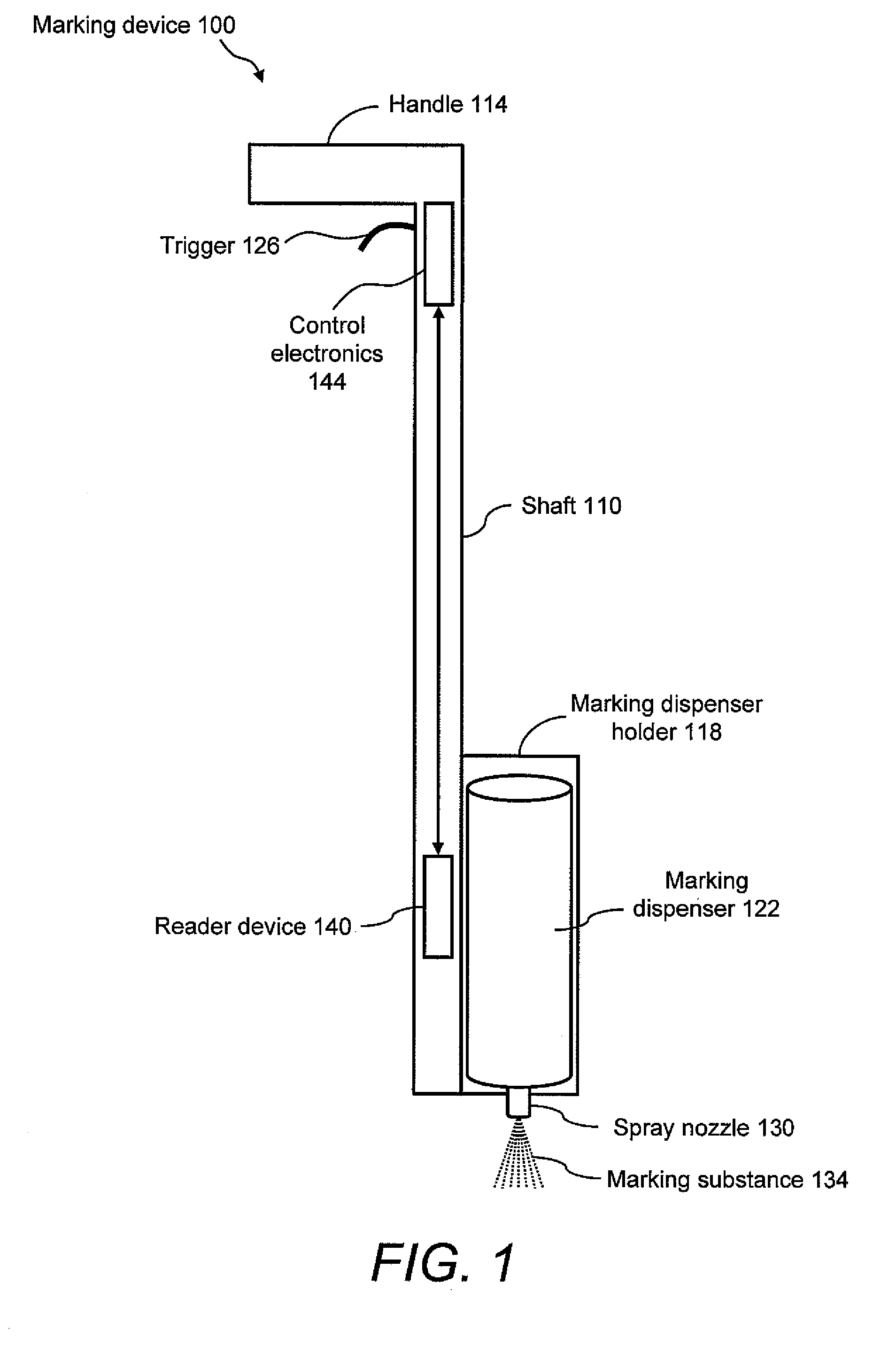
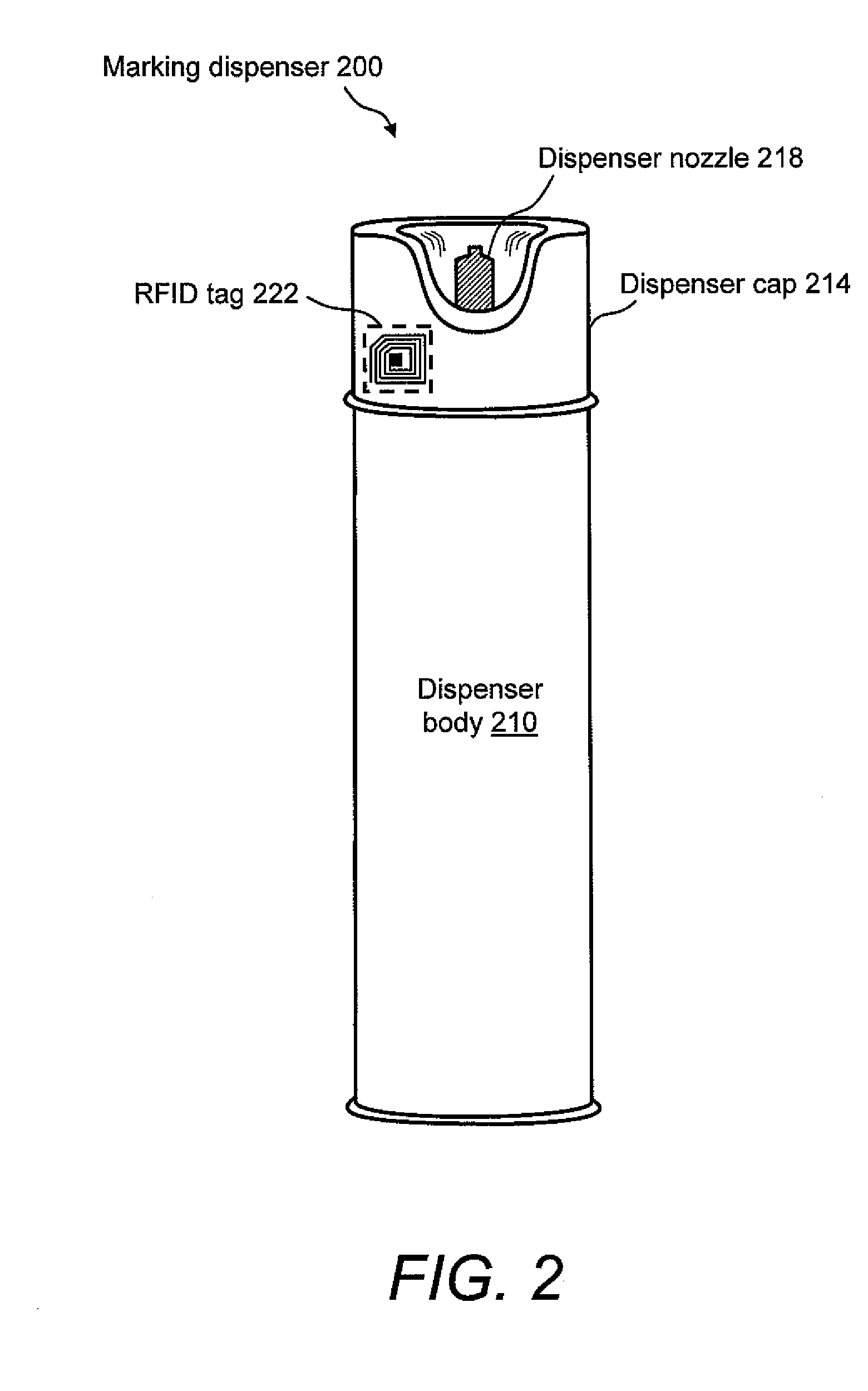
Marking apparatus and marking methods using marking dispenser with machine-readable id mechanism
Marking devices for dispensing markers on the ground and marking methods are provided. The marking devices and marking methods use a marking dispenser having a machine-readable ID mechanism. The ID mechanism has data storage capability. In one embodiment, the marking dispenser may be provided with a radio-frequency identification (RFID) tag. In another embodiment, the marking dispenser may be provided with a barcode. The type of information that may be encoded in the ID mechanism may include, but is not limited to, product-specific information, user-specific information, other predetermined information of interest, and any combination thereof. The ID information encoded in the ID mechanism may be collected and used for various purposes, such as, but not limited to, real-time product verification, tracking which user location received a batch of marking paint, tracking marking paint inventory, tracking marking paint problems, and tracking marking paint usage.
Owner:CERTUSVIEW TECH LLC
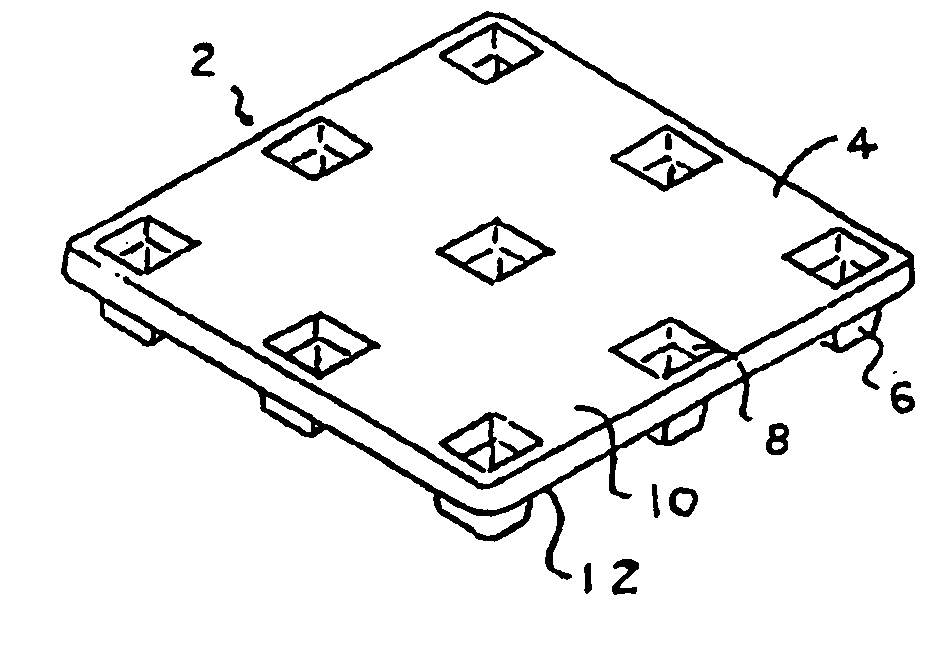
RF-enabled pallet
InactiveUS20050237184A1Small sizeContainer decorationsLevel indicationsElectrostatic couplingEngineering
The inventions involve material handling apparatus developed to operate in radio frequency rich environments. Articles are provided having at least one large compartment capable of enclosing at least one electronic device or a package populated with a plurality of devices. RFID tags are also provided having three antenna arrays situated on three planes to improve electromagnetic and electrostatic coupling with an external distributed network of devices. Pallets are provided with cellular communications devices to provide track and trace functionality. Sensors and actuators are used in connection with the material handling apparatus.
Owner:NEXTREME
Food preparation
ActiveUS20090236333A1Improve efficiencyIncrease net powerContainer decorationsLevel indicationsEngineeringIngested food
Owner:JOLIET 2010 LTD
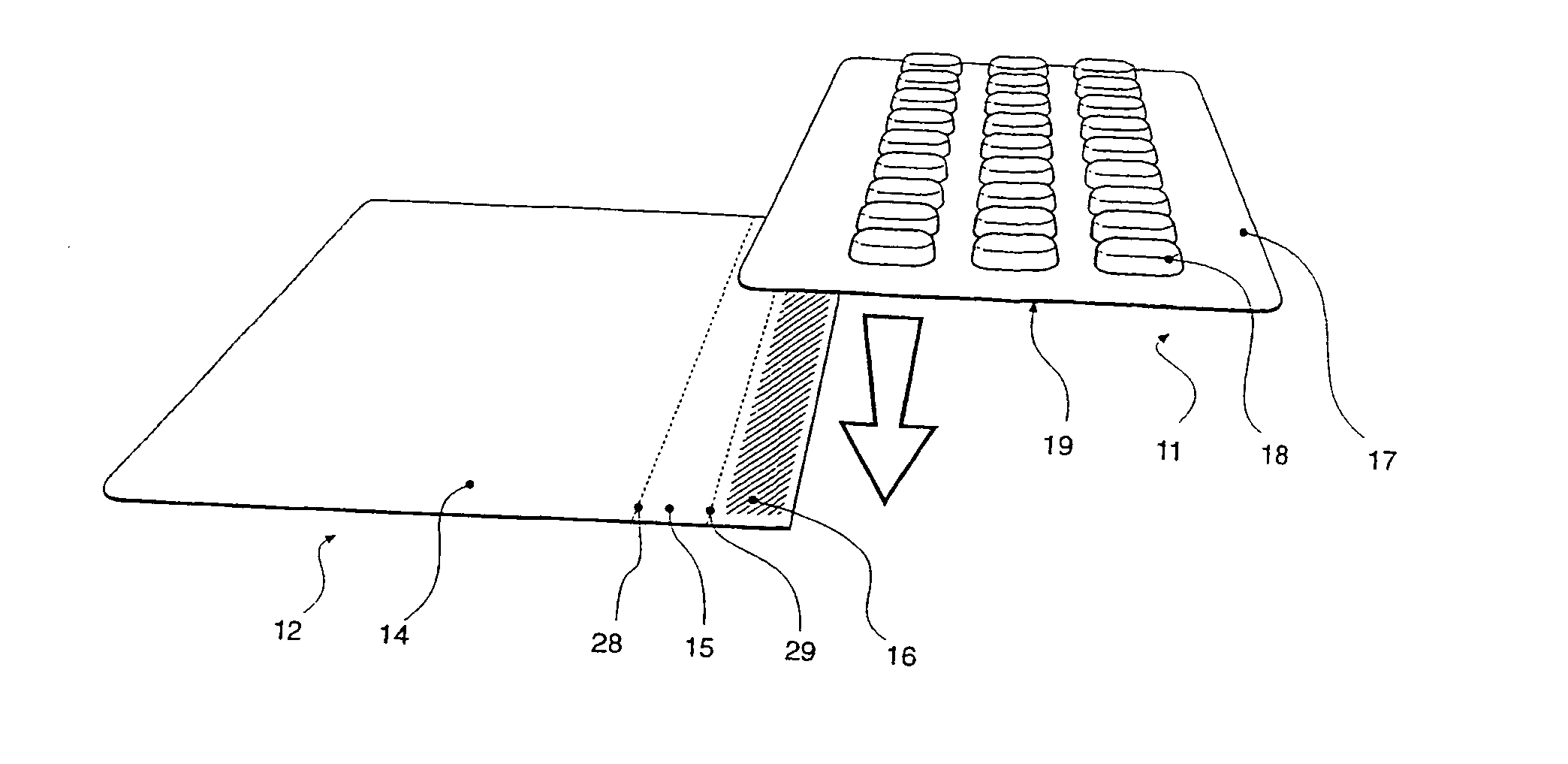
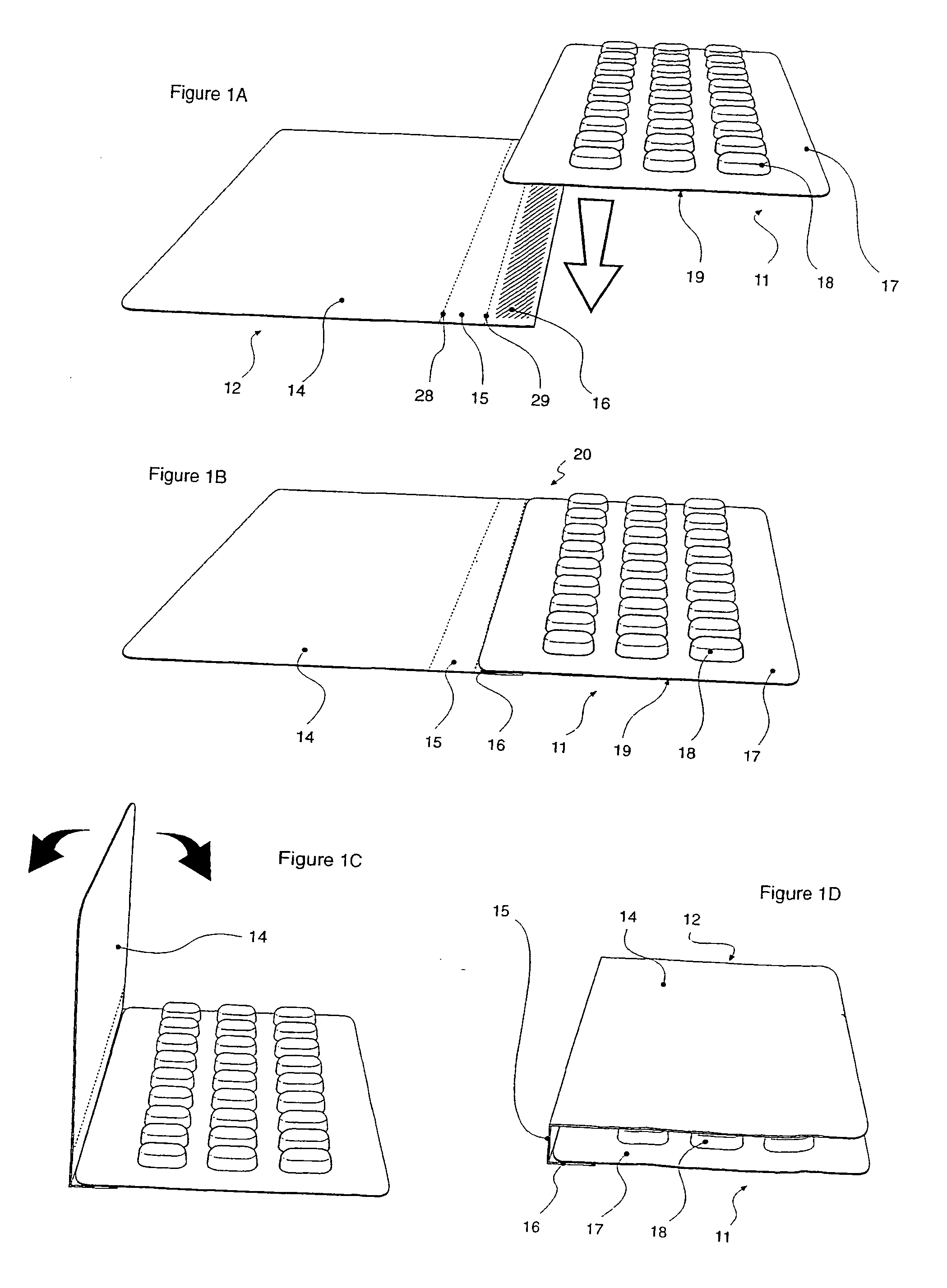
Carded blister pack
InactiveUS20040026293A1Easy to foldSmall article dispensingContainer decorationsBlister packAssembly machine
A blister pack (20) folded wallet has a mounting card (12), with a [hinged] spine segment (15), and an adhesive edge strip (16). Overlaid by a pre-formed blister pack (11), for mutual (edge) entrainment; a dedicated assembly machine entrains mounting cards and blister packs stored in respective magazines. Child resistance is available through cover latching and / or paper reinforced foil laminate (161), with through apertures (166) and perforations (163, 165) for selective localised paper patch (169) removal, over individual blister pockets (168).
