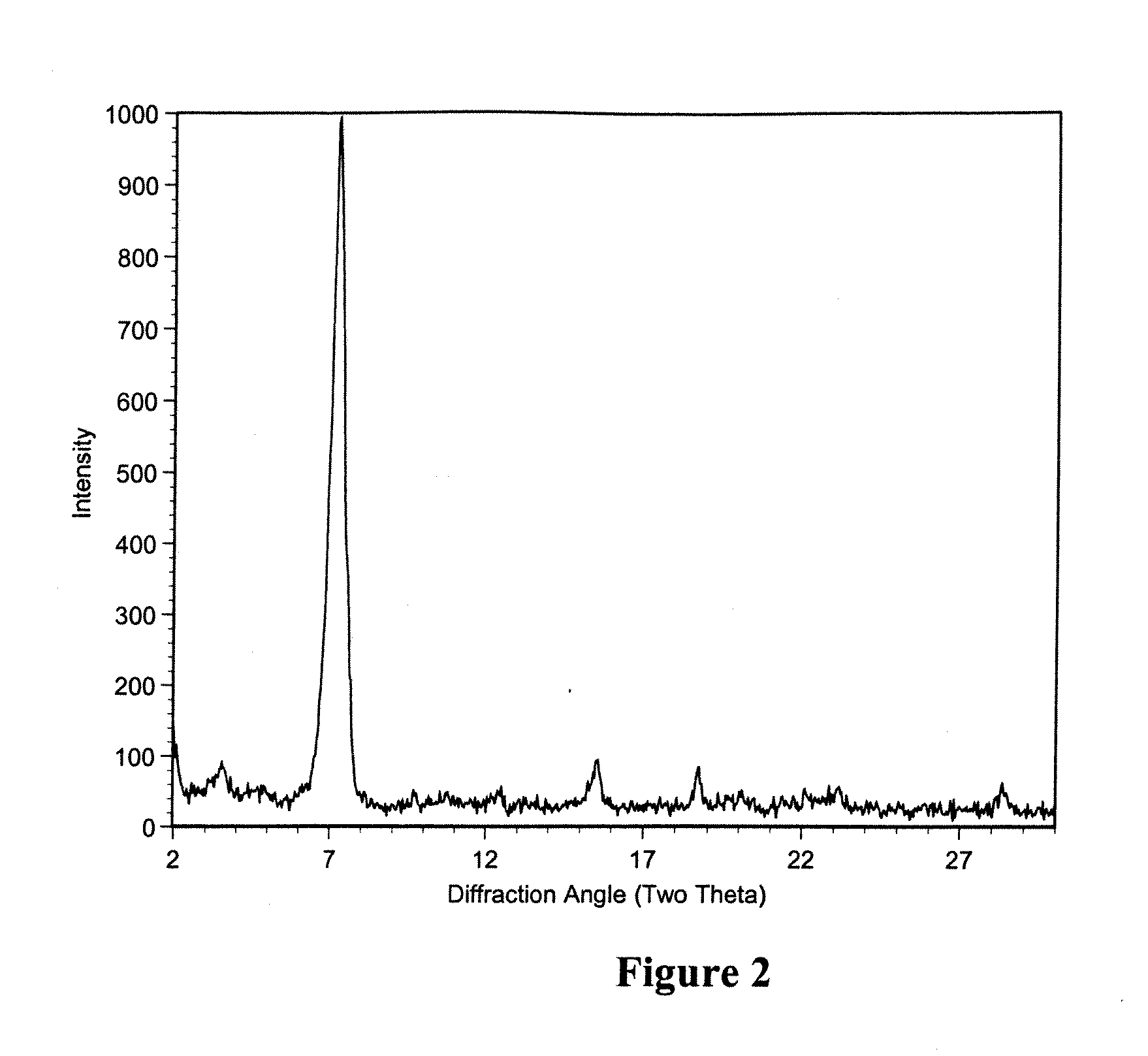
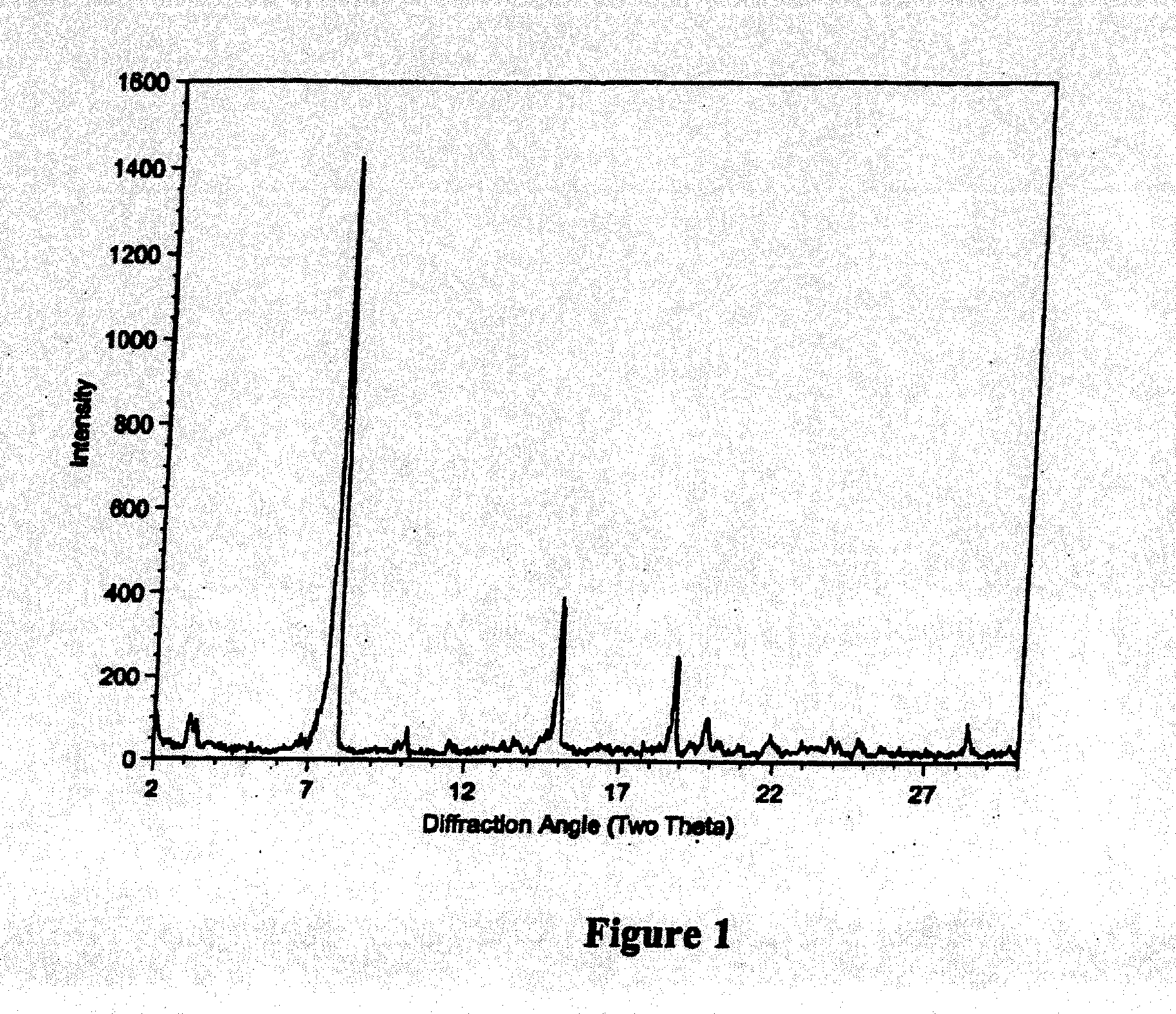
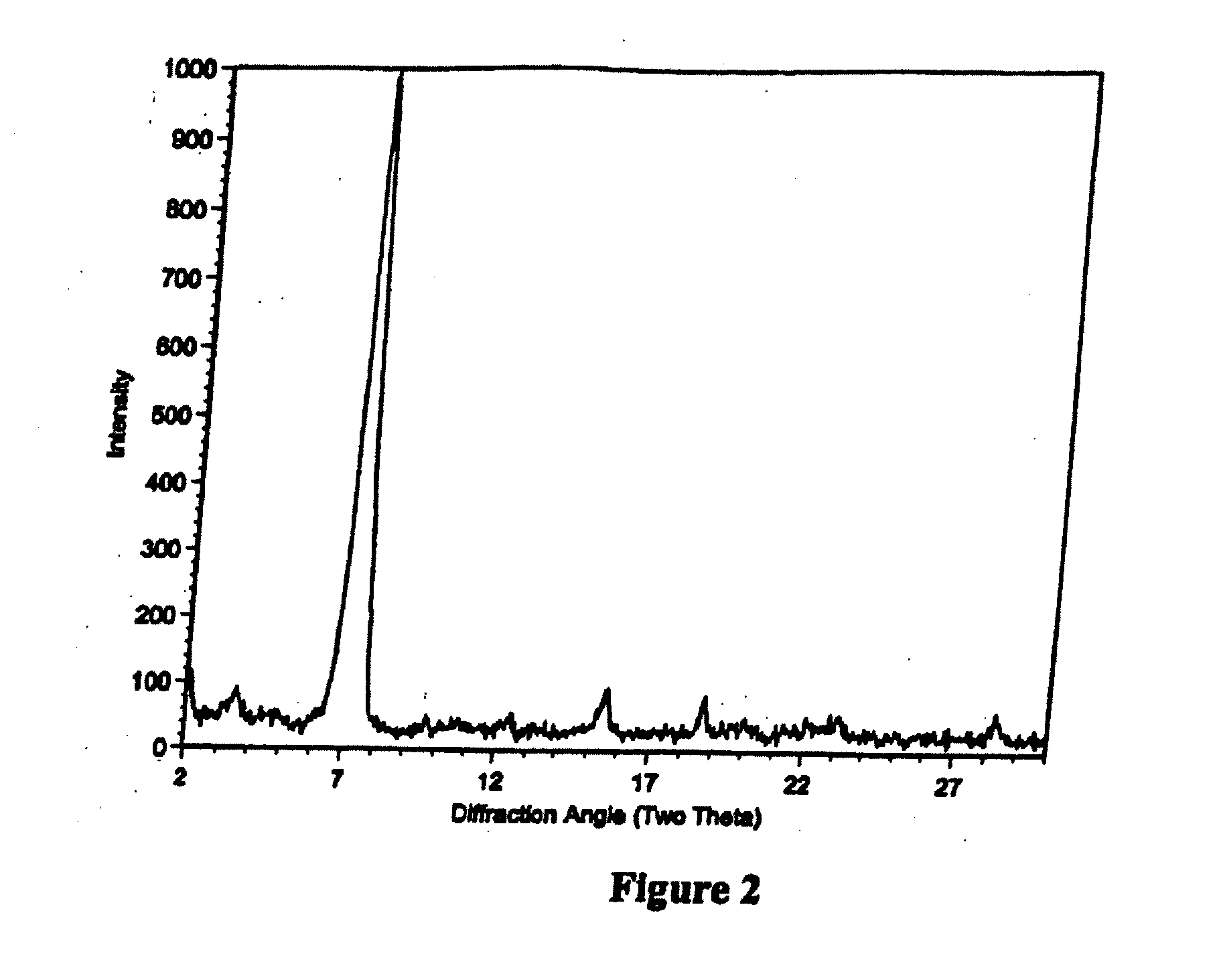
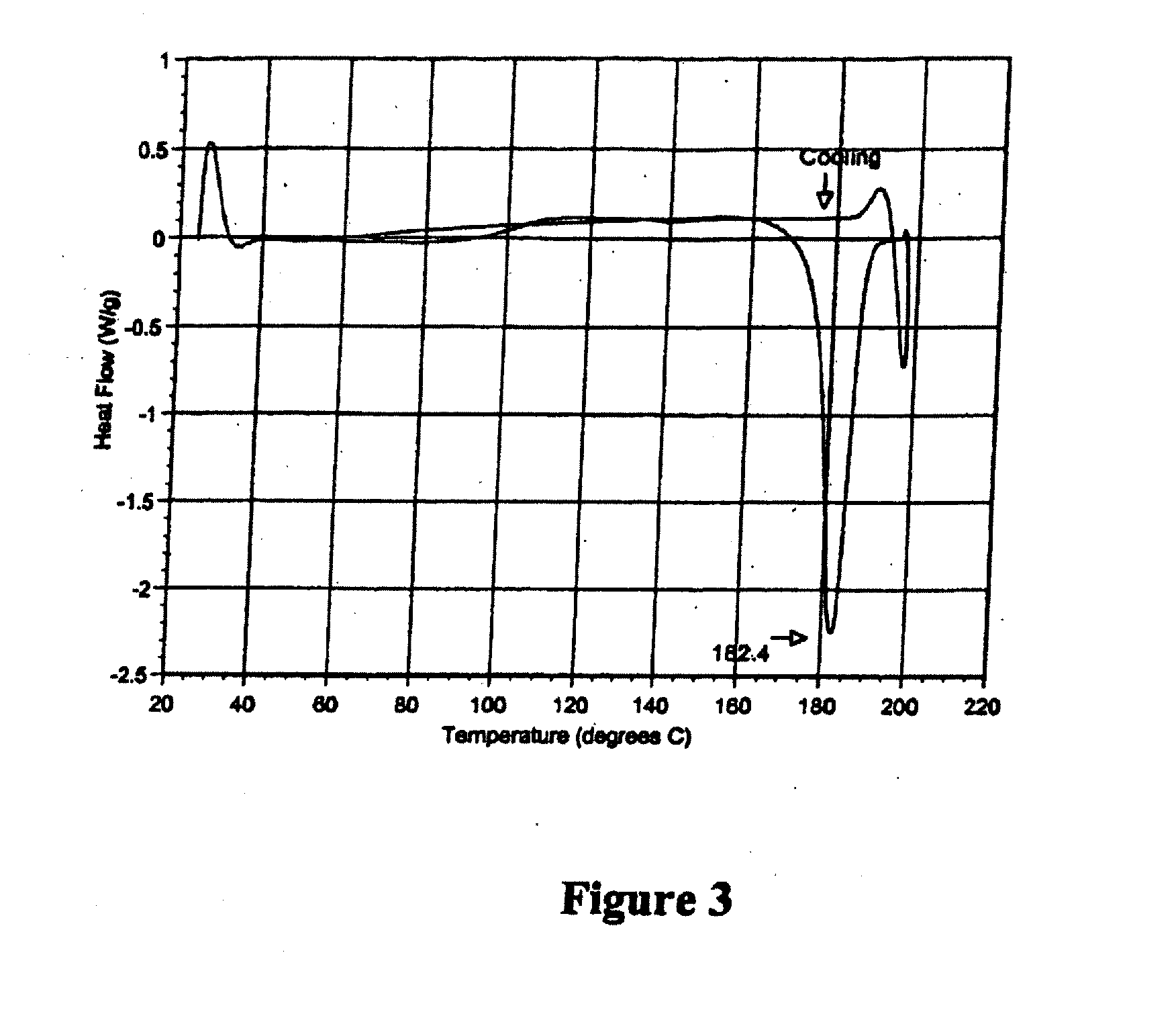
Patents
Literature
39 results about "Antibiotic-associated diarrhea" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
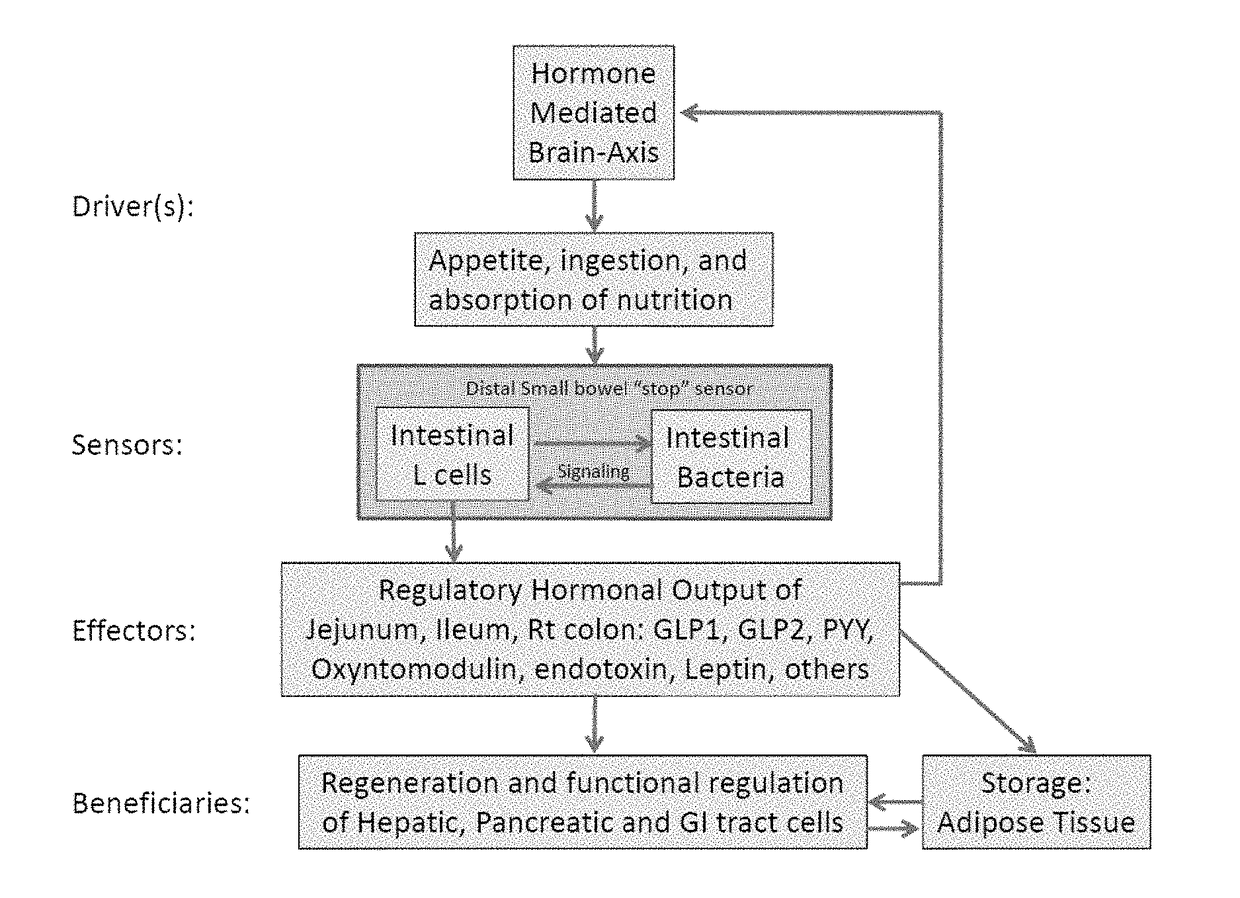
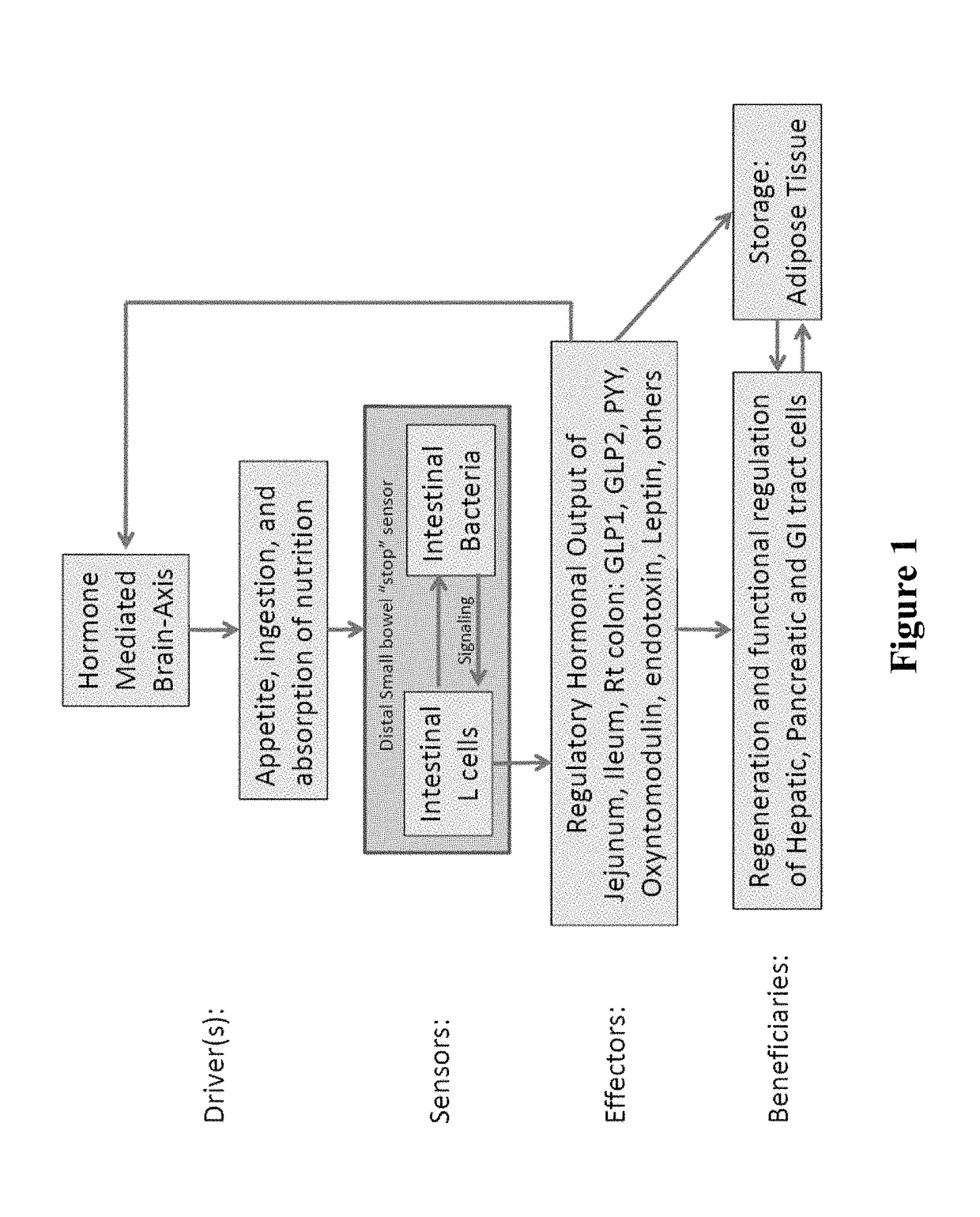
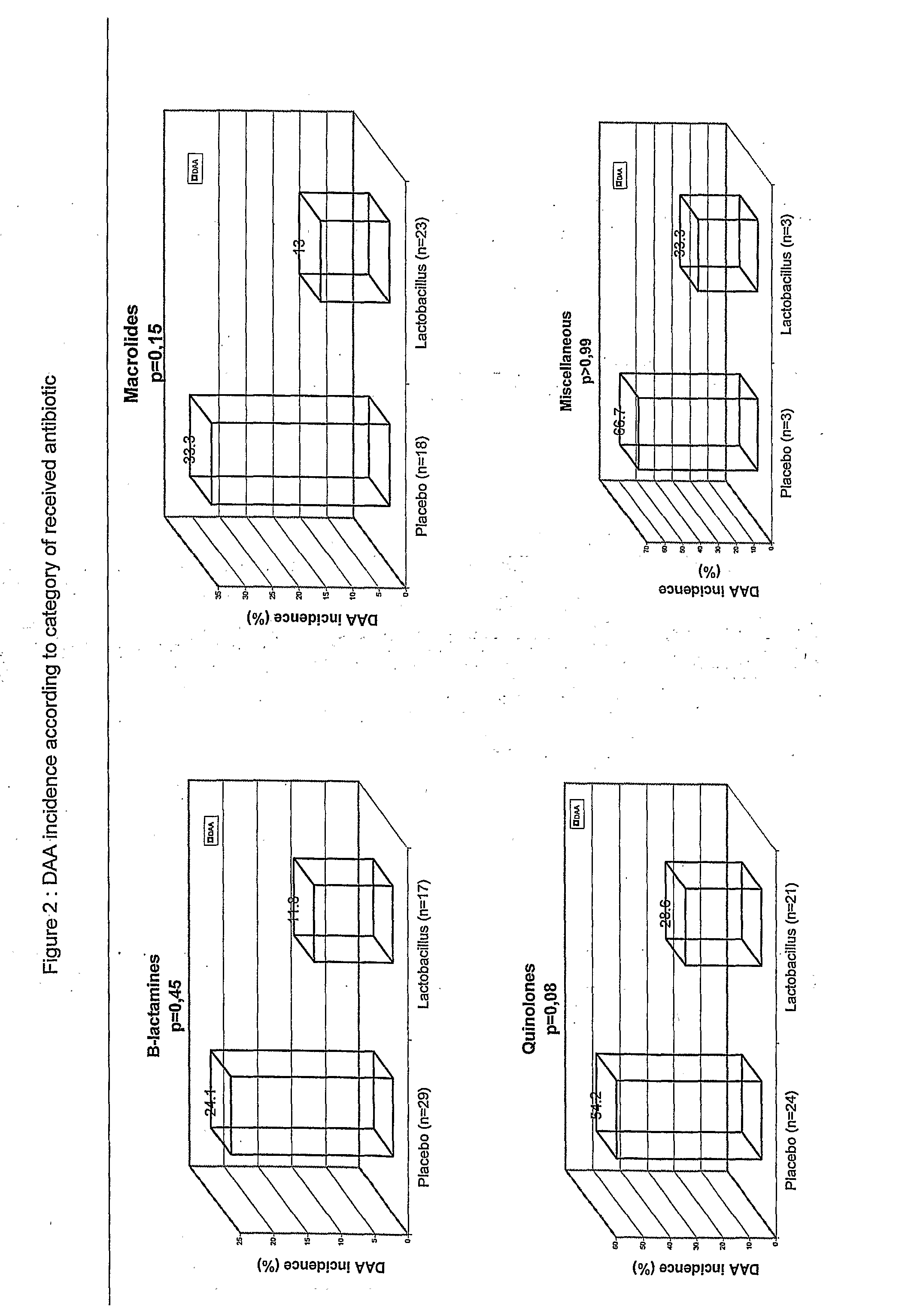
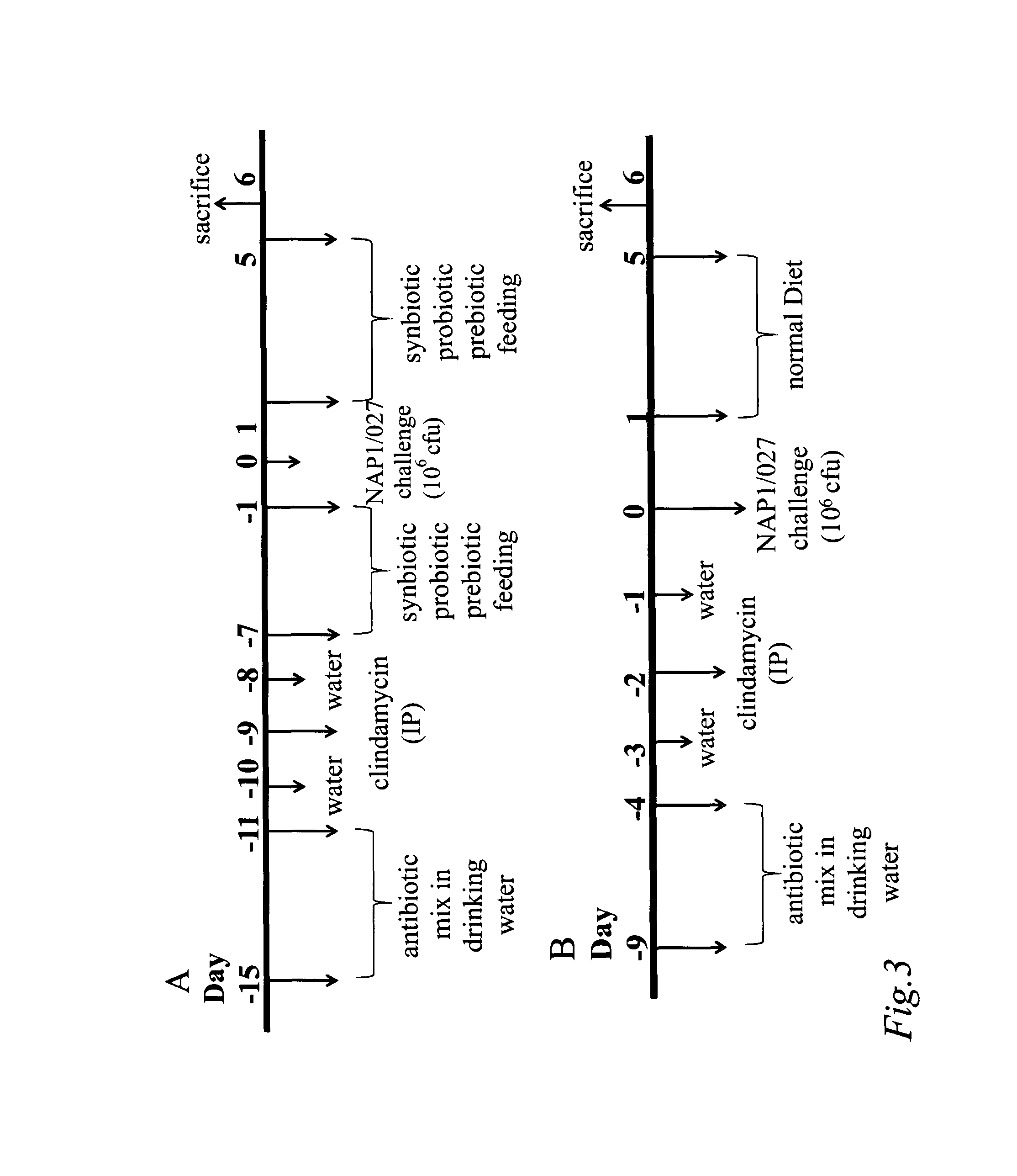
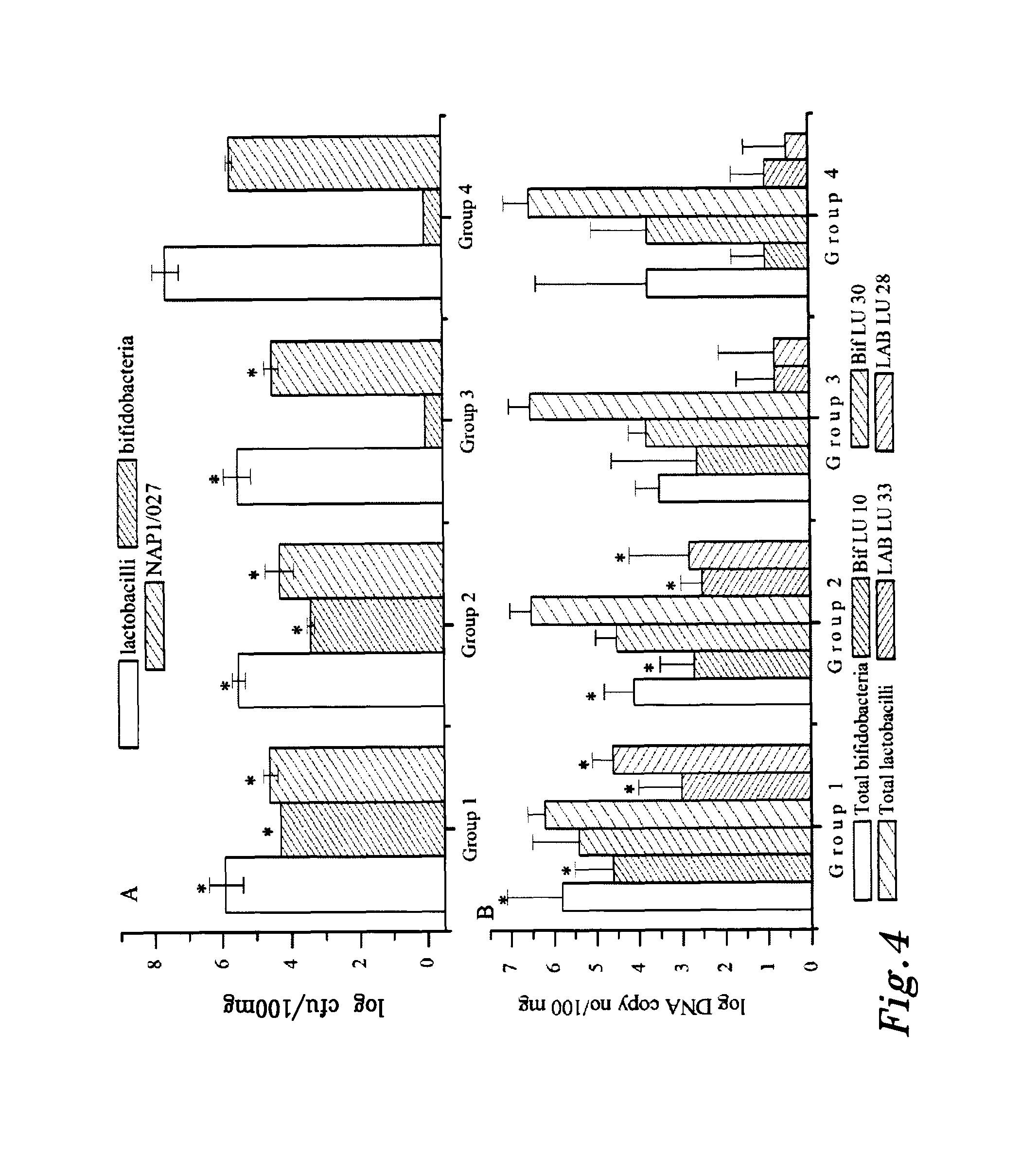
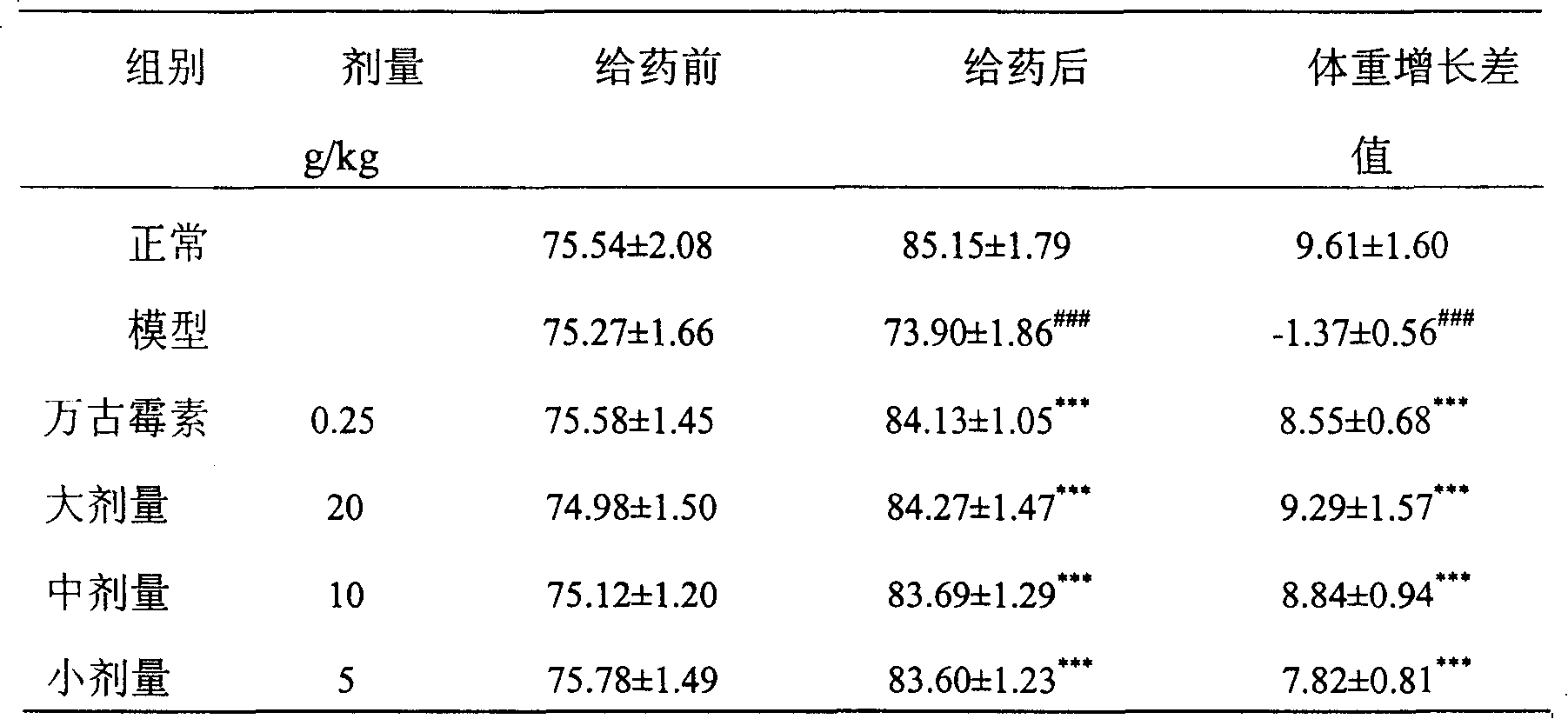
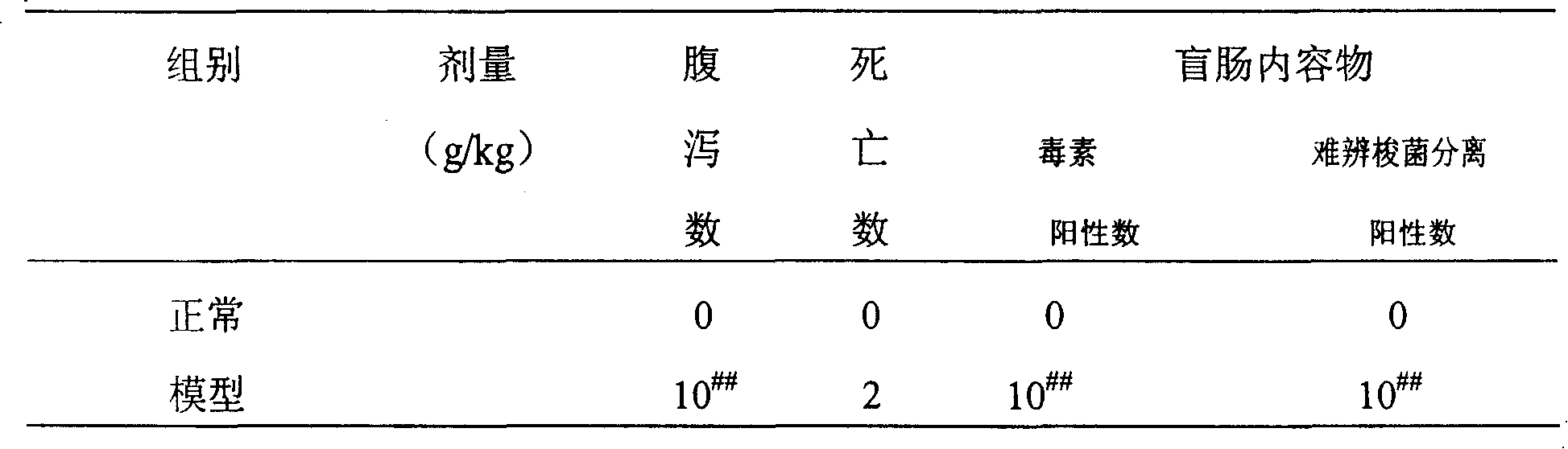
Antibiotic-associated diarrhea (AAD) results from an imbalance in the colonic microbiota caused by antibiotics. Microbiotal alteration changes carbohydrate metabolism with decreased short-chain fatty acid absorption and an osmotic diarrhea as a result. Another consequence of antibiotic therapy leading to diarrhea is overgrowth of potentially pathogenic organisms such as Clostridium difficile. It is defined as frequent loose and watery stools with no other complications.
