Patents
Literature
134results about How to "Realized benefits" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Perfume delivery systems for consumer goods
InactiveUS20070275866A1Low vapor pressureRealized benefitsCosmetic preparationsContainer decorationsEngineeringDelivery system
Owner:THE PROCTER & GAMBLE COMPANY
LED based light engine
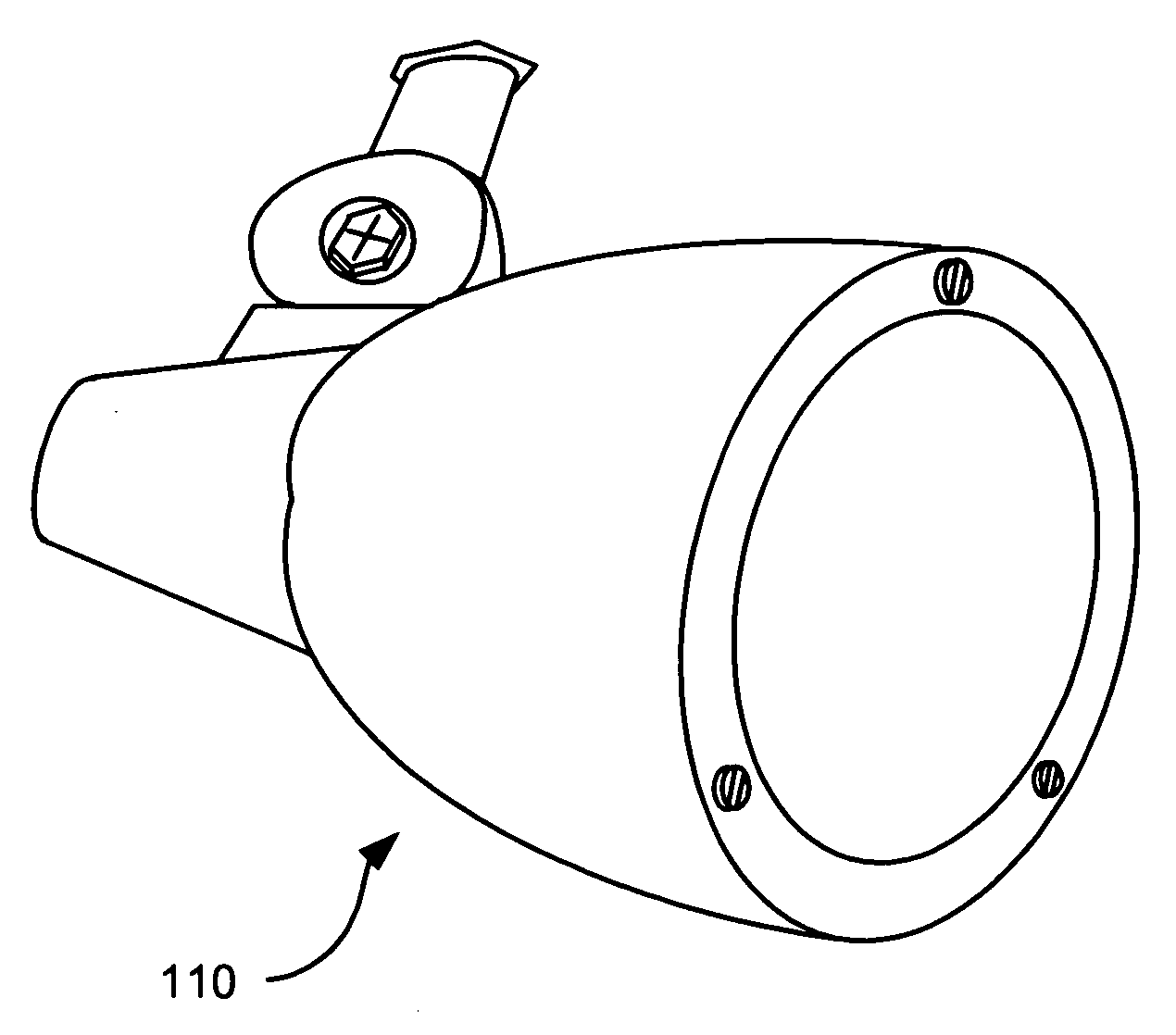
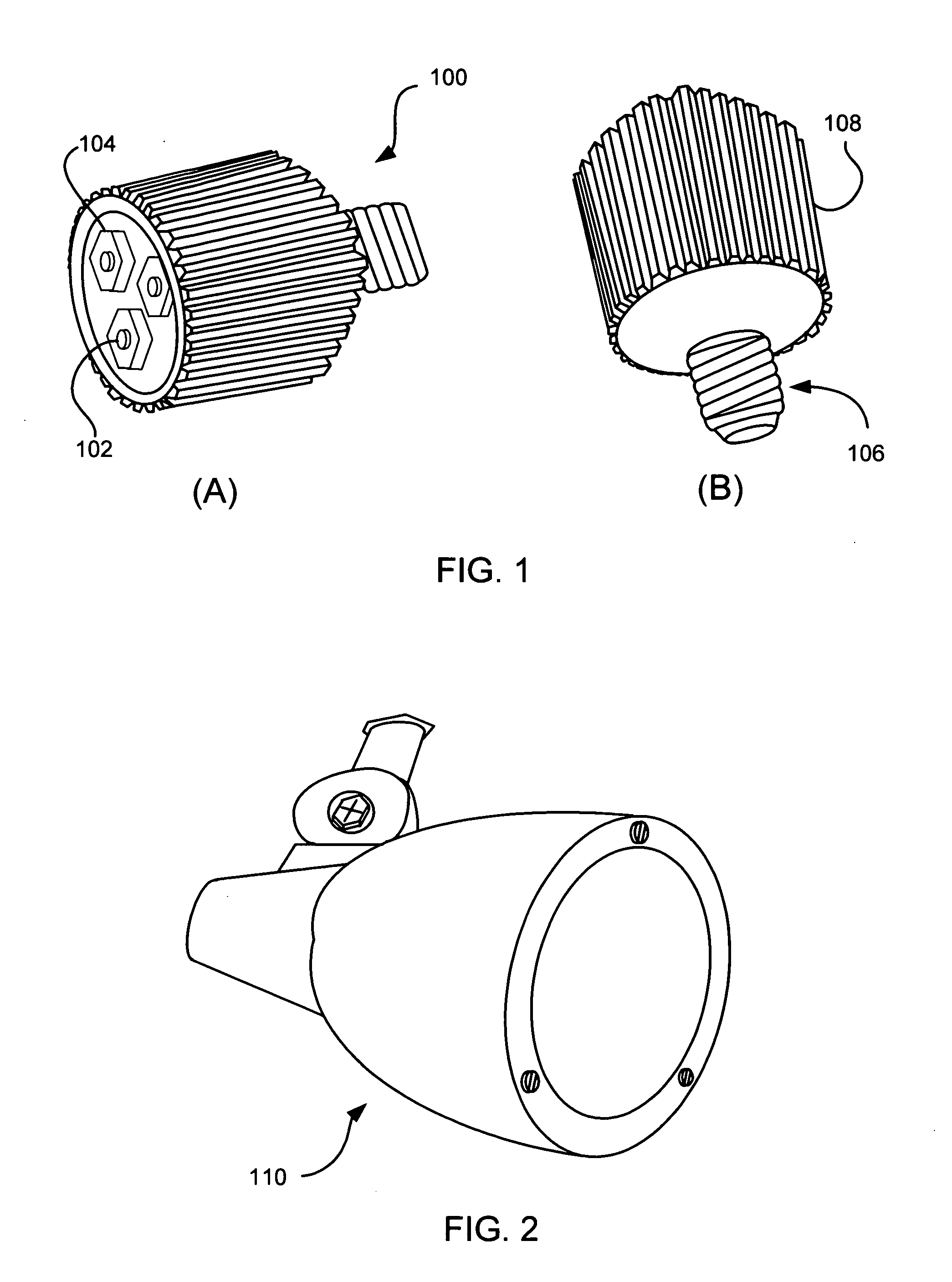
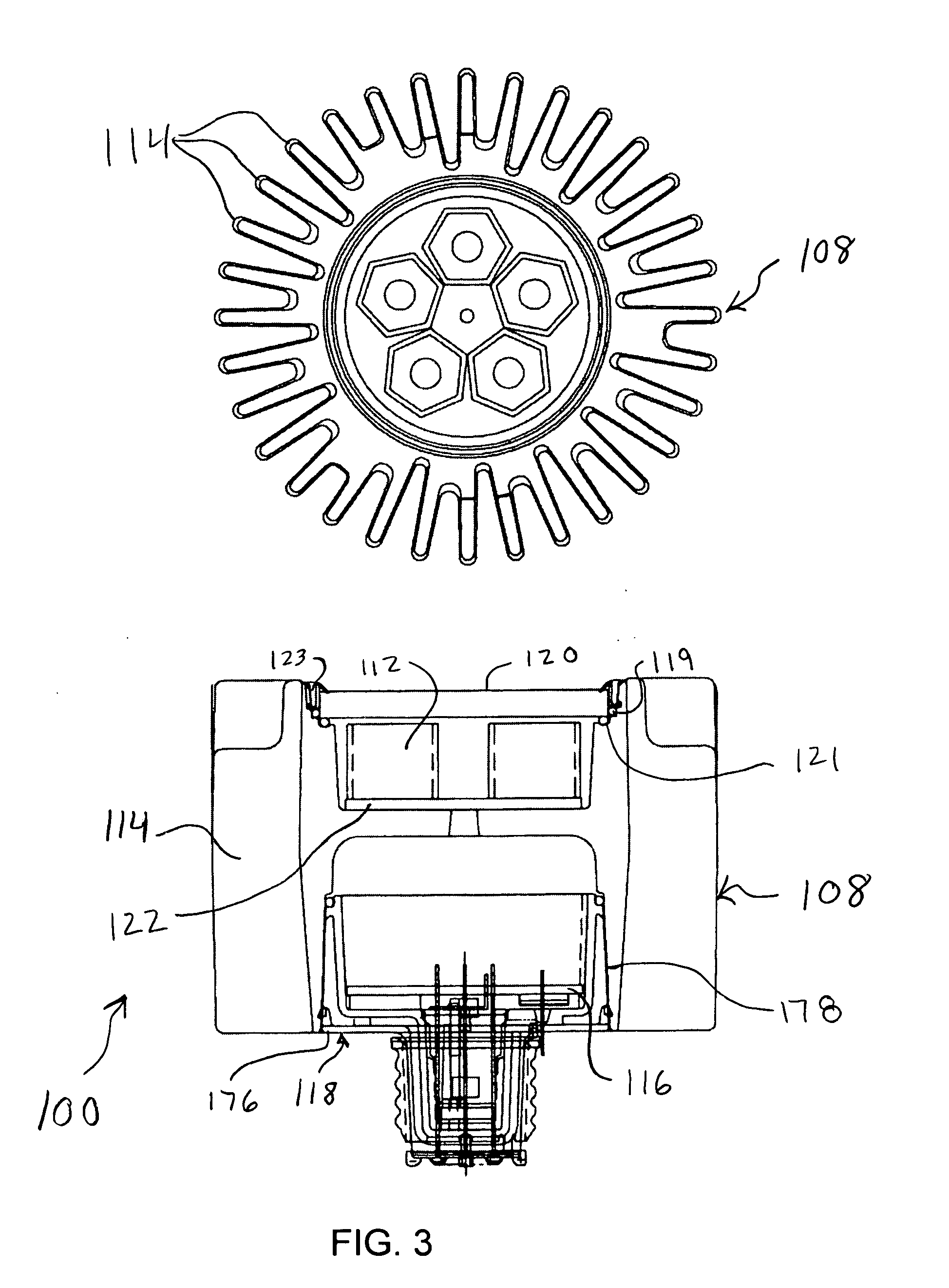
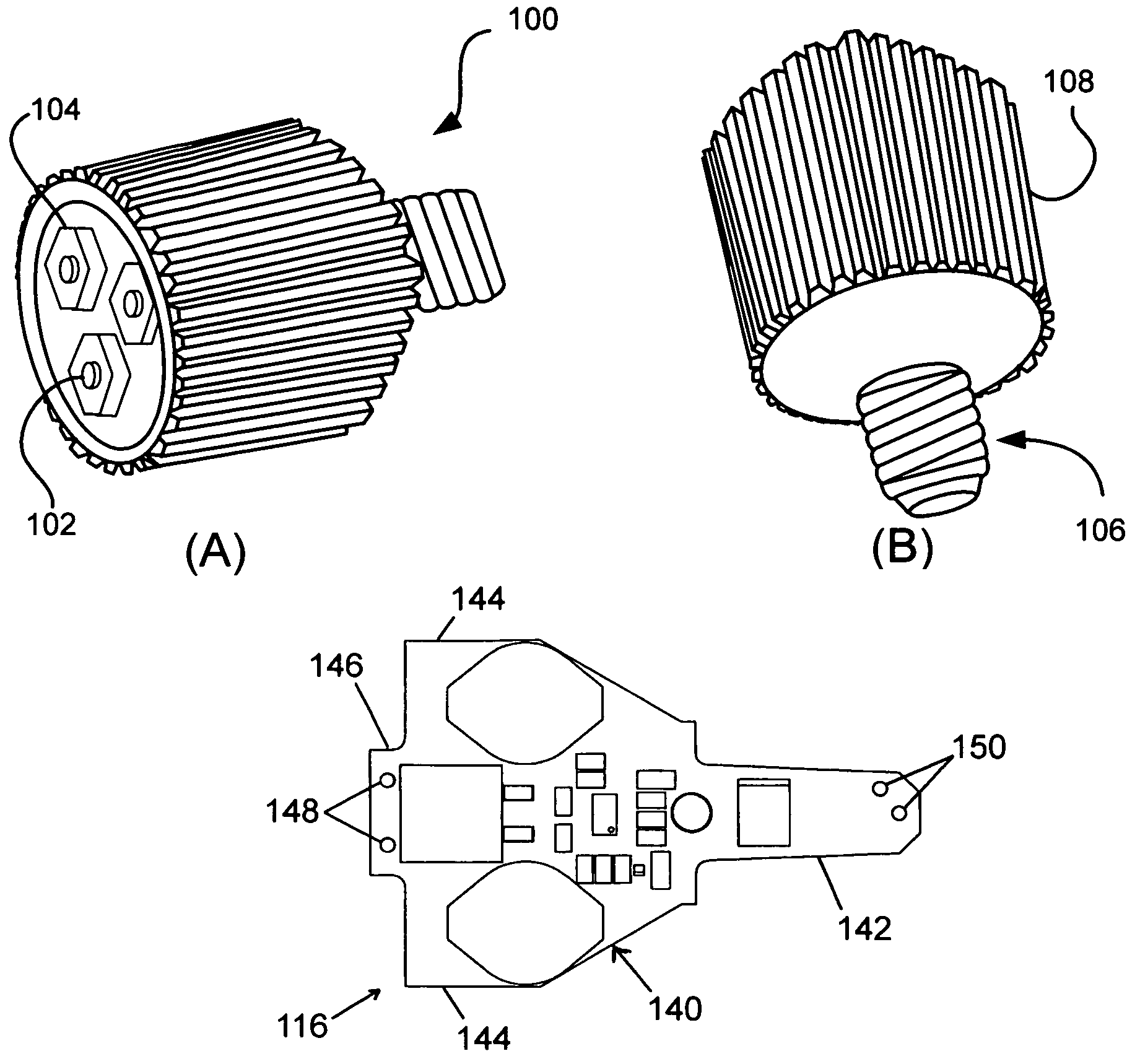
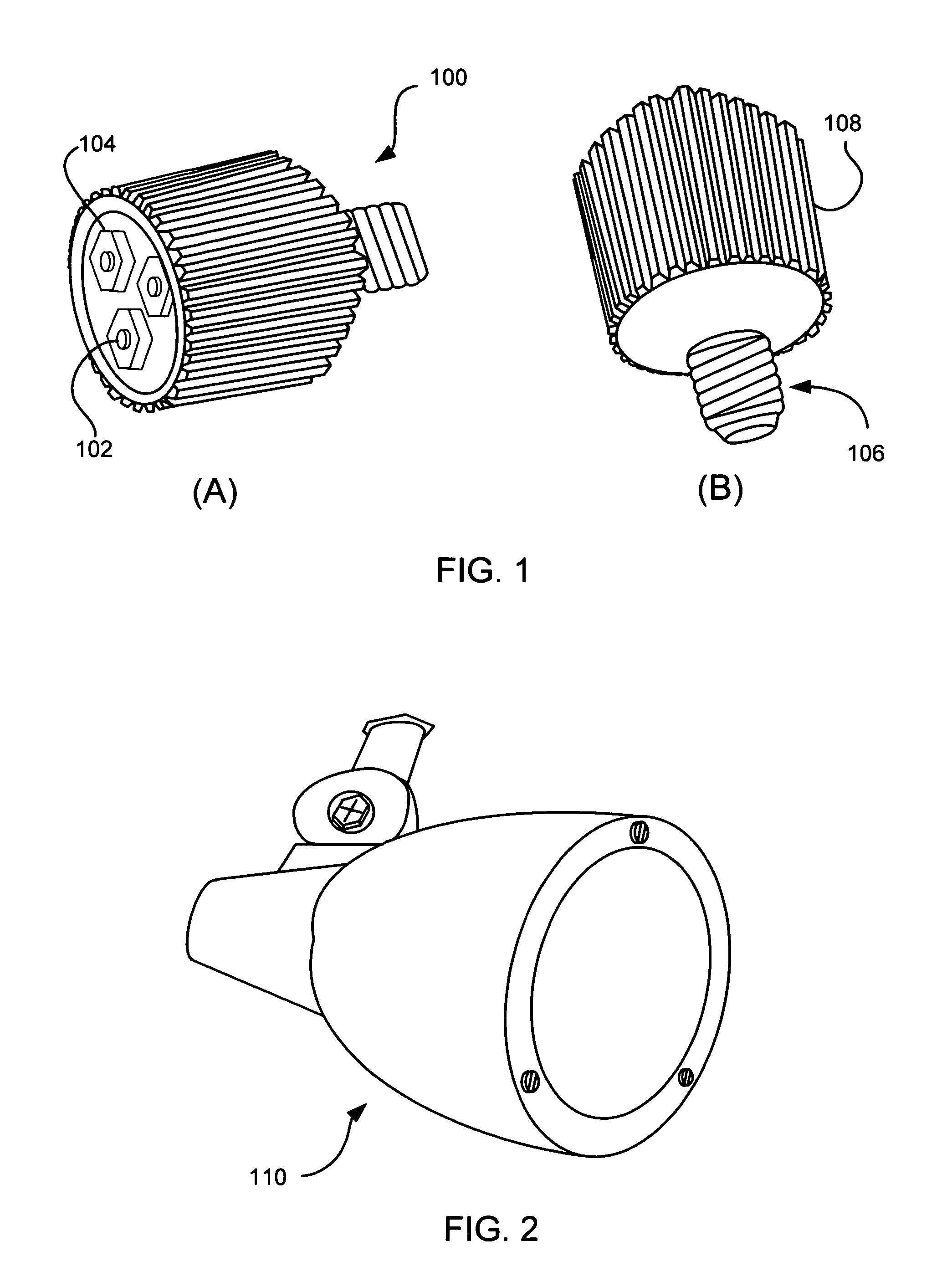
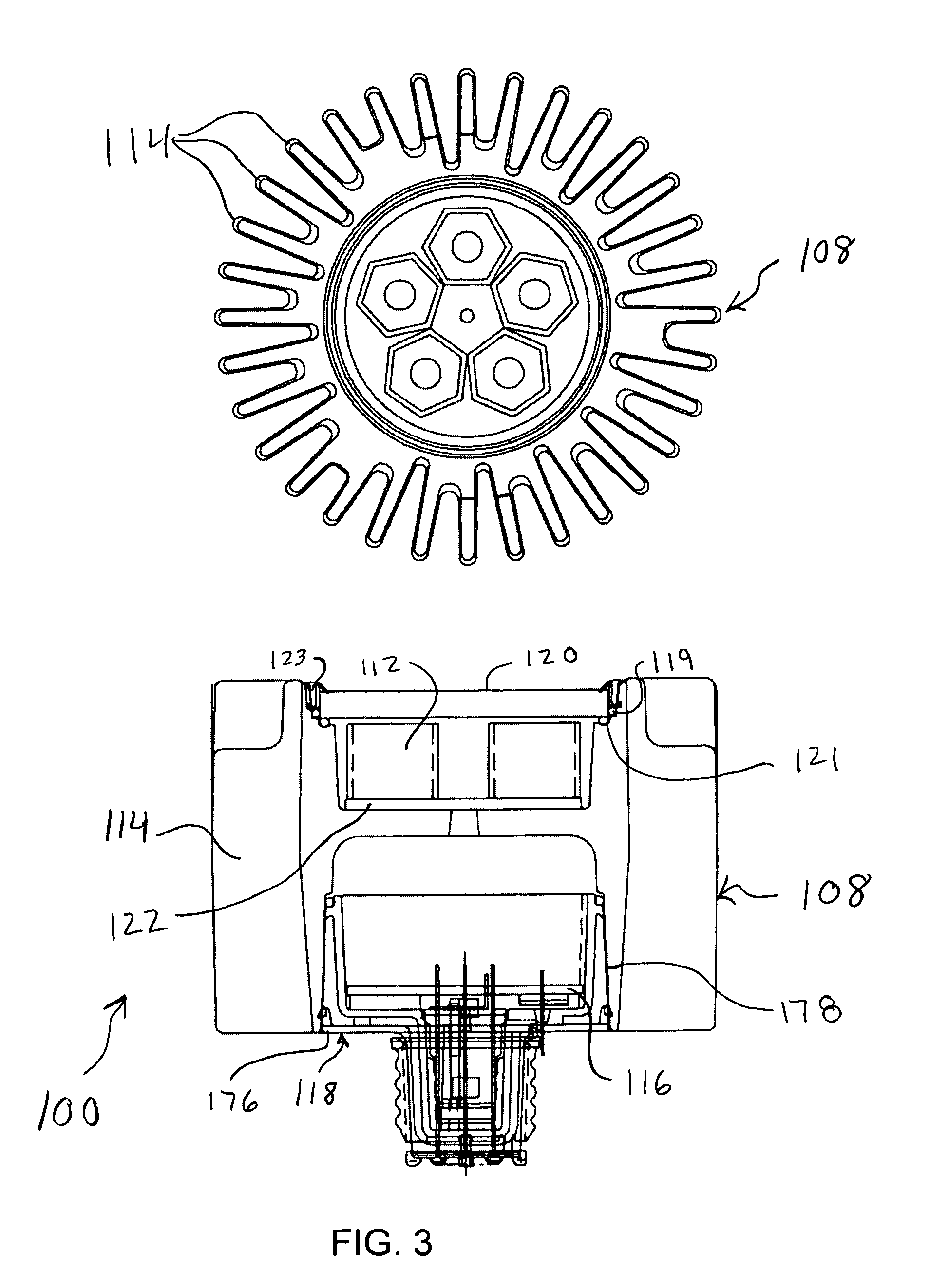
InactiveUS20070242461A1Efficient thermal management designDissipate generated heatCoupling device connectionsPlanar light sourcesElectricityOptical Module
A light emitting diode (LED) based light engine that can replace a conventional PAR type bulb with no modifications to a host lighting fixture is provided. The light engine includes a thermally conductive housing including a generally cylindrical wall defining a cavity, an outer surface of the wall includes a plurality of axially radiating fins and disposed on an inner surface of the wall is an annular center wall dividing the cavity into a first, upper cavity and a second, lower cavity; a light module including at least one LED is disposed in the first, upper cavity and configured to contact the annular center wall, wherein heat generated by the at least one LED is conducted to the housing; and a current driver circuit arranged on a substrate is disposed in the second, lower cavity and electrically coupled to the light module.