Owner:HUGHES DAVID
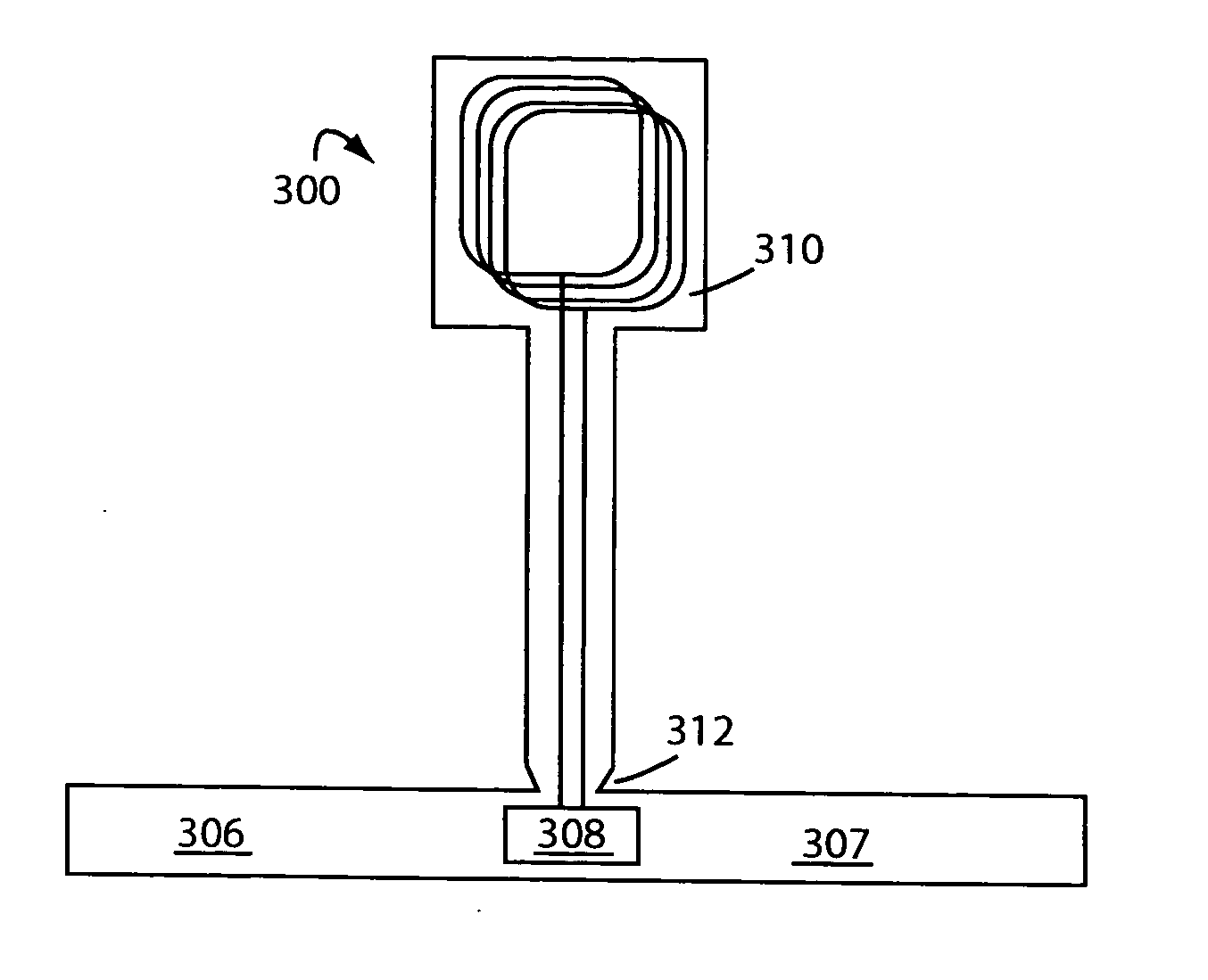
Package identification system
<heading lvl="0">Abstract of Disclosure< / heading> The present invention is embodied in a package for protecting and displaying goods, the package includes a wireless smart package assembly and a Radio Frequency Identification (RFID) tag. The assembly comprises a package having at least one sheet of material adapted to support a product during the shipment and storage of the product. An electrically conductive material distributed along a portion of the sheet so as to form a non-planar antenna. The non-planar antenna may be configured to form a corner antenna or an array of spaced-apart dipole antennas. The invention when used with a passive RFID tag includes a power source within the RFID tag that allows for a one-time transmit capability.
Owner:INT PAPER CO
Application of radio frequency identification
InactiveUS20050068182A1Easy to getElectric signal transmission systemsDigital data processing detailsMemory chipEngineering
Improved plastic article having an integral radio frequency identification (RFID) as an integral, permanent part of the article. The RFID can be molded onto an article; as a memory chip-antenna combination molded onto a desired location on an article.