Bacteroides fragilis and applications thereof
ActiveCN106399141AEffective for diarrheaGood probiotic propertiesBacteriaBacteria material medical ingredientsAntibiotic-associated diarrhoeaFood additive
The invention relates to Bacteroides fragilis and applications thereof, particularly to Bacteroides fragilis ZY-312 having the preservation number of CGMCC No.10685, and applications of the Bacteroides fragilis ZY-312 in preparation of medicines, pharmaceutical compositions, foods, health products and food additives for prevention and / or treatment of antibiotic-associated diarrhea, wherein the Bacteroides fragilis ZY-312 is preserved in the China general microbiological culture collection center on April 2, 2015, has the preservation number of CGMCC No.10685, does not contain enterotoxin gene bft, has characteristics of significantly-improved cholate resistance and significantly-improved gastric acid resistance compared to the existing Bacteroides fragilis, and can effectively prevent and / or treat antibiotic-associated diarrhea.
Owner:GUANGZHOU ZHIYI PHARMA INC
Targeted gastrointestinal tract delivery of probiotic organisms and/or therapeutic agents
ActiveUS20160022592A1Improve imbalanceAntibacterial agentsBiocideAntibiotic-associated diarrhoeaClostridium difficile infections
The present invention relates to the development of a targeted delivery system for the oral delivery of probiotics or therapeutic agent for various indications, including and not limited to active and prophylaxis treatment of Clostridium difficile infection, antibiotic associated diarrhea, irritable bowel syndrome, Crohn's disease, intestinal flora replacement, supplemental flora treatments for patients taking antibiotics, and for restoration of balance and signaling between the intestinal microbiome and the intestinal cells in patients under treatment of metabolic syndrome manifestations, specifically diabetes, insulin resistance, obesity, hyperlipidemia and hypertension.