Owner:TALL TOWER LED
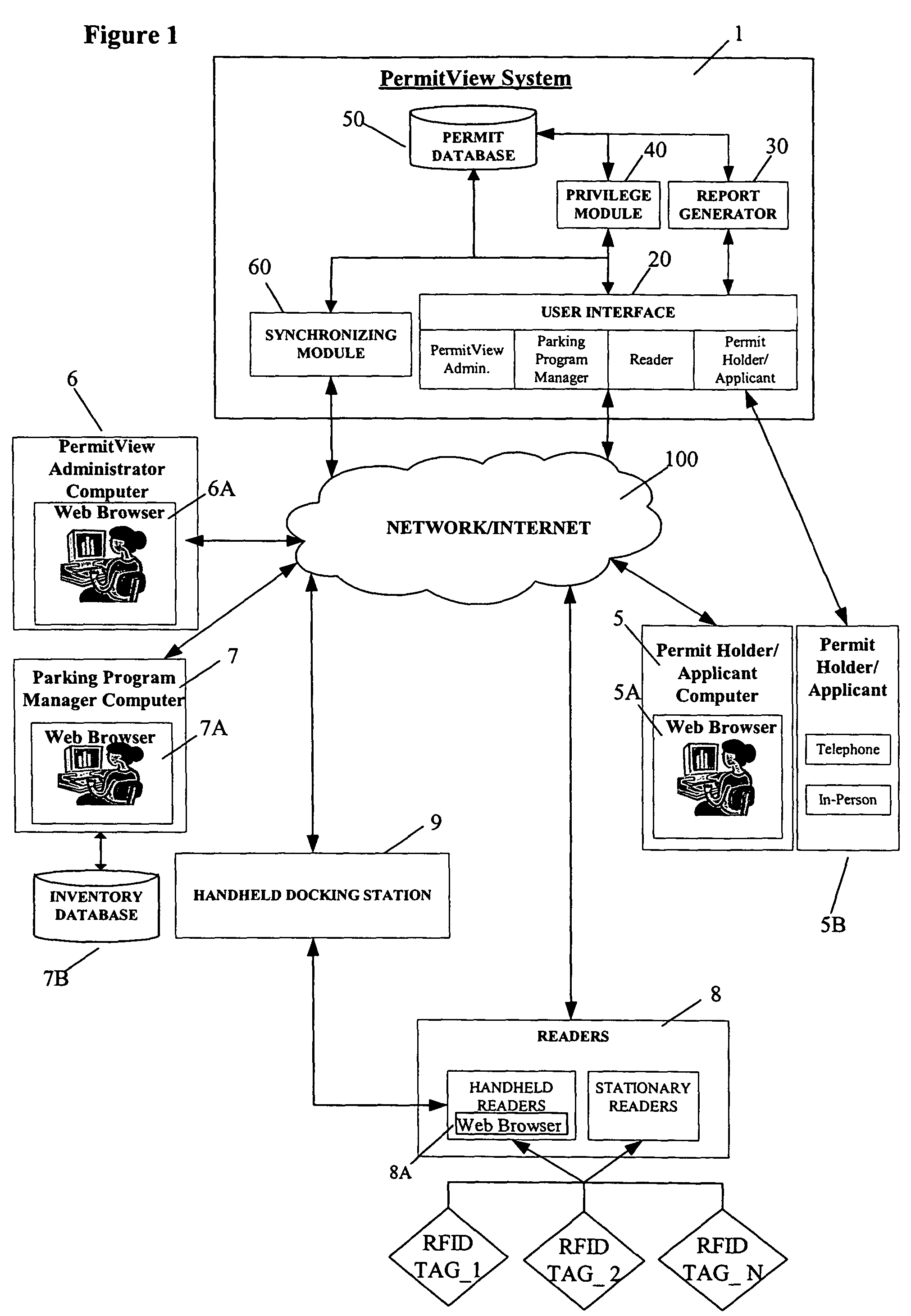
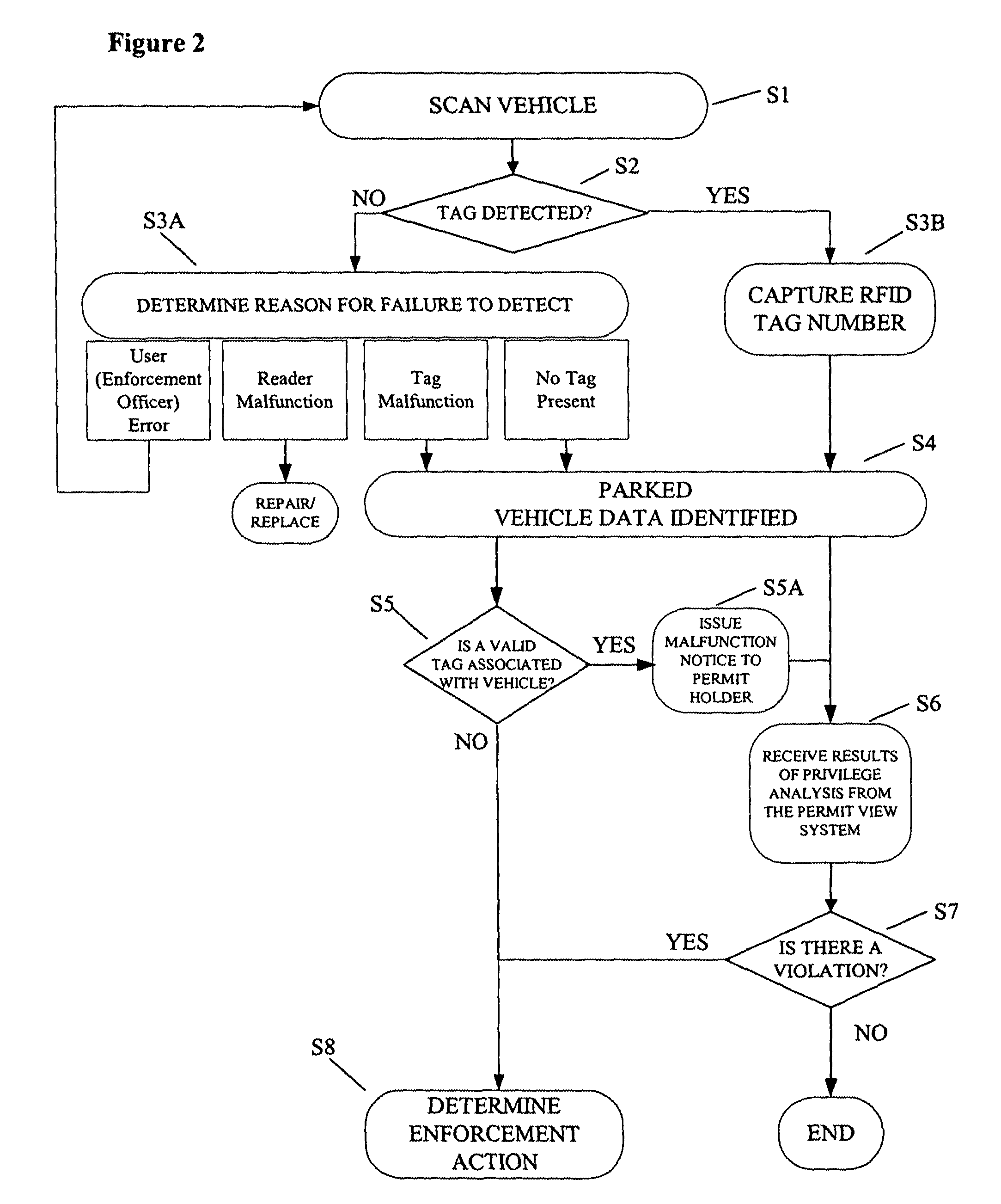
Parking environment management system and method
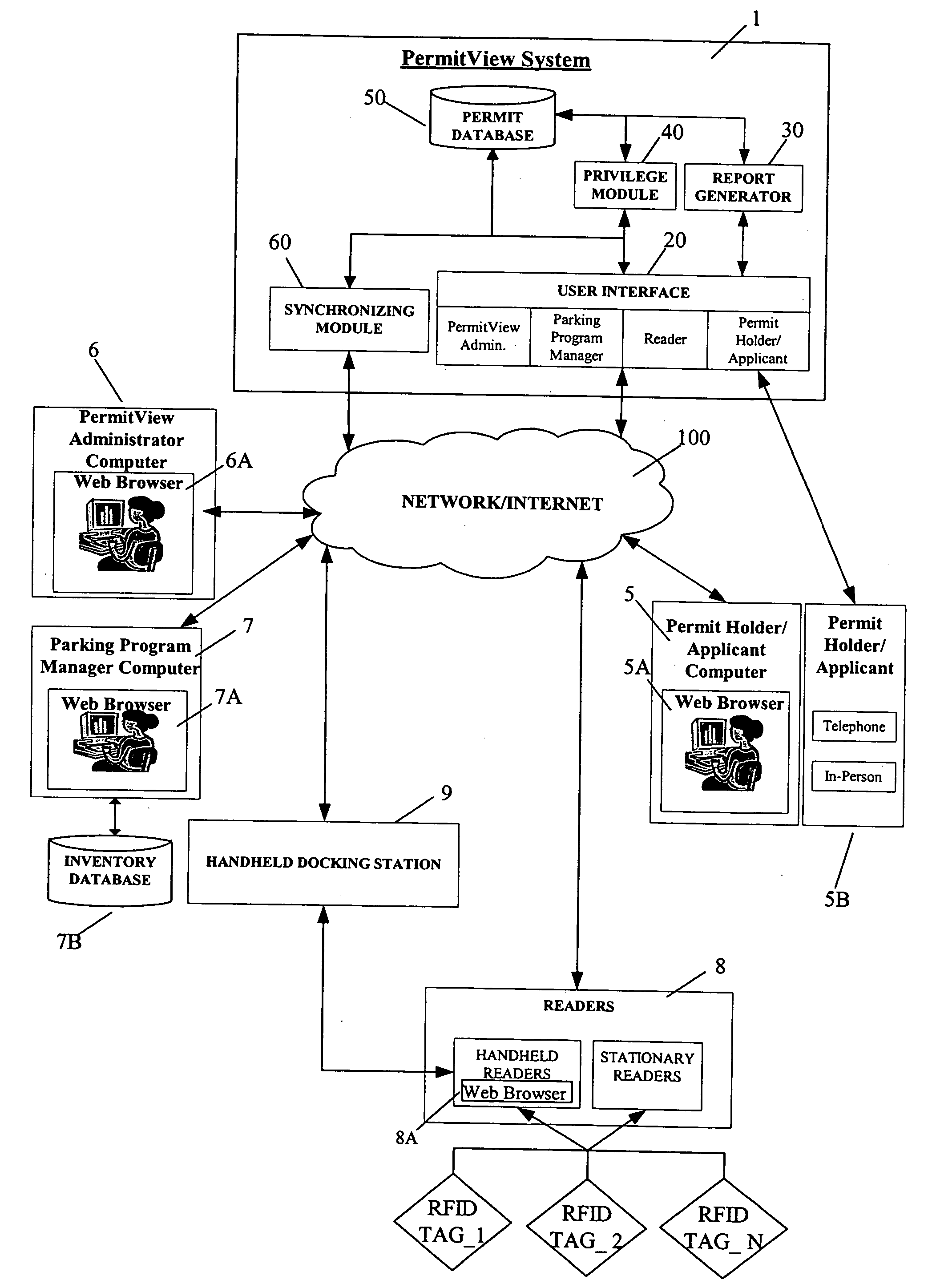
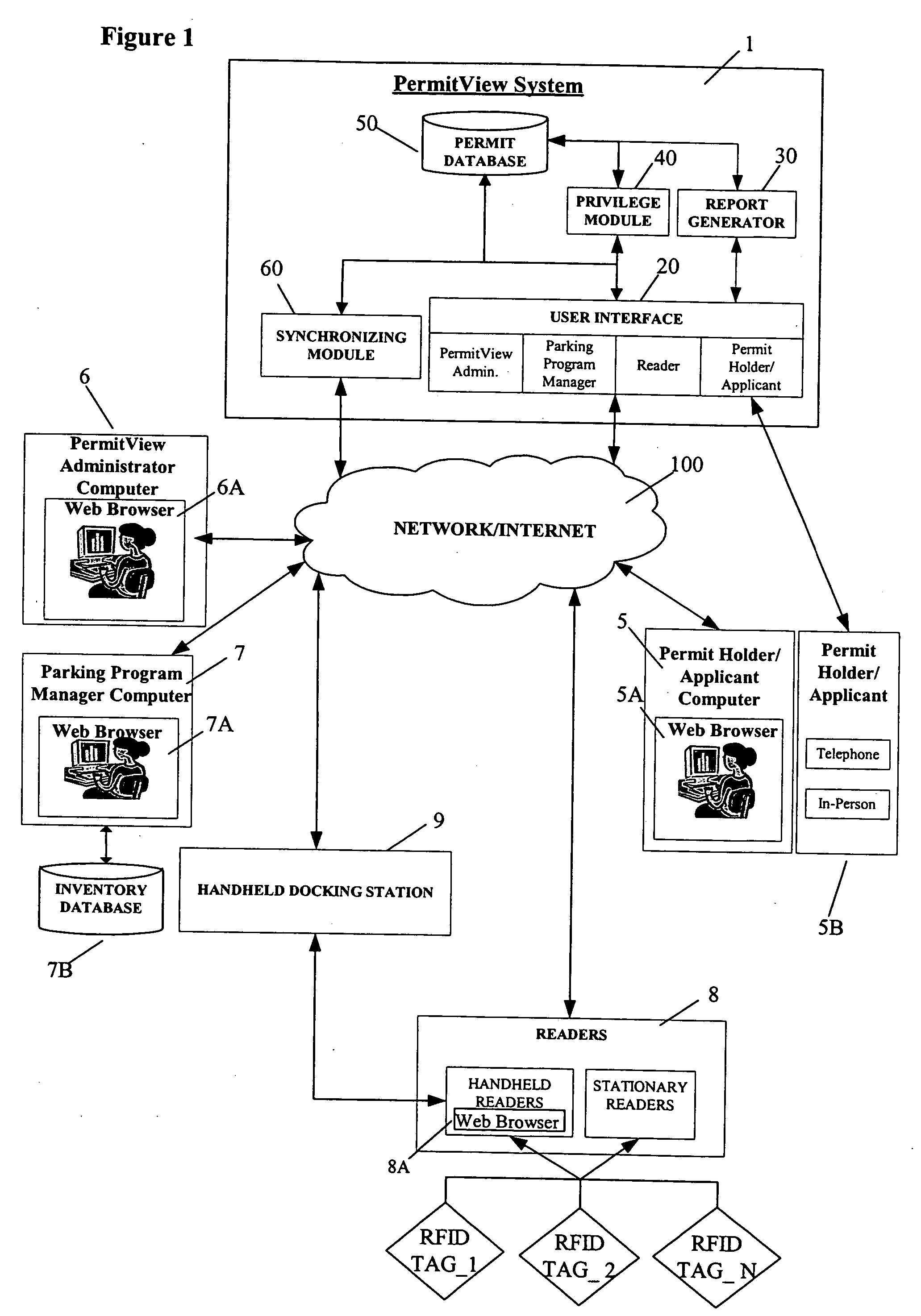
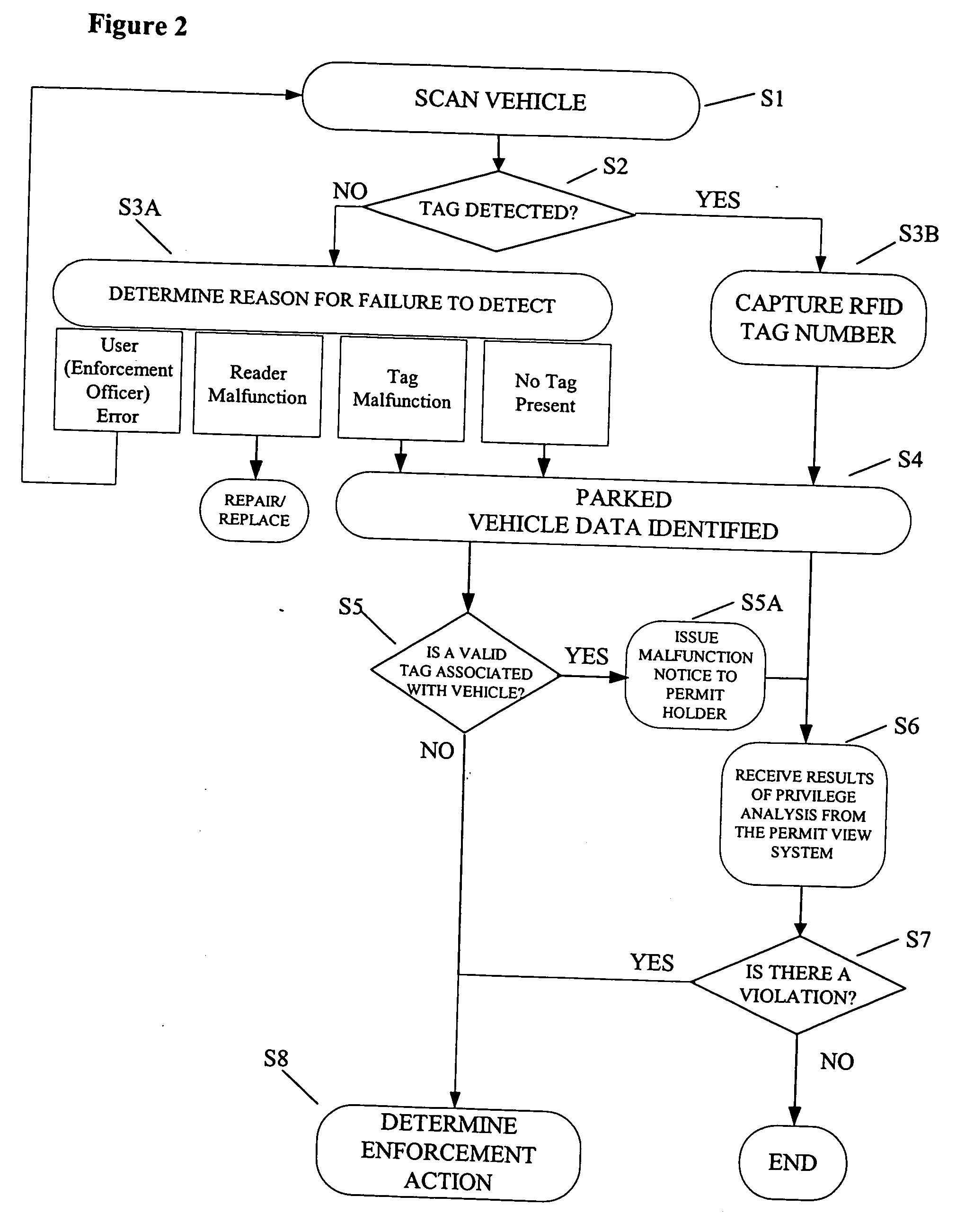
ActiveUS20060255119A1Realized benefitsGood release performanceAnti-theft cycle devicesBuilding locksProgram managementManagement system
A system and method for managing a permit-based parking environment governed by a parking program. The permit-based parking environment includes a number of parking permits each including a unique RFID tag and tag number. One or more RFID readers are used to scan the vehicles parked in the parking environment to determine if a RFID tag is associated with the parked vehicle. The results of the scan along with information related to the parked vehicle are provided to a permit management system to determine if the vehicle is parked within the scope of privileges pre-defined for that vehicle, pursuant to the parking program governing the parking environment. The permit management system stores, manages, and monitors data related to the permits controlled under the parking program.
Owner:IPT
LED lamp module
ActiveUS7488097B2Easy to modifyMinimize impactLighting heating/cooling arrangementsDisplay meansElectricityPower flow
Owner:TALL TOWER LED
Server-centric customized interactive program guide in an interactive television environment
InactiveUS6904610B1Enjoyable viewer experienceReduce search timeTelevision system detailsPulse modulation television signal transmissionInteractive televisionVideo sequence
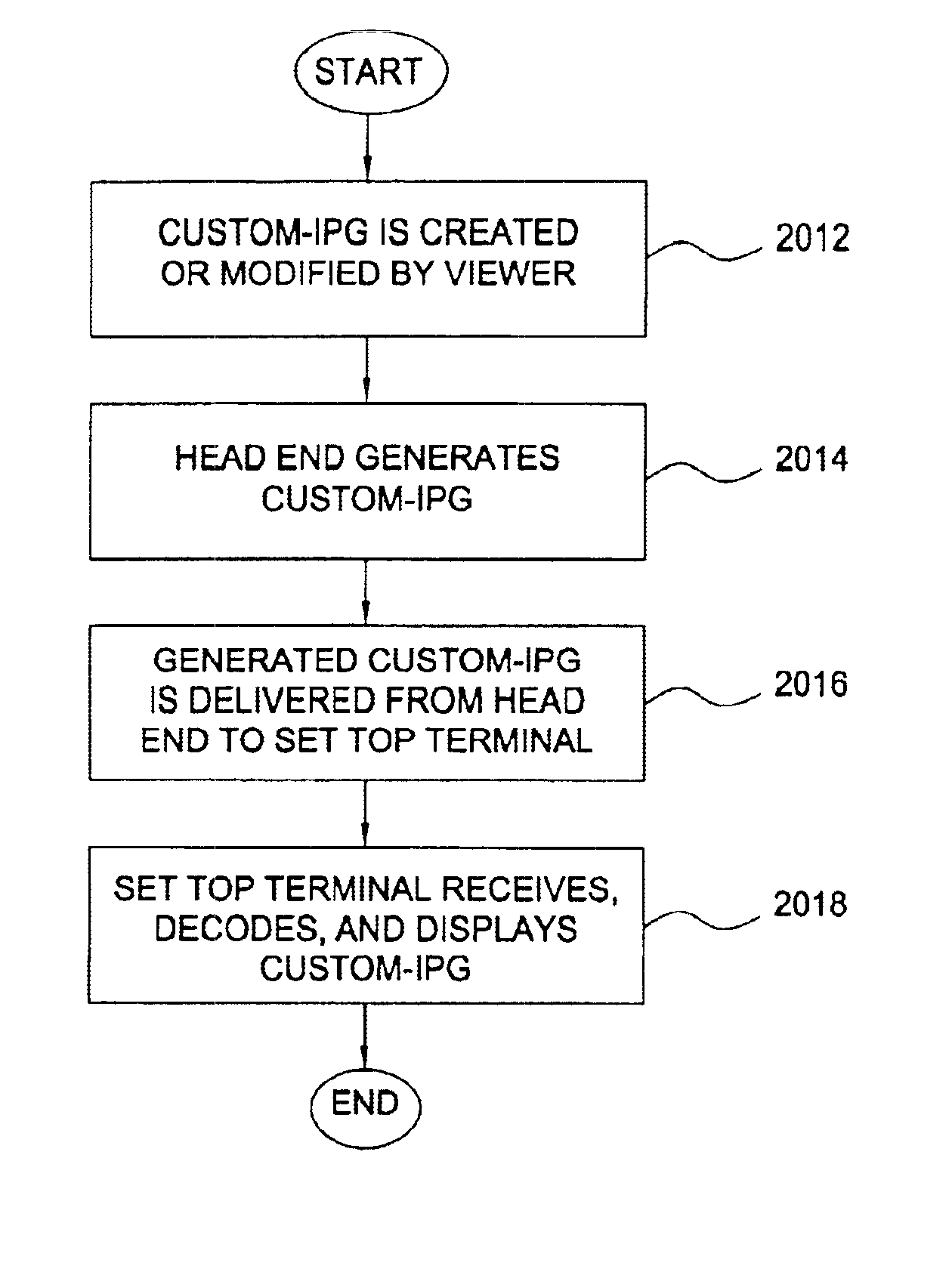
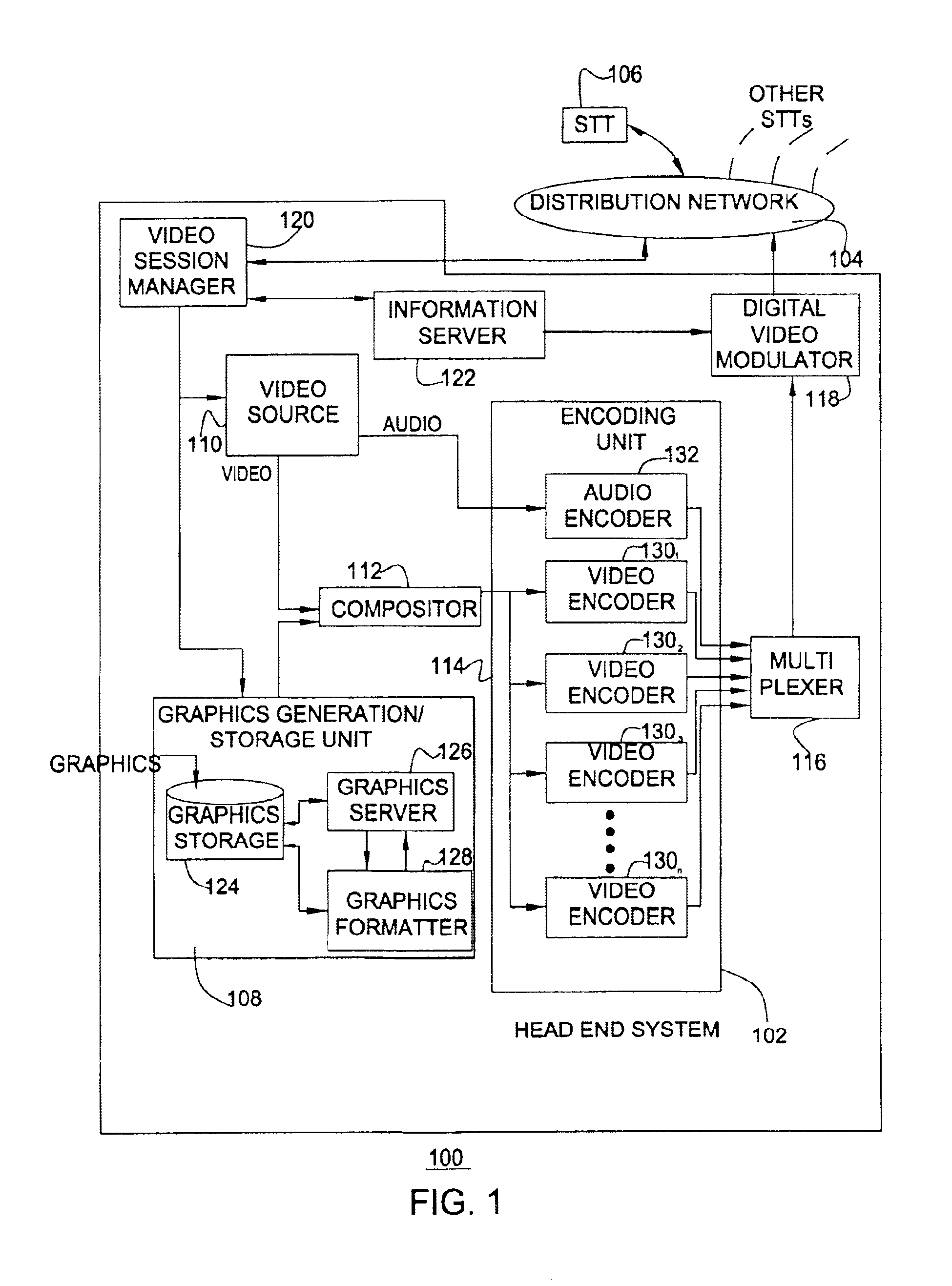
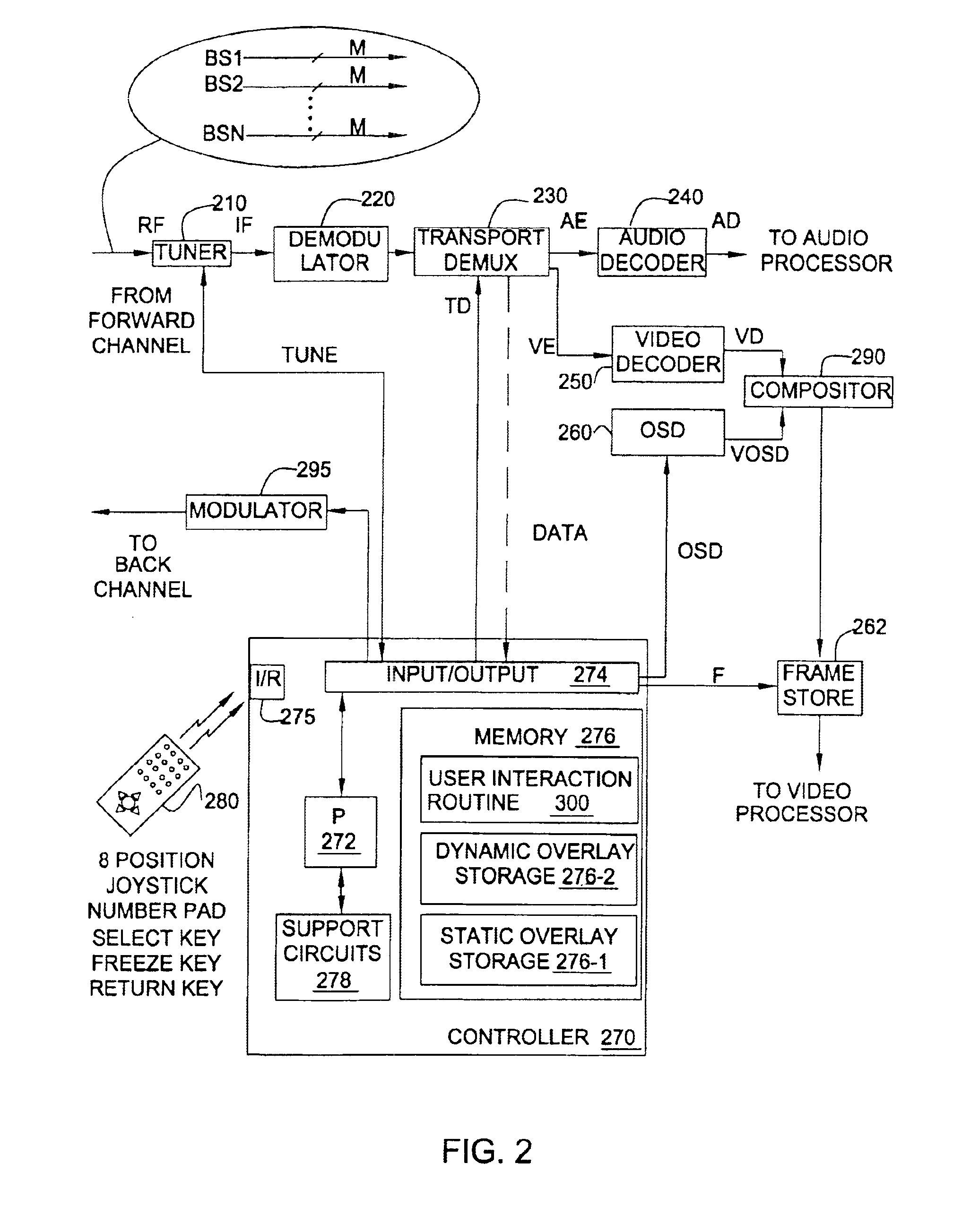
Techniques to create, generate, and deliver customized interactive program guide (custom-IPG). In one method, selections indicative of a set of channels to be included in the custom-IPG are received. In response, one or more custom-IPG screens having included therein the set of selected channels are rendered at a head end. The custom-IPG screens can be formed as subsets of the regular IPG screens, or as new screens. The rendered custom-IPG screens are provided from the head end to a set top terminal upon receiving a viewer request for the custom-IPG. Command indicative of a particular location at which to overlay the custom-IPG screens may also be received. In this case, the custom-IPG screens are re-rendered at the indicated location. The custom-IPG screens can be overlaid on a video sequence provided on a particular channel, which can be the channel currently being viewed, the channel used to carry regular program guide, or a channel that is independent of the channels used to carry regular programming and program guide. For example, either the custom or regular IPG can be provided on the program guide channel, depending on the viewer's selection.
Owner:TIVO CORP
Perfume delivery systems for consumer goods
InactiveUS20100305021A1Low vapor pressureRealized benefitsCosmetic preparationsContainer decorationsEngineeringOrganoleptic
Owner:DYKSTRA ROBERT RICHARD
Ferroelectric memory cells and methods for fabricating ferroelectric memory cells and ferroelectric capacitors thereof
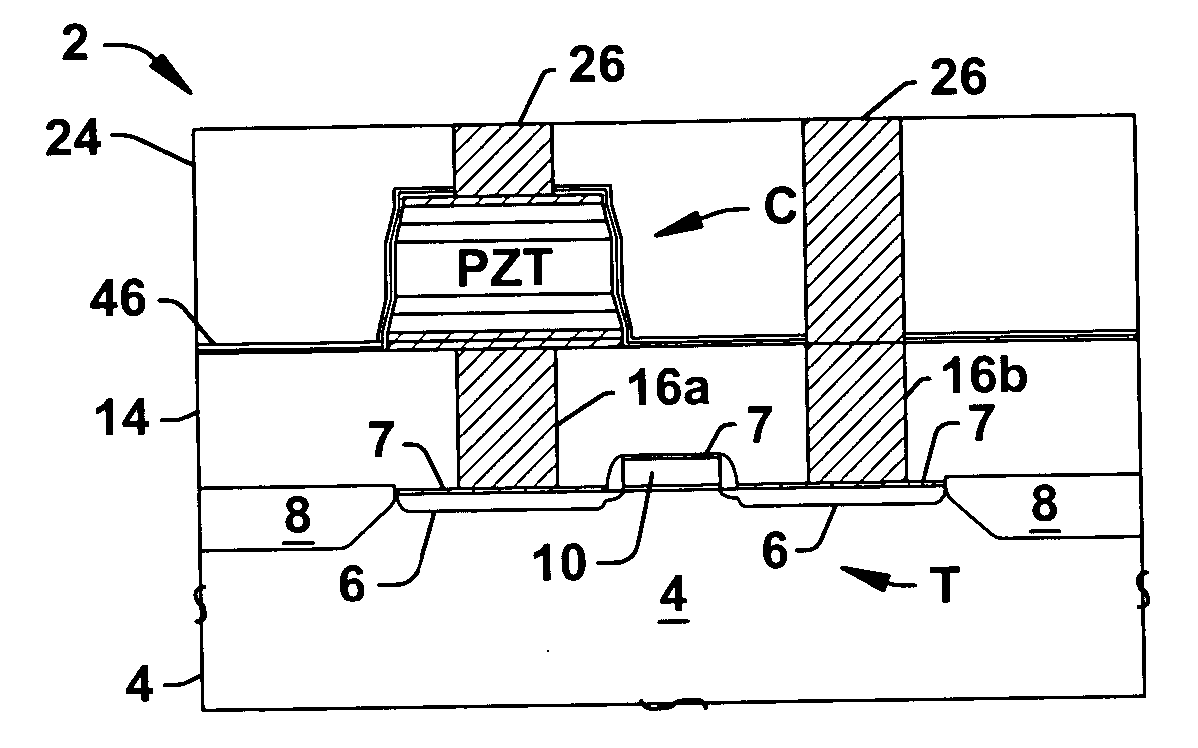
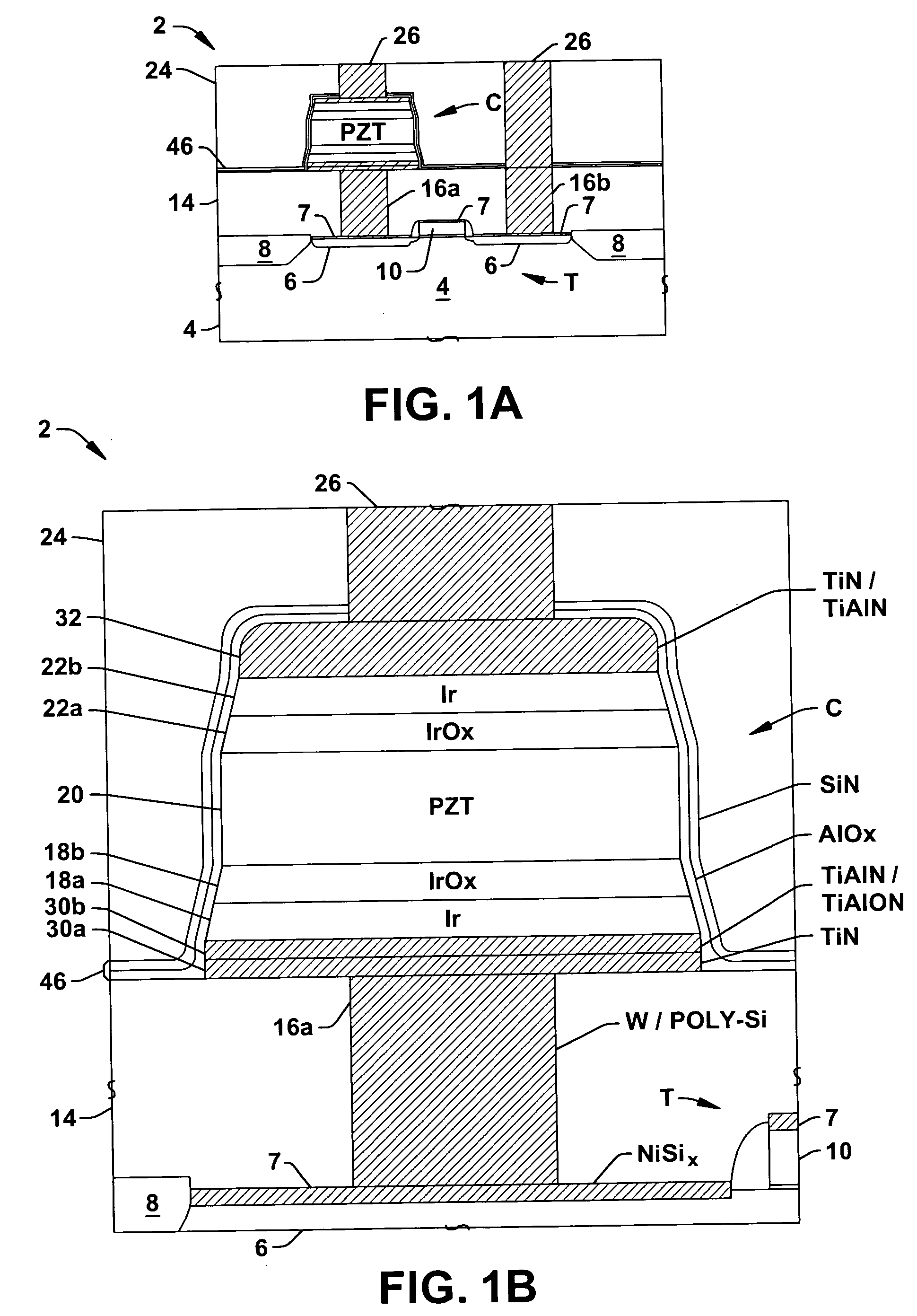
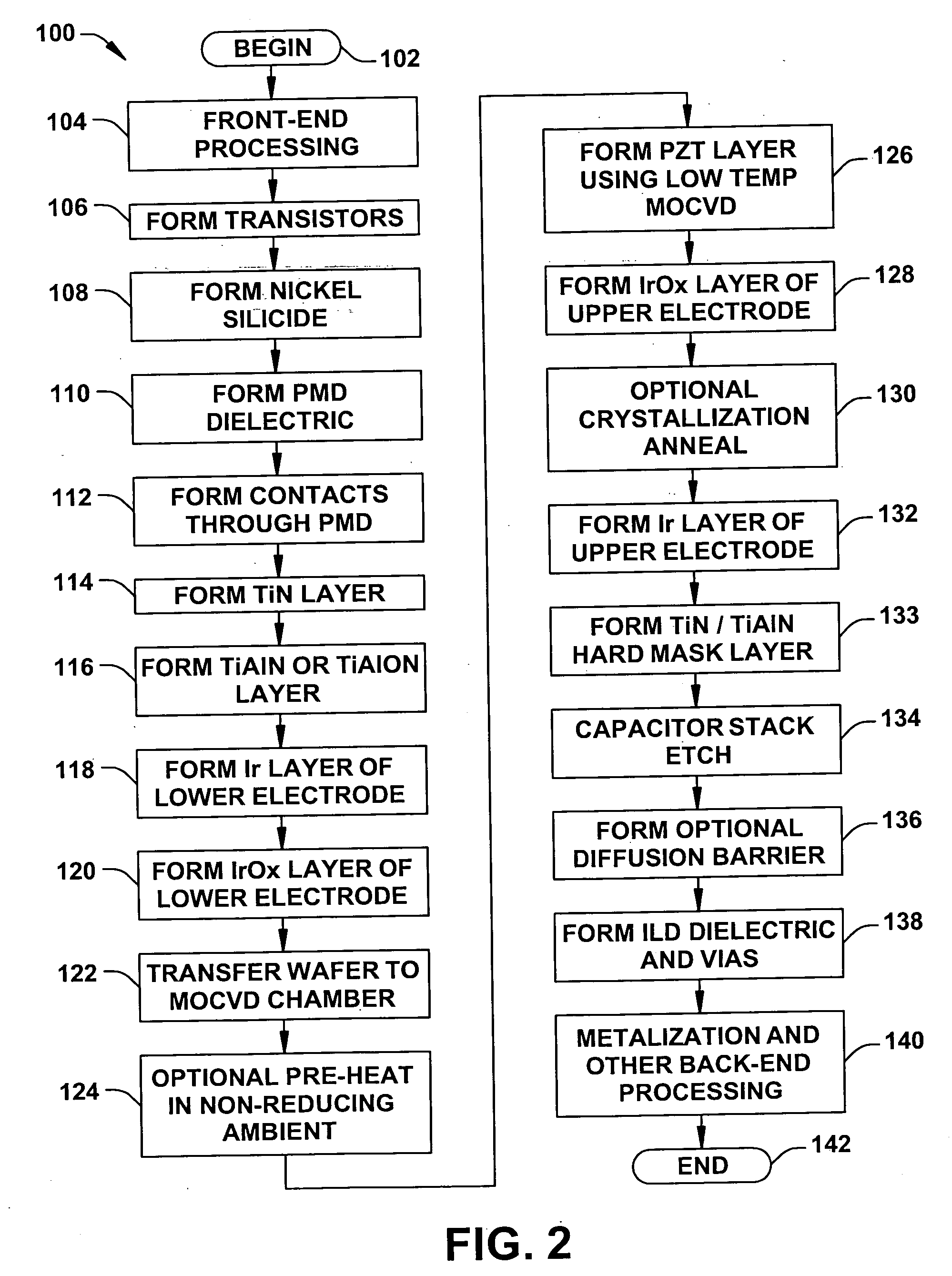
InactiveUS20060073613A1High crystallinityReduce pruningSolid-state devicesSemiconductor/solid-state device manufacturingDielectricDevice material
Methods (100) are provided for fabricating a ferroelectric capacitor in a semiconductor device wafer, comprising forming (118) a lower electrode, depositing (126) PZT ferroelectric material on the lower electrode at a temperature below 450 degrees C., and forming (128) an upper electrode on the PZT. Methods are also provided for fabricating a ferroelectric memory cell in a semiconductor device wafer, comprising forming (106) a transistor in the wafer, forming (108) a nickel silicide structure on the gate or a source / drain of the transistor, forming (110) a dielectric over the transistor, forming (112) a conductive contact extending through the dielectric to the silicide structure, forming (114, 116, 118, 120) a lower electrode on at least a portion of the conductive contact, forming (126) PZT ferroelectric material above and in contact with the lower electrode at a temperature below 450 degrees C., forming (128, 132) an upper electrode above and in contact with the PZT, and patterning (134) the upper electrode, the PZT, and the lower electrode to form a patterned ferroelectric capacitor.