Owner:PLASTIPAK PACKAGING
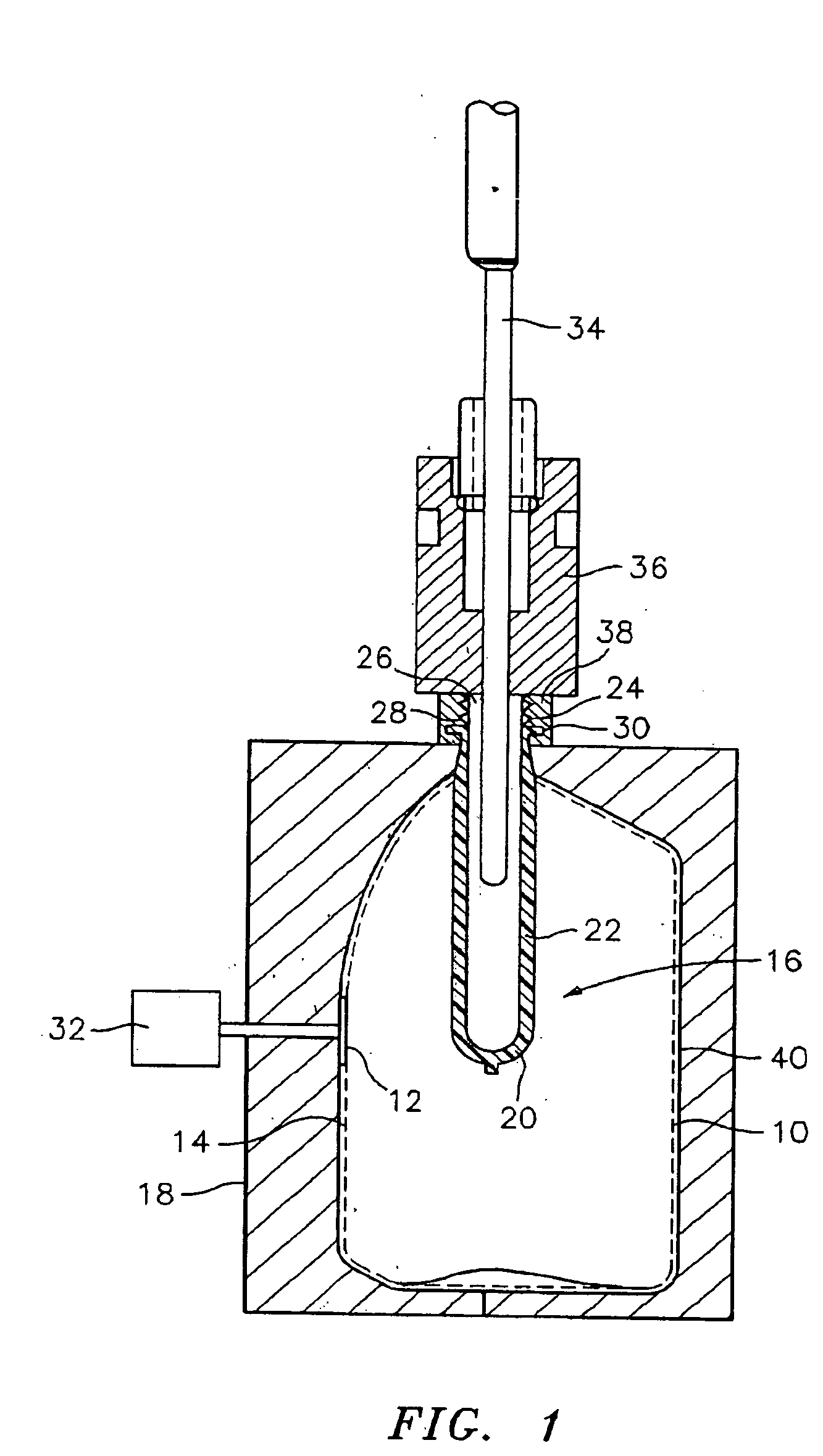
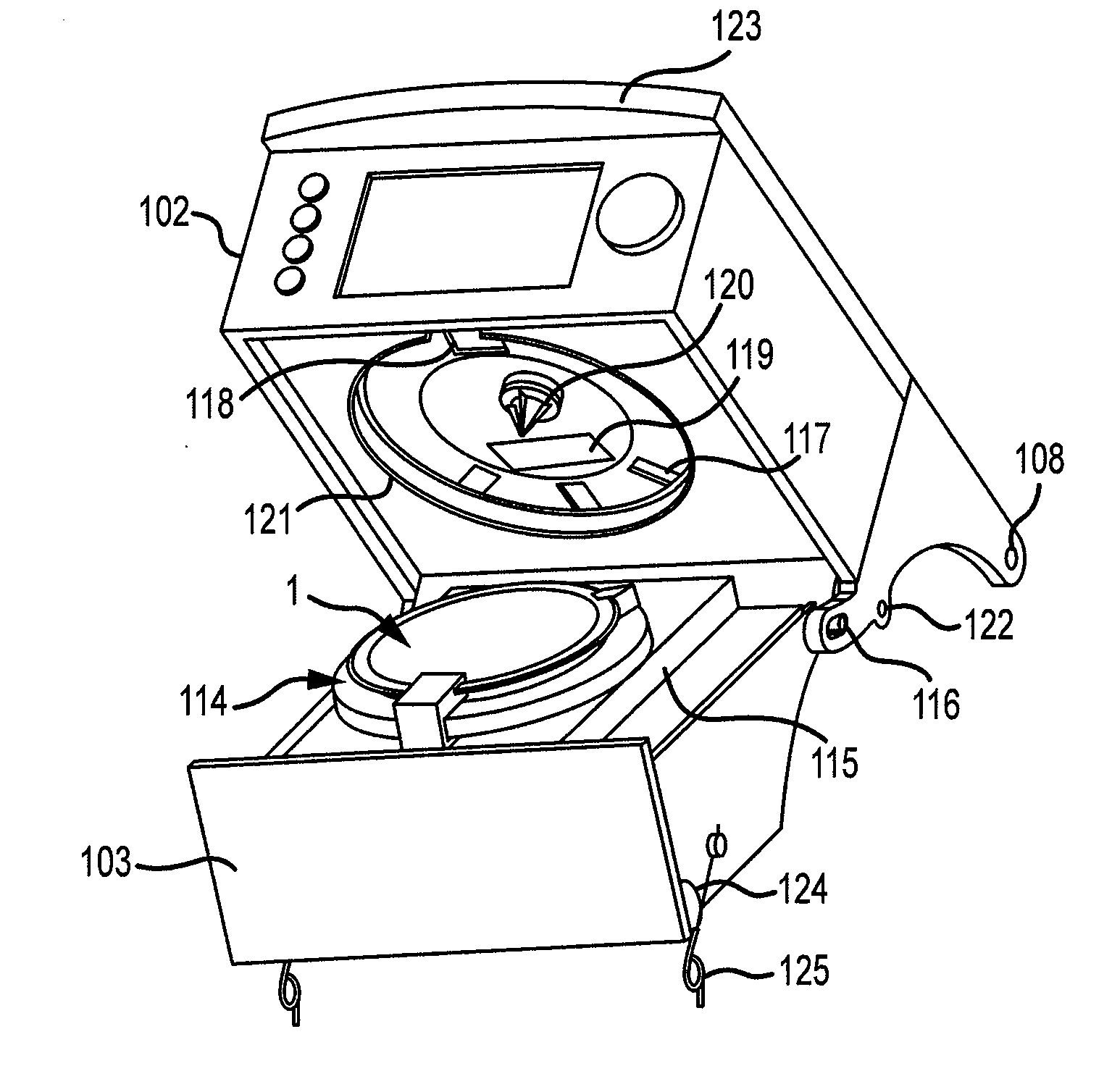
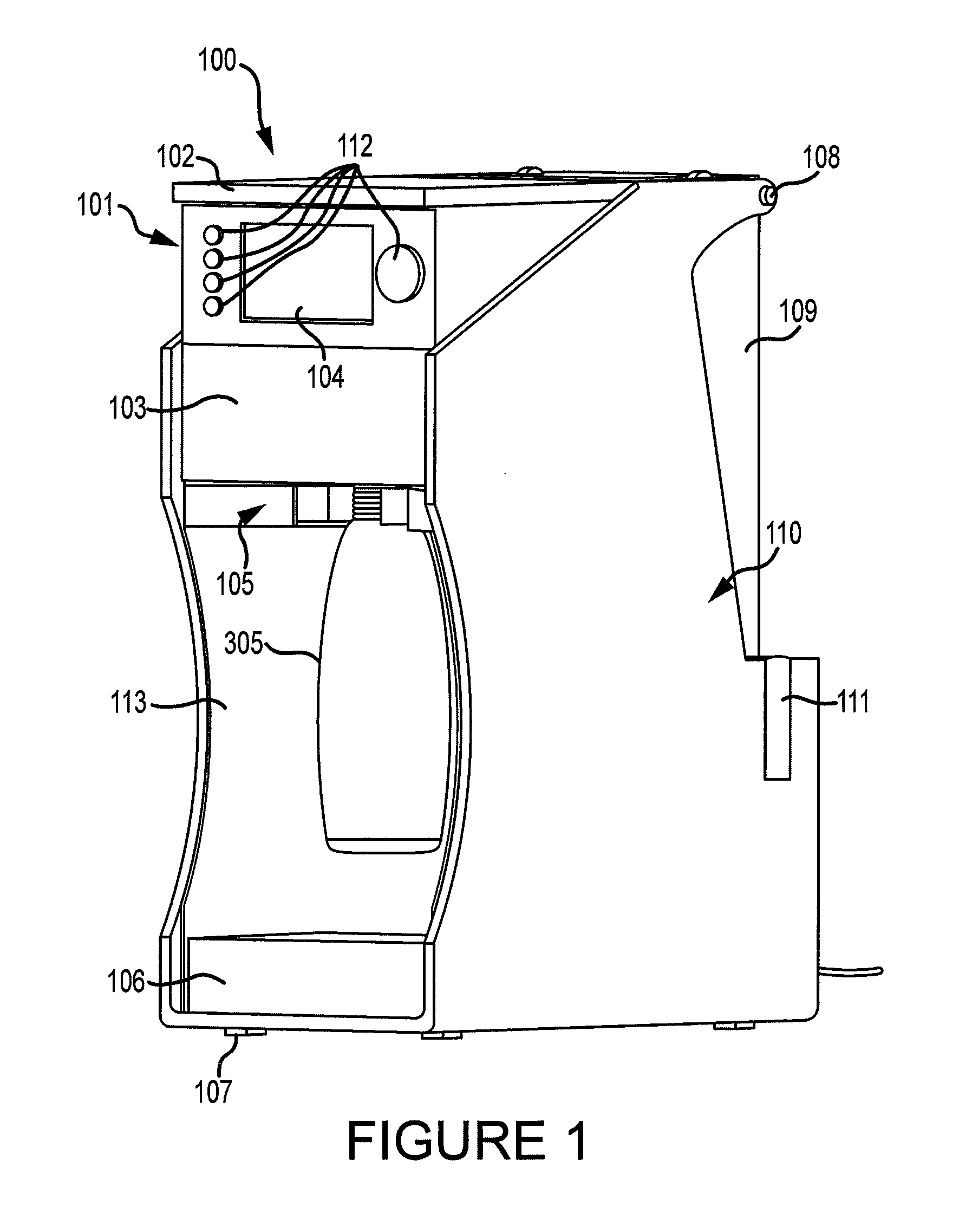
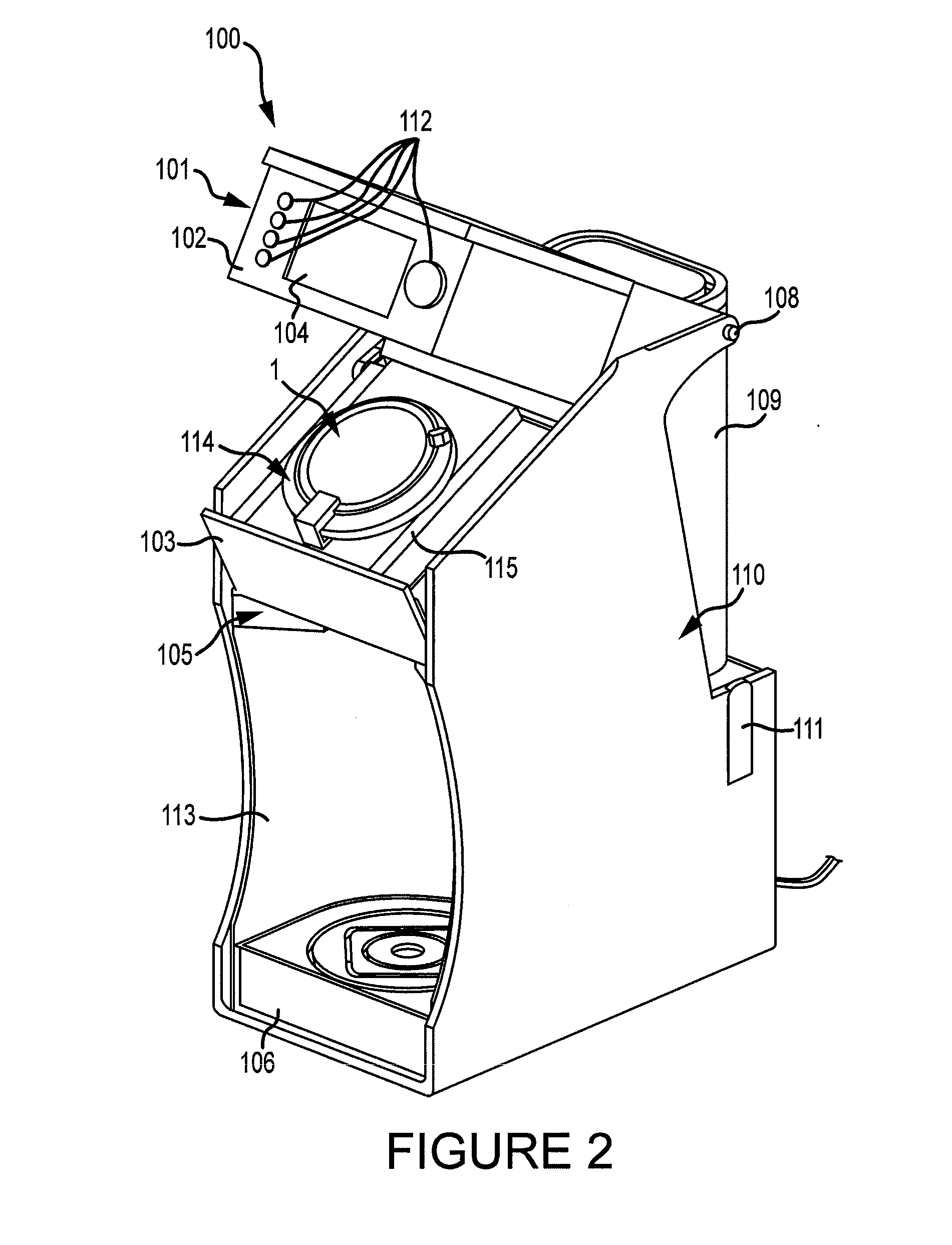
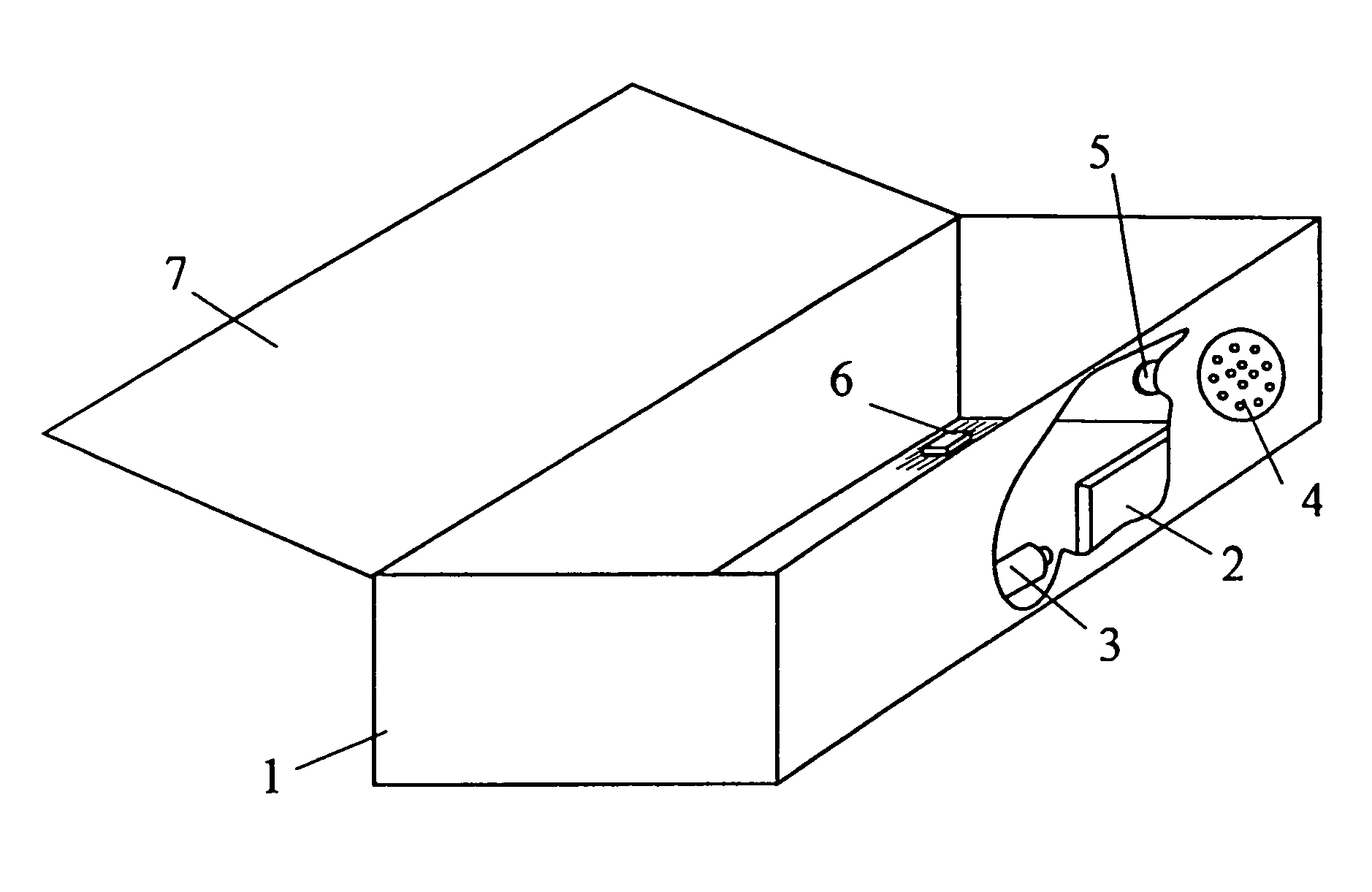
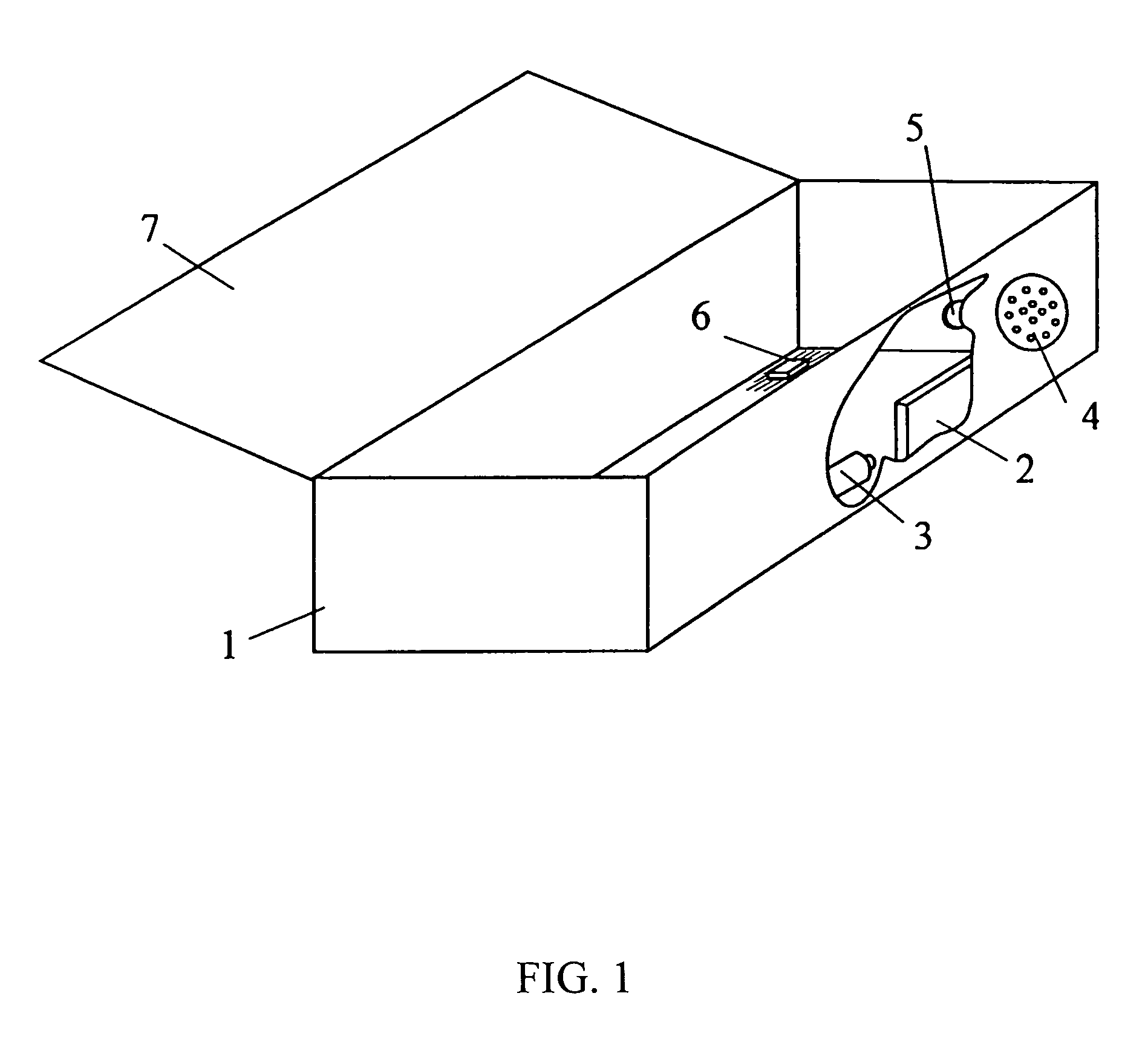
Apparatus and method for preparing a liquid mixture
A mixing apparatus, puncturing mechanism, and cartridge are disclosed. The mixing apparatus has a housing and a drawer with a recess. Corresponding cartridges may be inserted into the drawer and slid into the housing to facilitate mixing a liquid with contents of the cartridge. The liquid may originate from a reservoir in the mixing apparatus or a direct line. Also inside the housing of the mixing apparatus is the puncturing mechanism. The puncturing mechanism has a nozzle configured to puncture a lid of a cartridge and inject liquid to mix with the contents of the cartridge. The puncturing mechanism is further configured to drive an internal puncturing unit inside of the cartridge through a lower portion of the cartridge to allow liquid from the nozzle and contents of the cartridge to be dispensed into a receptacle.