Owner:THERABIOME
Synbiotic compositions for restoration and reconstitution of gut microbiota
ActiveUS20130336931A1Promote growthAntibacterial agentsBiocideAntibiotic-associated diarrhoeaIntestinal microorganisms
The present invention relates to synbiotic compositions comprising probiotic bacterial strains and prebiotic substances that, when combined exhibit synergistic behavior. The synergetic compositions will stimulate the indigenous microflora to restore and reconstitute in vivo gut like conditions after antibiotic associated diarrhea (AAD), and / or other gut infections caused by gastrointestinal pathogens, and relapses thereof, as well as the prevention of said disorders.
Owner:SYNBIOTICS CORP
Compositions and methods for the delivery of therapeutic peptides
ActiveUS20130259834A1Promotes therapeutic responseReduce mucosal damageAntibacterial agentsBiocideAntibiotic-associated diarrheaClostridium difficile infections
Methods and compositions for targeted delivery of biotherapeutics are provided. The compositions comprise bile-sensitive St. thermophilus bacteria modified to release a biotherapeutic agent following bile exposure. Biotherapeutic agents released by the St. thermophilus bacteria disclosed herein include AQ and AQR rich peptides. Methods of the invention comprise administering to a subject a St. thermophilus bacterium modified to release a biotherapeutic agent following bile exposure. Administration of the St. thermophilus bacterium promotes a desired therapeutic response. The bacterium may be modified to express and release AQ or AQR rich peptides which subsequently inhibit cellular apoptosis or reduce mucosal damage. Thus, methods of the invention find use in treating or preventing a variety of gastrointestinal disorders including C. difficile infection and antibiotic-associated diarrhea.