Owner:TEXAS INSTR INC
Parking environment management system and method
ActiveUS7950570B2Improve performanceEffective enforcementAnti-theft cycle devicesDetection of traffic movementProgram managementEnvironmental management system
A system and method for managing a permit-based parking environment governed by a parking program. The permit-based parking environment includes a number of parking permits each including a unique RFID tag and tag number. One or more RFID readers are used to scan the vehicles parked in the parking environment to determine if a RFID tag is associated with the parked vehicle. The results of the scan along with information related to the parked vehicle are provided to a permit management system to determine if the vehicle is parked within the scope of privileges pre-defined for that vehicle, pursuant to the parking program governing the parking environment. The permit management system stores, manages, and monitors data related to the permits controlled under the parking program.
Owner:IPT
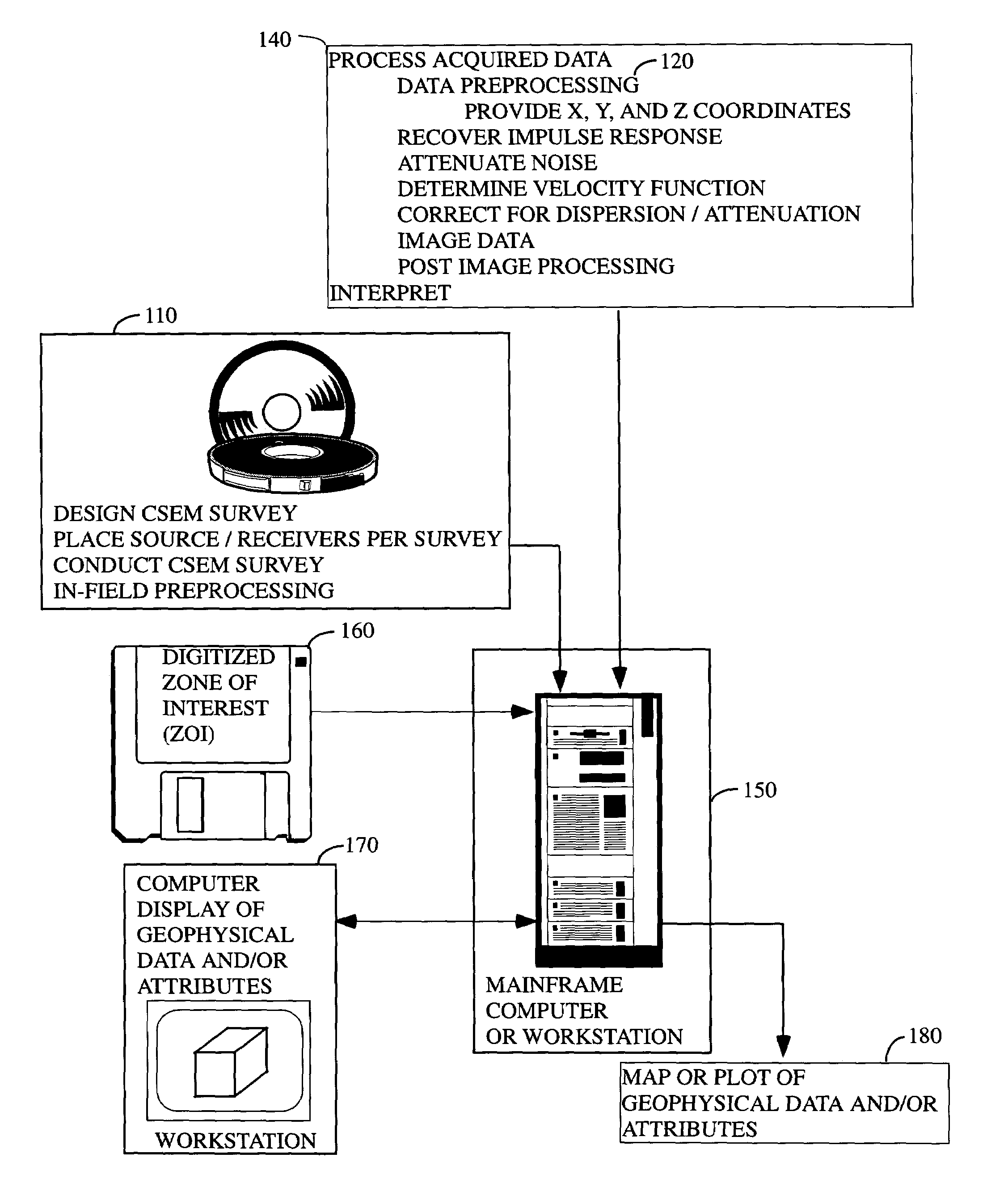
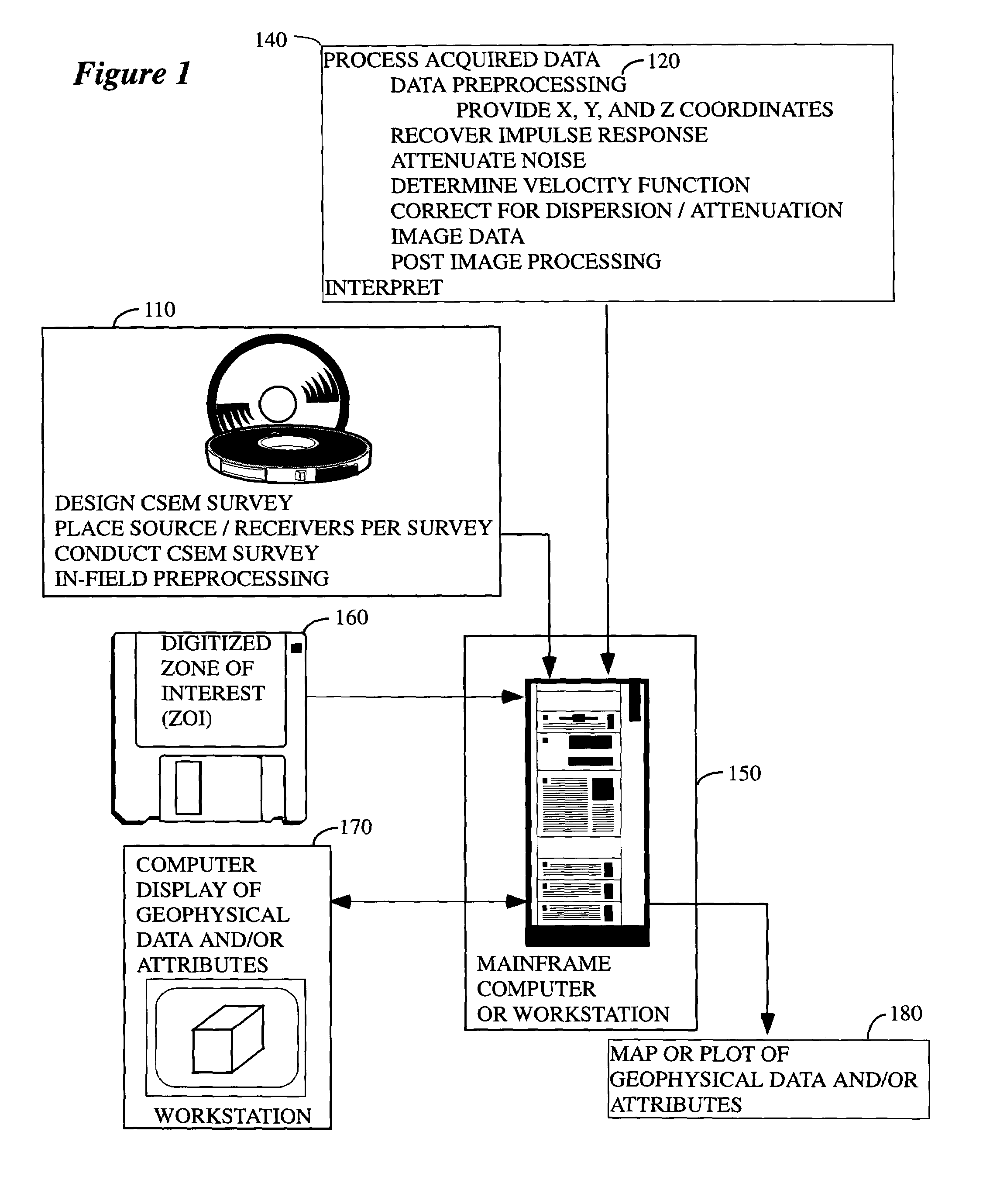
System and method for using time-distance characteristics in acquisition, processing, and imaging of t-CSEM data
InactiveUS7502690B2Enhance the imageEasy to processSeismic signal processingSeismology for water-covered areasUltrasound attenuationControlled source electro-magnetic
There is provided herein a system and method of acquiring, processing, and imaging transient Controlled Source ElectroMagnetic (t-CSEM) data in ways that are similar to those used for seismic data. In particular, the instant invention exploits the time-distance characteristics of t-CSEM data to permit the design and execution of t-CSEM surveys for optimal subsequent processing and imaging. The instant invention illustrates how to correct t-CSEM data traces for attenuation and dispersion, so that their characteristics are more like those of seismic data and can be processed using algorithms familiar to the seismic processor. The resulting t-CSEM images, particularly if combined with corresponding seismic images, may be used to infer the location of hydrocarbon reservoirs.
Owner:BP CORP NORTH AMERICA INC
Network safety optimum attacking and defending decision method for attacking and defending game
ActiveCN103152345ASimple calculationRealized benefitsData switching networksNetwork managementReal-time computing
The invention relates to a network safety attacking and defending method for a state attacking and defending map model, and belongs to the technical field of network safety attacking and defending. The method comprises the following steps of modeling an attacking and defending scene of a network system by a state attacking and defending map, then calculating the cost and income of atomic attacking, further calculating utility matrixes of different attacking and defending strategies adopted by an attacking party and a defending party under different network safety states, and finally solving a Mash balance on the basis of a non-cooperated type non-zero-sum game model, so as to obtain an optimum attacking and defending strategy. The method has the significant advantages that 1, the attacking scene is modeled by the state attacking and defending map, and the attacking and defending strategies of the network system under different network safety states are visually and clearly described; 2, the calculation of the cost and income of the attacking and defending is converted into the calculation of the attacking and defending success probability and the hazard index, and the calculation of the attacking and defending utilities is simplified; and 3, in the attacking and defending process of the network system, the costs and the incomes of the attacking party and the defending party are considered, the rational decision is made for a network management person, and the maximum income is realized for the network management.
Owner:BANK OF BEIJING CONSUMER FINANCE CO
LED based light engine
InactiveUS7784969B2Easy to modifyMinimize impactCoupling device connectionsPlanar light sourcesOptical ModuleEffect light
Owner:TALL TOWER LED
Electrolysis cell and internal combustion engine kit comprising the same
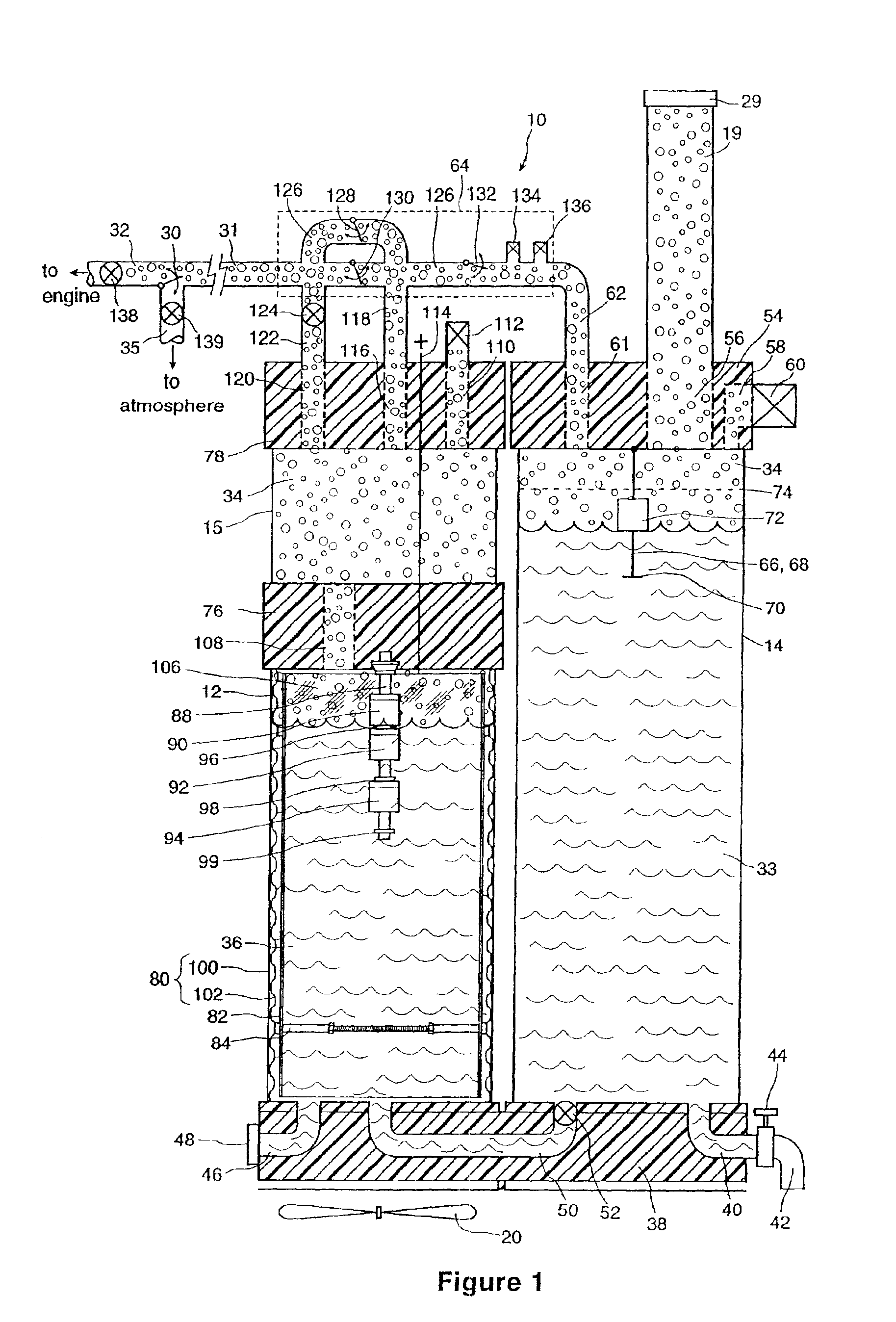
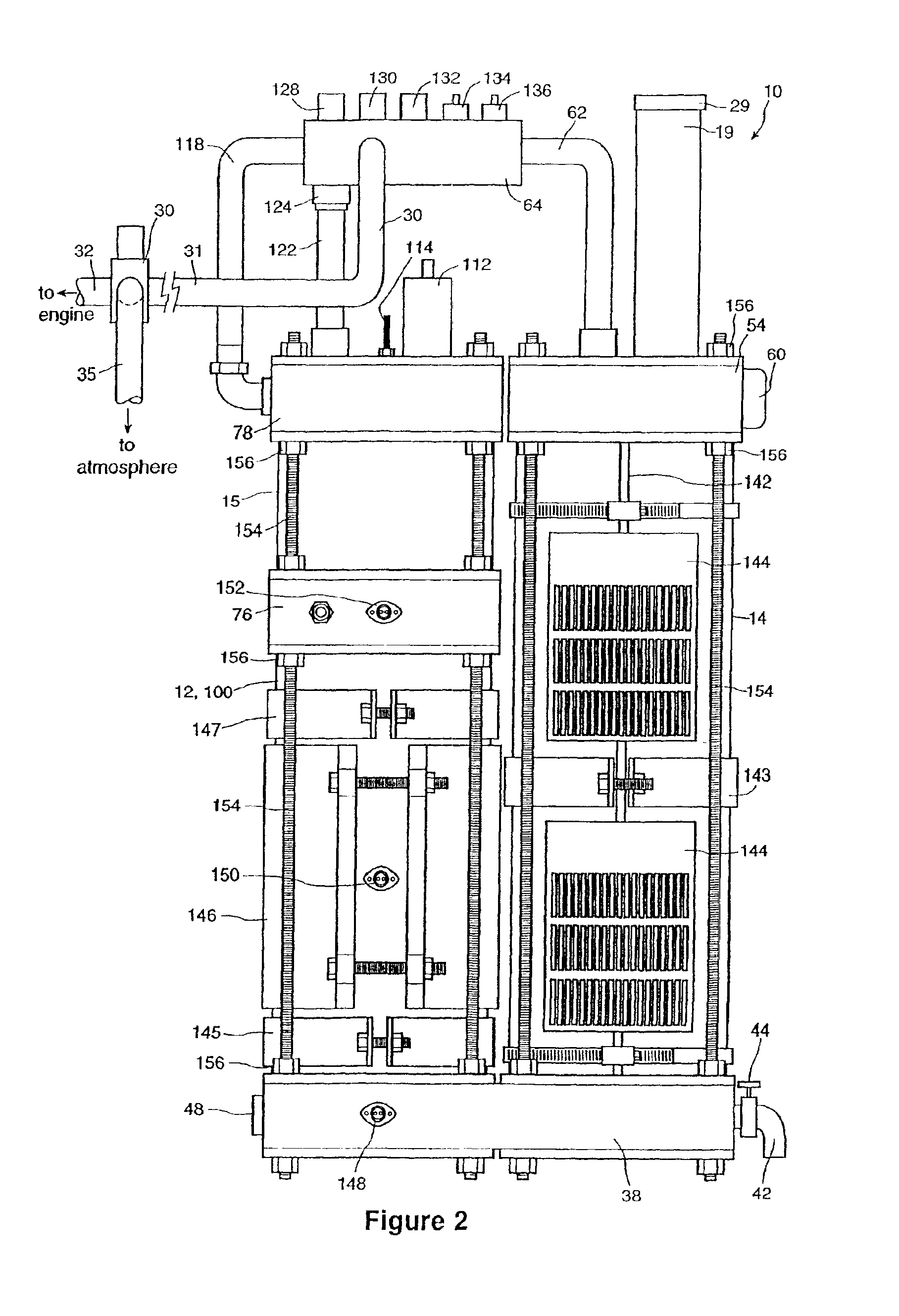
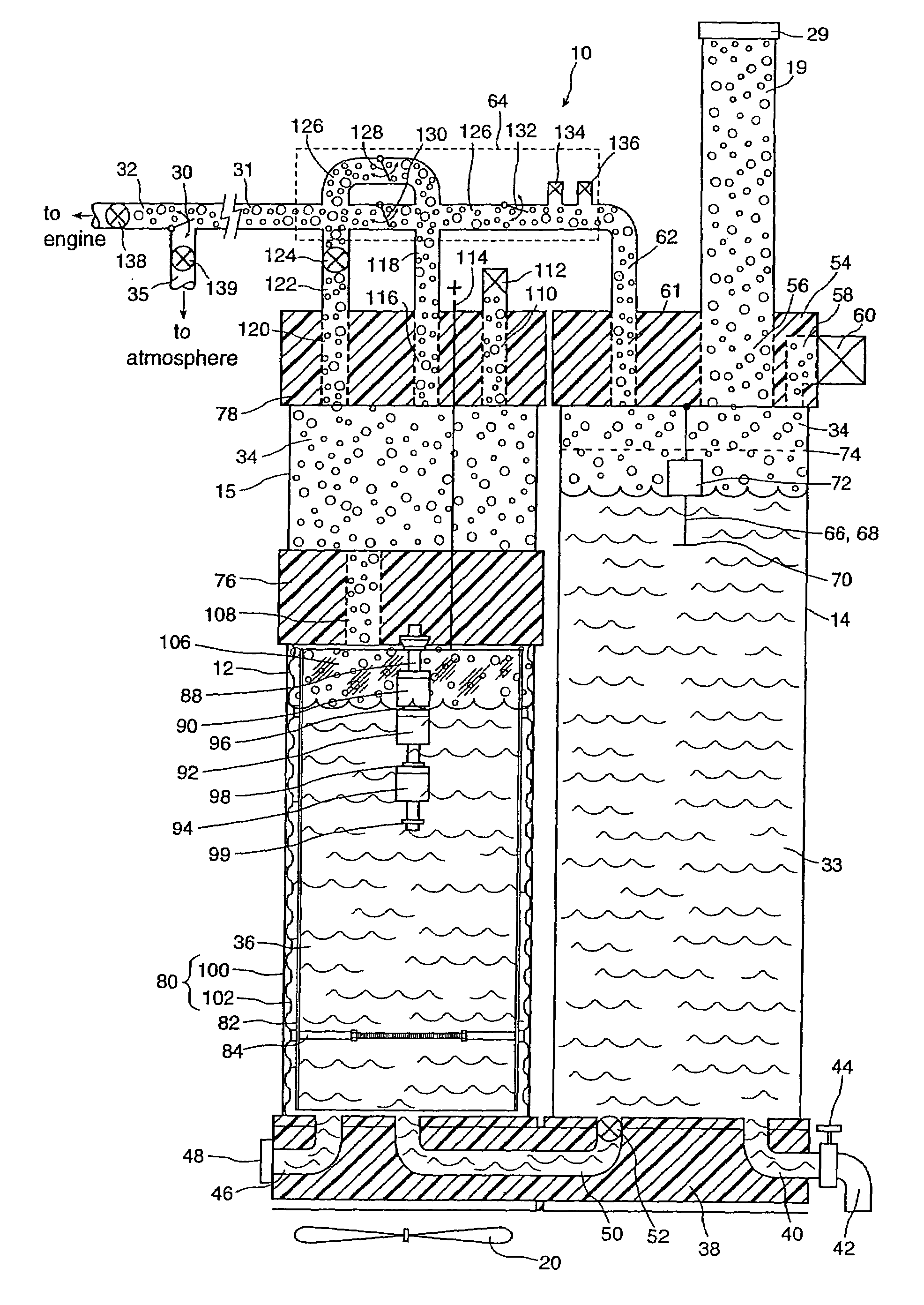
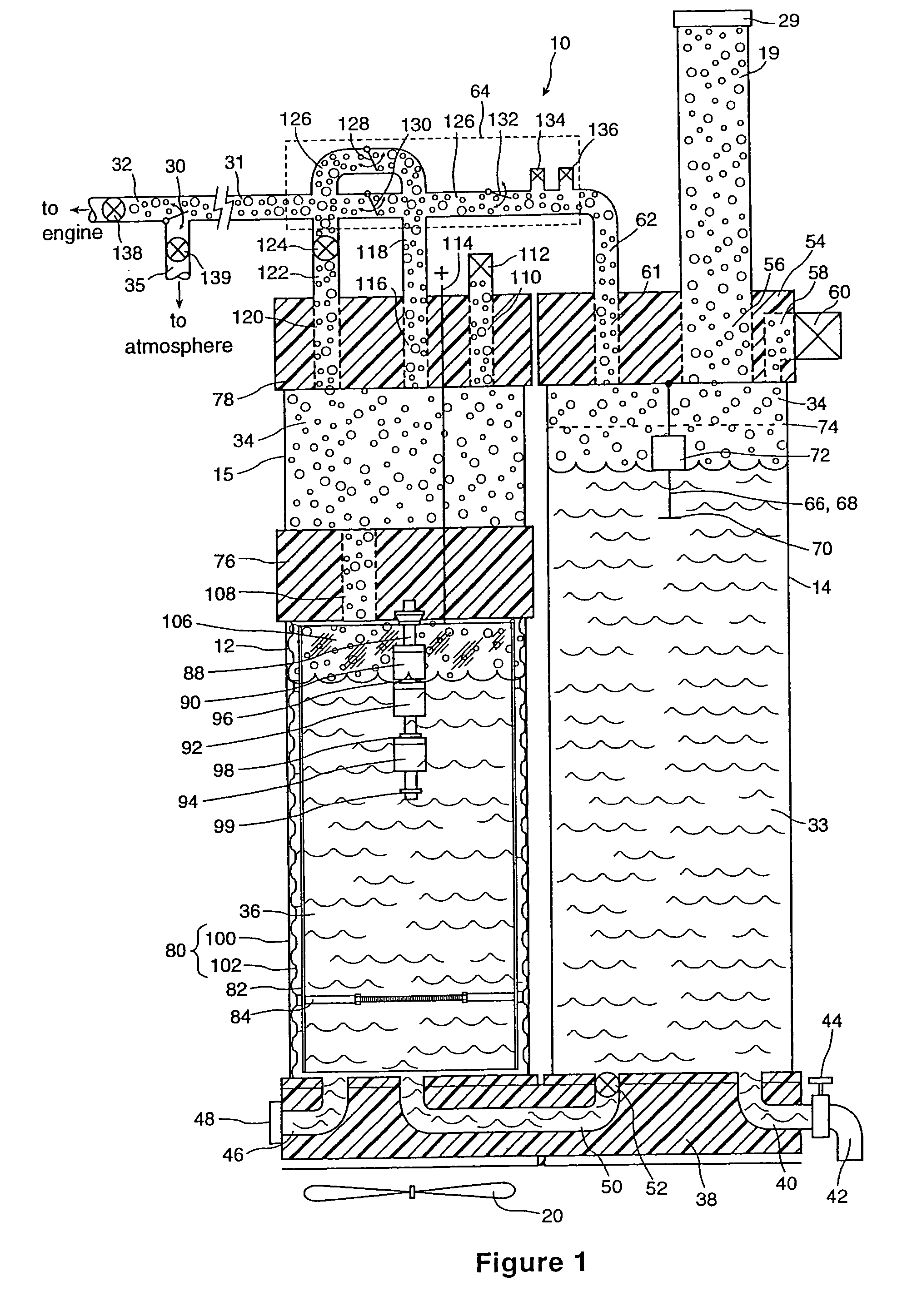
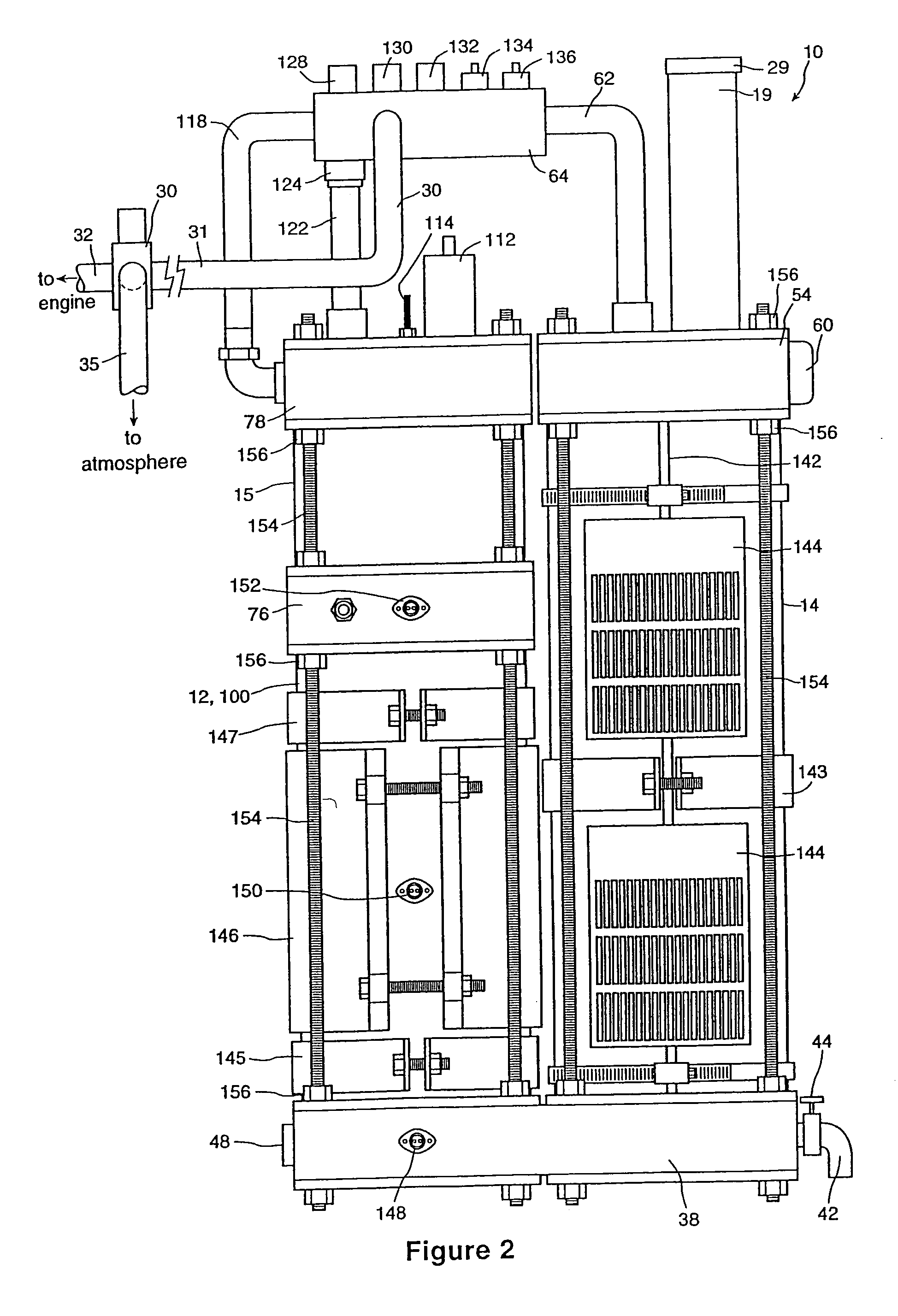
InactiveUS6896789B2Improve combustion efficiencyRealized benefitsCellsPhotography auxillary processesElectrolysisCombustion
A system for producing one or more gases for enhancing combustion in an internal combustion engine, the engine having an intake, the system comprising: an electrolysis cell, for generating one or more combustion enhancing gases under pressure; a gas conduit, for connecting the electrolysis cell to the internal combustion engine; and a flow regulator, operatively connected between the electrolysis cell and the intake of the engine, for regulating a flow of the combustion enhancing gases to the engine.