Owner:FORMULANOW
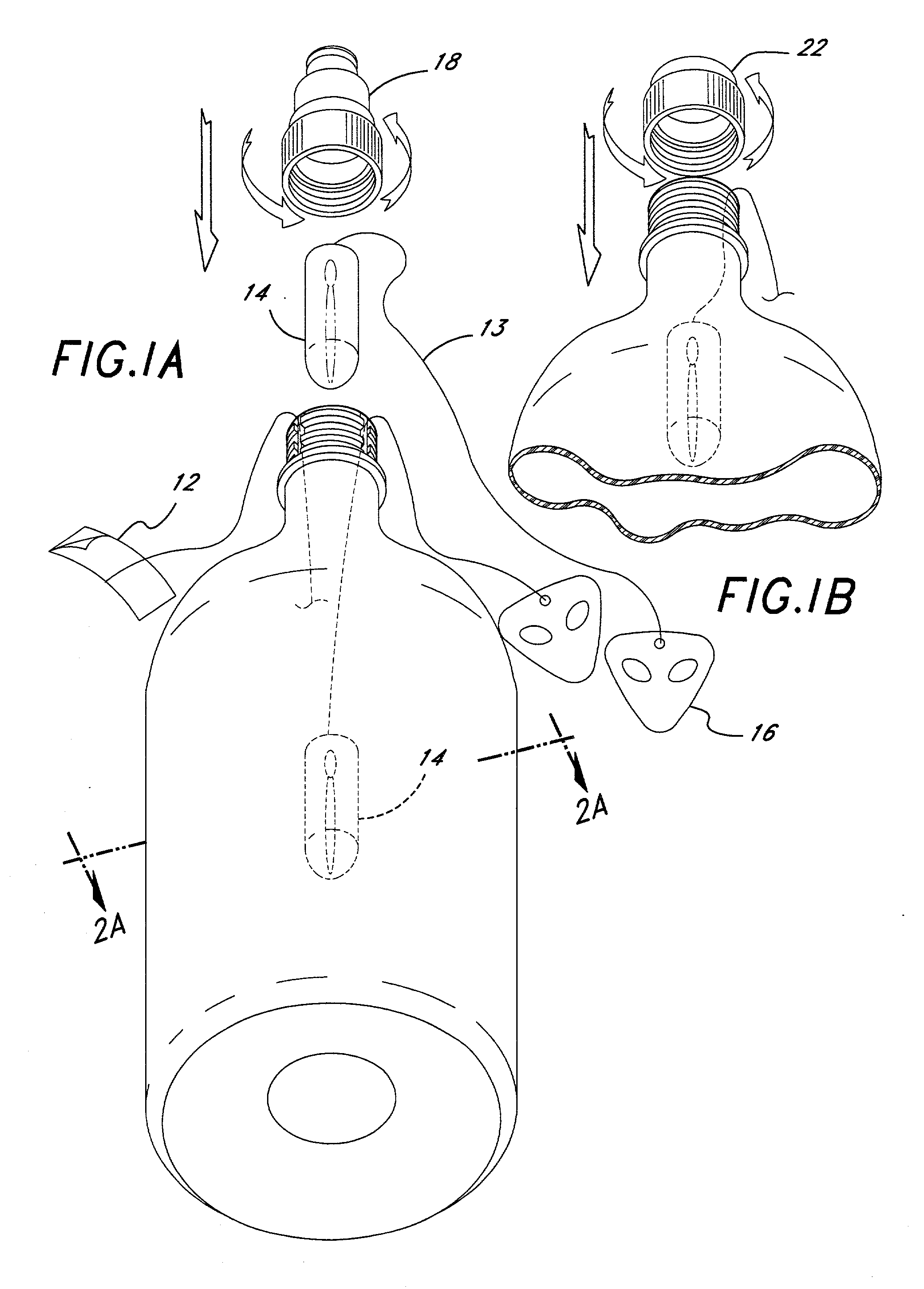
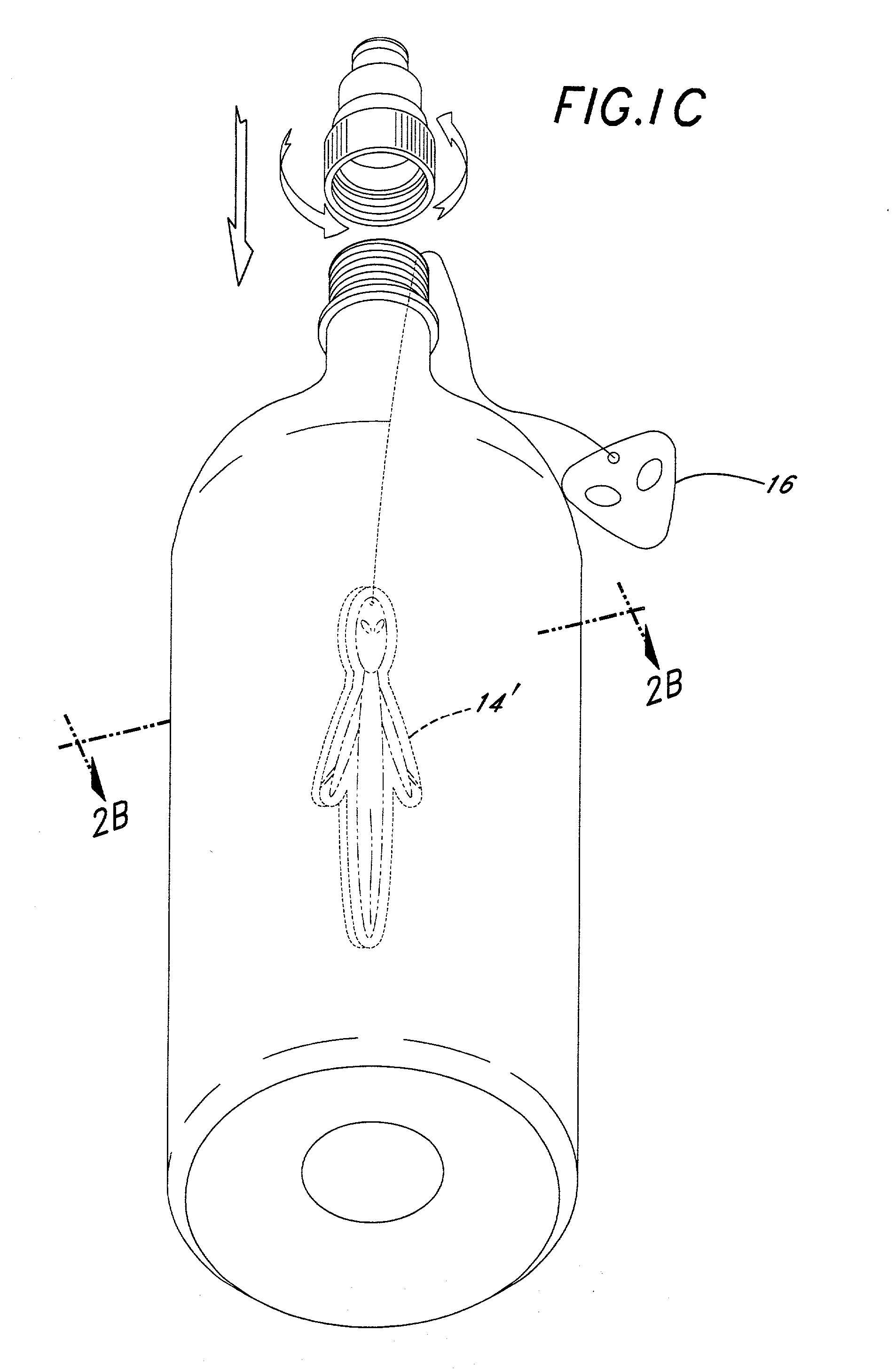
Method of hydration; infusion packet system(s), support member(s), delivery system(s), and method(s); with business model(s) and Method(s)
InactiveUS20020012689A1Constant deliveryUniform deliveryBiocideOrganic active ingredientsDiagnostic Radiology ModalityDietary supplement
Liquid activated infusion packet(s) / system, promoting hydration, containing active and / or inactive ingredients and / or a support member(s). Infusion Packet(s) / System is one or more individual compartments, and / or group(s), whereby the enveloping material(s) may be totally or partially dissolvable, edible, transparent, opaque, decorated, etc. Further, including of one or more: color(s), flavor(s), aroma(s), pharmaceutical(s), nutraceutical(s), dietary supplement(s), enzyme(s), pre / pro-biotic(s), amino-acid(s), soluble-fiber(s), diagnostic agent(s) etc. regardless of form, + / - effervescence, + / - uniform / controlled-release encapsulations into liquid for humans and / or animals. Enveloping material may be in whole and / or in combination; non-synthetic / porous, and / or synthetic porous / non-porous with deliberate perforations. Infusion Packet(s) / System + / - tag, support member for assistance, consumer compliance: promotion, advertising, education, entertainment, (toy / game), etc. Manual and / or power operated parts, lights, noise, etc. Additionally incorporated; unique business modalities with test market opportunities and / or the ability to provide income and / or esteem for the health challenged.
Owner:STILLMAN SUZANNE JAFFE
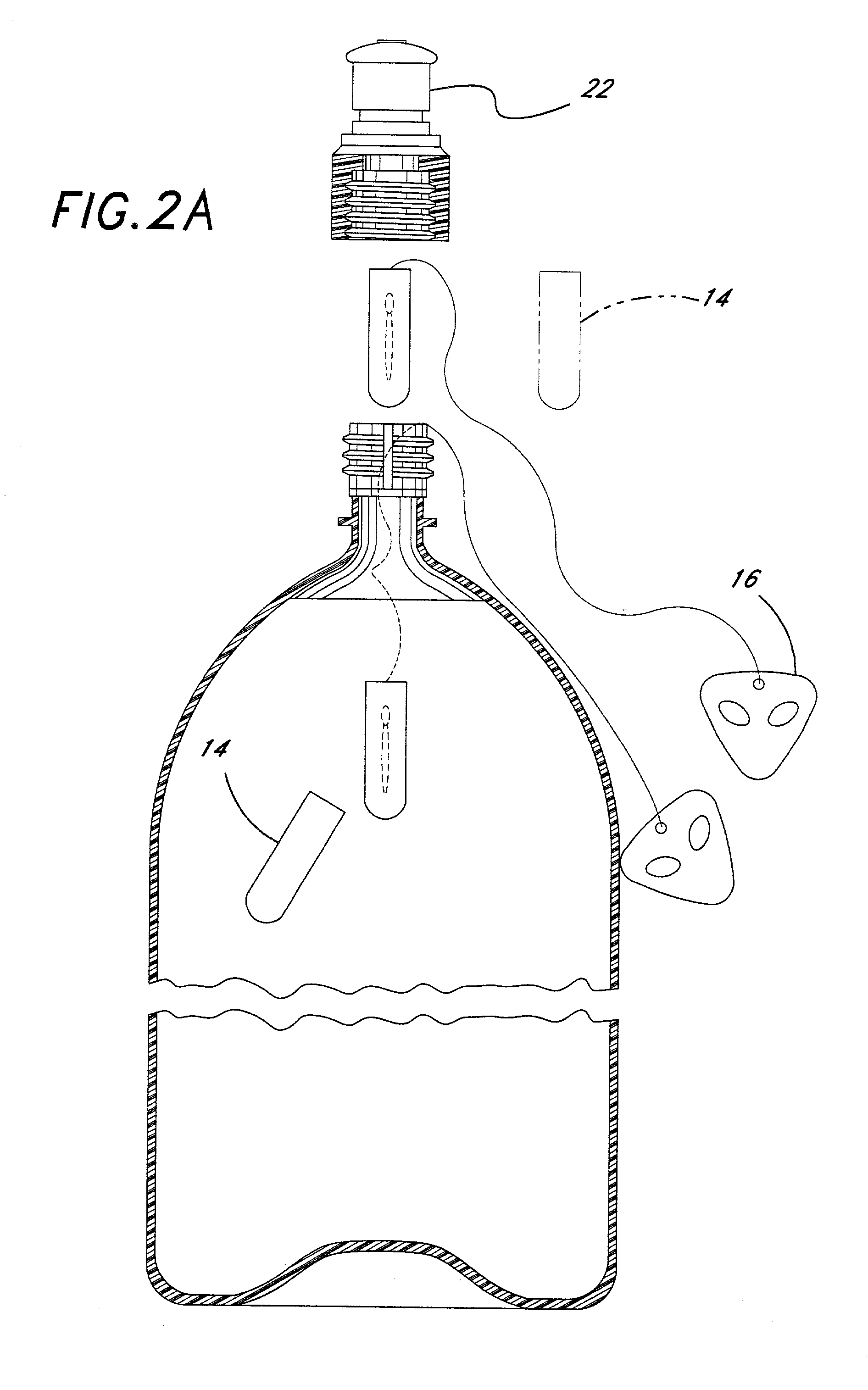
Secure product packaging system
A product security system includes an RFID chip and antenna that are polymerized onto separable parts of a commercial product package. The RFID chip includes a unique serial number that can be interrogated by a wireless reader. A database of such unique serial numbers associated with particular manufacturing production runs is used in a method to detect counterfeiting. The RFID chip and antenna are embedded such that attempts to remove or transfer them will be obvious to an inspector.
Owner:TEPLITXKY BERTRAND +1
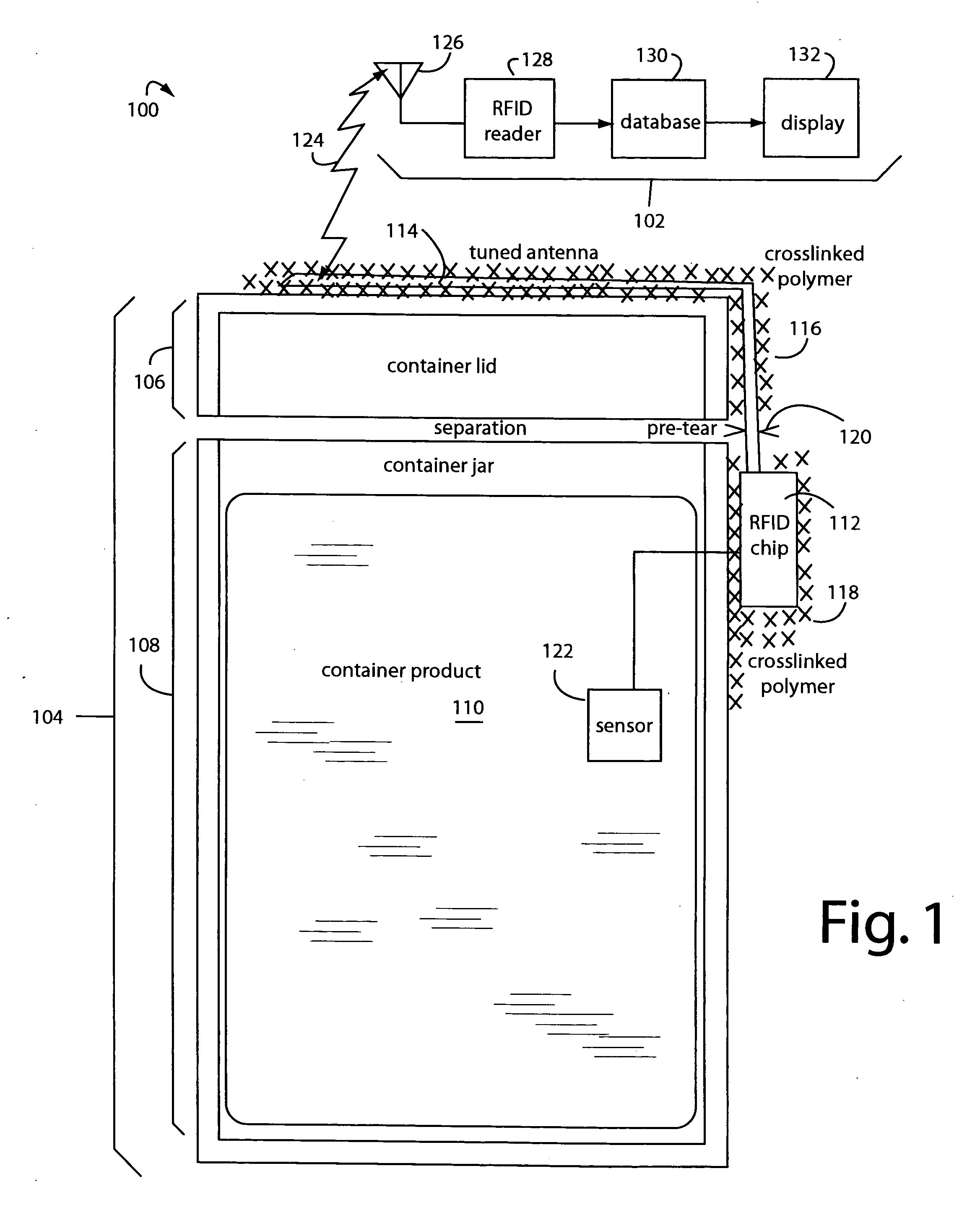
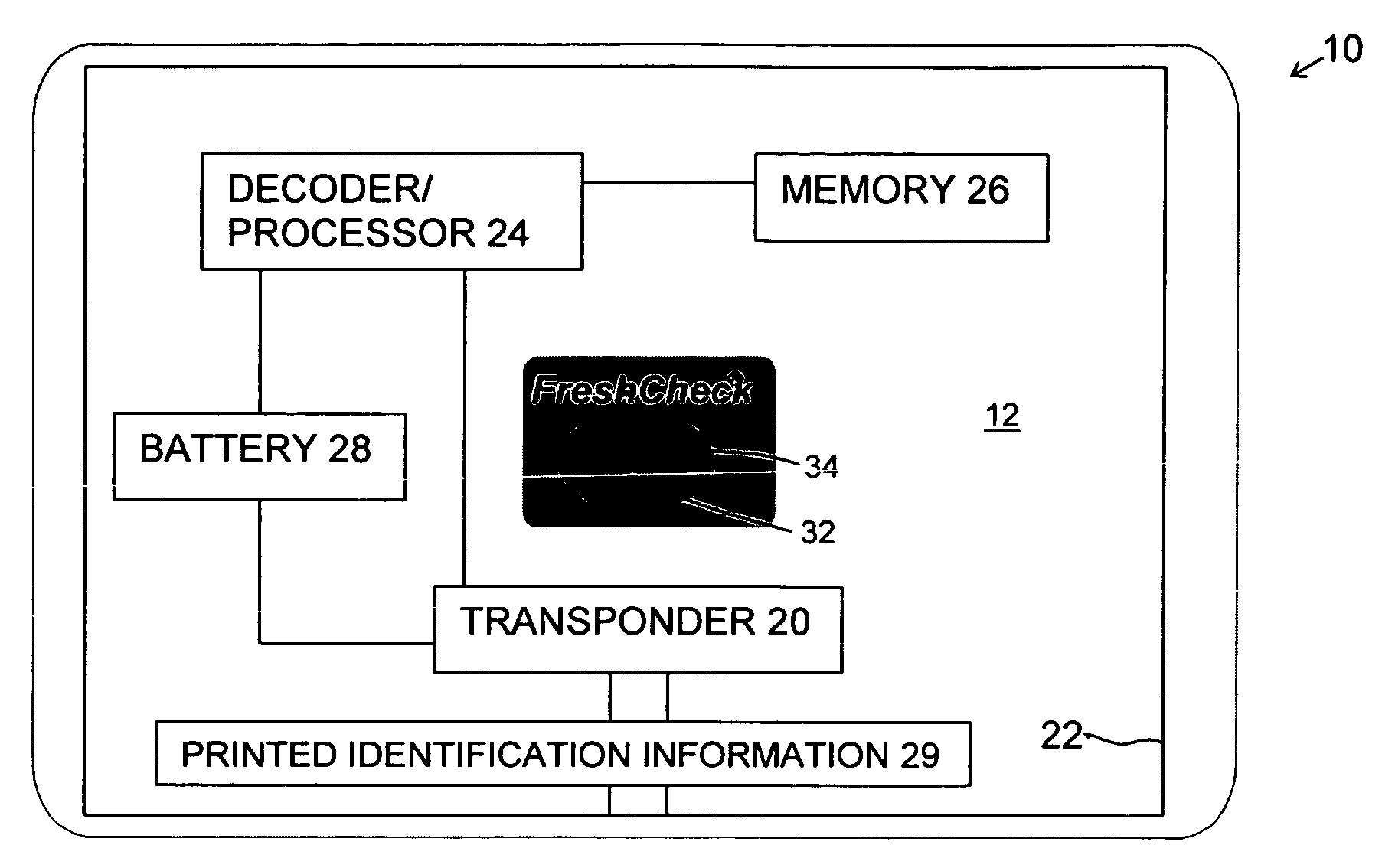
RFID tag with visual environmental condition monitor
ActiveUS20060145863A1Easy to processEasy to readContainer decorationsLevel indicationsStatistical analysisColor changes
A combination RFID tag intended to be associated with a host product, for example by being secured to the outside of a package containing a perishable product, e.g. foodstuffs or vaccines, which RFID tag is provided with a visually readable environmental condition exposure indicator. The visual indicator can sense the exposure of the RFID tag to an environmental condition e.g. temperature, experienced by the host product providing a visual indication, e.g. a color change, readable externally of the RFID tag of the sensed environmental condition. The visual indicator can be chemically active, for example an acetylenic agent, and may be responsive to cumulative temperature excursions over time. The novel RFID tag 11 and tag inspection methods of the invention permit an efficacious harnessing of information about the condition exposure history of a specific inventory item including product identification and related data. The information from multiple items can be compiled into a database that may be audited or statistically analyzed to reveal useful information regarding the handling of the items.