Owner:NORTH CAROLINA STATE UNIV +1
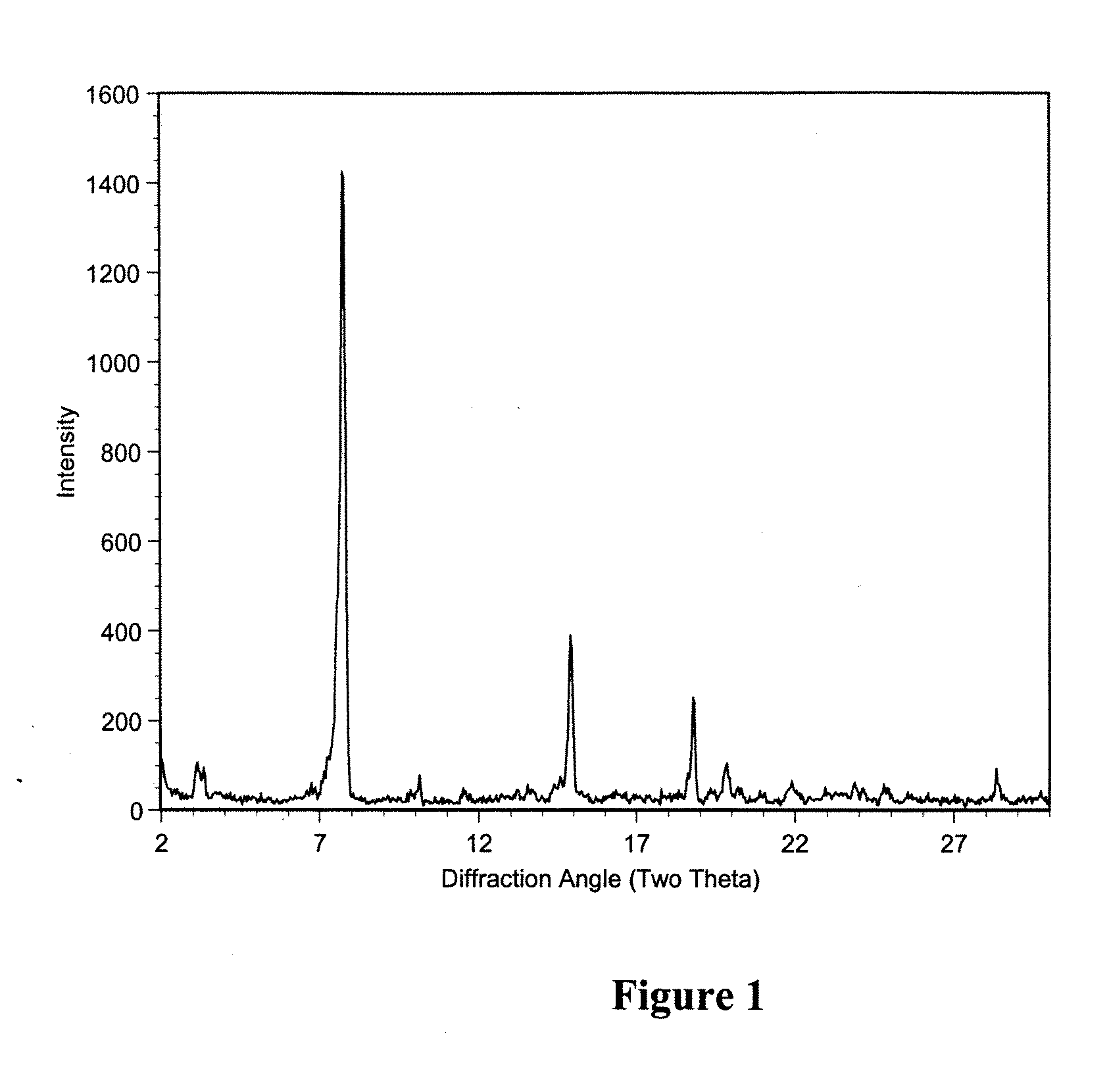
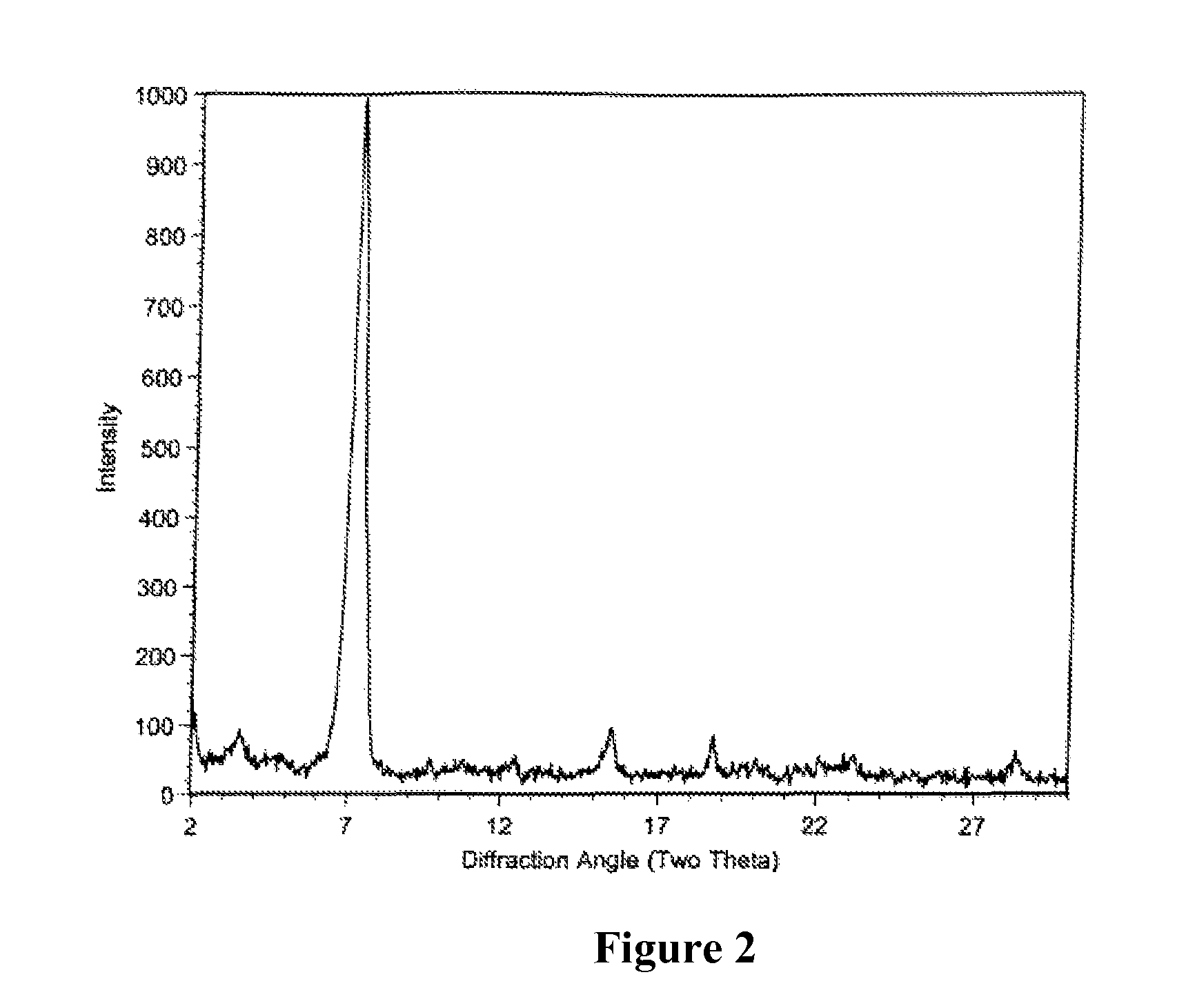
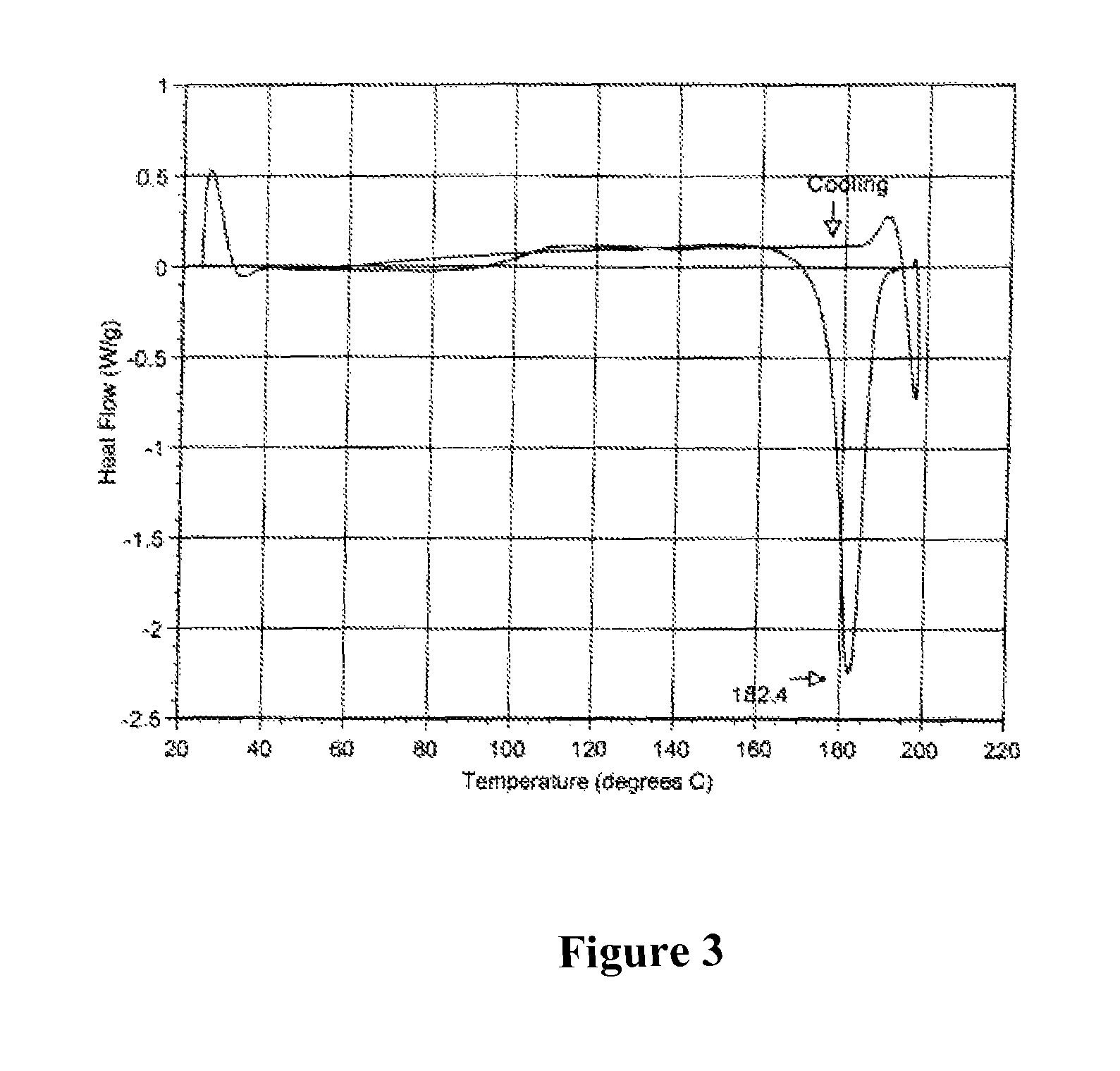
Polymorphic crystalline forms of tiacumicin B
ActiveUS7378508B2Antibacterial agentsOrganic active ingredientsAntibiotic-associated diarrhoeaDisease
The invention relates to novel forms of compounds displaying broad spectrum antibiotic activity, especially crystalline polymorphic forms and amorphous forms of such compounds, compositions comprising such crystalline polymorphic forms and amorphous forms of such compounds, processes for manufacture and use thereof. The compounds and compositions of the invention are useful in the pharmaceutical industry, for example, in the treatment or prevention of diseases or disorders associated with the use of antibiotics, chemotherapies, or antiviral therapies, including, but not limited to, colitis, for example, pseudo-membranous colitis; antibiotic associated diarrhea; and infections due to Clostridium difficile (“C. difficile”), Clostridium perfringens (“C. perfringens”), Staphylococcus species, for example, methicillin-resistant Staphylococcus, or Enterococcus including Vancomycin-resistant enterococci.