Owner:CANADIAN HYDROGEN ENERGY CO LTD
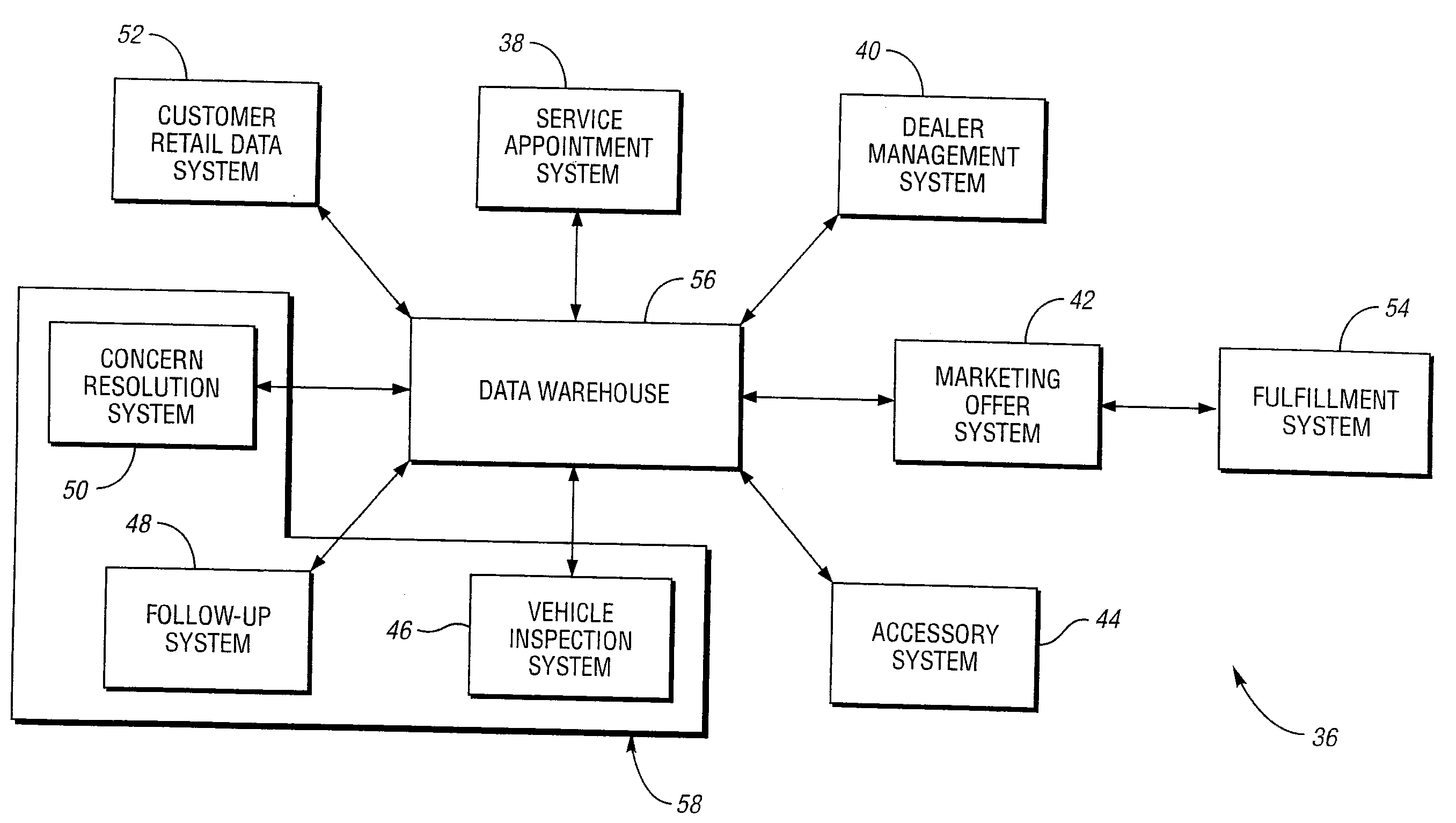
Vehicle sales and service data integration system and method
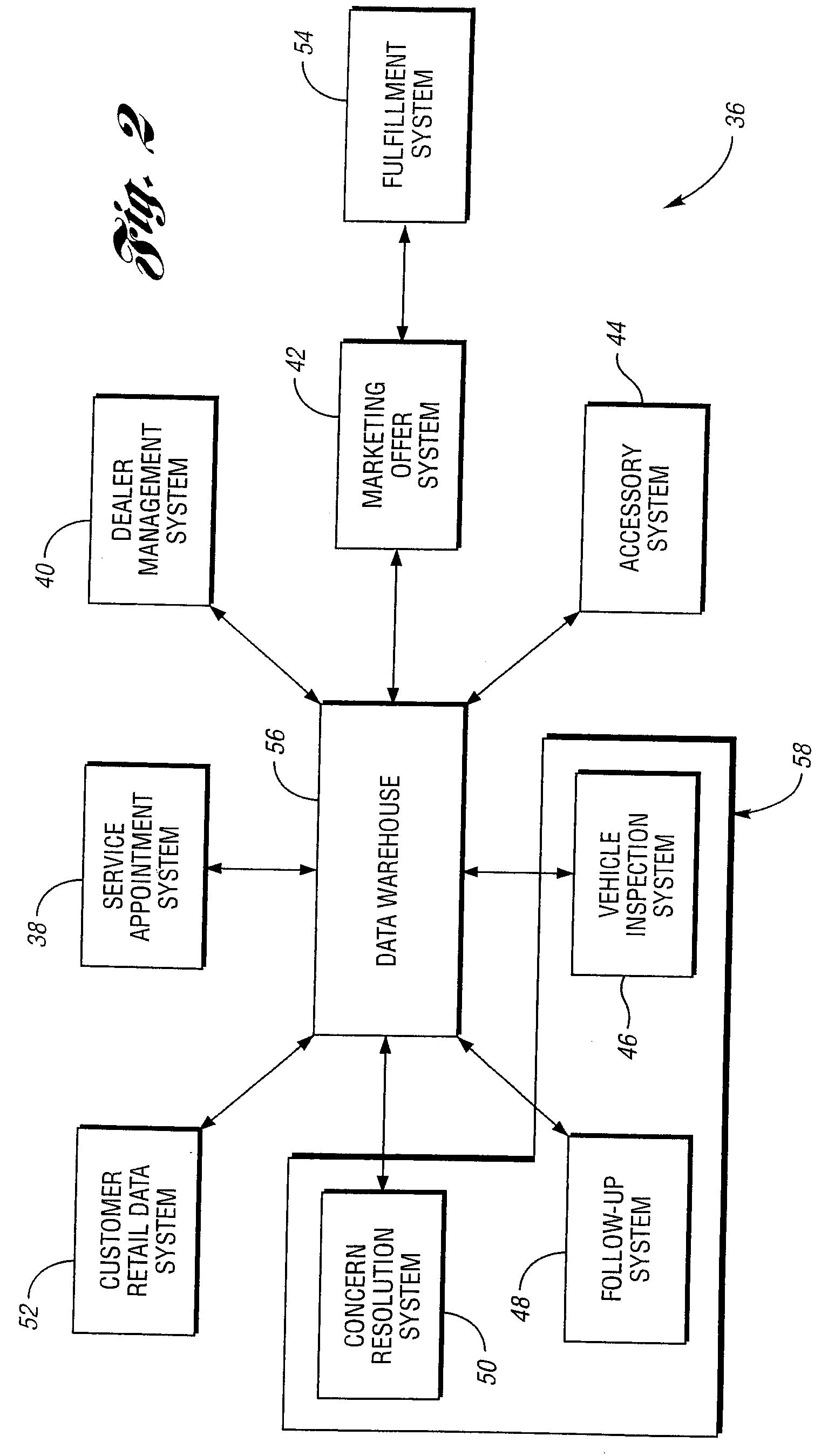
A computer-implemented method for integrating data output from a relationship between a customer and a service or product provider. The method includes receiving one or more customer vehicle attributes from two or more computer systems selected from the group consisting of: a service appointment system, a dealer management system, a marketing offer system, an accessory system, a vehicle inspection system, a follow-up system, and a concern resolution system; storing the one or more customer vehicle attributes into a data warehouse; and transmitting the one or more customer vehicle attributes stored in the data warehouse based upon an electronic request. Advantageously, the one or more customer vehicle attributes are integrated across the two or more computer systems to facilitate management of an after vehicle sales delivery relationship between a customer and a service or product provider.
Owner:FORD MOTOR CO
Electrolysis cell and internal combustion engine kit comprising the same
InactiveUS7143722B2Improve combustion efficiencyRealized benefitsCellsNon-fuel substance addition to fuelElectrolysisCombustion
A system for producing one or more gases for enhancing combustion in an internal combustion engine, the engine having an intake, the system comprising: an electrolysis cell, for generating one or more combustion enhancing gases under pressure; a gas conduit, for connecting the electrolysis cell to the internal combustion engine; and a flow regulator, operatively connected between the electrolysis cell and the intake of the engine, for regulating a flow of the combustion enhancing gases to the engine.
Owner:CANADIAN HYDROGEN ENERGY CO LTD
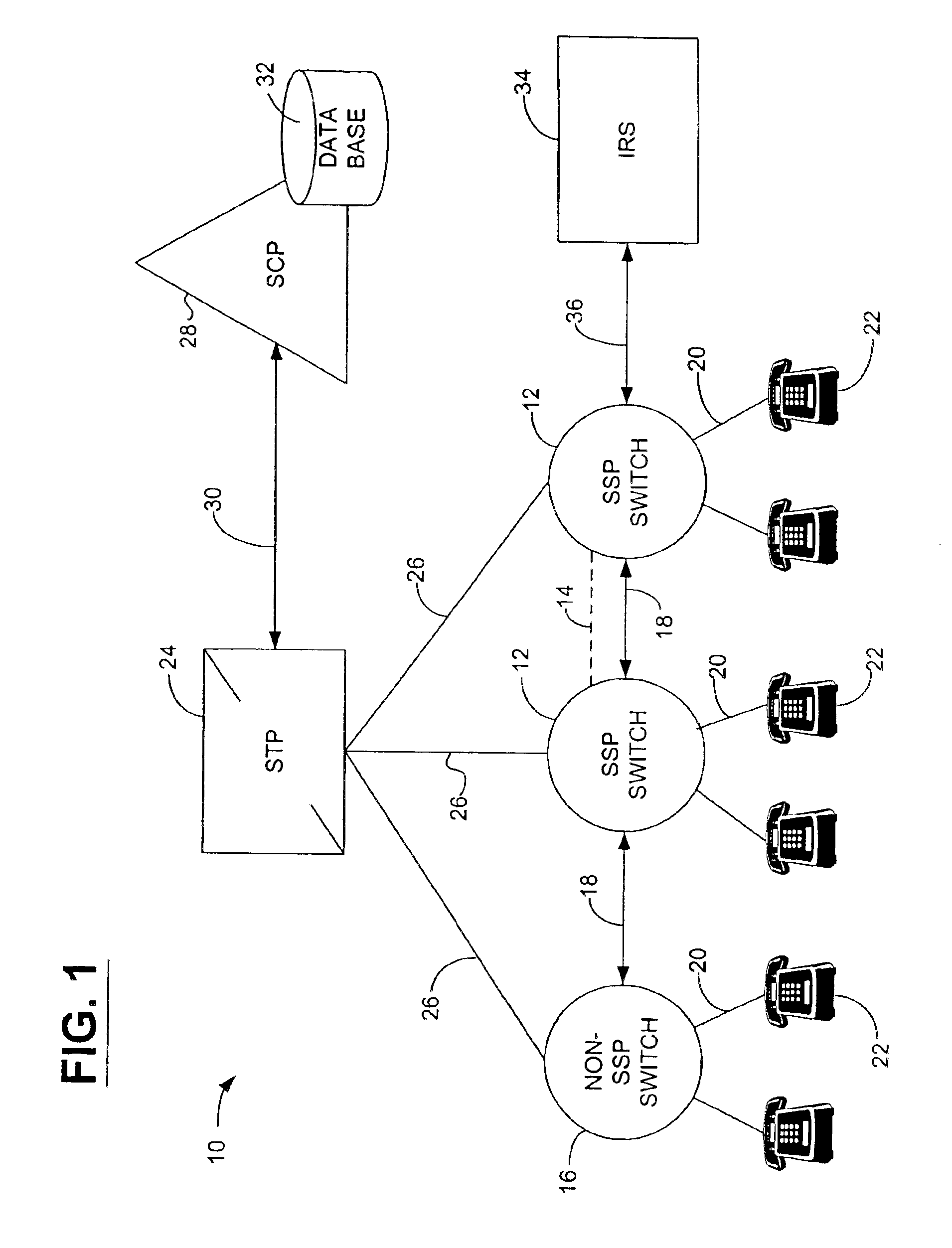
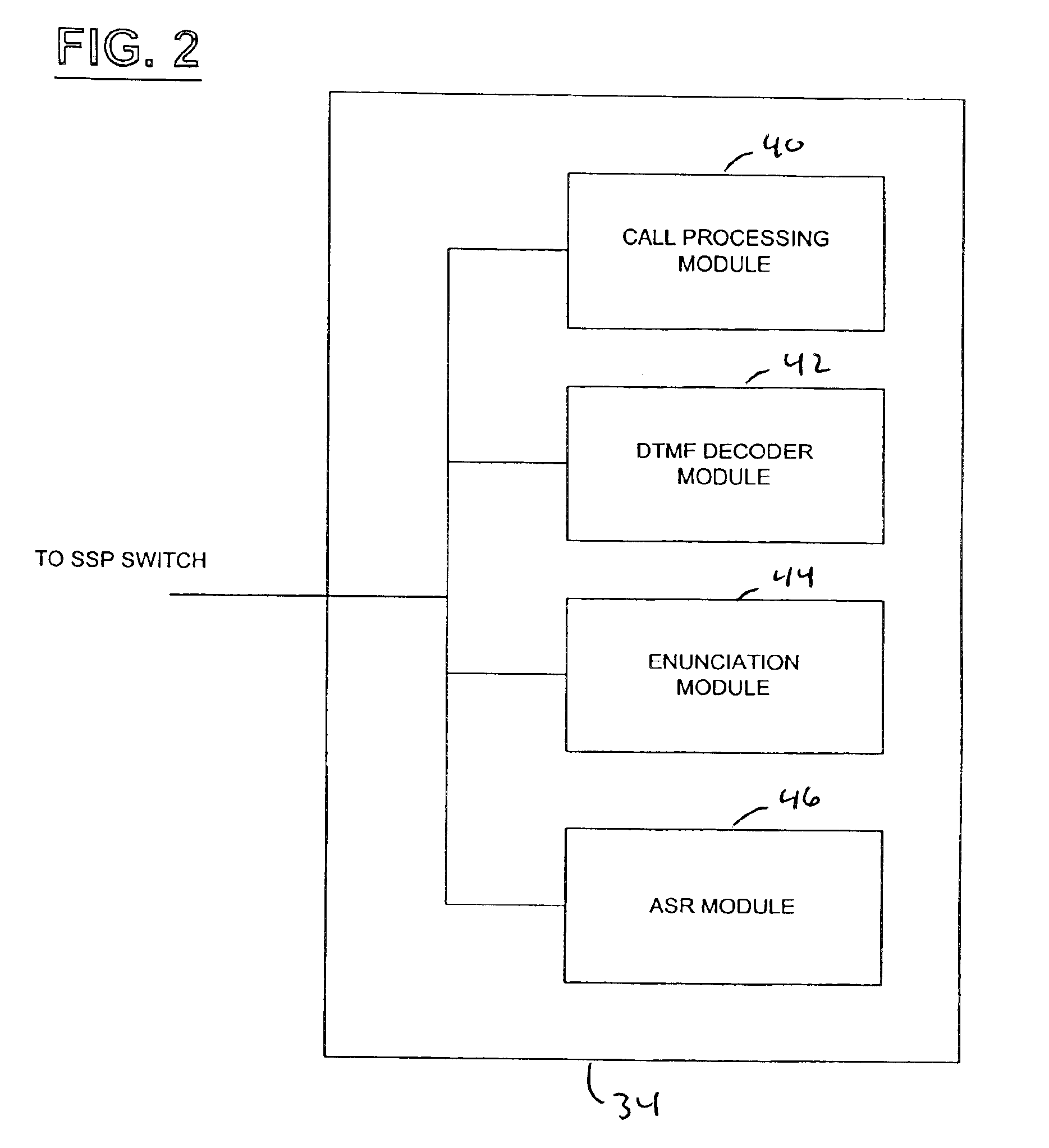
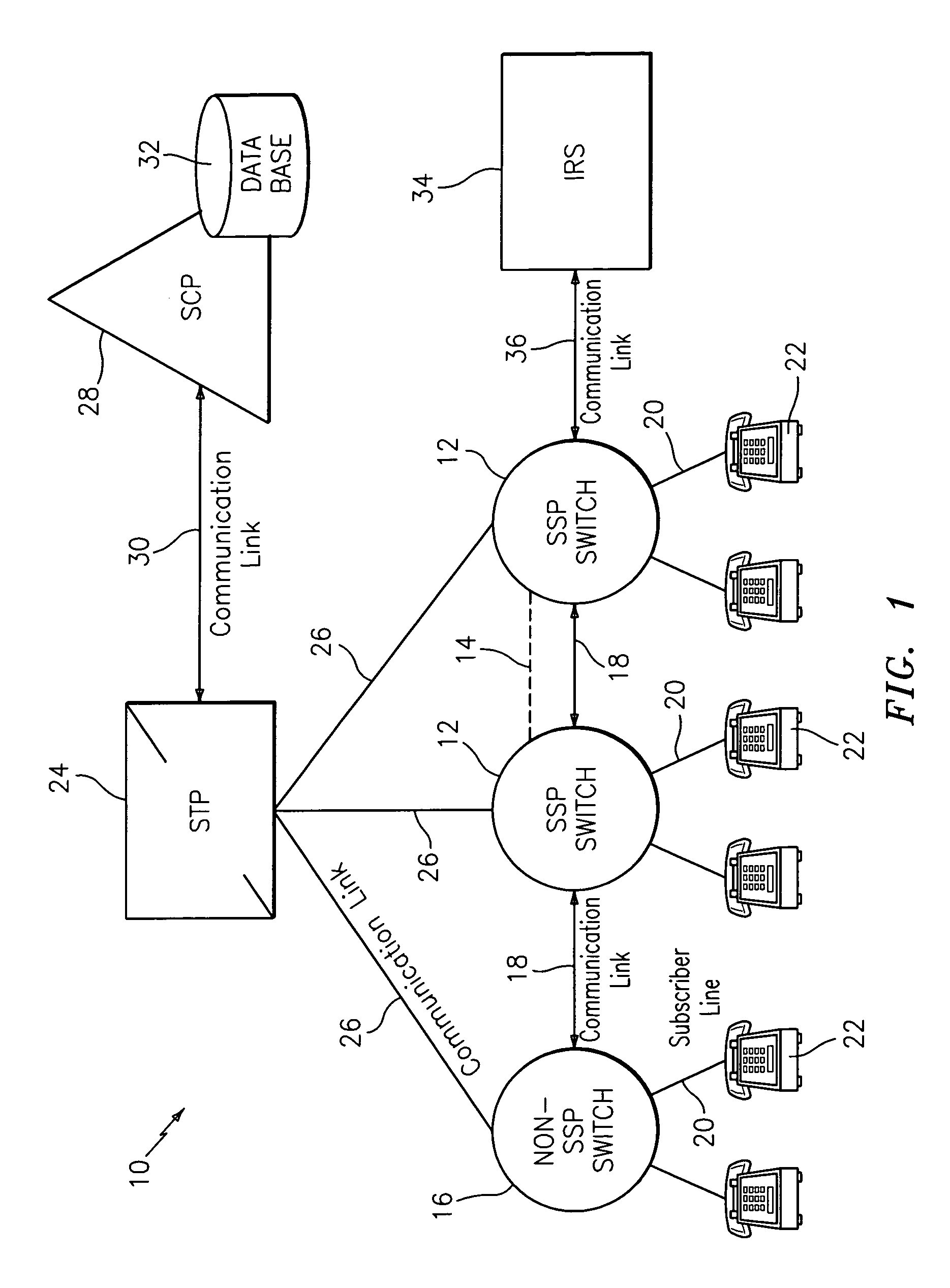
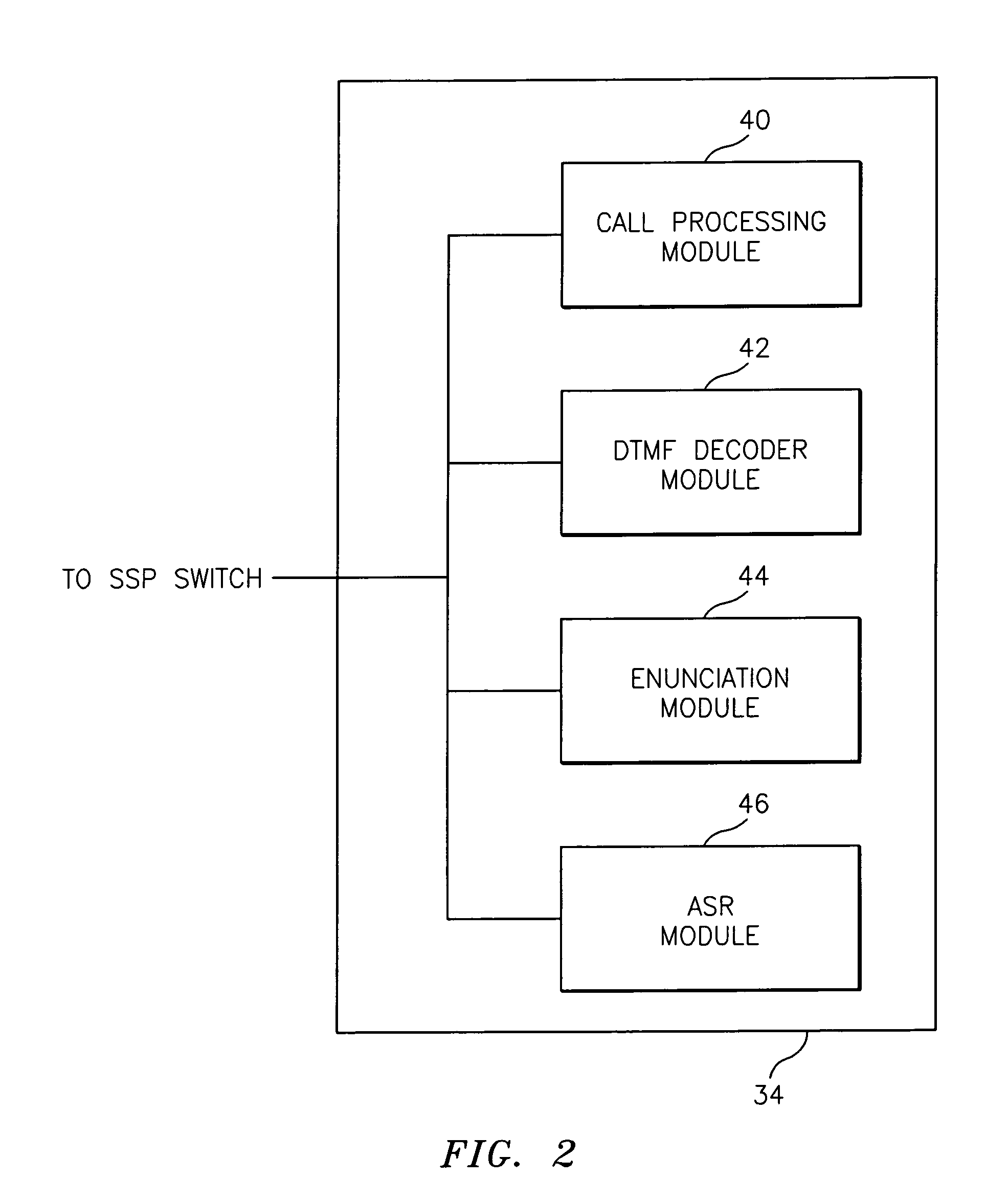
Network and method for providing a name and number delivery telecommunications services with automatic speech recognition capability
InactiveUS6907111B1Add featureGood serviceInterconnection arrangementsSpecial service for subscribersTelecommunications linkSpeech identification
A network for providing a telecommunications service with automatic speech recognition to a telecommunications user, including a switch in communication with a telecommunications device associated with the telecommunications user for detecting a trigger specific to the service in response to a communication from the telecommunications device and for routing the communication to an operator services system in response to detection of the trigger, and an intelligent resource server in communication with the switch for receiving via the switch the communication from the operator services system with a message including information regarding a party requested by the telecommunication user from the operator services system, for playing an audible message for the telecommunications user in response to receiving the communication, the audible message containing the information regarding the party and prompting the telecommunications user to place an outgoing communication to the party, and for automatically recognizing a predetermined keyword spoken by the telecommunications user in response to the audible message.