Owner:TEMPTIME CORP
Apparatus and method for detecting tampering with containers and preventing counterfeiting thereof
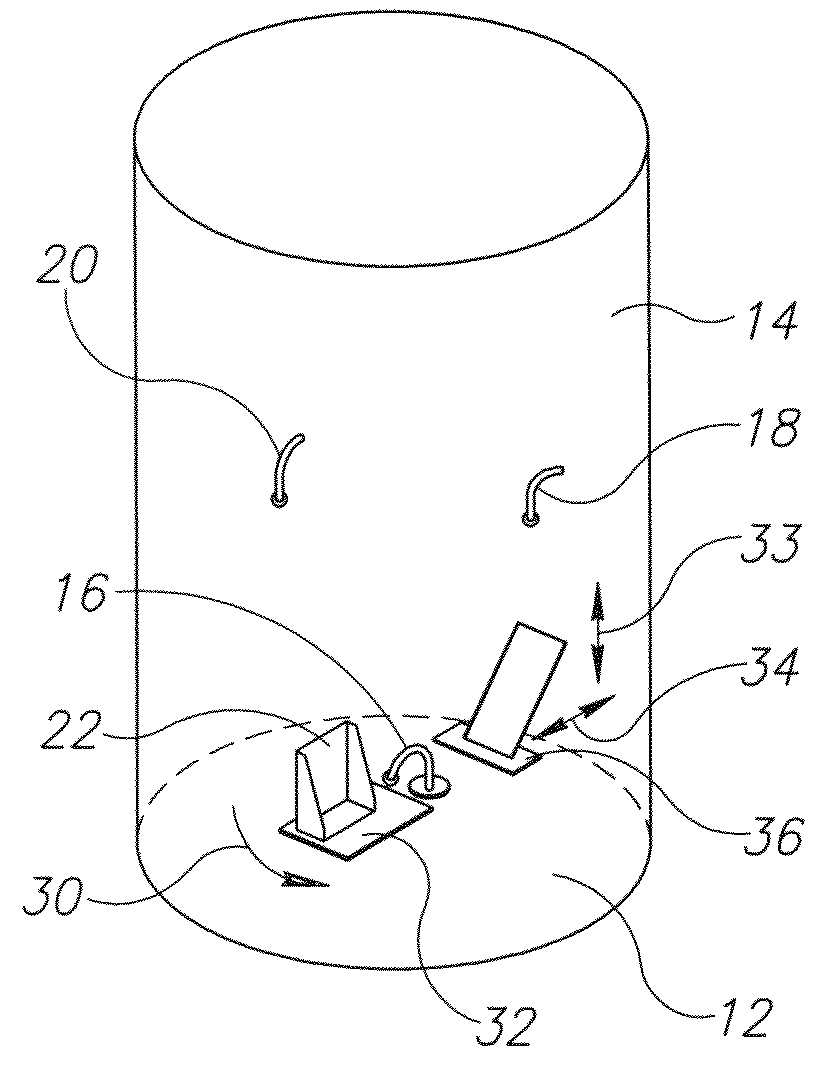
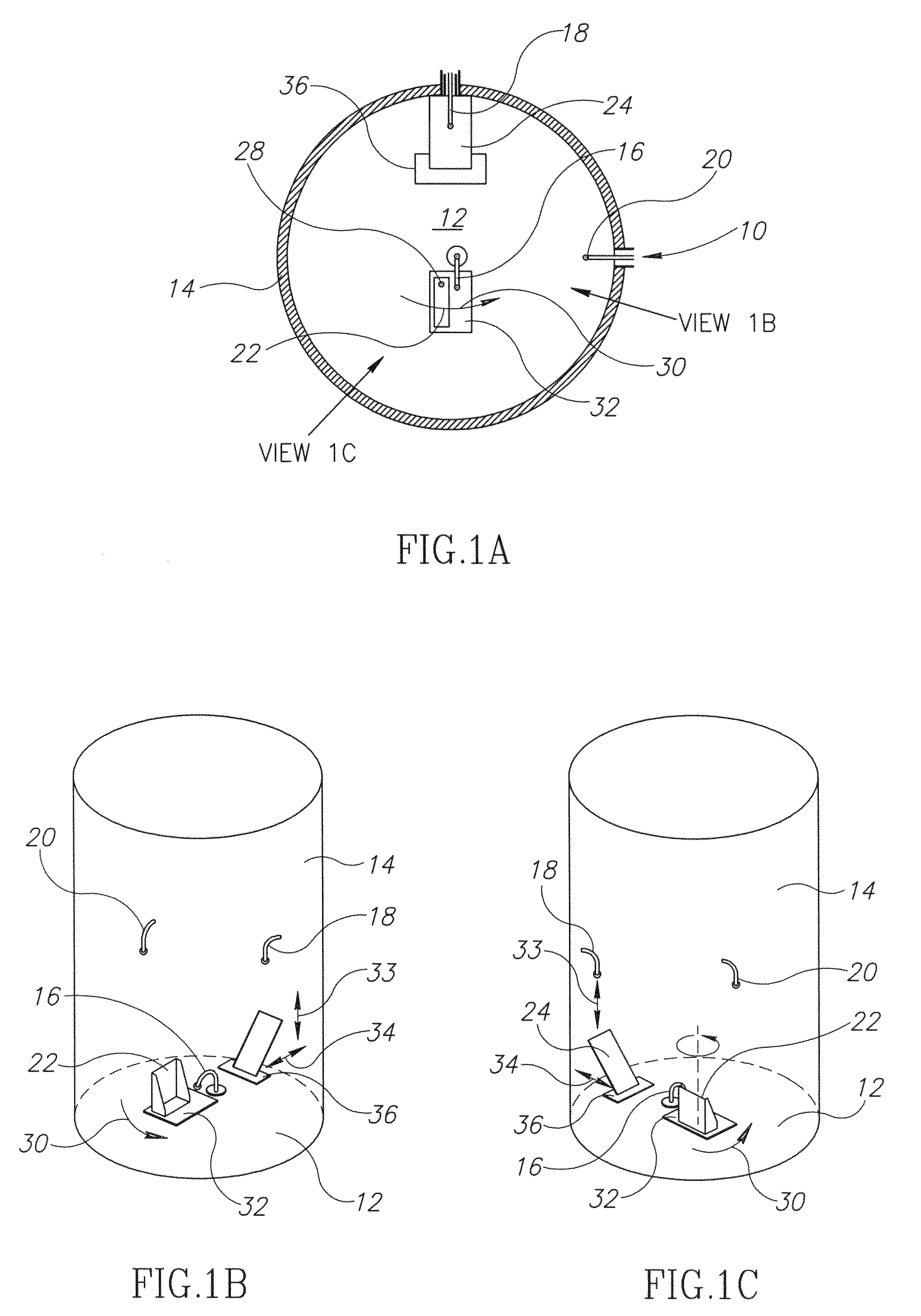
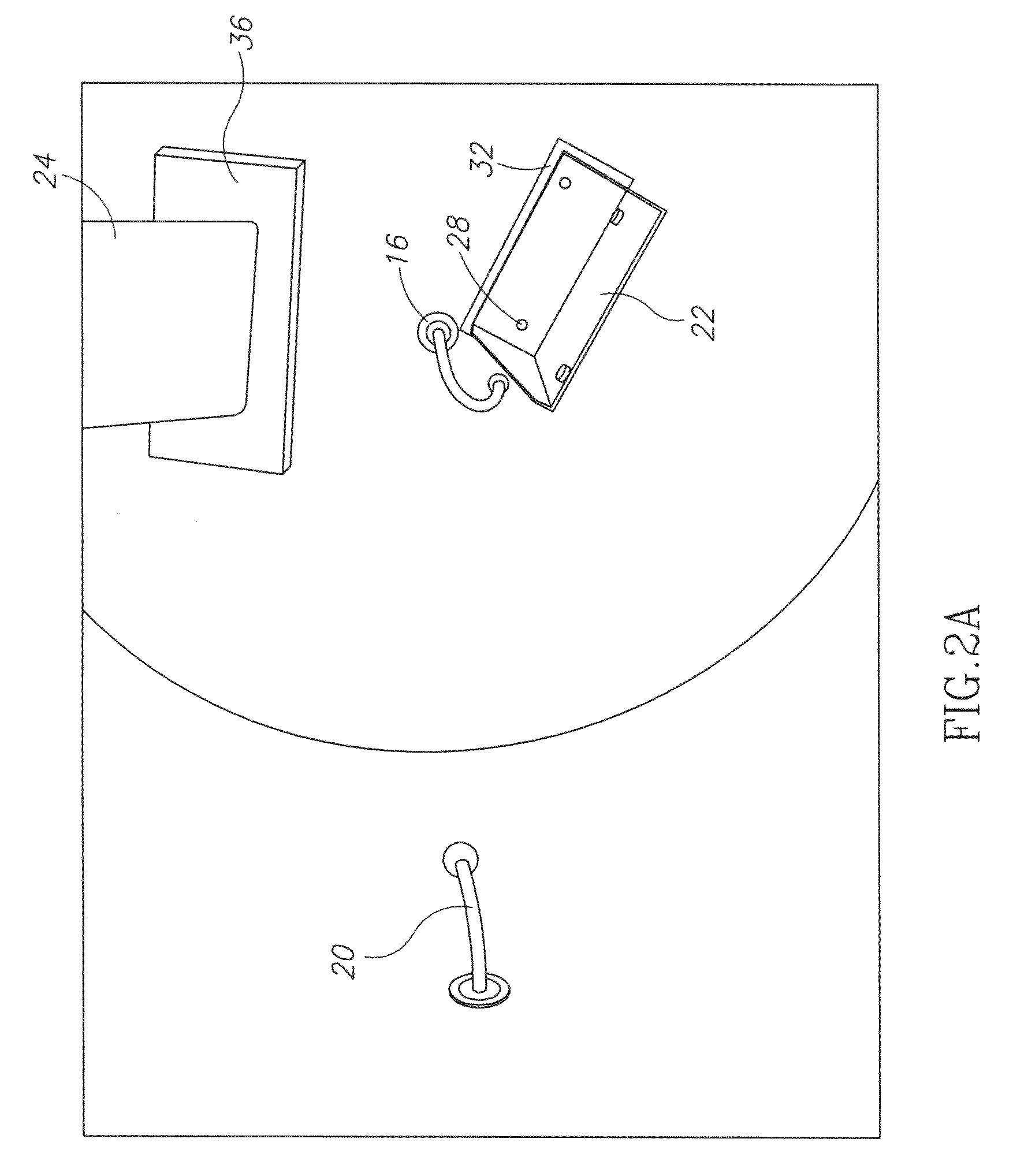
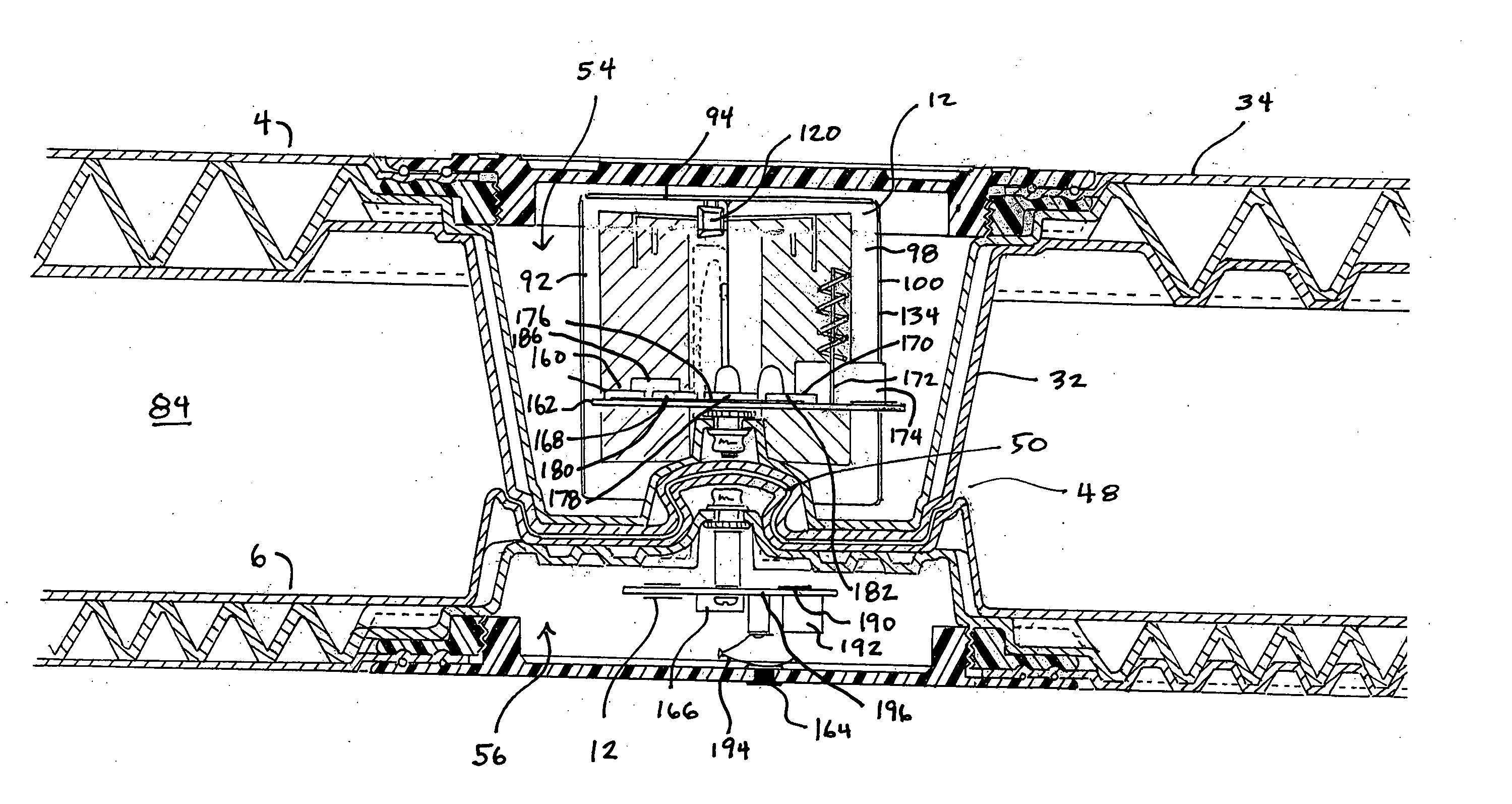
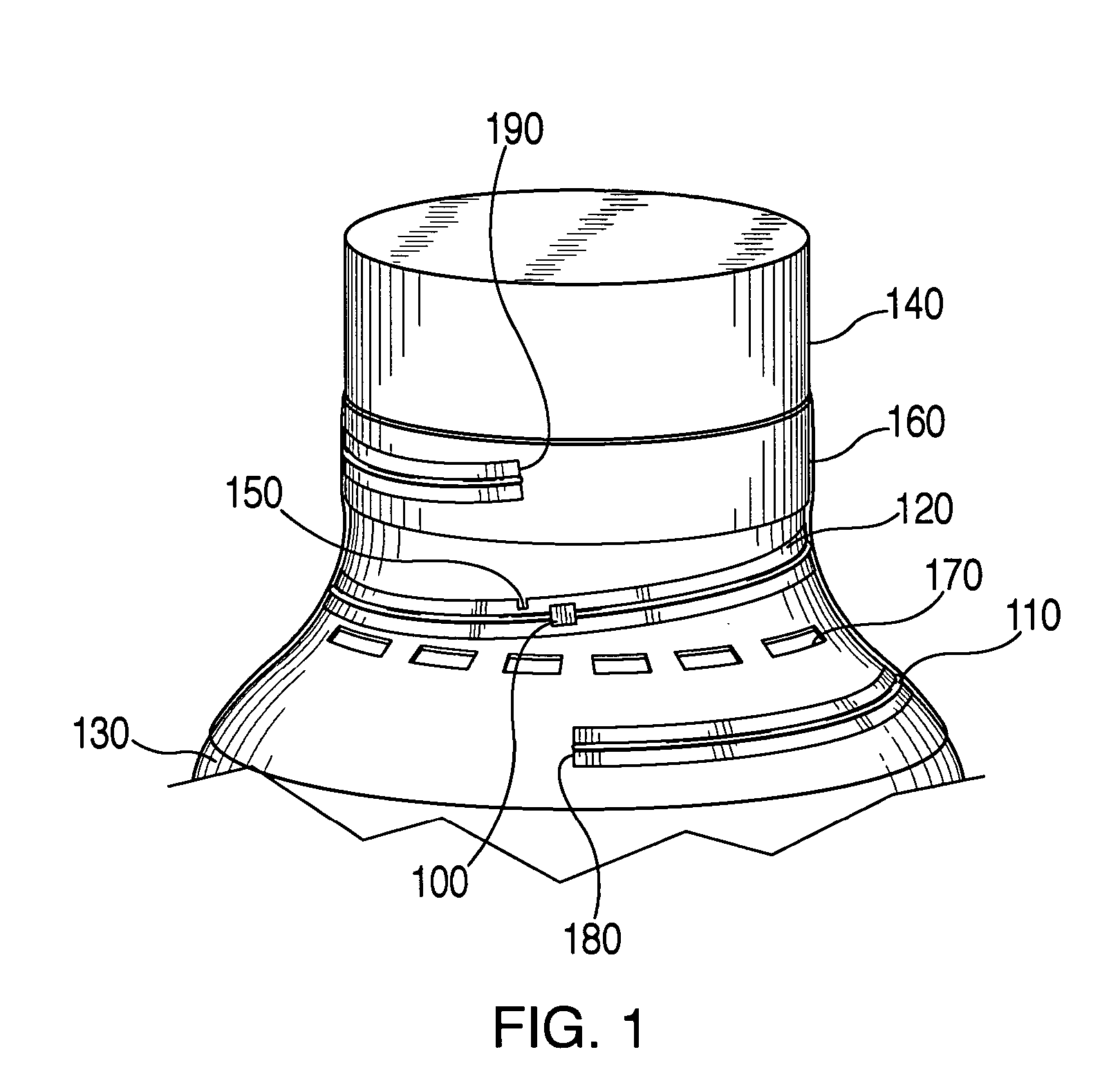
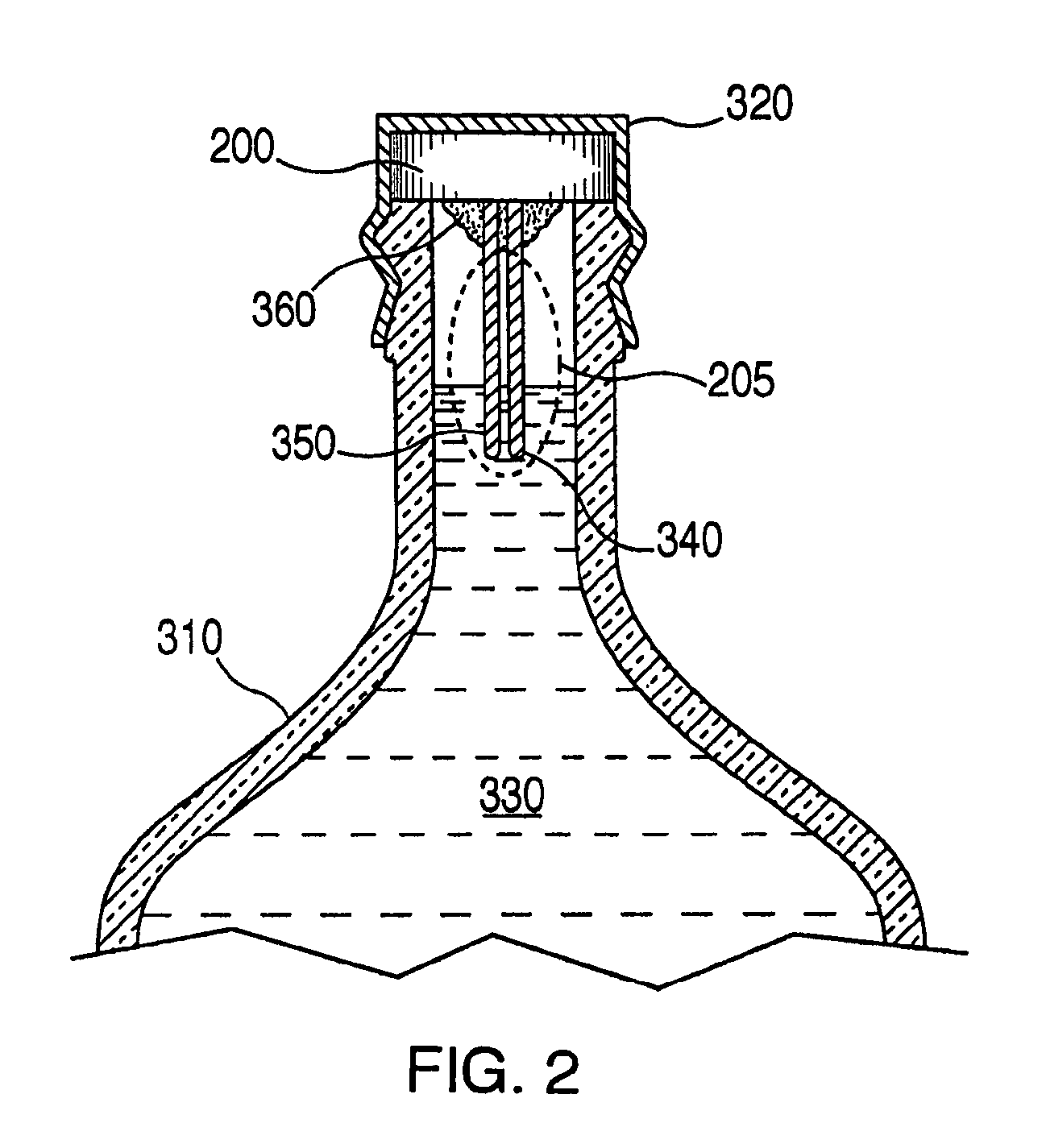
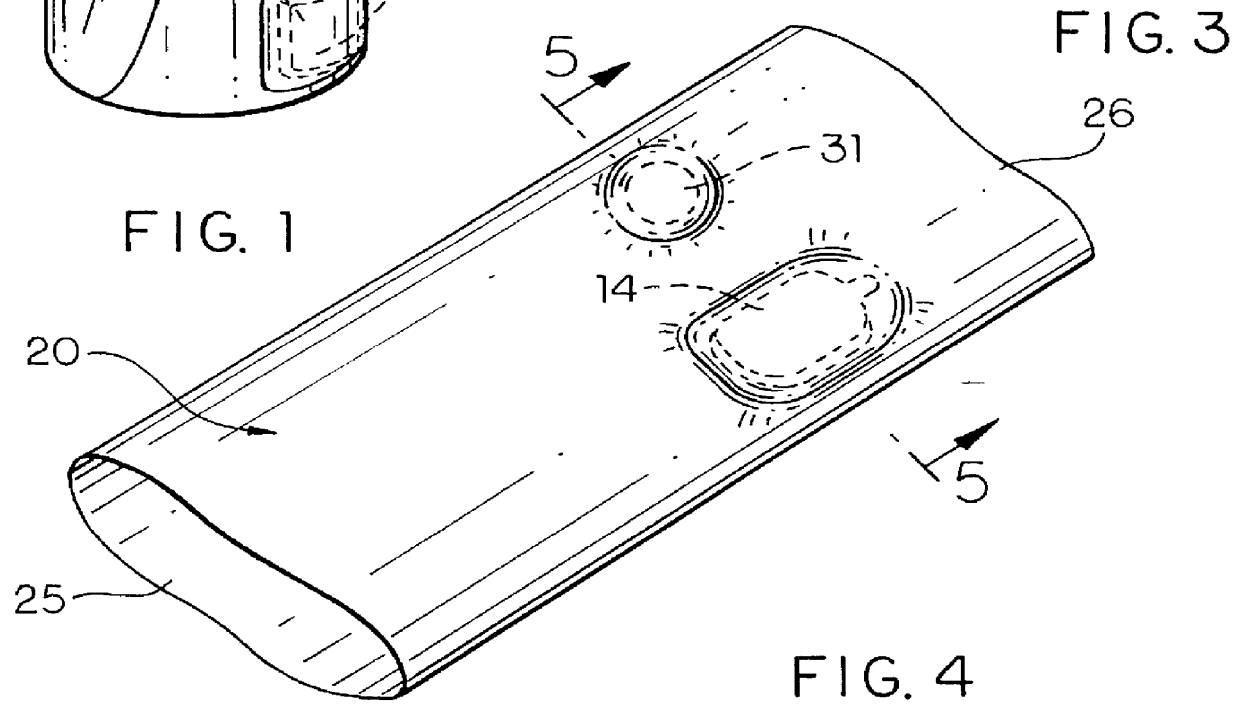
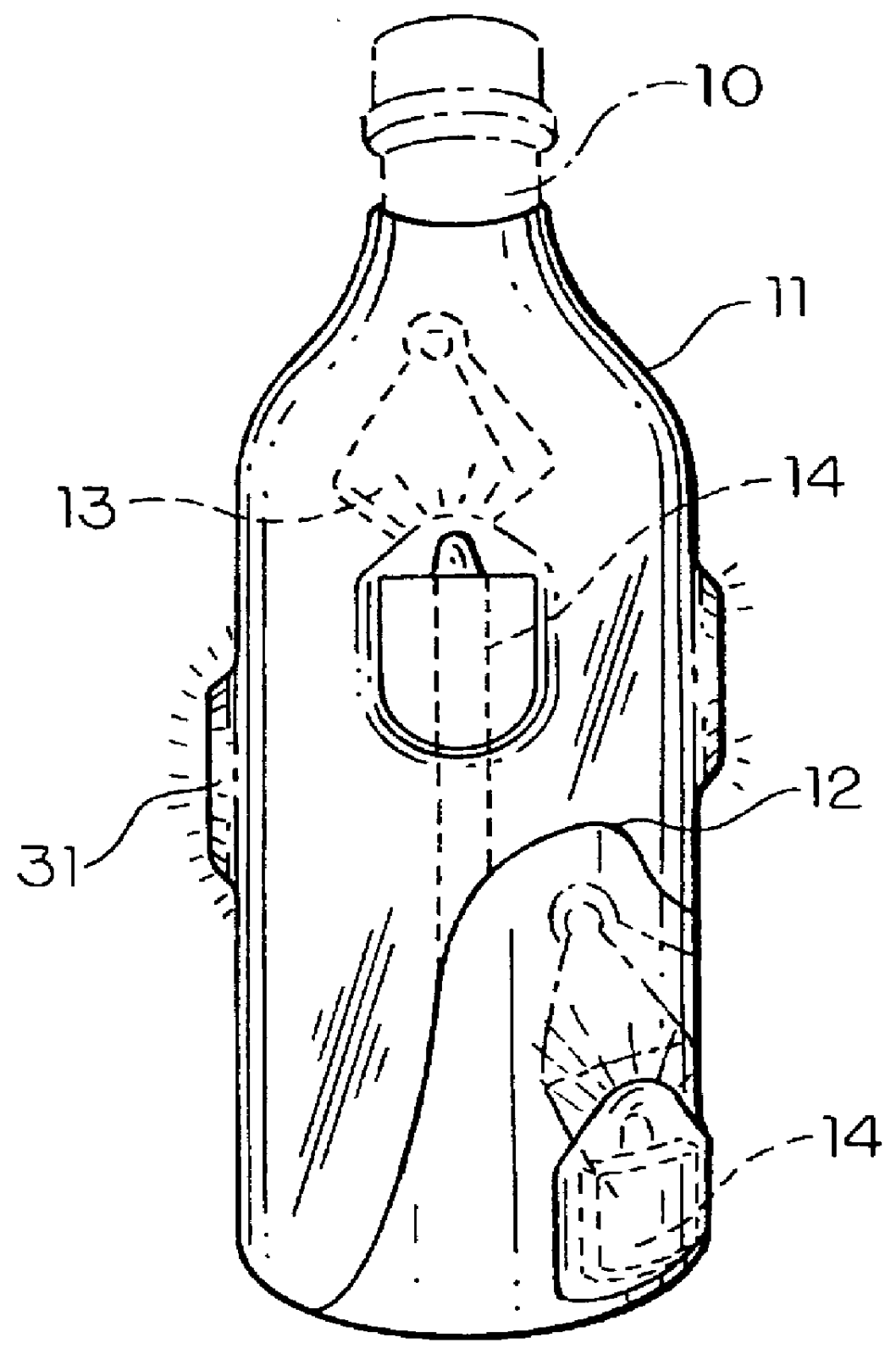
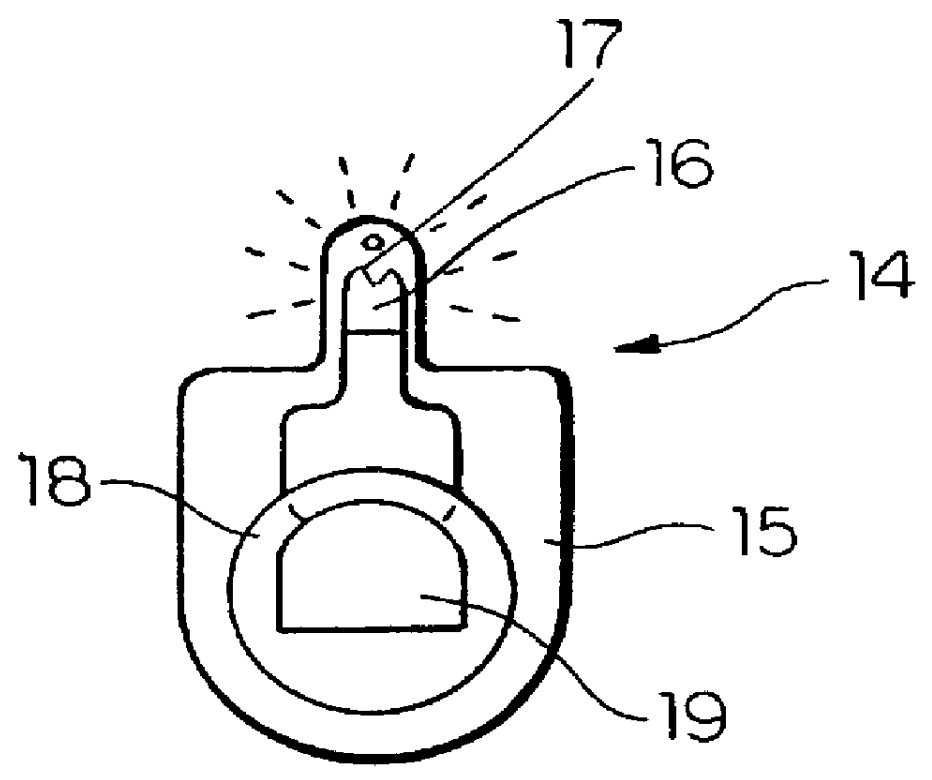
An apparatus for detecting tampering with a container sealed with a cap. The apparatus is comprised of a radio frequency transceiver for transmitting a signal upon receipt of a transmit command and an antenna attached thereto. In a first embodiment, the radio frequency transceiver and the antenna are mounted on a substrate attached to the container and the cap. The radio frequency transceiver is disabled when the antenna is separated into two portions upon breaking the seal between the container and the cap. In a second embodiment, the apparatus is mounted within a cap and is also comprised of a logic circuit and a probe that detects changes in the level of contents within the container. The probe produces an output relative to the level of contents in the container. The logic circuit then either prevents the radio frequency transceiver from communicating or causes it to transmit an alternative signal.