Owner:MERCK SHARP & DOHME LLC
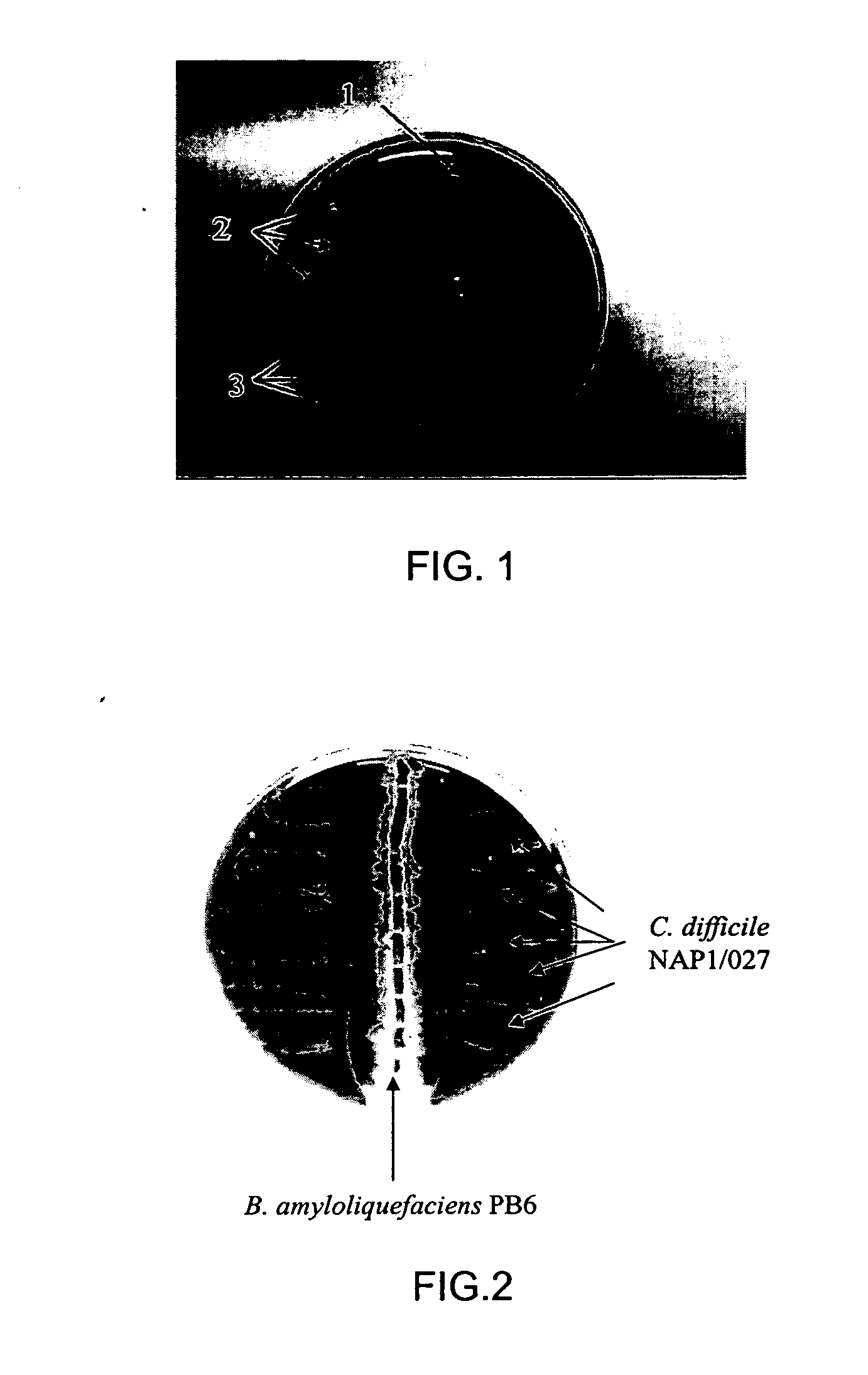
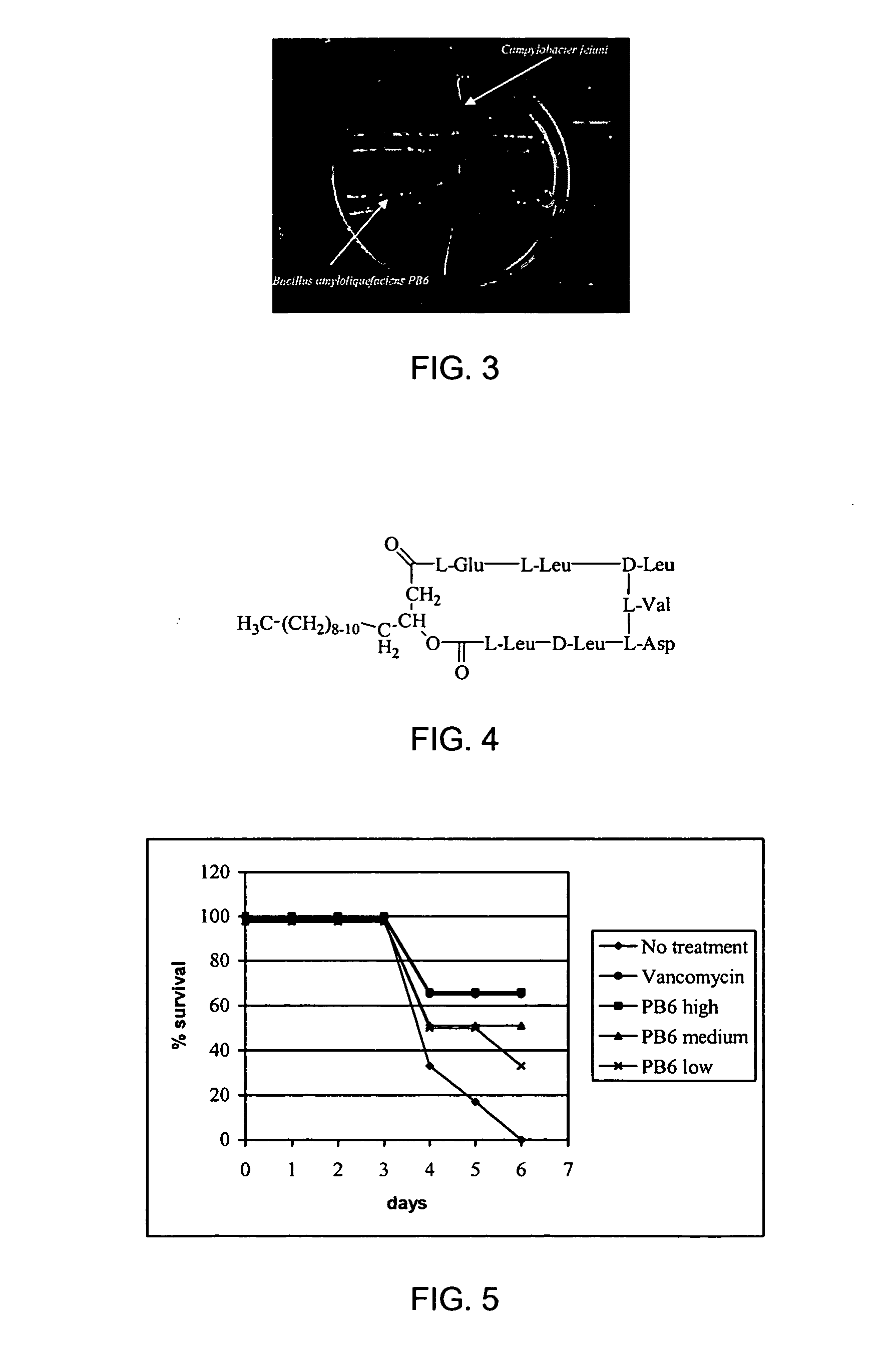