Owner:IBM CORP
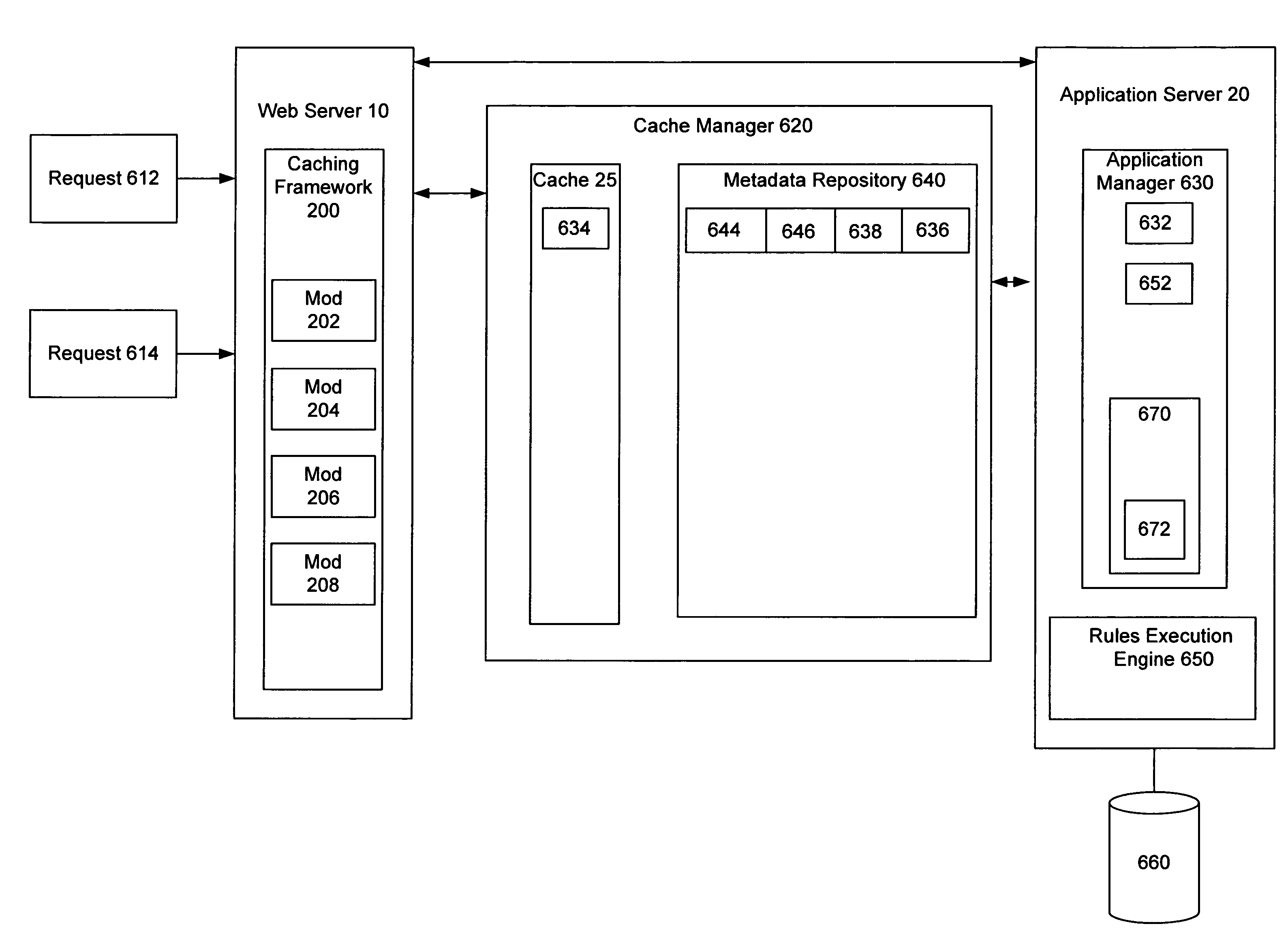
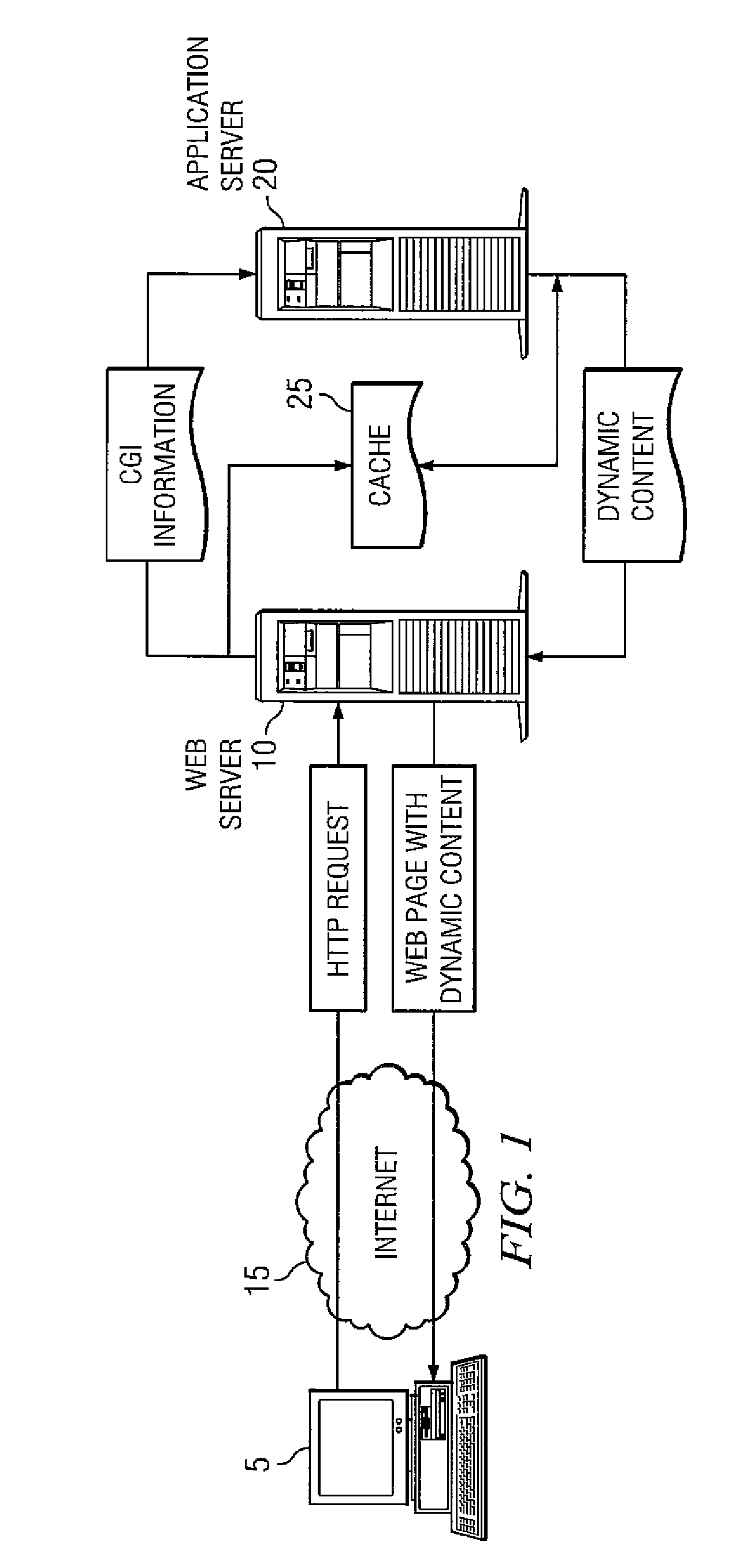
Method and system for cache management
ActiveUS7818506B1Realized benefitsEasy to updateDigital data information retrievalMultiple digital computer combinationsCache managementWorld Wide Web
Owner:OPEN TEXT SA ULC
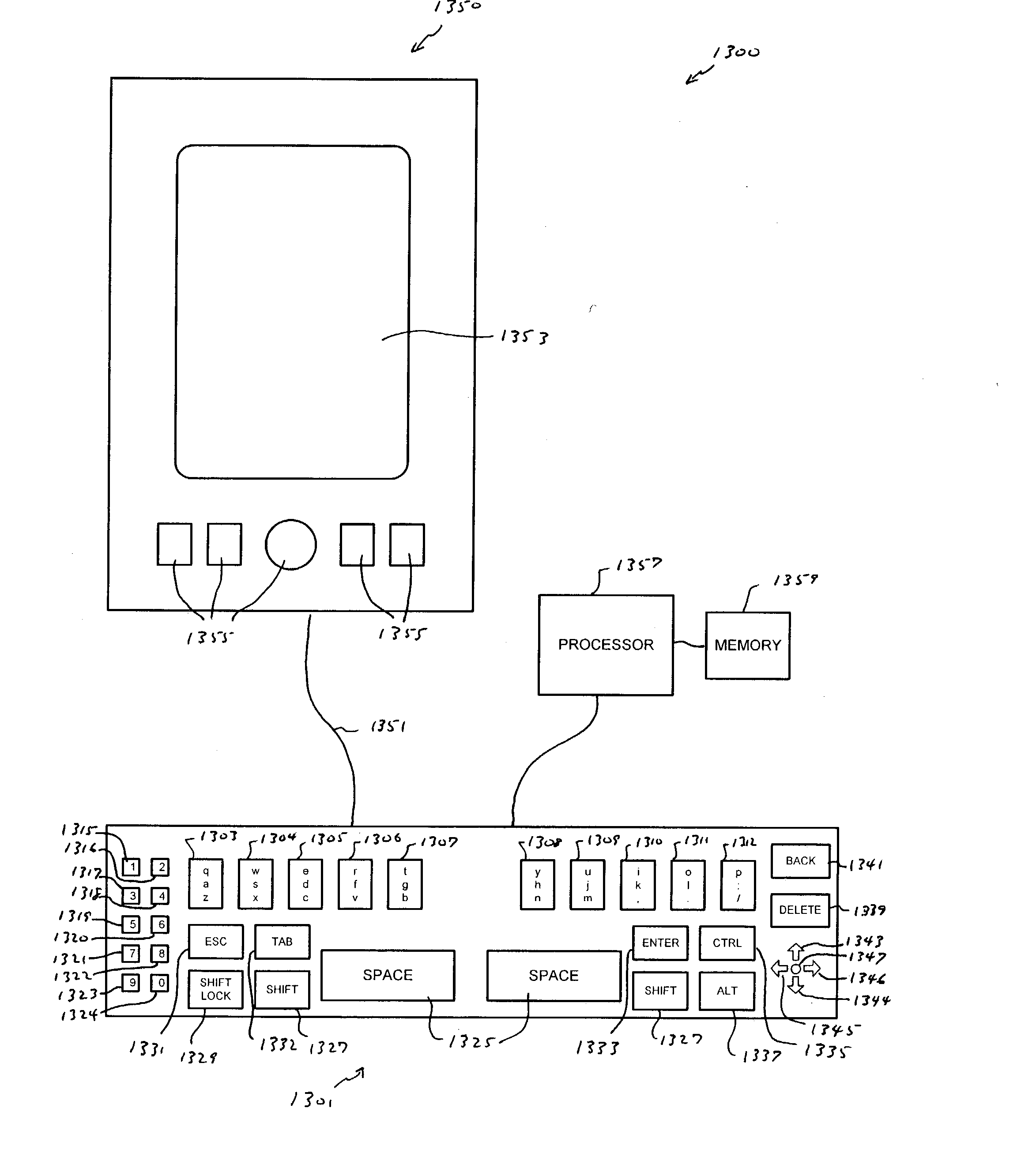
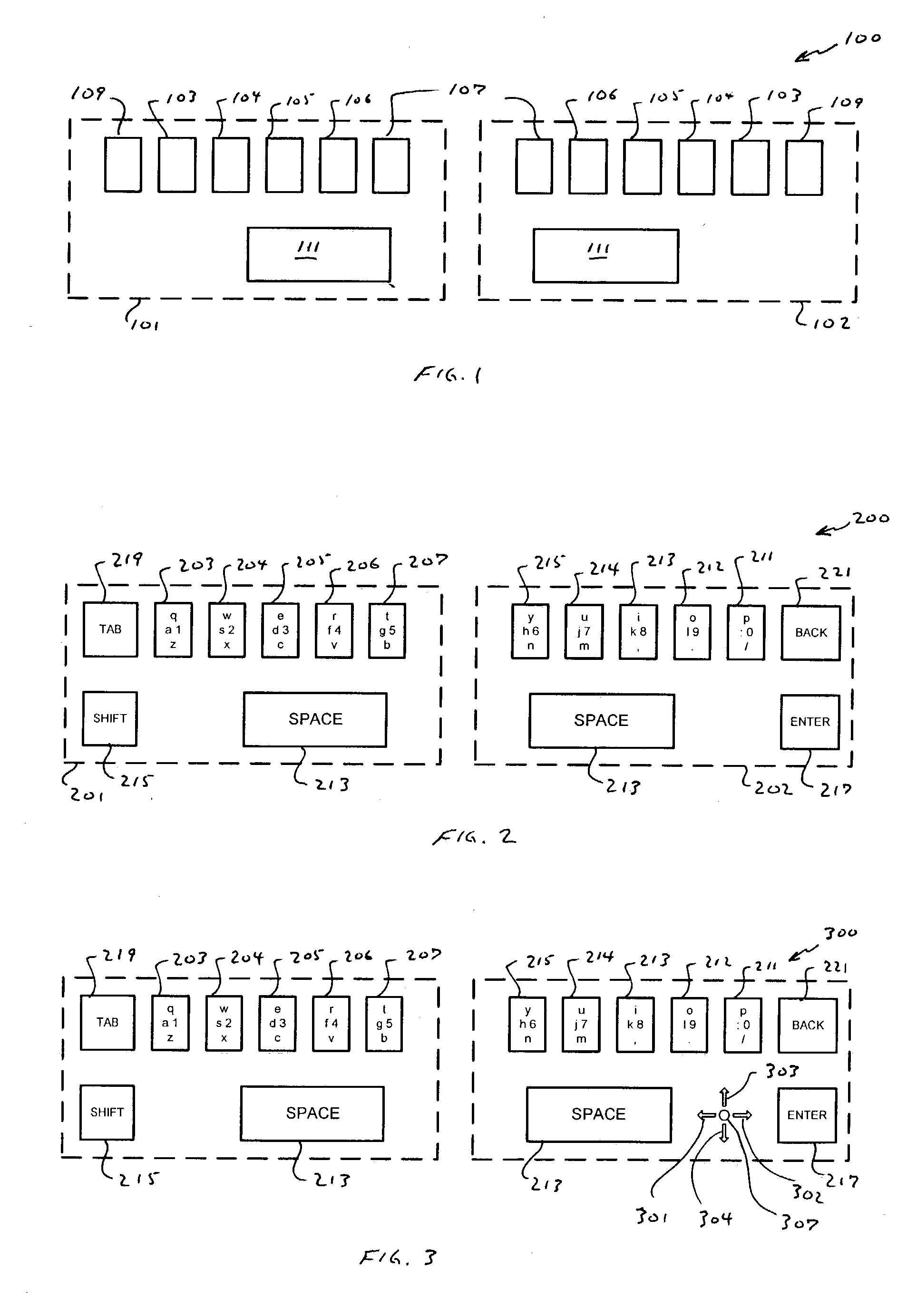
Compressed standardized keyboard
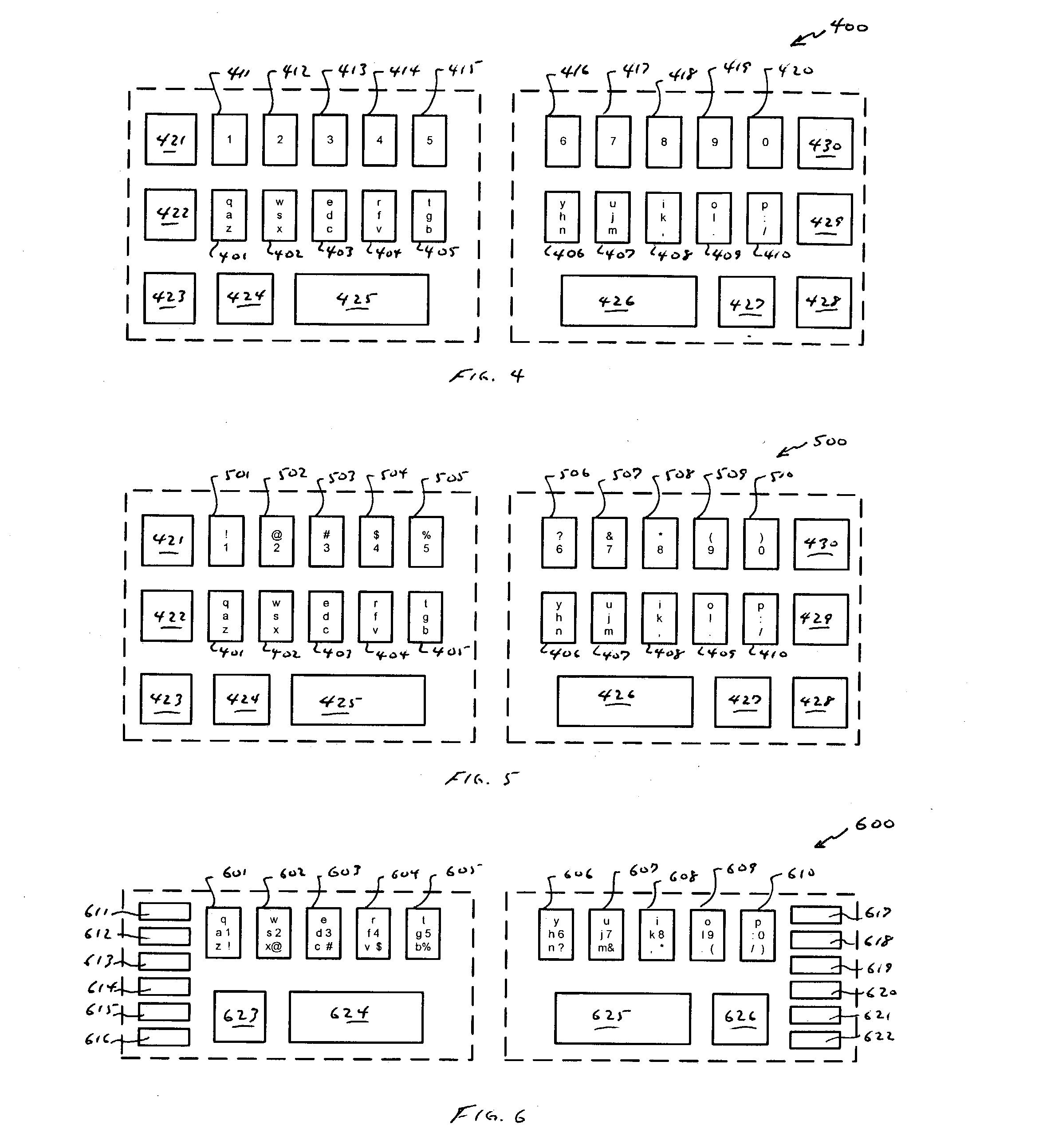
InactiveUS20040239533A1Small sizeRealized benefitsInput/output for user-computer interactionInterconnection arrangementsReduced sizeComputer science
A keyboard of reduced size is provided in which multiple characters are assigned to each of the primary keys. The selection of the characters to be assigned to each specific primary key is based on the touch typing rules associated with a specific standardized keyboard, preferably allowing at least three rows of keys to be reduced to a single row of keys. A disambiguating system is used to interpret which of the characters and / or symbols assigned to a particular key is intended, typically by applying a set of disambiguating rules to generated input sequences.
Owner:BOLLMAN TAYLOR
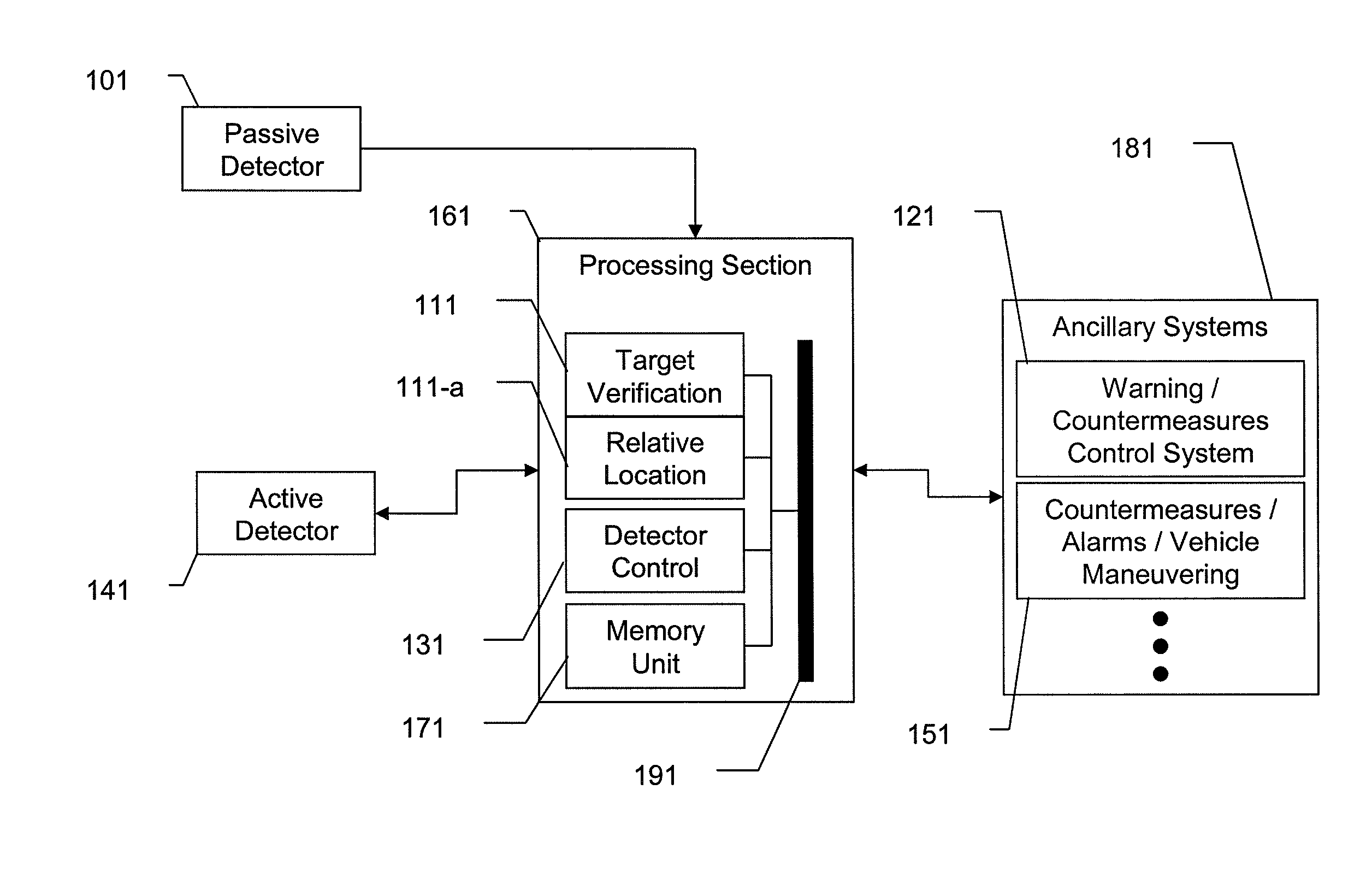
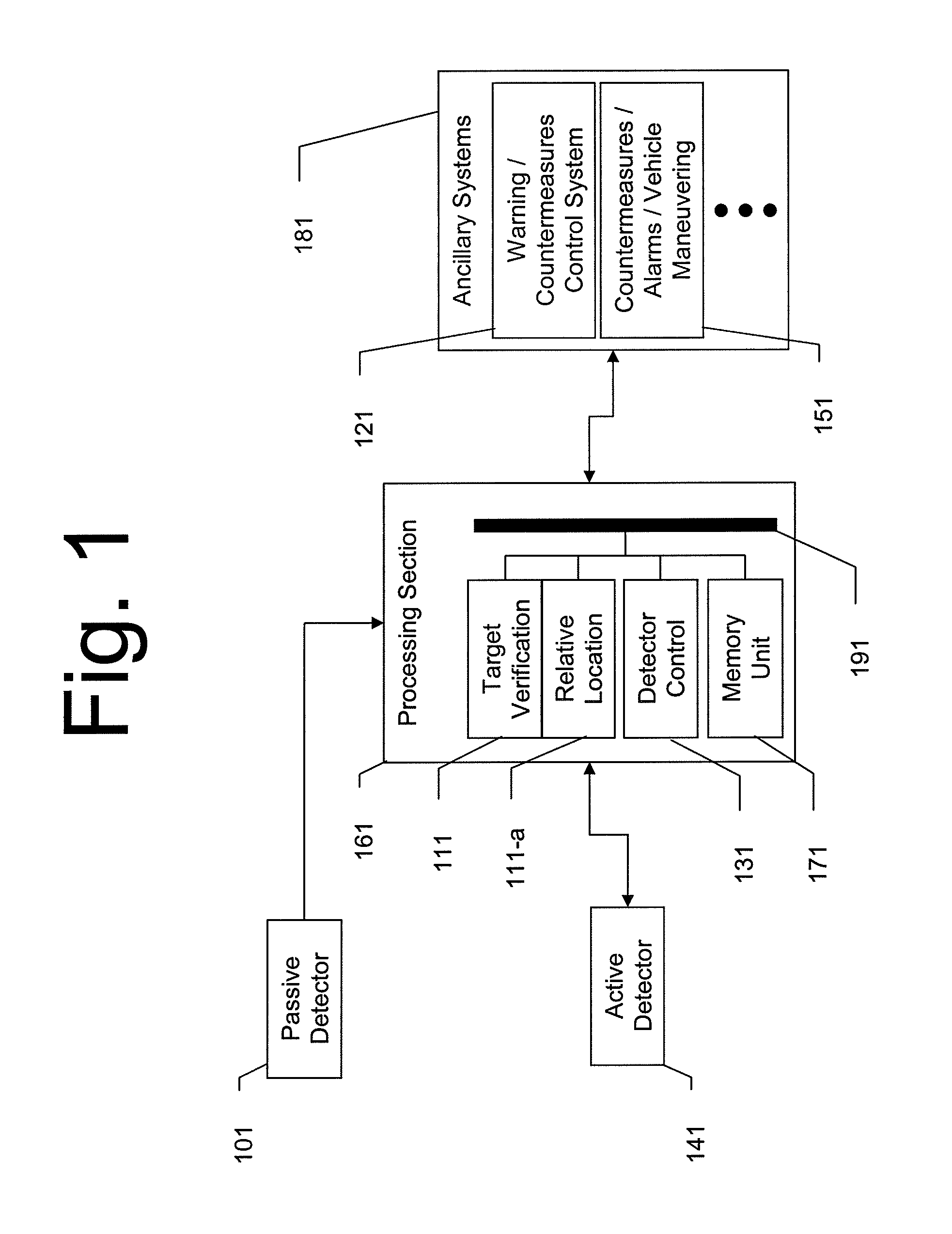
Dual Band Threat Warning System
InactiveUS20110127328A1Reduce false alarm rateAccurately detectDefence devicesWeapon control systemsActive detectionPassive detection
A system and method for dual-band detection of incoming threats using an initial passive detection system and a selectively activated active detection system. Advantages include improved threat detection accuracy, reduction of false alarms, and reduced radiation emission from the active system, thereby making the active system more difficult to detect and reducing irradiation levels of users and bystanders. Variations include systems employing passive optical or electro-optical detectors, systems employing active RADAR or LADAR detectors, and systems connected to alarm signal / threat mitigation systems. The system may be configured for use on ground vehicles or small watercraft. Variations of the system may be specifically configured to detect incoming munitions launches.
Owner:LOCKHEED MARTIN CORP
Method for enhanced performance training
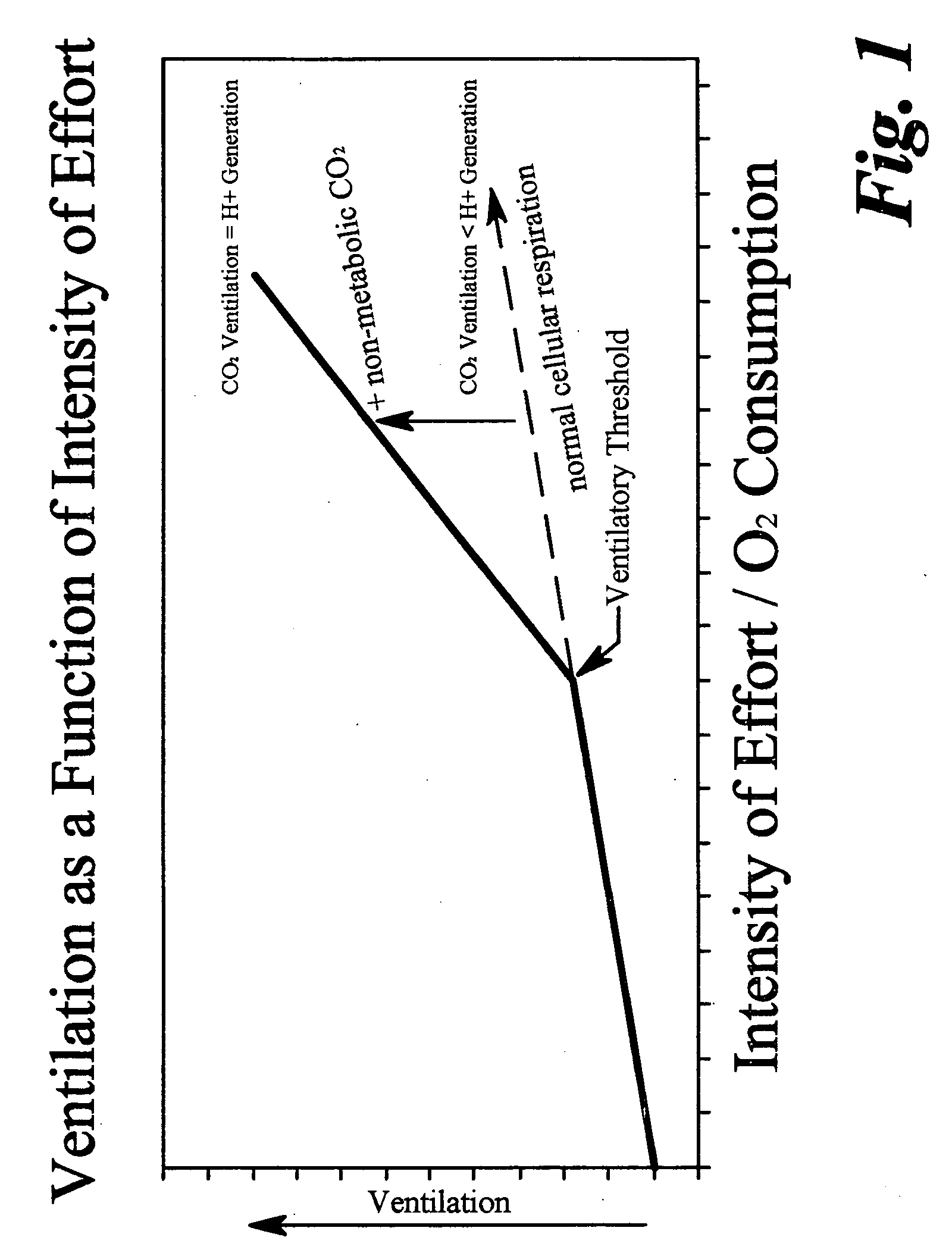
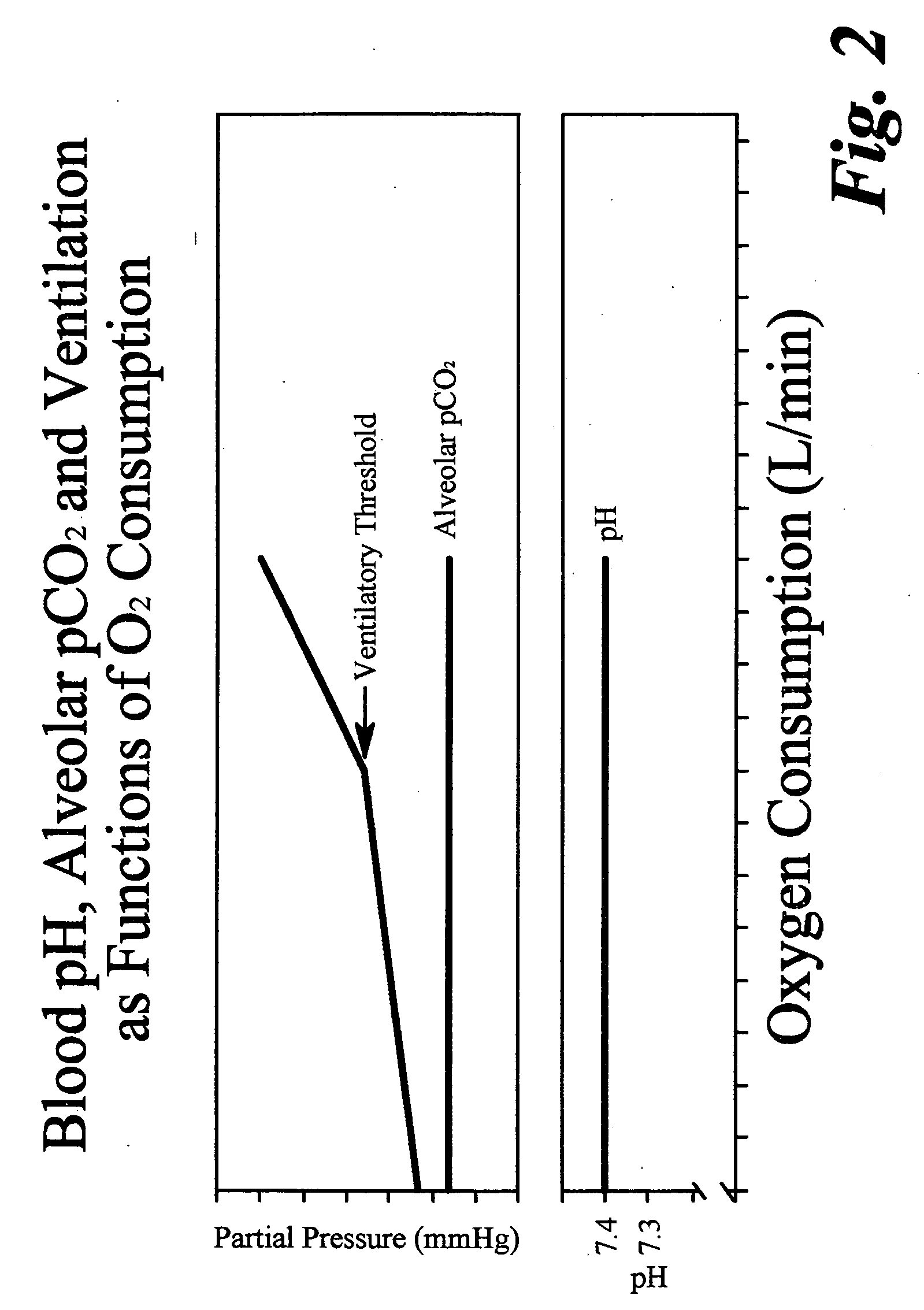
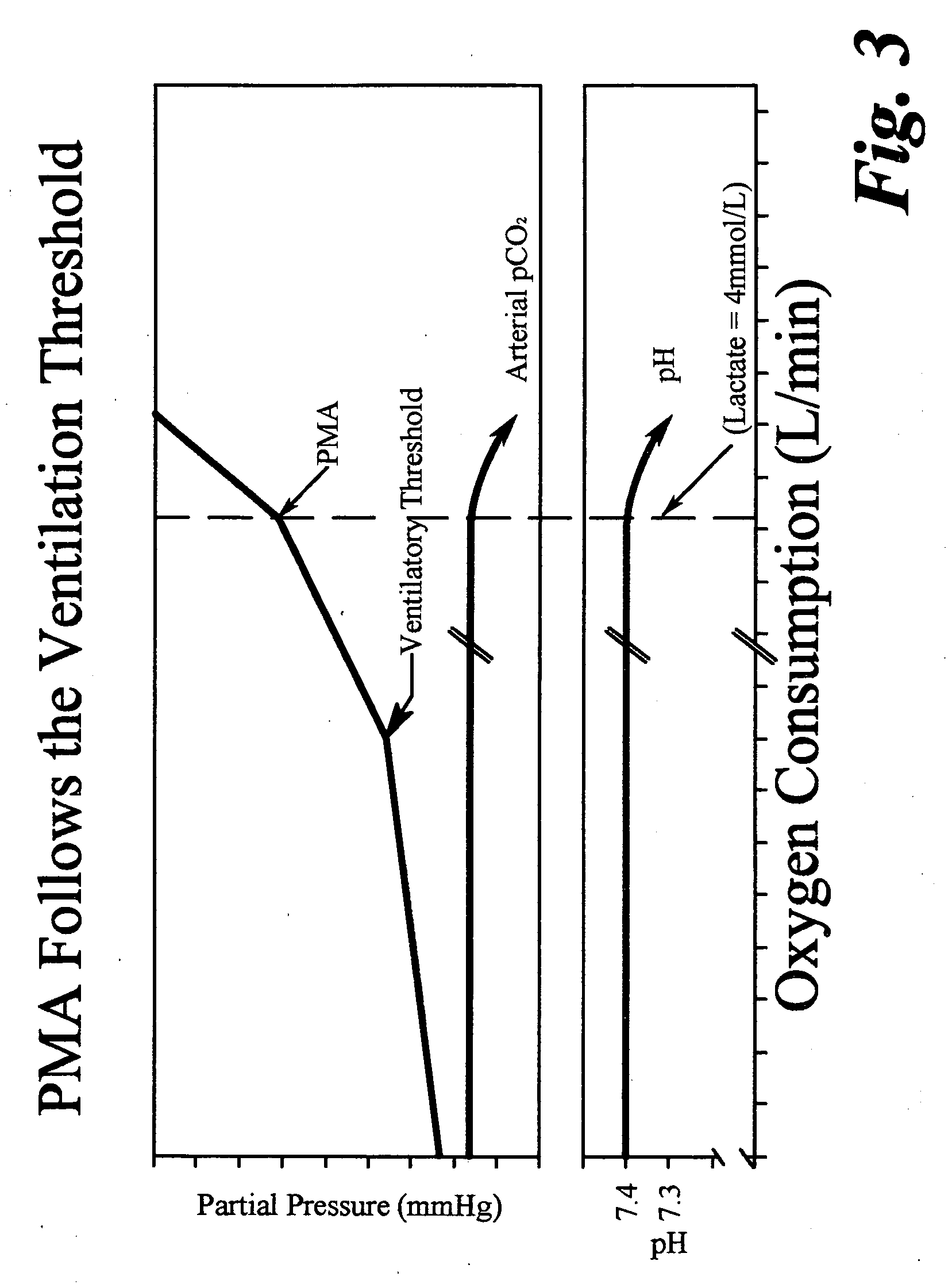
ActiveUS20070179350A1Decreased level of CO2Alter pHGymnastic exercisingCatheterBlood lactatePhysical exercise
A method for enhanced exercise training or performance utilizing intentional controlled tachypnea and somatic sensory alkalosis biofeedback training to maintain an essentially non-acidic state during exercise. A trainee is instructed to decrease measured transcutaneous CO2 levels by increased ventilation and to correlate measured transcutaneous CO2 levels with subjective somatic symptoms. Studies under exercise conditions measure the intensity of exercise correlating to an onset in blood lactate accumulation in the trainee and such level of intensity is in turn correlated with a predetermined heart rate. The trainee is then instructed to use heart rate as a guide to the need for increased ventilation to lower blood CO2. In another embodiment, the method of the instant invention utilizes intentional controlled tachypnea to increase maximum breath holding time.