Owner:CLAESSENS FRANCIS M +1
Array of packages having relative size indicators
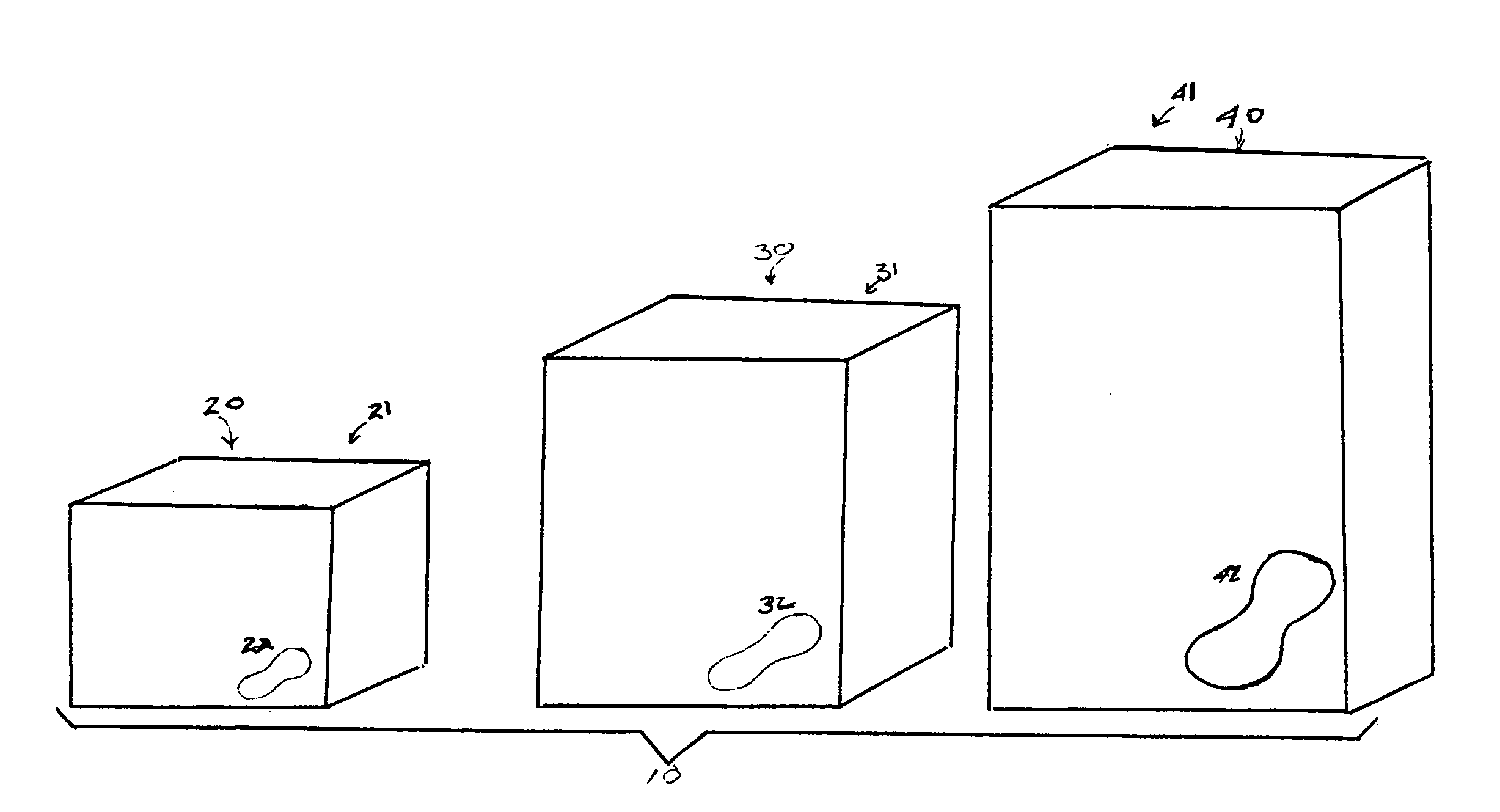
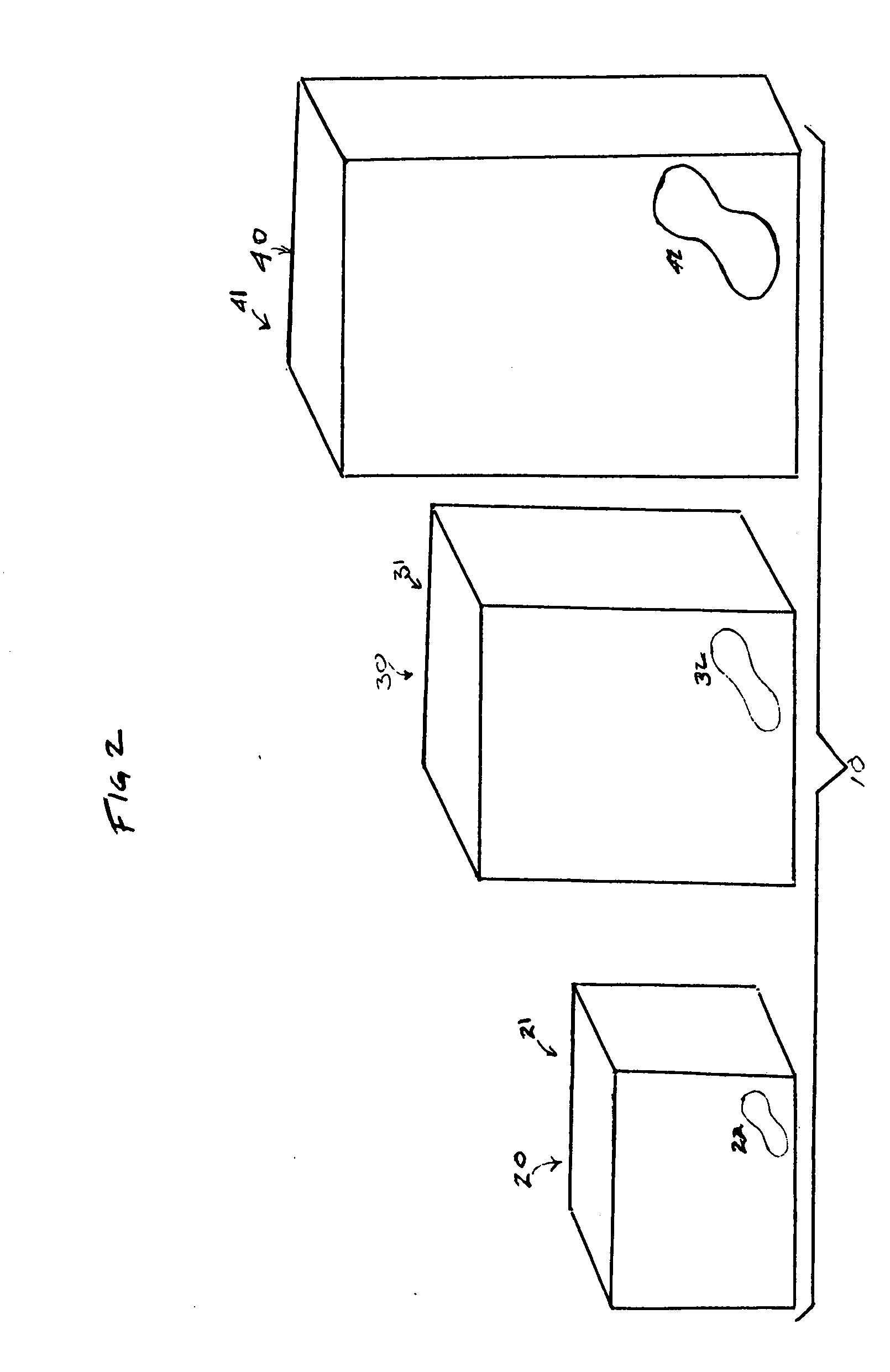
An array of packages wherein each package has relative size indicators is provided. The relative size indicators can be any indicator suitable for visually communicating to a consumer, the relative size of the absorbent articles contained in a package, in relation to other absorbent articles contained in other packages within an array of packages. The packages can comprise an interior surface and an exterior surface, the interior surface defining an interior space. Absorbent articles can be disposed within the interior space and the exterior surface can include relative size indicators.
Owner:THE PROCTER & GAMBLE COMPANY
Decorative packaging with special effects
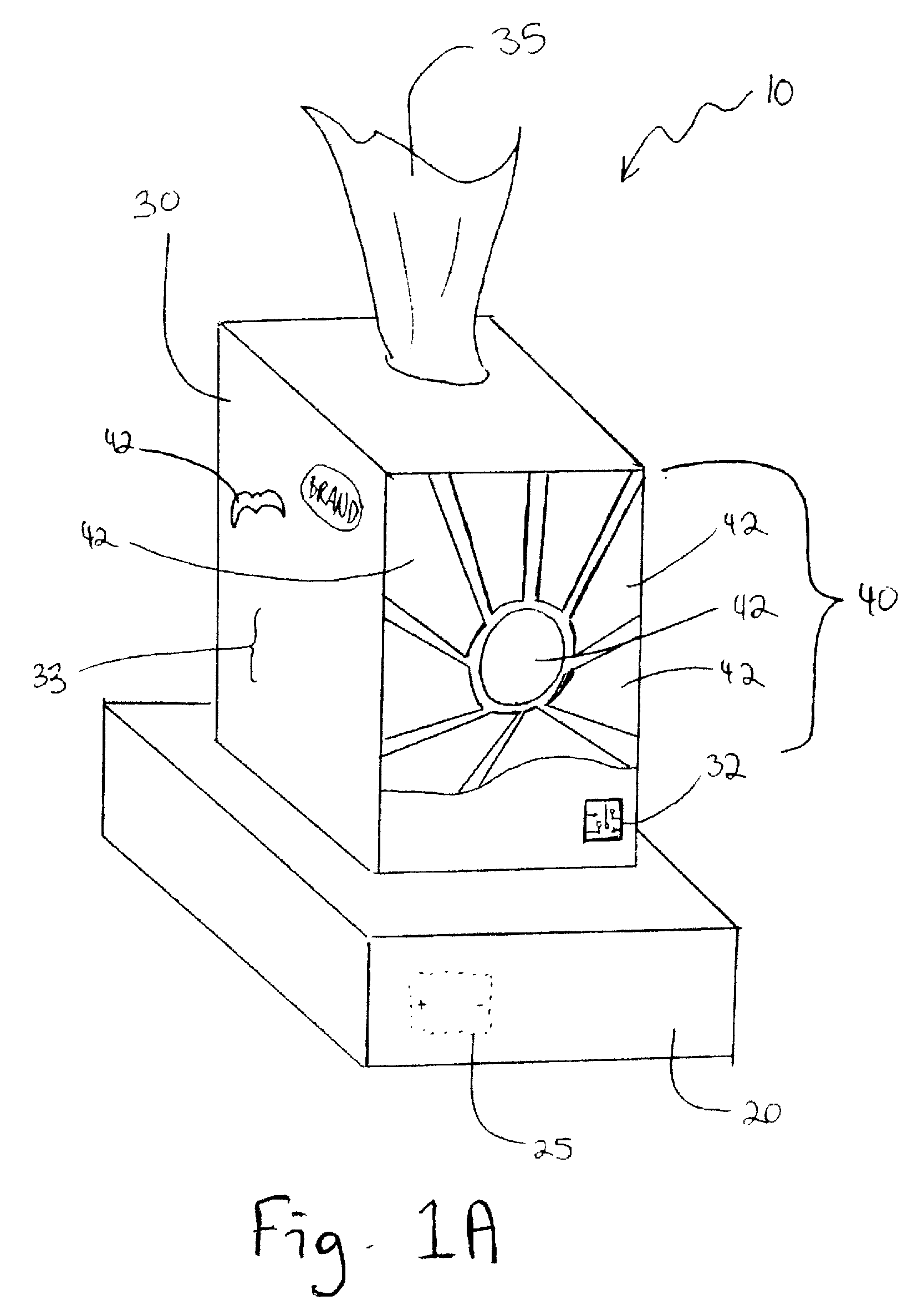
A package wrap for decoratively covering a container having side walls defining an interior space for carrying a product therein, includes a flexible thin sheet of polymeric material adapted to be shrink-wrapped onto the exterior of the container. An illuminating or a sound producing device is positioned between the flexible thin sheet of polymeric material and the exterior of the container so that when the flexible sheet of polymeric material is shrink-wrapped onto the container, the illuminating device or the sound device will be held securely in place. The illuminating device is adapted to be illuminated upon activation by a pressure, motion, or acoustically sensitive switch. The sound device is adapted to produce an audible signal upon activation by a pressure or motion sensitive switch. When the illuminating device is activated, illumination therefrom will be visible through the flexible thin sheet of polymeric material to enhance and highlight a printed pattern on the exterior of the package wrap.