Owner:NADEAU GARY
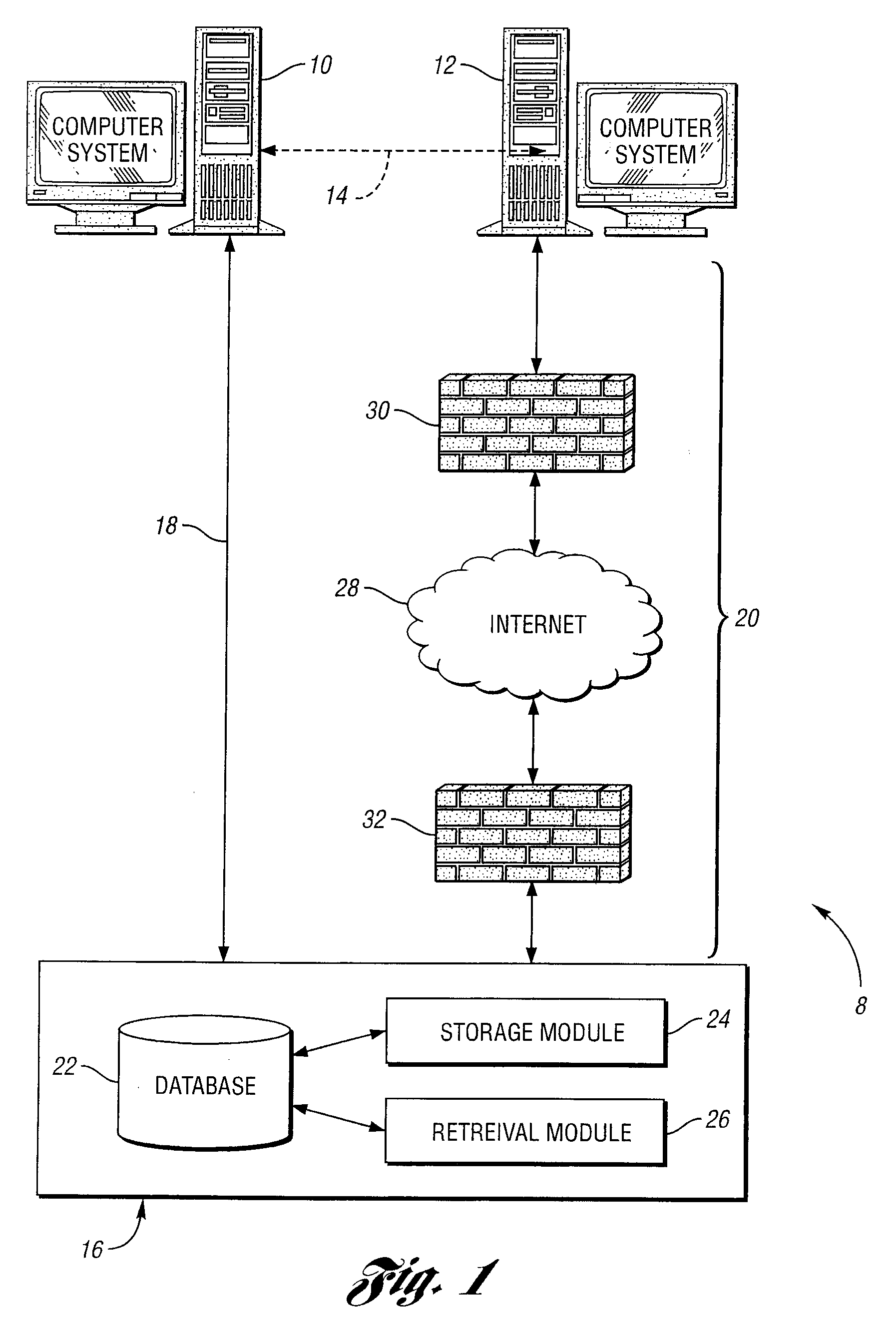
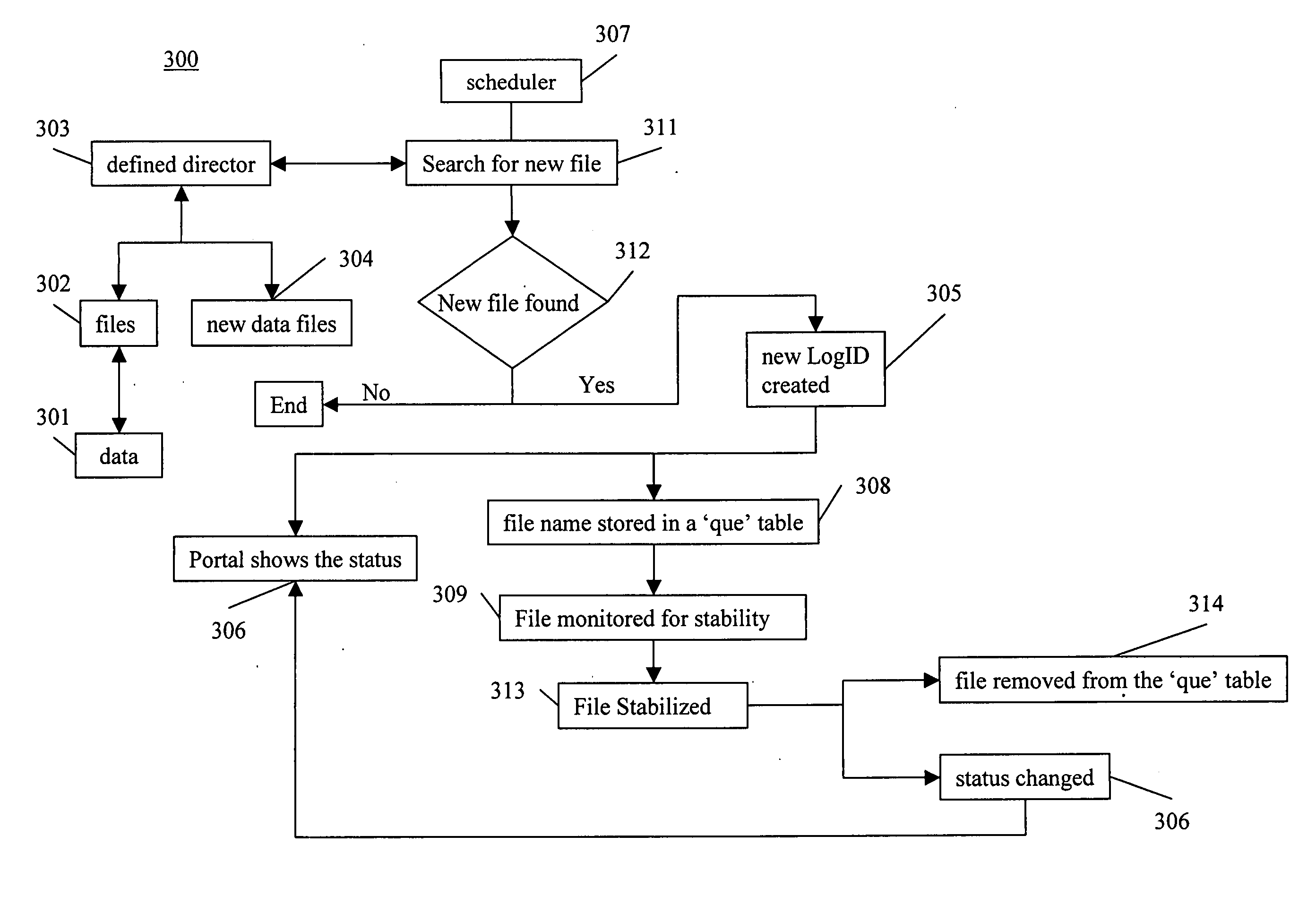
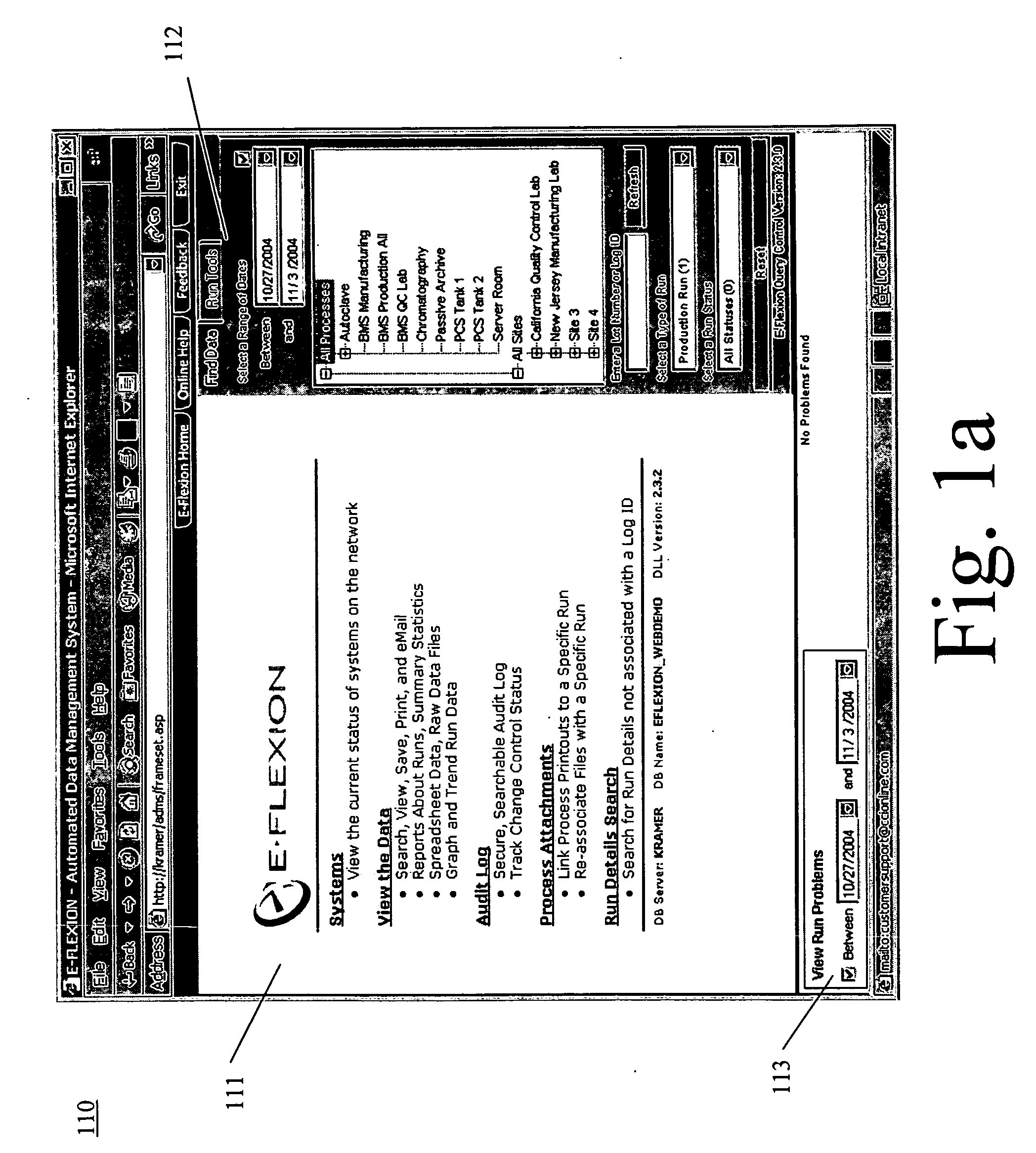
Method of batching and analyzing of data from computerized process and control systems
InactiveUS20060122812A1Time consumeError proneError detection/correctionDigital computer detailsData formatControl system
The present invention is a solution for problems associated with batching, analyzing and viewing data from computerized and process control systems used in the manufacture of pharmaceutical products. Utilizing the hardware and software networking standards already implemented by a corporate IS / IT group as a platform, the present invention automatically captures (via 3rd party software), processes, archives, reports on and distributes data for any computerized equipment at a facility. Data formats for all equipment regardless of manufacturer, plant location, or hardware / software platform used, are standardized. A single data repository is provide so that any authorized user connected to the company / facility network will have the ability to query this secure data and can generate any number of different reports. Once data is collected, it is batched or grouped according to user defined rules. A batch is given a distinct number, which will then be used to identify the data. Within seconds of batching, data is automatically analyzed using process macro code. This code can be simple or complex as needed to provide only the most important details about a batch. Such analyzation eliminates the manual processing of data, which is usually complex, time-consuming and prone to error. Via the Intranet or Internet, authorized users can view or trend data and access reports including adding electronic signatures. Additional process information such as standard operating procedures, and cycle descriptions may be uploaded and attached to a batch. Any action a user performs that creates, modifies or deletes data in the data repository will be audited.
Owner:TINSETH LANCE DAVID
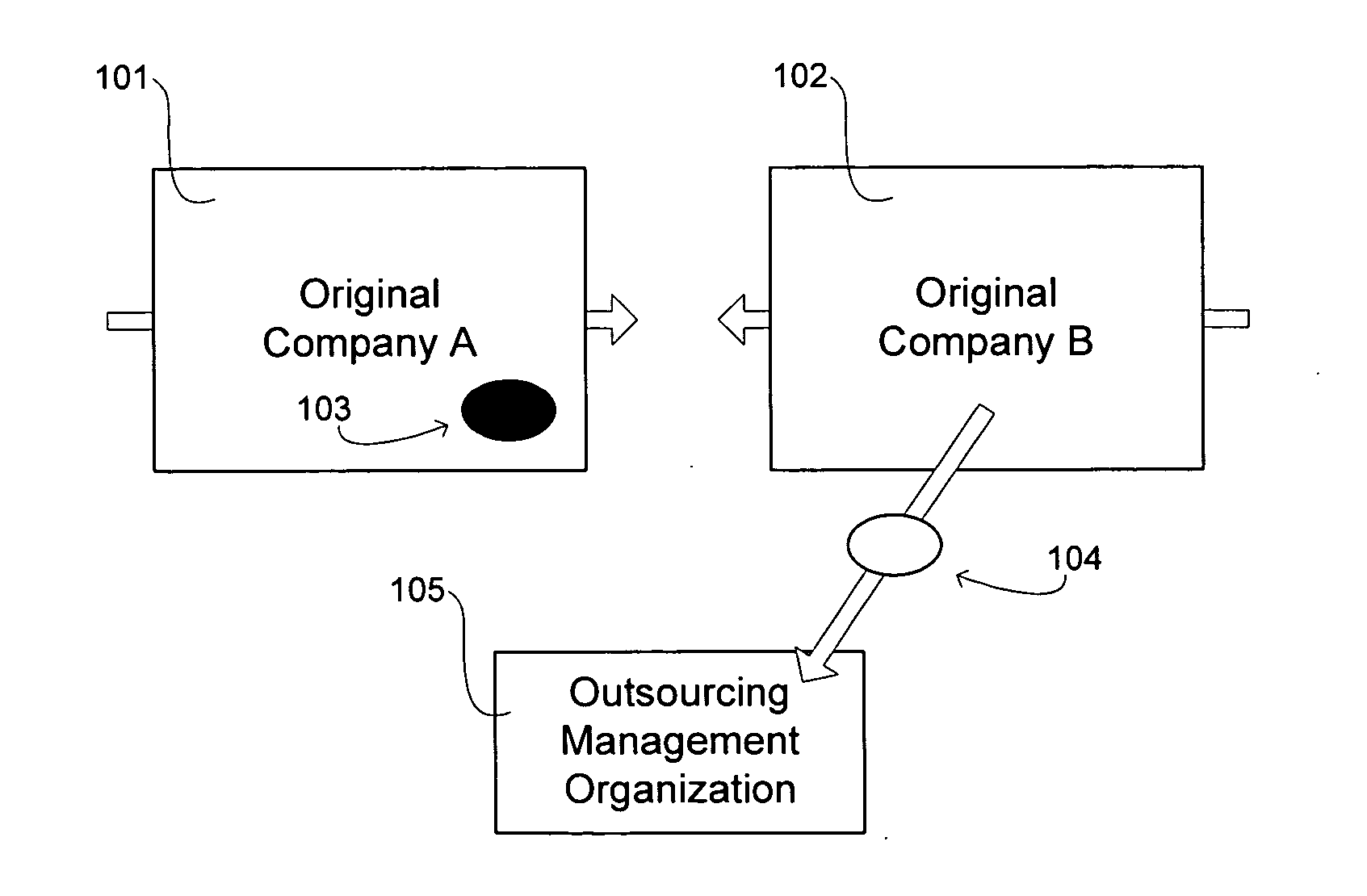
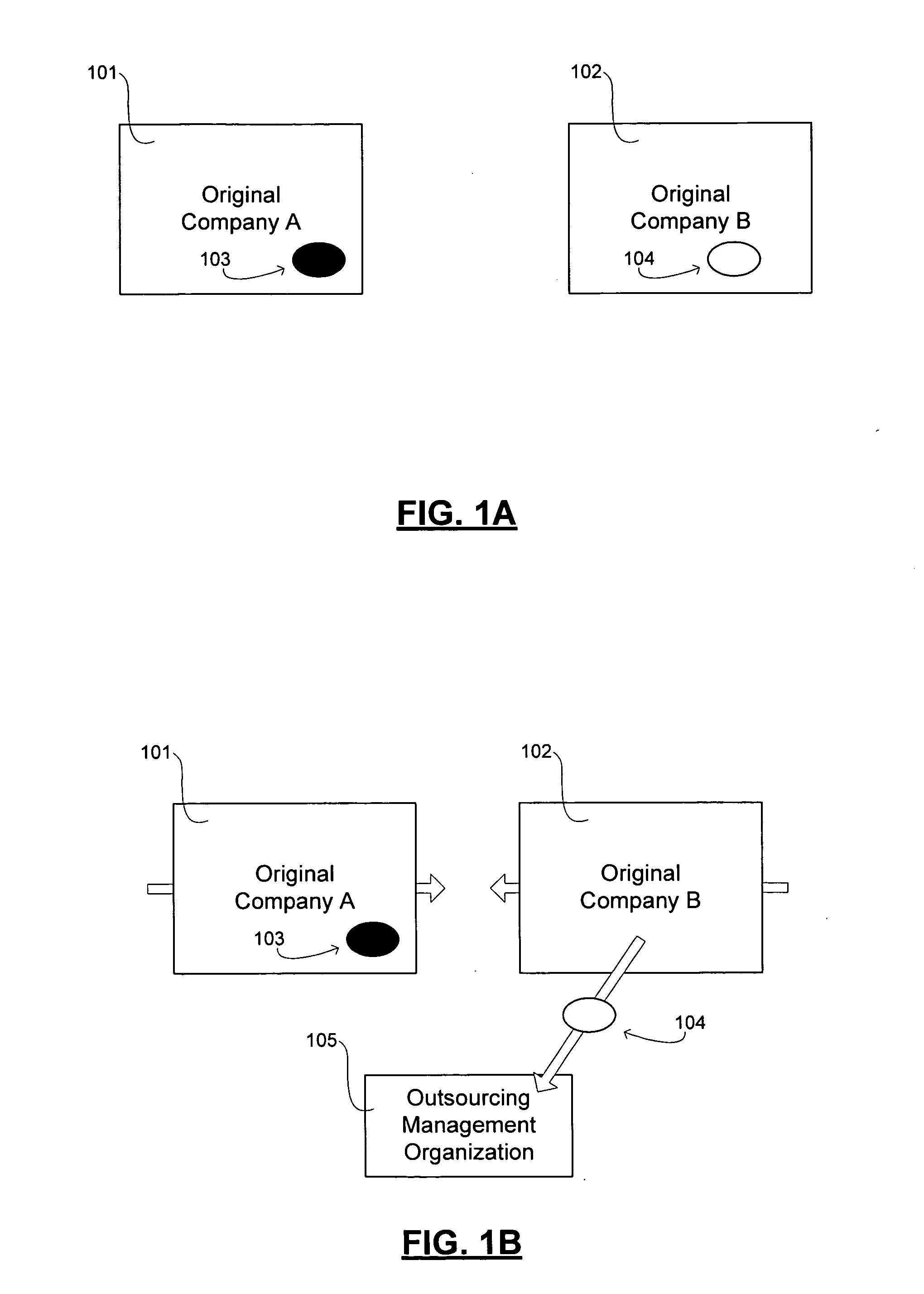
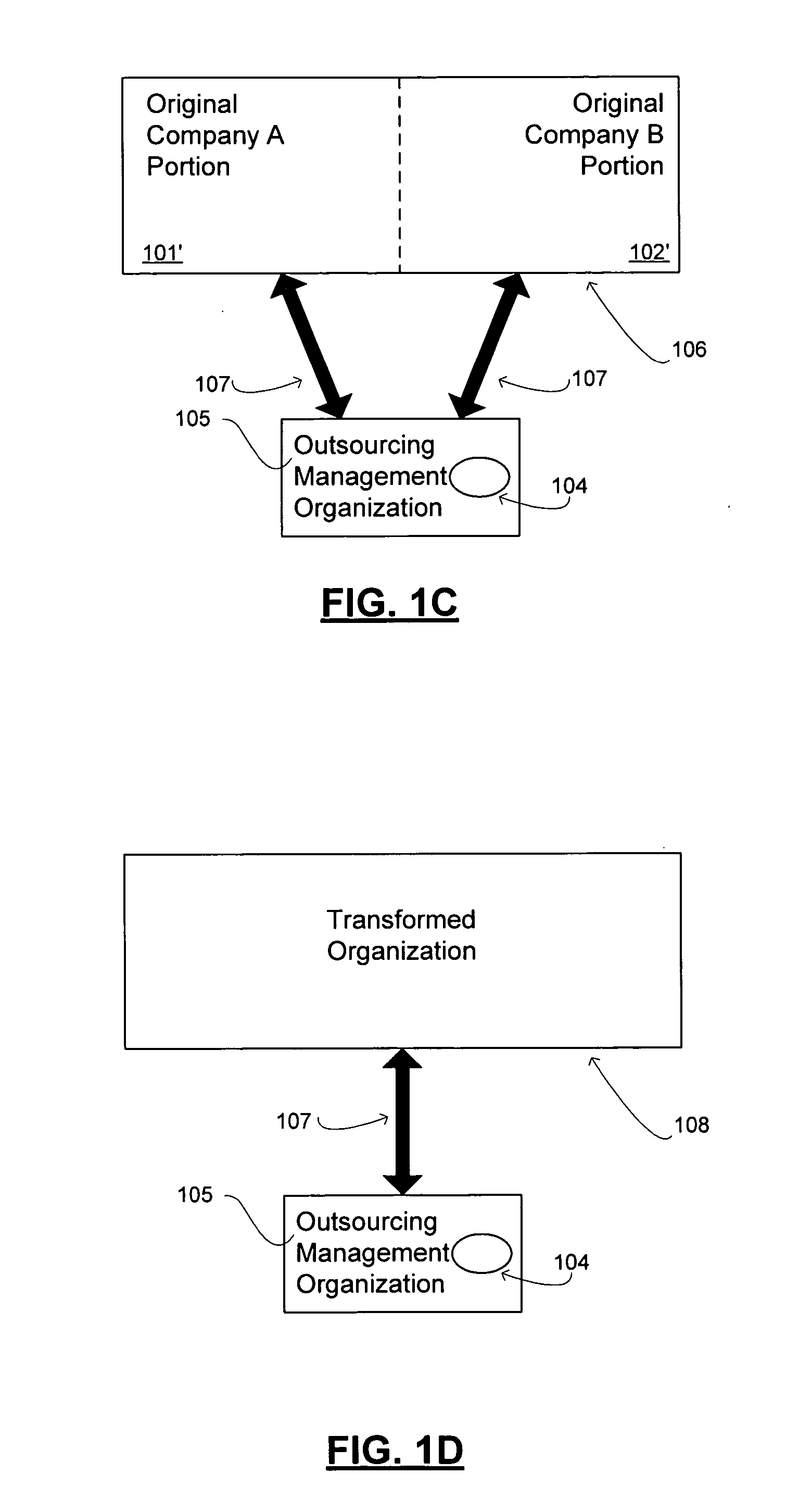
Transformation of organizational structures and operations through outsourcing integration of mergers and acquisitions
InactiveUS20060069607A1Suppress destructionRealized benefitsHardware monitoringOffice automationEngineeringSkill sets
Disclosed are tools and related methods for business organizations to quickly obtain, preserve and exploit new or improved assets, skills or capabilities that are important to growth and success. The tools and processes disclosed are adapted to preserve one or more target elements of an acquired target business organization by outsourcing those target elements during the integration period that follows the merger or acquisition. This outsourcing of one or more target elements during the integration period that necessarily follows a merger or acquisition deal creates various inherent advantages over the traditional merger, acquisition, or outsourcing approaches as described herein, and these advantages help to deliver benefits of the target element in speedy fashion and with undiminished quality.
Owner:ACCENTURE GLOBAL SERVICES LTD
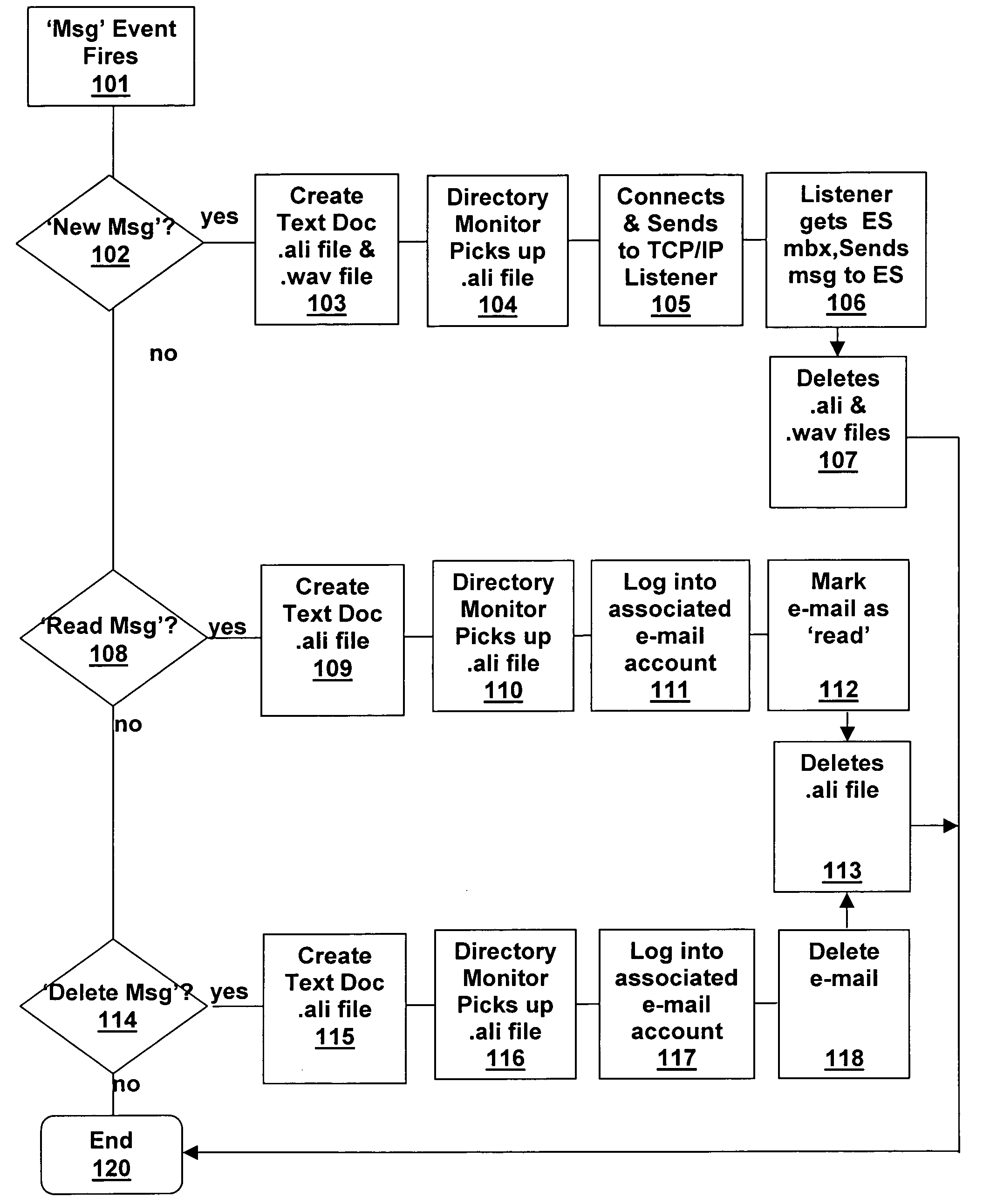
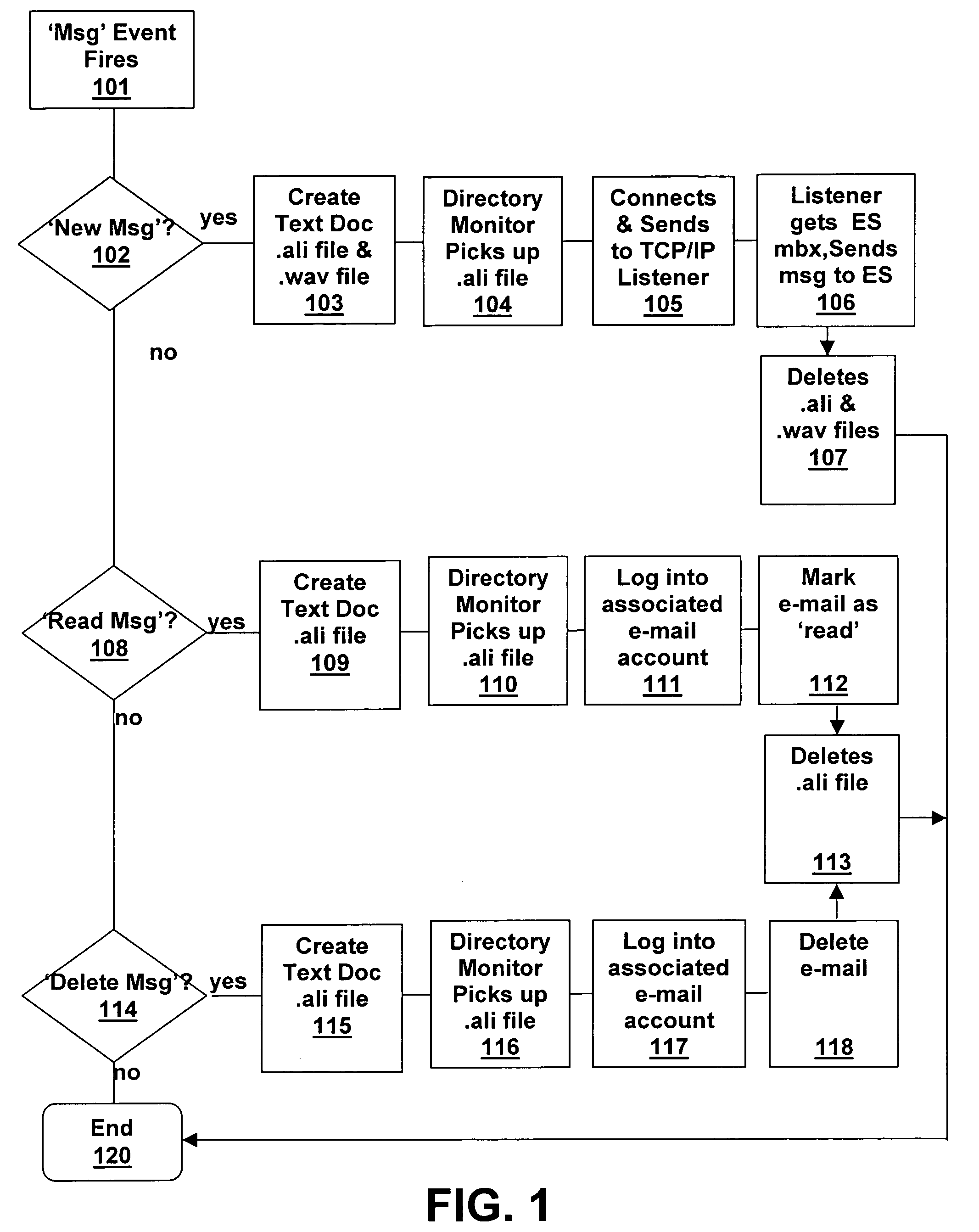
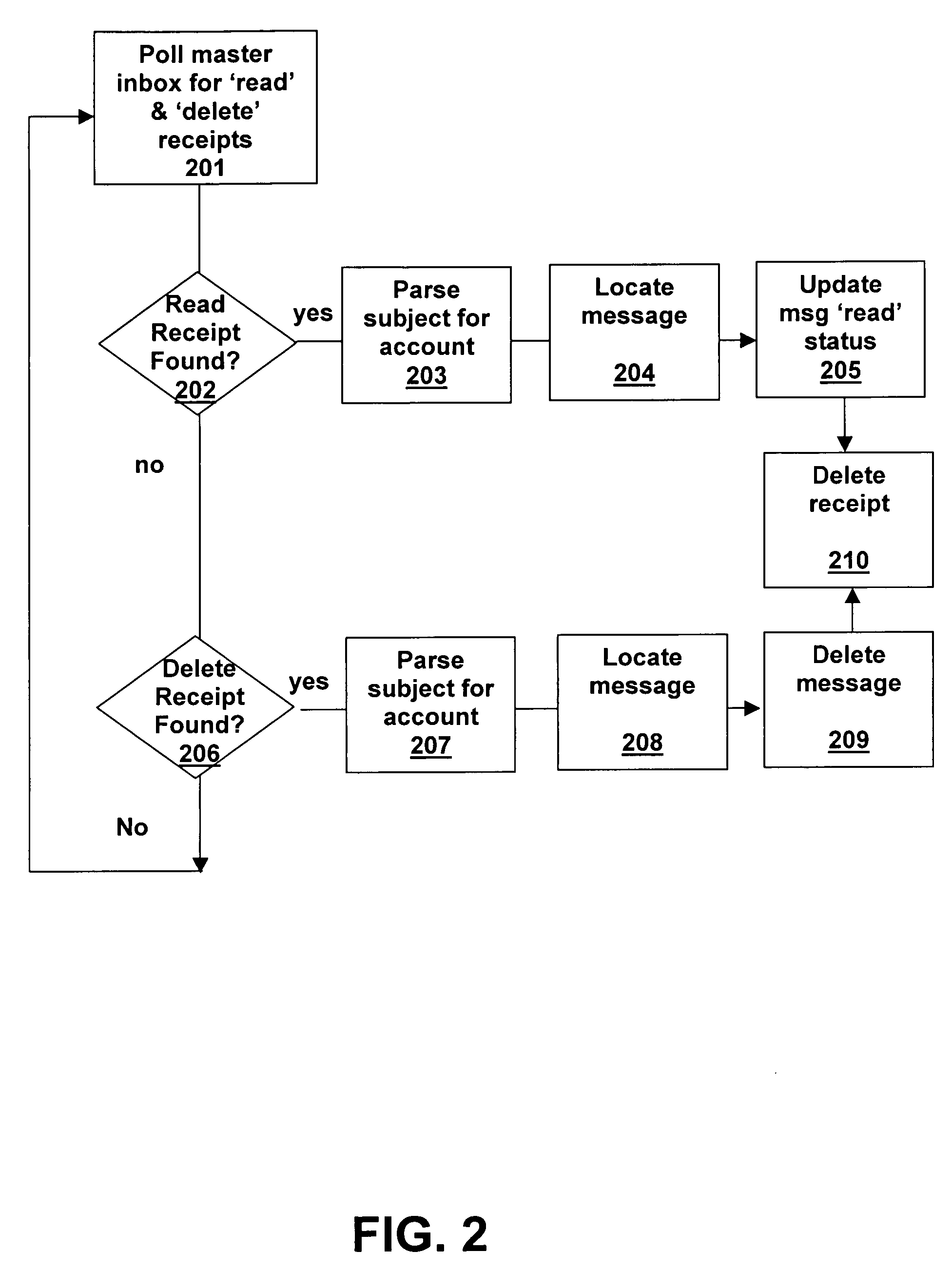
System and method for voice-mail and e-mail synchronization
InactiveUS20060018444A1Less-intensiveMinimize effortInterconnection arrangementsAutomatic call-answering/message-recording/conversation-recordingFile systemFail-safe
Disclosed is a unified messaging system and method for combining a voice-mail system with an e-mail system. The present invention is a hybrid event driven system that polls a universal e-mail box for receipts, with the voice-mail system being fully event driven. The present invention maintains two persistent log-ins to the universal e-mail box, one to deliver messages and one to read message receipts. Thus, the system and method does not need to log-in and -out or read the entire list of messages. The present invention uses a file system instead of a state database. The system and method acts in real time such that messages in the unified messaging systems are synchronized within a pre-determined real-time. The system and method uses a “fail-safing” technique in which, after copies of messages are made and stored by each messaging component, allows one system to work if another is down.
Owner:ADVANCED LOGIC INDS
Competitive and effective bacterial strains
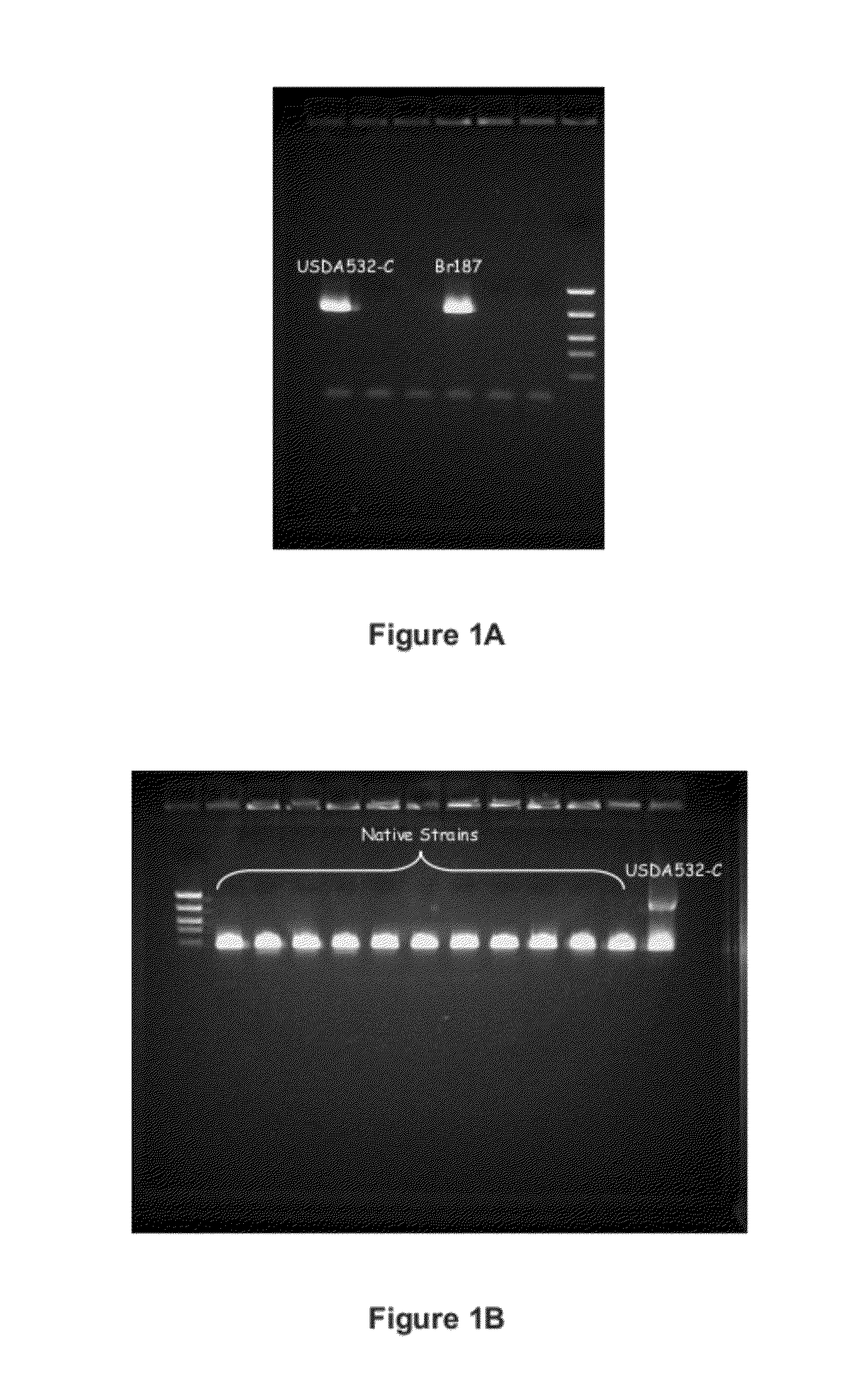
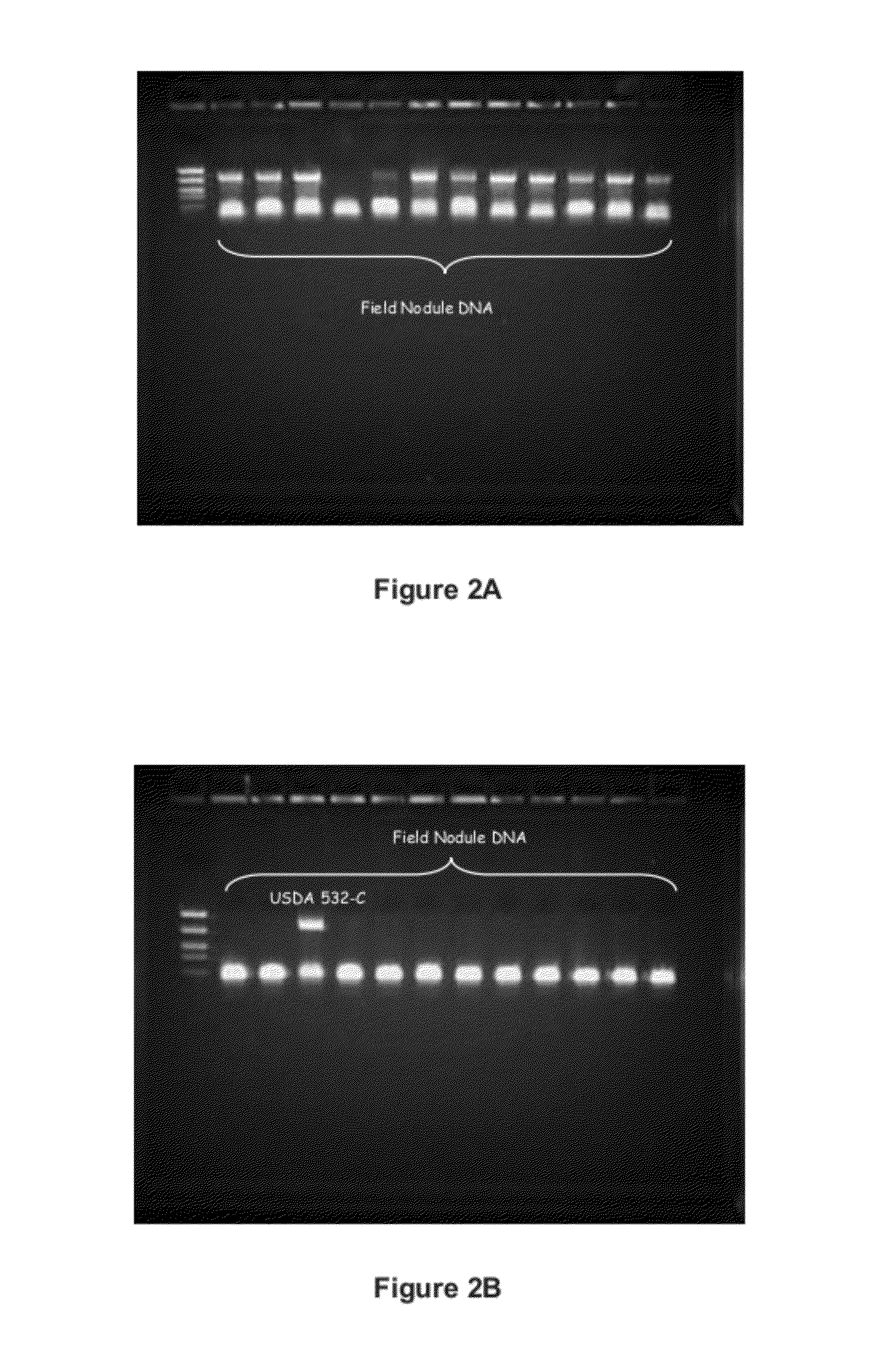
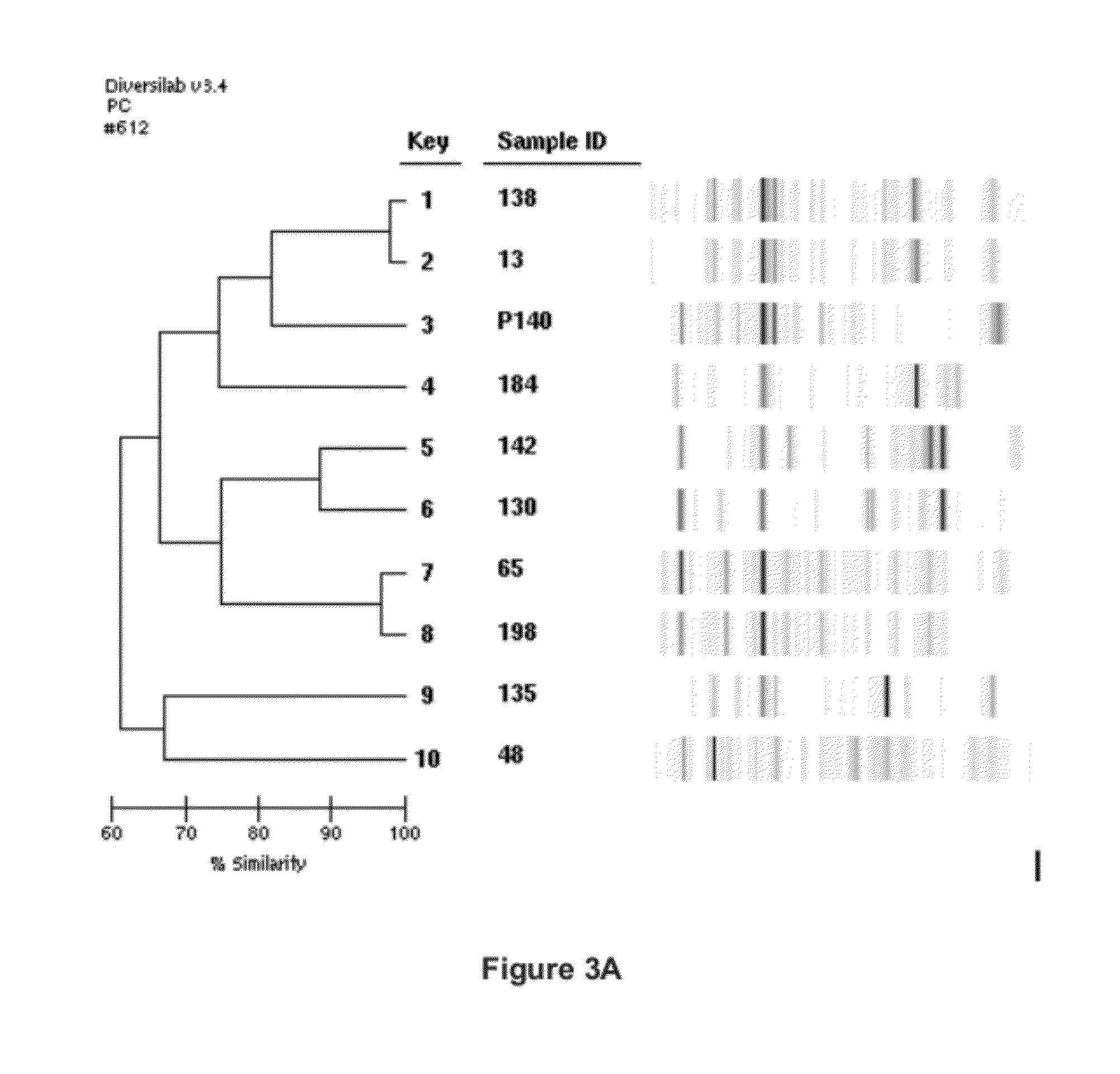
ActiveUS8999698B2Superior at colonizing plantsImprove availabilityBiocideFungiRhizobacteriaMicrobiology
Owner:NOVOZYMES BIOLOGICALS
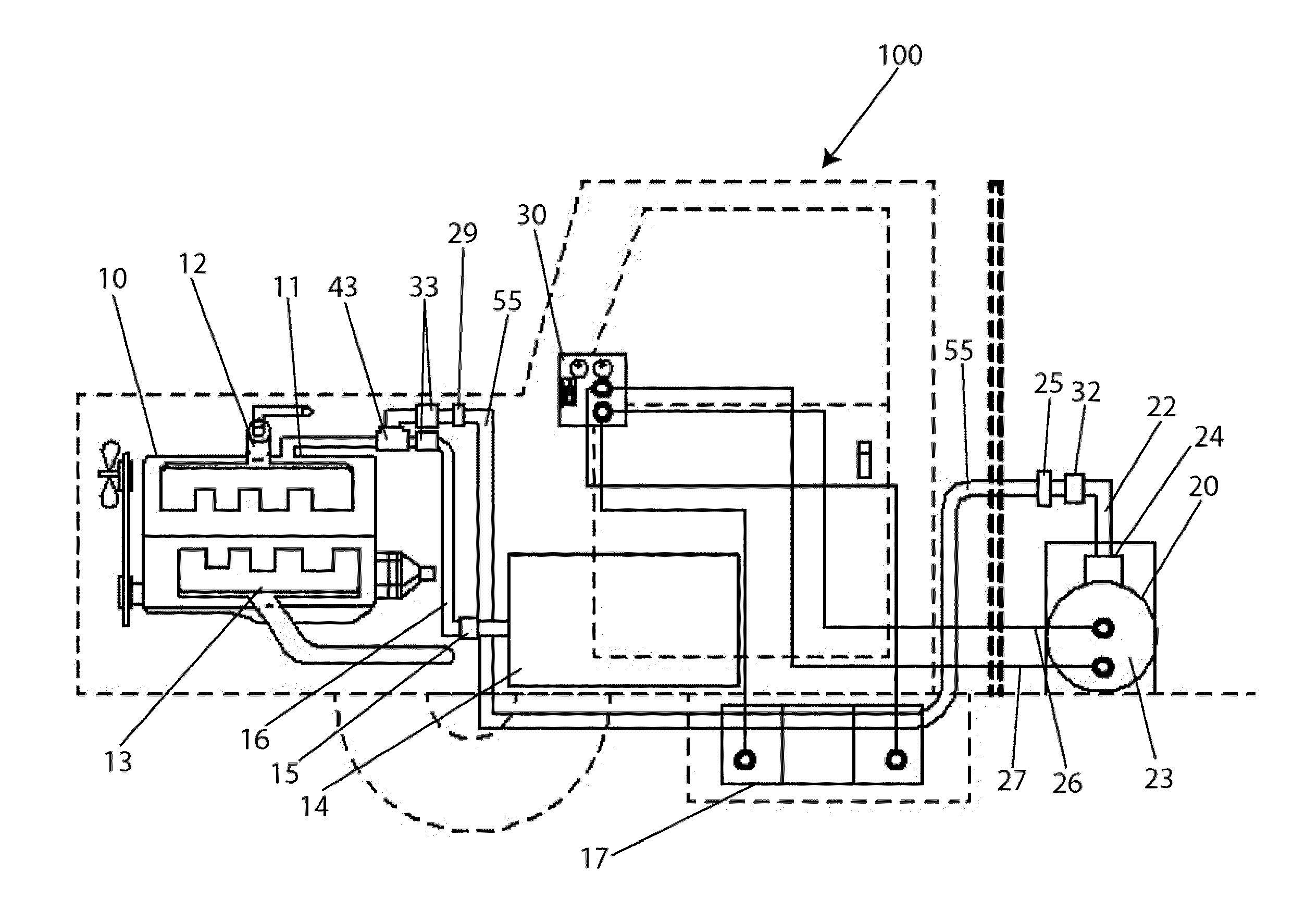
Hydrolysis system to produce hydrogen-oxygen gas as a fuel additive for internal combustion engines
InactiveUS20110220039A1Overcome problemsImprove combustion efficiencyCellsInternal combustion piston enginesElectrolysisHydrogen
Internal combustion engines operate by igniting a mixture of liquid fuel and air inside its combustion chamber. The energy from the ignition is converted to mechanical energy that is used to power a vehicle. Research indicates that adding hydrogen gas into the combustion chamber improves the efficiency of the engine. The present invention is an electrolysis system that produces hydrogen and oxygen gases and injects them into the fuel line of the engine to create a mixture of the gases and the liquid fuel that is subsequently introduced into the combustion chamber for ignition. The operating temperature of the engine is lower if the gases are injected into the fuel line rather than directly into the combustion chamber.
Owner:NOWICKI RICHARD +3
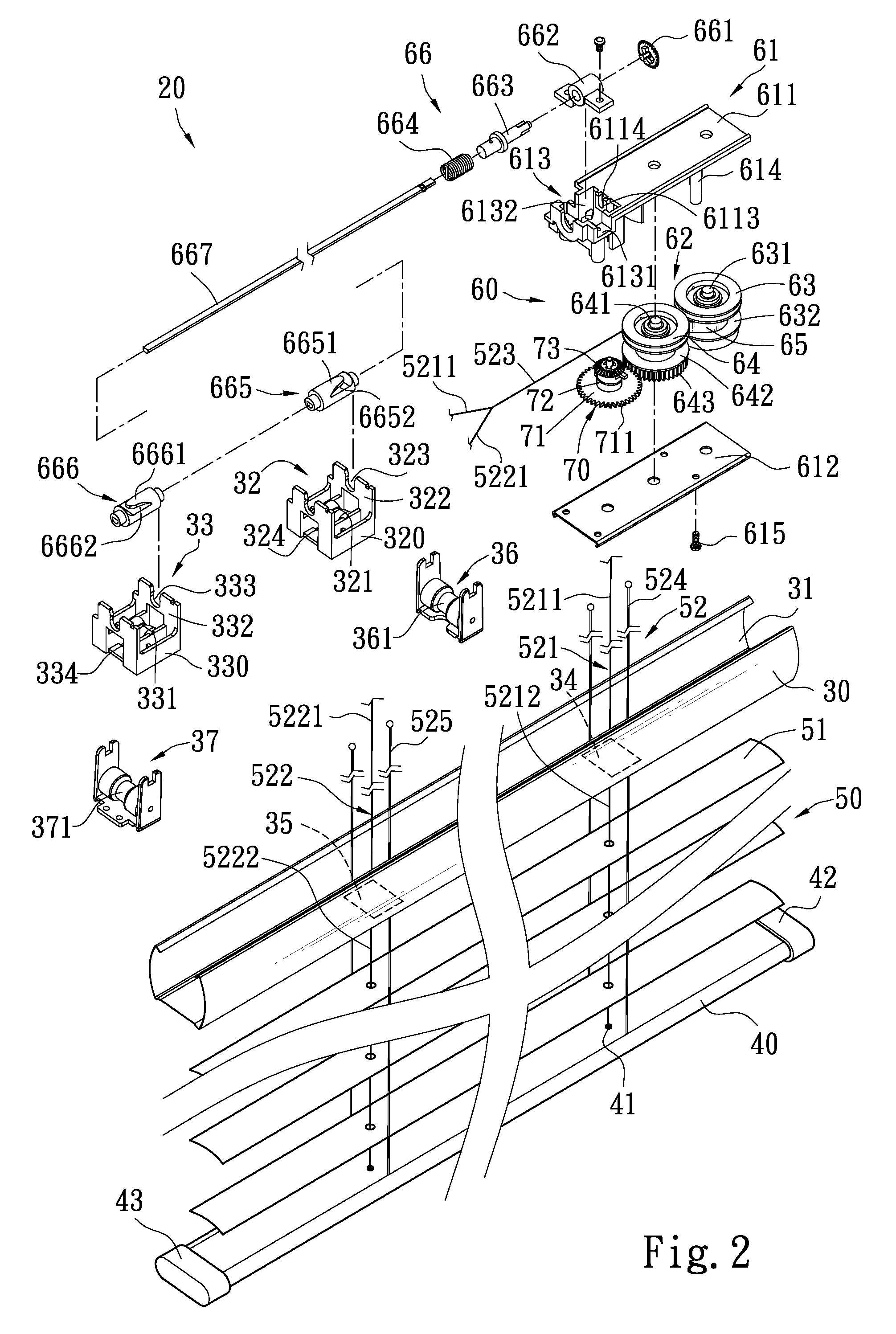
Venetian blind
InactiveUS8002012B2Easy to operateSimple and consistent mannerLight protection screensVenetian blindsMechanical engineering
A Venetian blind includes an upper rail, a lower rail, a slat assembly, an automatic retraction means and a slat opened / closed control means equipped with an adjustment member. The automatic retraction means is installed on one side of the upper rail which has a first cord wheel set and a second cord wheel set near two sides to direct a first lift cord and a second lift cord. The automatic retraction means has a retaining set to pivotally couple a transmission set inside that has a first transmission wheel and a second transmission wheel. The first transmission wheel and second transmission wheel are linked by an elastic element which provides an elastic force to drive the transmission set. The second transmission wheel controls the slat opened / closed control means. When the lower rail is pulled downwards or pushed upwards, the slat assembly can be opened or closed at different angles.
Owner:CHENG LI MING
Augmented accuracy using large diameter shape fiber
ActiveUS20190183318A1Small diameterConvenient and accurateSurgical navigation systemsEndoscopesFiberEngineering
Where a flexible tool includes a tool body with a flexible portion, a distal end and a first optical fiber within the flexible portion, shape sensing can be achieved with increased accuracy by inserting or otherwise including a second optical fiber within the flexible portion. The increased accuracy can be achieved when the second optical fiber has a diameter larger than that of the first optical fiber. Once the shape of the flexible tool has been determined using at least the second optical fiber, the first optical fiber can be used for subsequent shape sensing. This may be particularly applicable where the tool includes an instrument such as an optical imaging device inserted in a channel of the tool, where not all of the width of the channel is occupied by functional components behind the operable end of the instrument.
Owner:INTUITIVE SURGICAL OPERATIONS INC
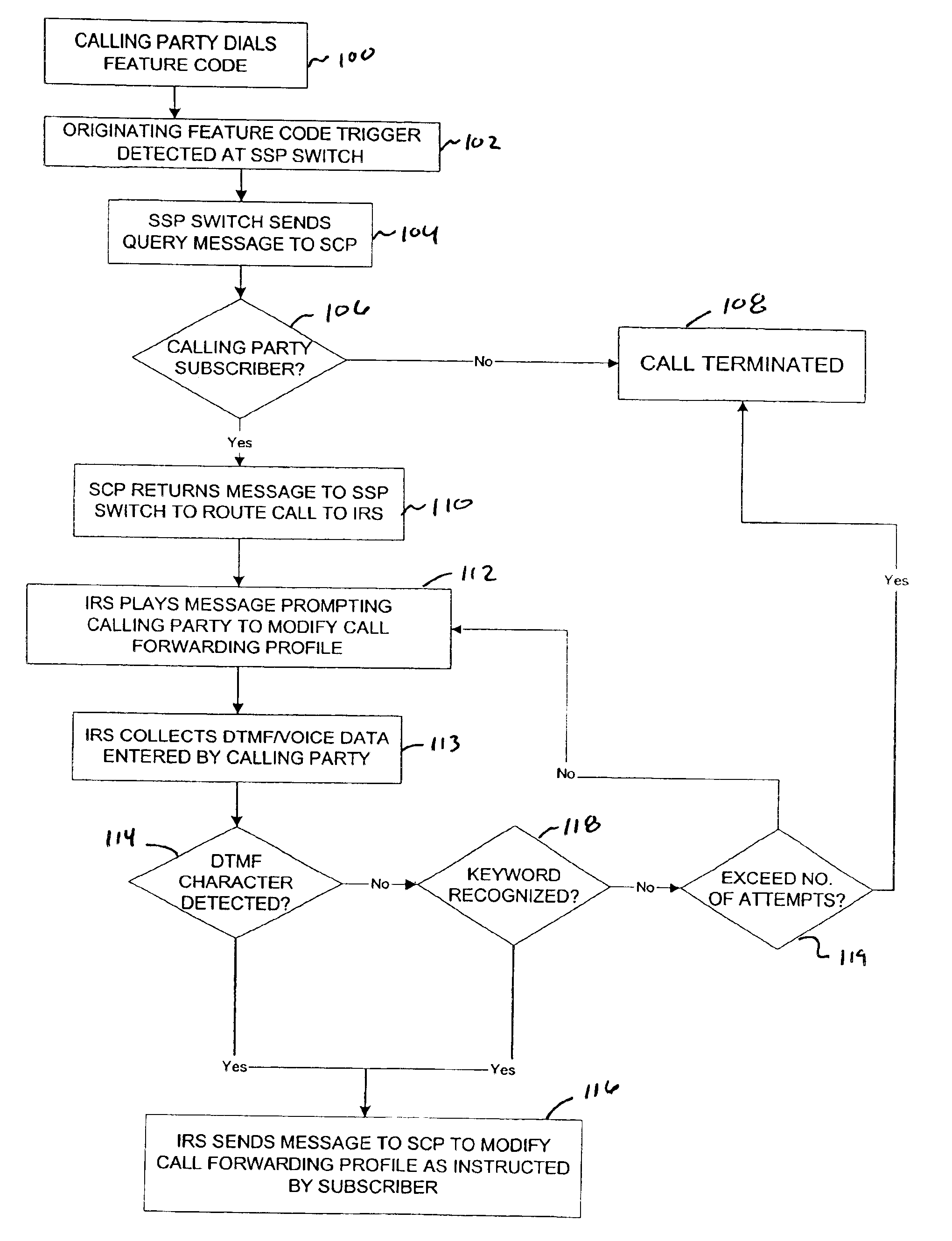
Network and method for providing a flexible call forwarding telecommunications service with automatic speech recognition capability
InactiveUS6993119B1Add featureGood serviceSpecial service for subscribersAutomatic call-answering/message-recording/conversation-recordingTelecommunications linkCall forwarding
A network for providing a telecommunications service with automatic speech recognition to a telecommunications user, including a switch in communication with a telecommunications device used by the telecommunications user for detecting a trigger specific to the telecommunications service in response to a communication from the telecommunications device, and an intelligent resource server in communication with the switch for receiving the communication from the telecommunications device via the switch, for playing an audible message for the telecommunications user in response to receiving the communication, the message prompting the telecommunications user to modify a call forwarding profile associated with the telecommunications user, and for automatically recognizing a predetermined keyword spoken by the telecommunications user in response to the message.