Owner:DECICCO RICHARD J
Package and merchandising system
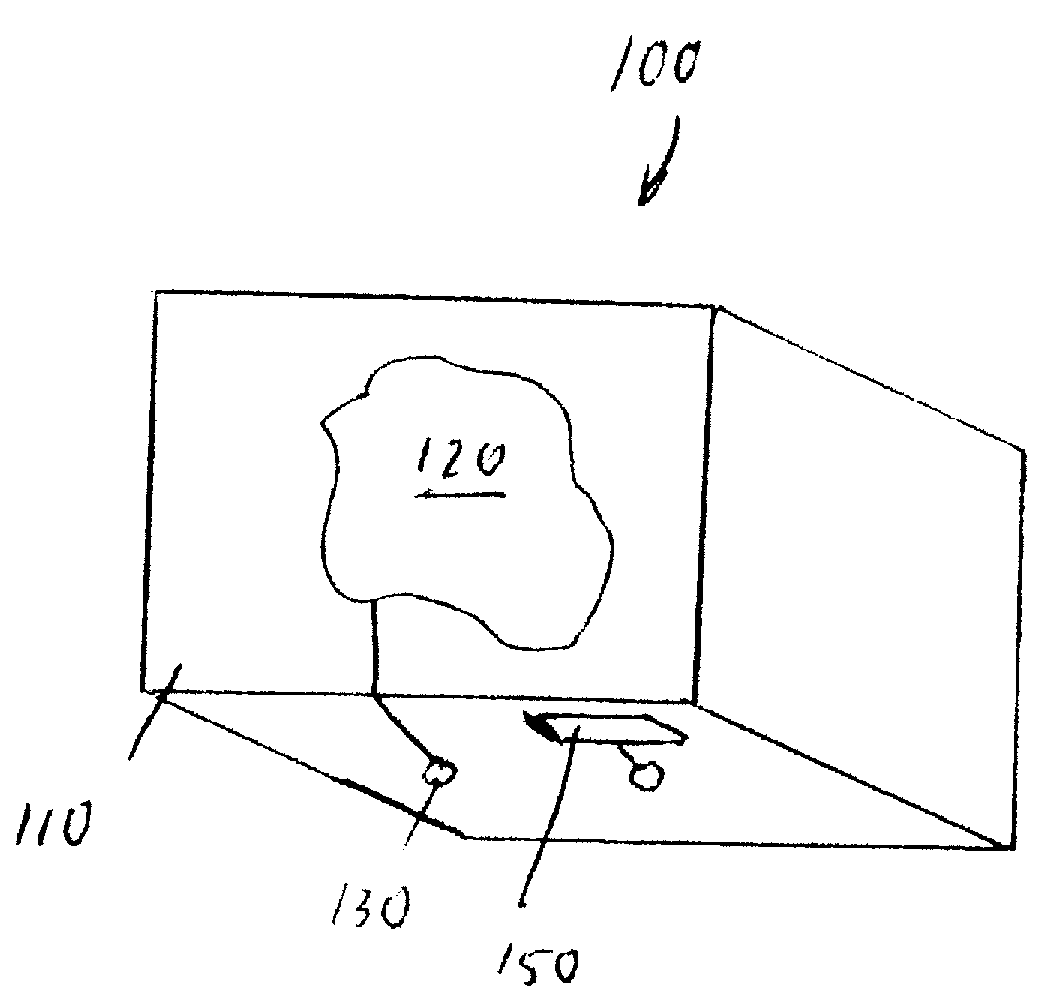
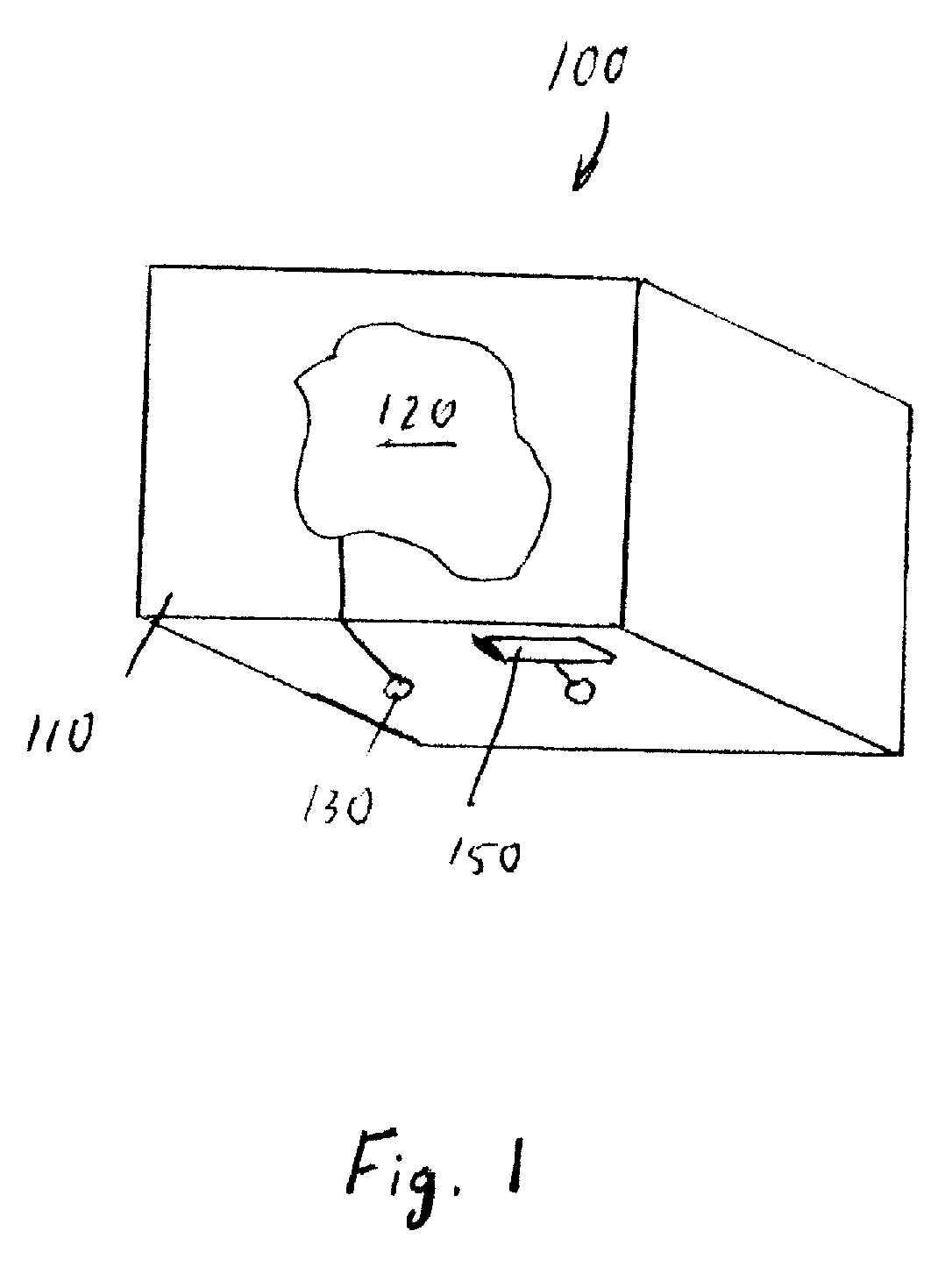
A package and product merchandising system comprising the package. The package includes a package wall which in turn includes a display element visible from the exterior of the package. The package includes a power receiving element operably connected to the display element. The system includes a sensor, a power distribution element, and a controller, operably connected to each of the sensor and to the power distribution element. The controller receives an output of the sensor as an input and provides a first output profile to the power distribution element, the power distribution element receives the output profile from the controller and provides a power profile to the power receiving element. The power receiving element provides a characteristic change profile to the display element. The display element changes at least one characteristic in response to the characteristic change profile.
Owner:THE PROCTER & GAMBLE COMPANY
RFID tag with visual environmental condition monitor
ActiveUS7209042B2Easy to processEasy to readContainer decorationsLevel indicationsTime responseStatistical analysis
A combination RFID tag intended to be associated with a host product, for example by being secured to the outside of a package containing a perishable product, e.g. foodstuffs or vaccines, which RFID tag is provided with a visually readable environmental condition exposure indicator. The visual indicator can sense the exposure of the RFID tag to an environmental condition e.g. temperature, experienced by the host product providing a visual indication, e.g. a color change, readable externally of the RFID tag of the sensed environmental condition. The visual indicator can be chemically active, for example an acetylenic agent, and may be responsive to cumulative temperature excursions over time. The novel RFID tag 11 and tag inspection methods of the invention permit an efficacious harnessing of information about the condition exposure history of a specific inventory item including product identification and related data. The information from multiple items can be compiled into a database that may be audited or statistically analyzed to reveal useful information regarding the handling of the items.
Owner:TEMPTIME CORP
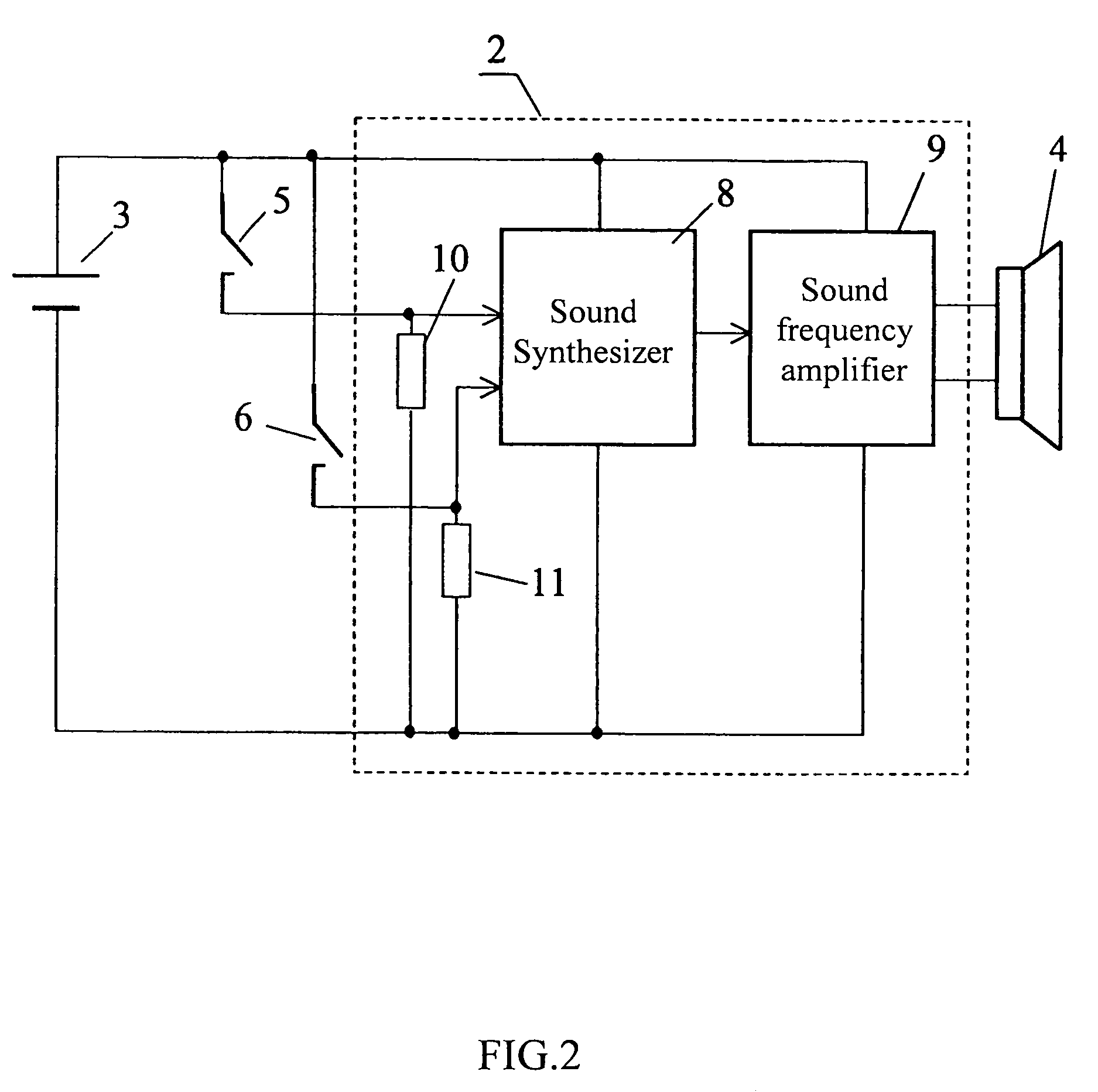
Package provided with a sound-reproducing device
InactiveUS7116233B2Function increaseAnimal feeding devicesAnimal housingEngineeringBiological activation
A sound-reproducing package comprises a housing for storing a consumer product, a disposed therein sound signal reproducing unit electronically connected to a power supply and a loudspeaker, at least one external activation sensor connected to the sound signal reproducing unit, and a package state sensor operative to form a signal corresponding to an open or closed state of the package, or the presence or absence of a consumer product in it. The package state sensor is connected to the sound signal reproducing unit operative to select from its memory sound signals for reproducing depending on the signal from the package state sensor.
Owner:MARS LLC
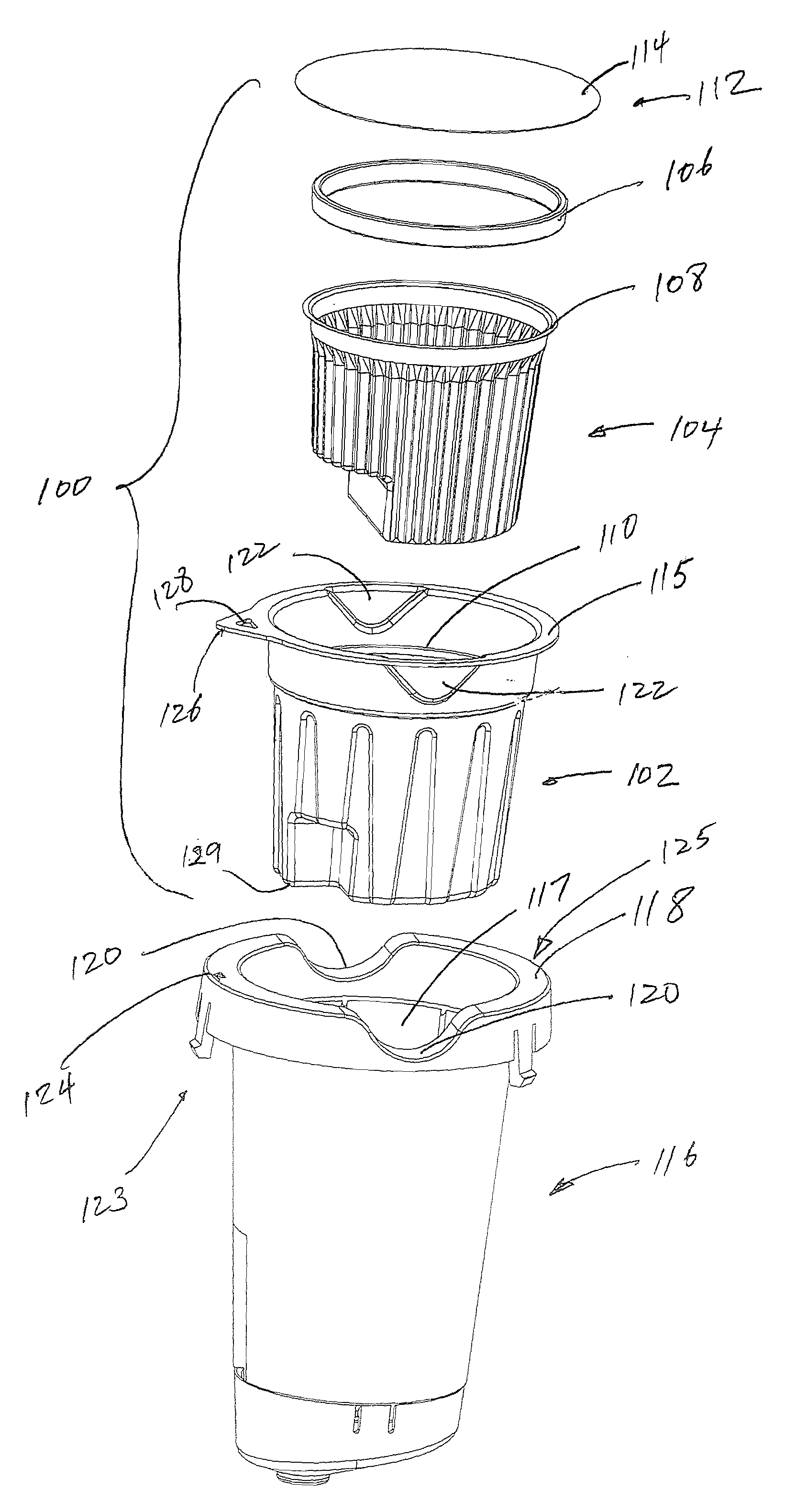
Beverage brewing system
InactiveUS20130340626A1Convenient amountSmooth tasteReady-for-oven doughsContainer decorationsGrindBiomedical engineering
A beverage cartridge system is adapted to brew a beverage through a brewer having a holder adapted to receive the cartridge system. The holder may have a deep well with one or more needles therewithin to pierce through the bottom of the cartridge system when inserted into the well. The cartridge system may include a short cup and a tall cup, where the tall cup is taller than the short cup to pack more beverage grind. The cartridge system may include a filter within an outer cup. The bottom of the filter may be deep enough to be juxtaposed to the bottom of the cup. The filter may be formed from a material that is substantially resistant to piercing by the needle within the holder such that when the outlet needle pierces through the bottom of the cup, the outlet needle raises the filter at a point of contact, and the filter substantially resists the outlet needle from piercing through the filter during a brewing process.
Owner:TOUCH COFFEE & BEVERA
Package and Merchandising System
A sensory interactable packaging assembly having a base and a container. The base has a power supply. The container may contain a consumer product. The container is removably attachable to the base. The container has an outer surface and a sensory interactable element disposed on the outer surface and a control system having an output. The control system is s in electrical communication with the sensory interactable element and the sensory interactable element is responsive to the output of the control system when the container is proximate to the base and the control system is in electrical communication with the power supply.
Owner:THE PROCTER & GAMBLE COMPANY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com