Owner:NUANCE COMM INC
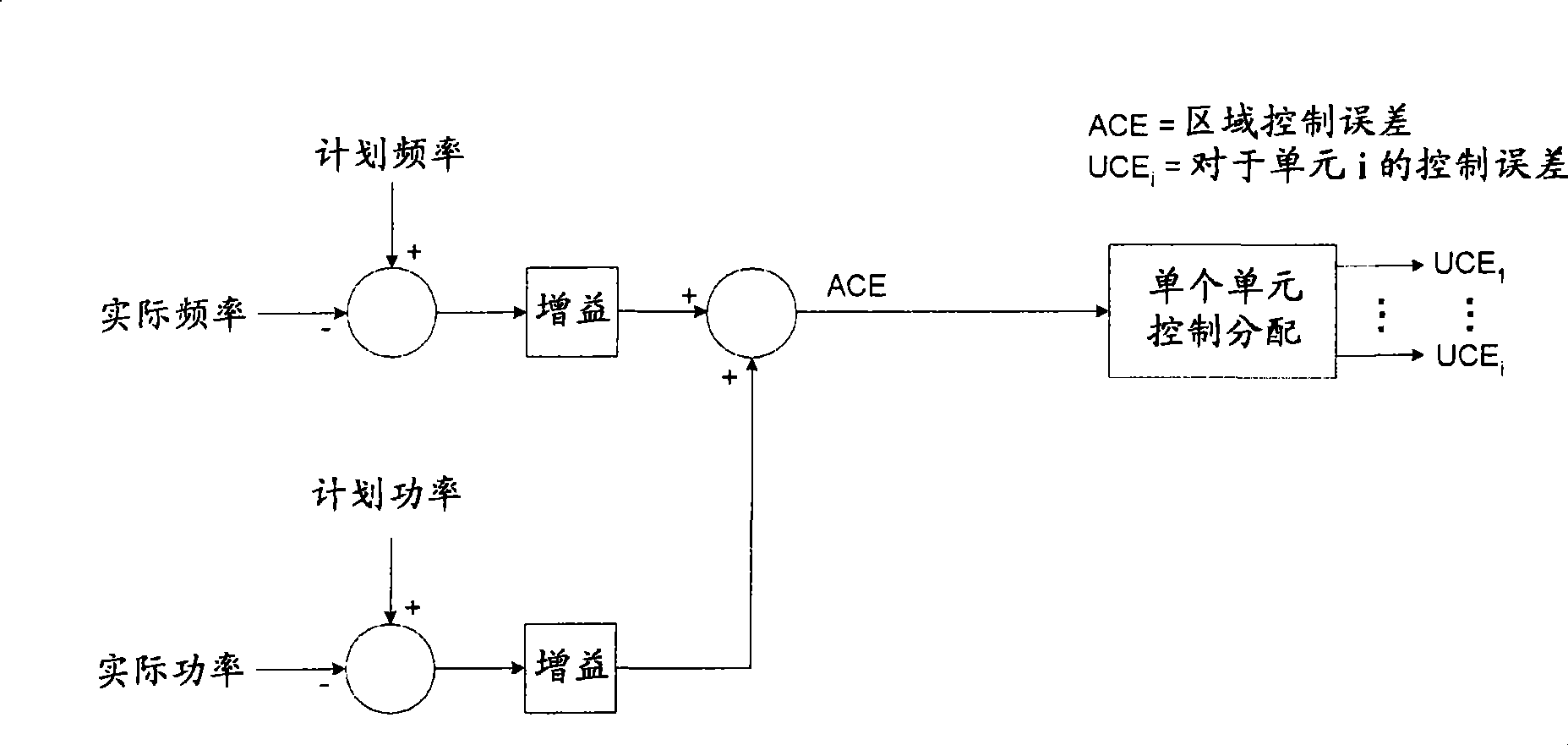
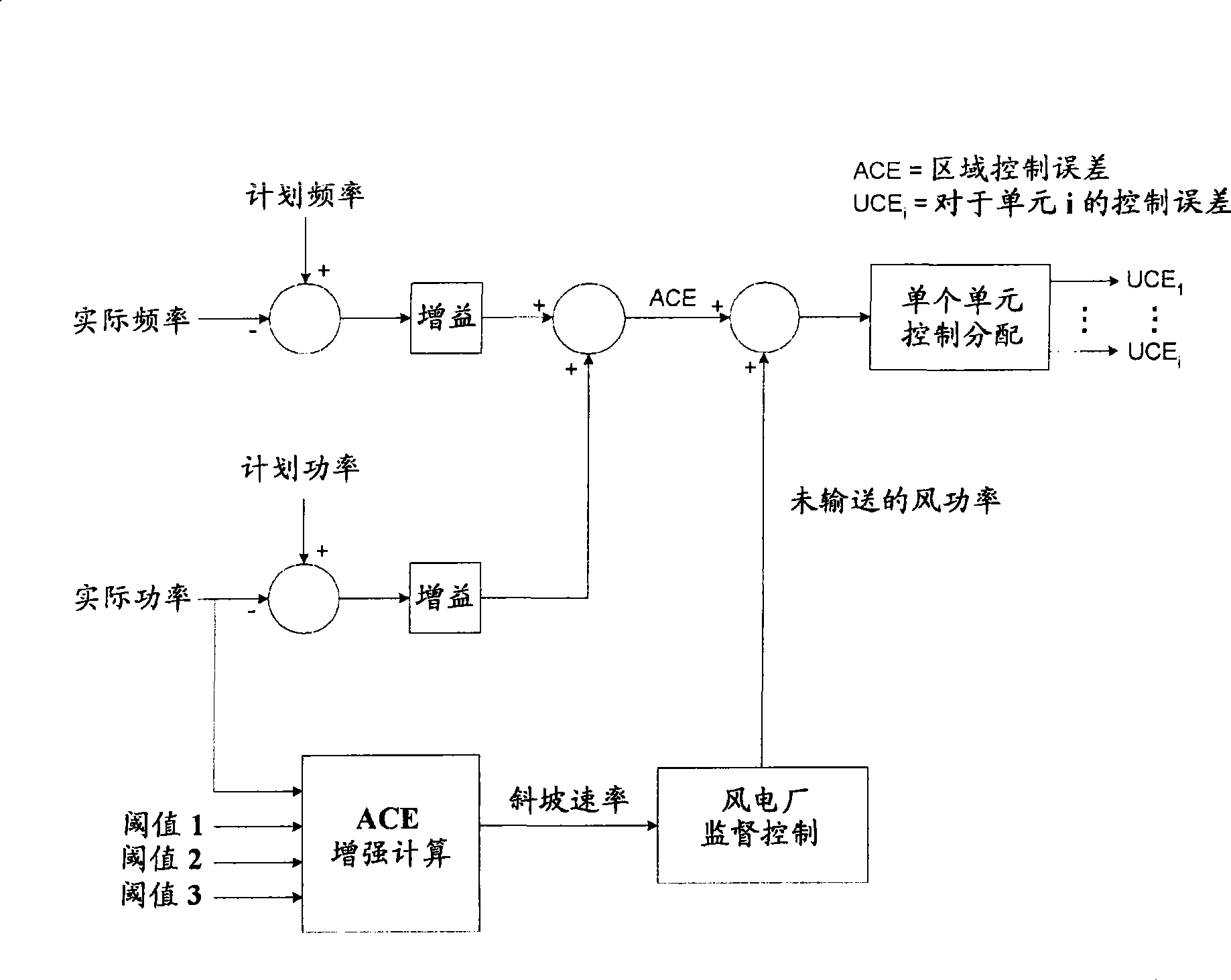
Automatic generation control augmentation for wind plant integration
ActiveCN101521380AIncreased power contributionRealized benefitsData processing applicationsWind motor controlAutomatic Generation ControlPower grid
An automatic generation control (AGC) augmentation system and method for wind power plant integration for controlling the power contribution to a grid by the wind plant is disclosed that provides for the calculation of the area control error (ACE) by actively communicating to the wind plant ramp rate limits and curtailment requests contributing to the ACE calculation. The augmented control of the ACE minimizes the amount of lost energy production by the wind plant.
Owner:GENERAL ELECTRIC RENOVABLES ESPANA SL
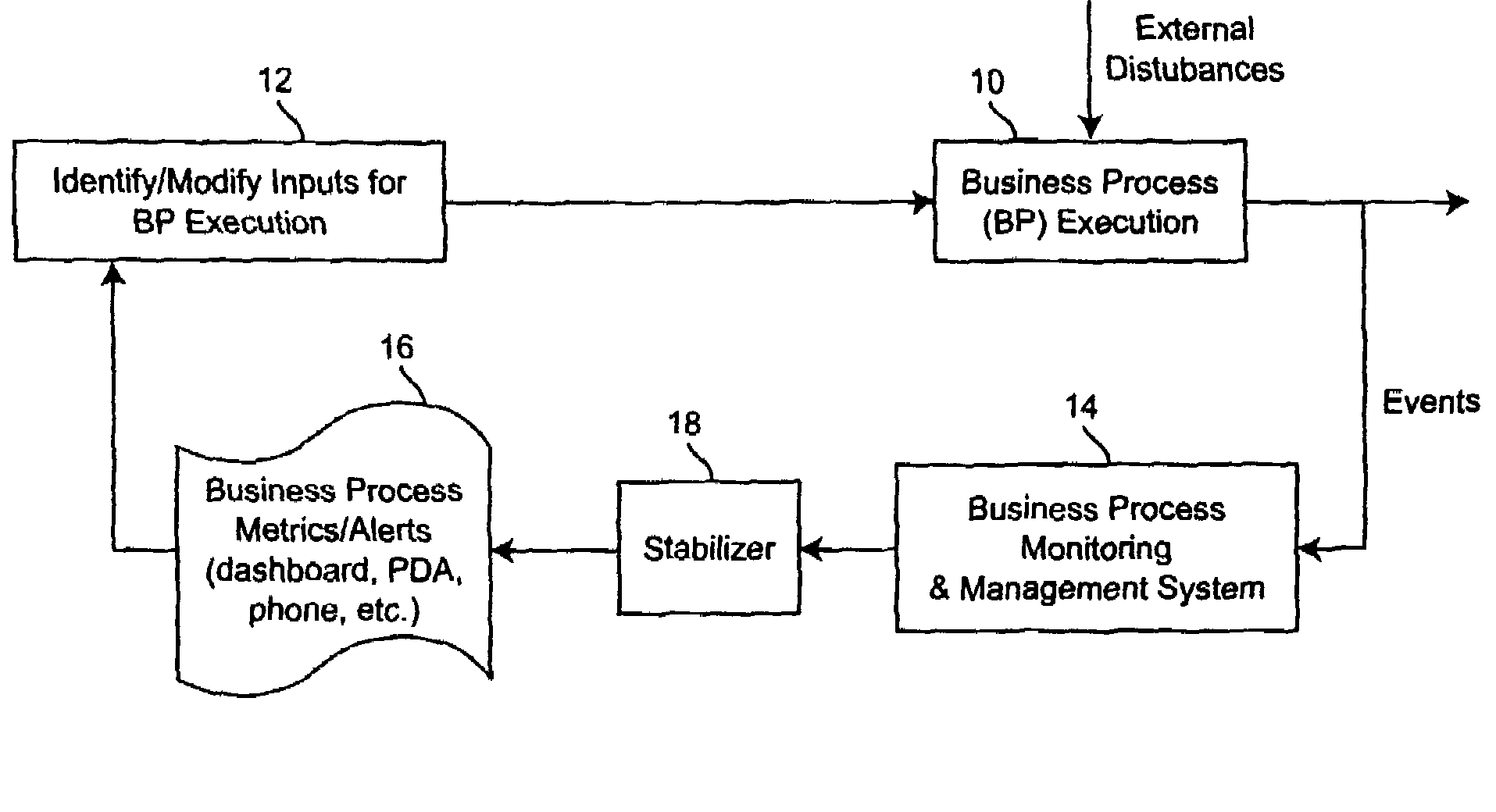
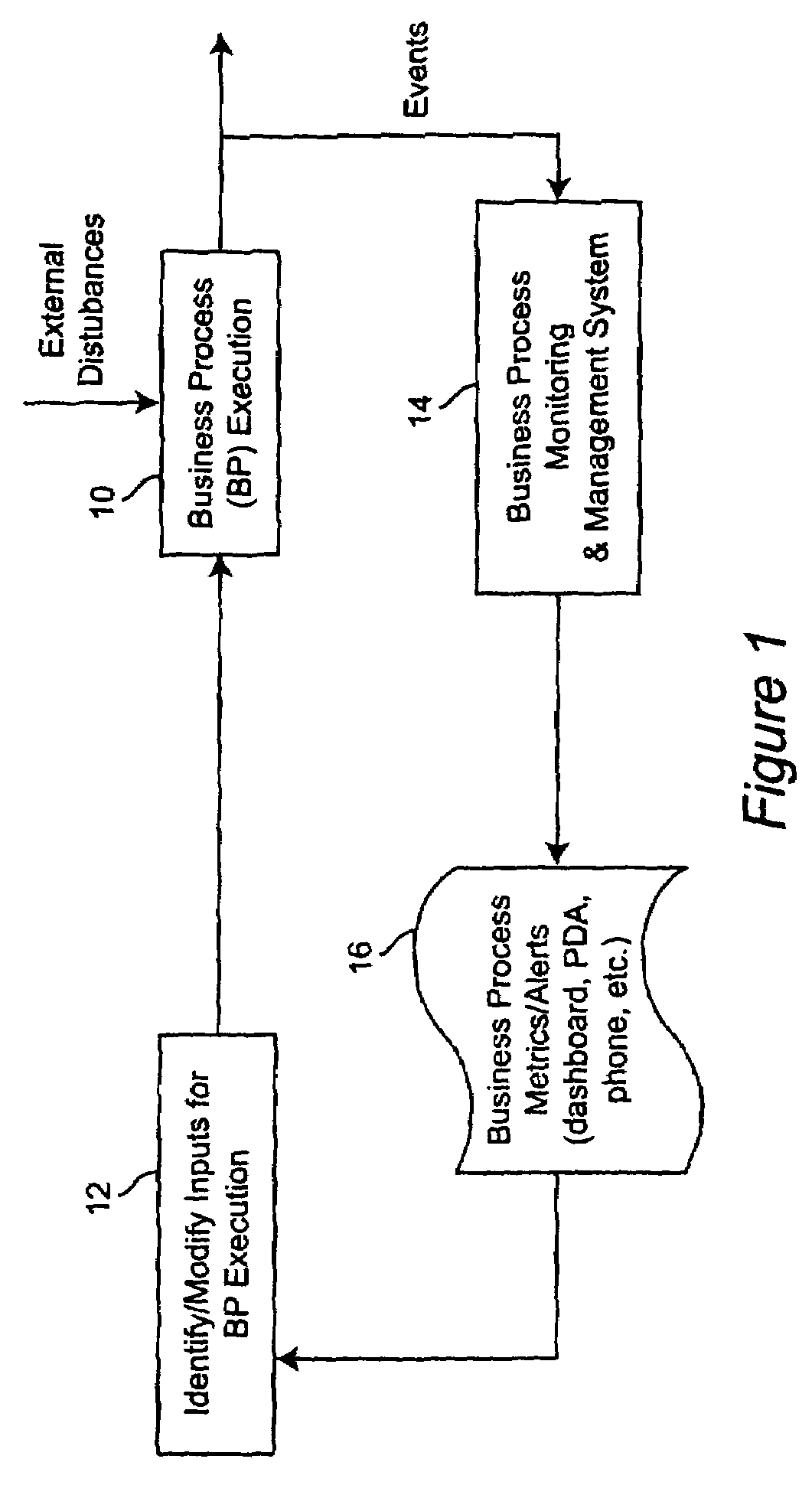
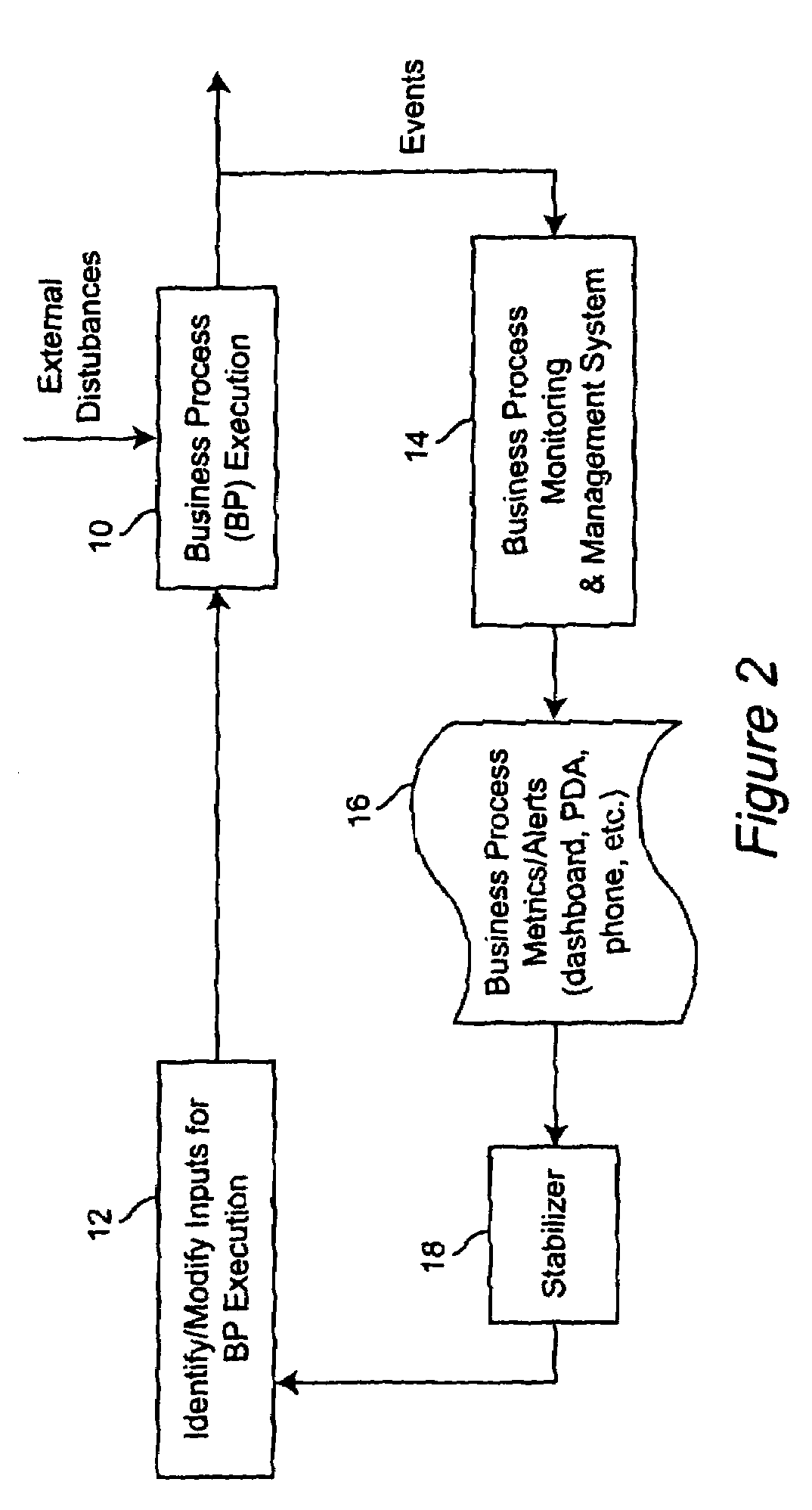
Method for managing and controlling stability in business activity monitoring and management systems
InactiveUS8126762B2Improve business performanceStabilizing the BAM systemFinanceResourcesEngineeringActivity monitoring
A stabilization methodology and system component in Business Activity Monitoring and Management systems. This enables firms to use Business Activity Management (BAM) systems to manage business activity by only responding to monitored data when the overall business performance can be improved. This enables firms to identify appropriate tradeoffs between potentially conflicting objectives while meeting business objectives. Information from BAM systems are analyzed based on models of the business process and different information filter criteria are assessed for their impact on business performance indicators. Based on this, a filter criterion is chosen which is executed by an information filter. The outputs from the information filter are used as the basis for deciding the inputs for business process execution.
Owner:INT BUSINESS MASCH CORP
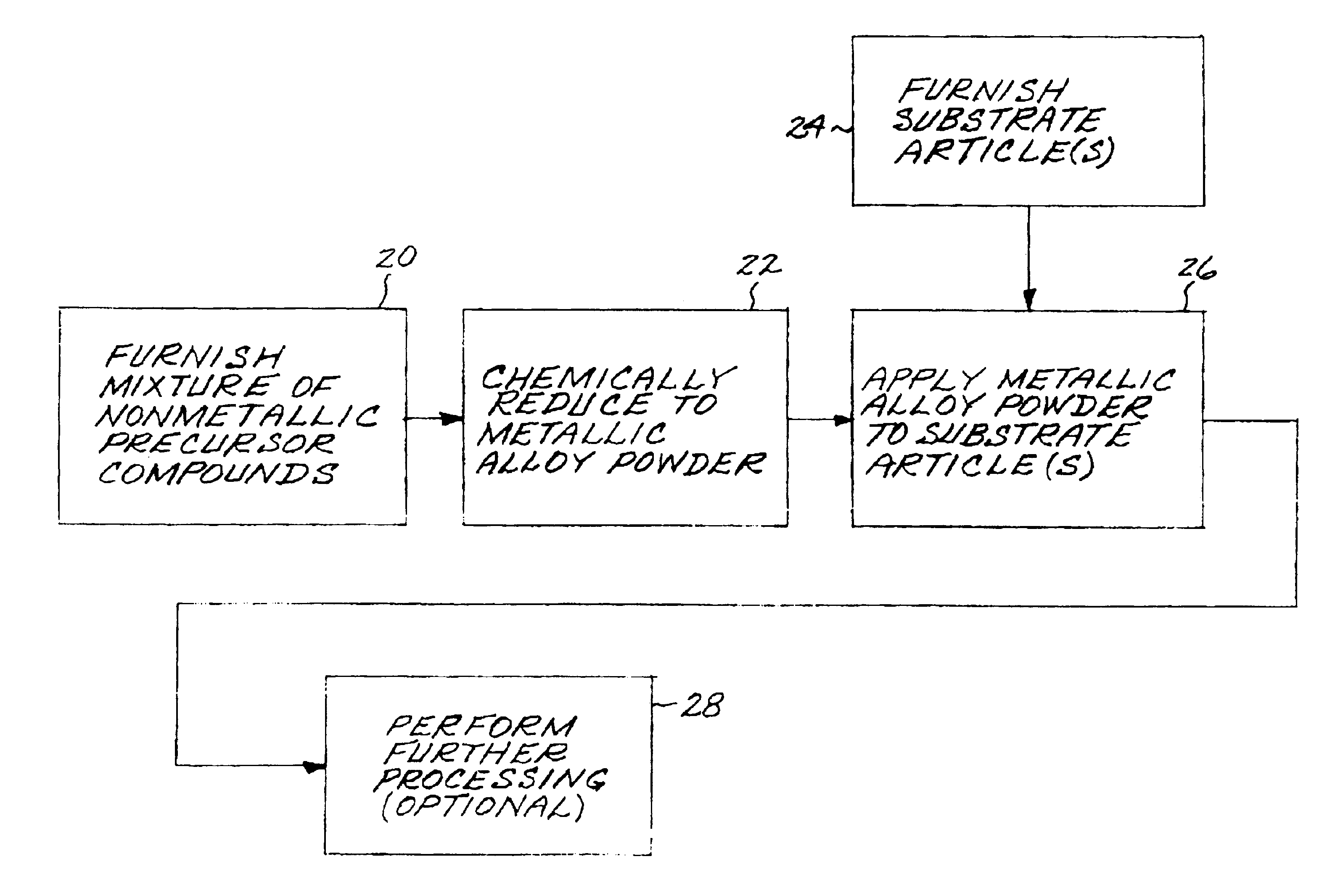
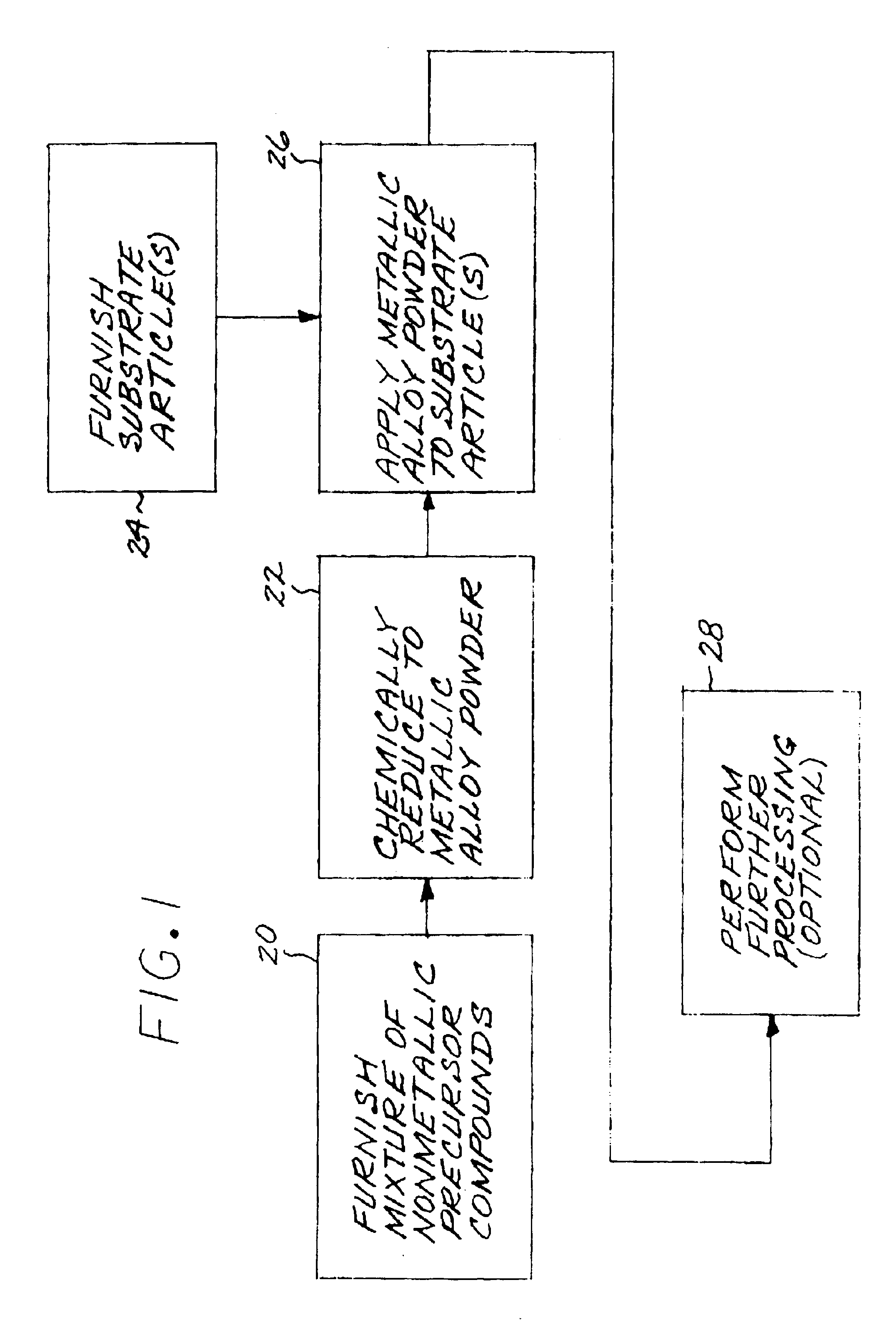
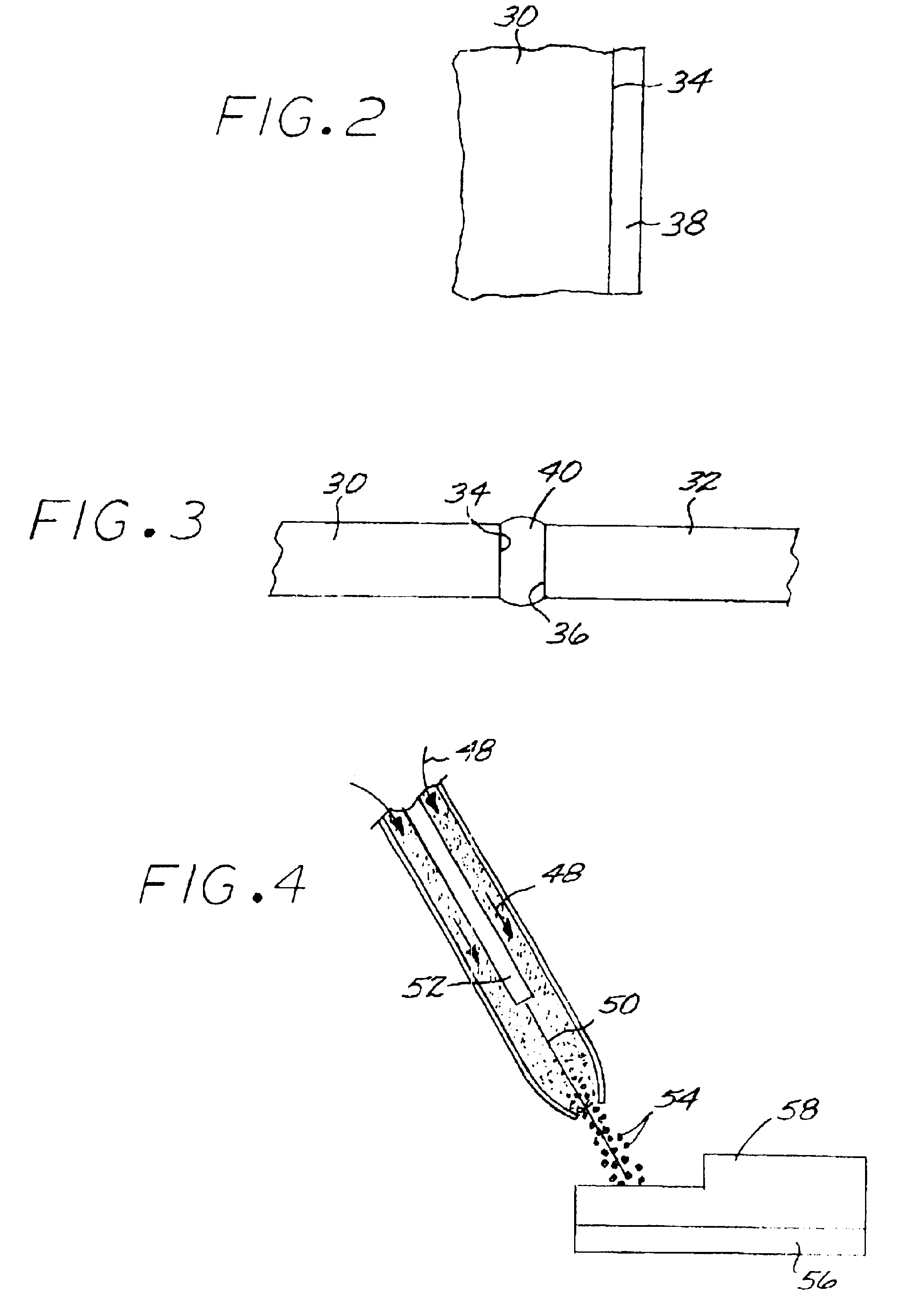
Fabrication and utilization of metallic powder prepared without melting
InactiveUS6968990B2Narrow size distributionImprove overall utilizationTurbinesMolten spray coatingMetal powderMetallic alloy
A metallic alloy made of metallic constituent elements is fabricated and utilized by first furnishing a mixture of nonmetallic precursor compounds of the metallic constituent elements, and thereafter chemically reducing the mixture of nonmetallic precursor compounds to produce a metallic alloy as a metallic alloy powder, without melting the metallic alloy. The metallic alloy powder is applied to a surface of a substrate article, preferably in a coating, joining, or deposition application.
Owner:GENERAL ELECTRIC CO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com