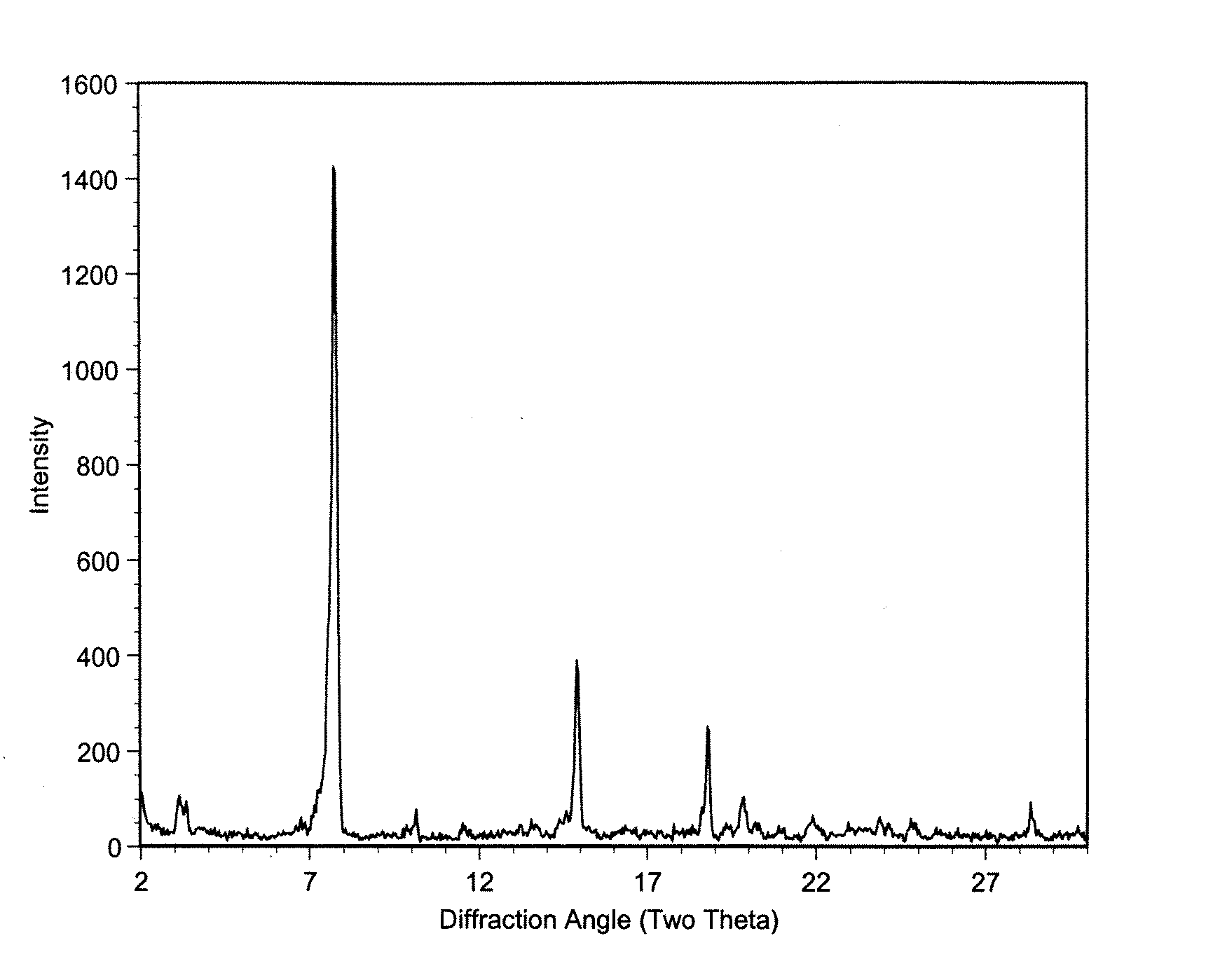
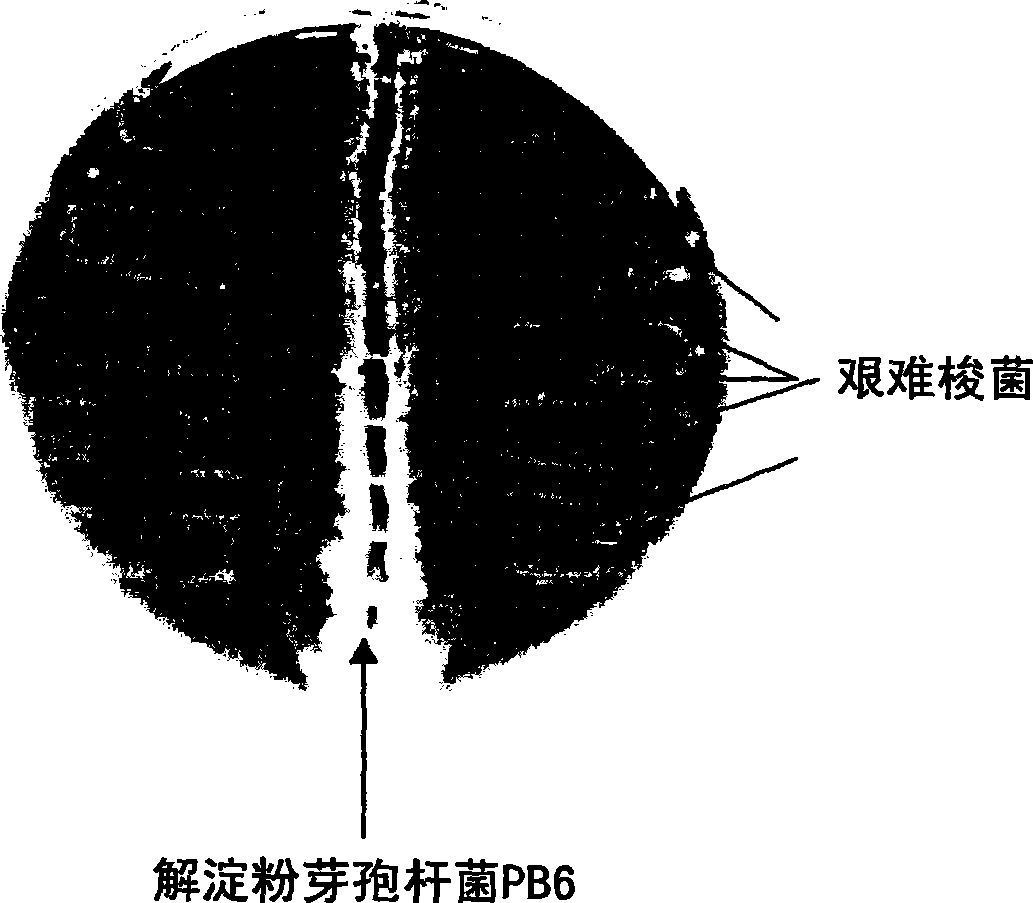
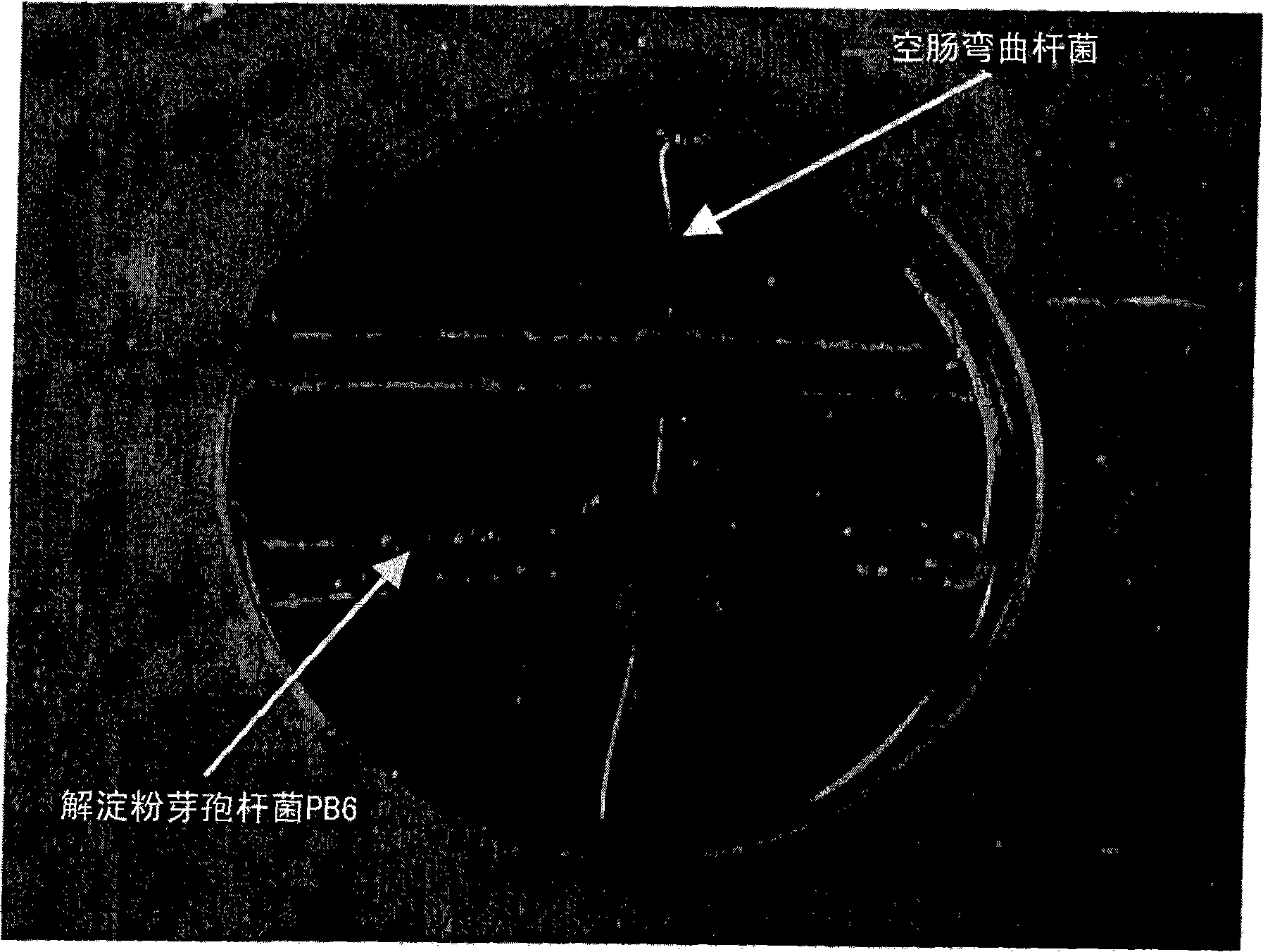
Use of bacillus amyloliquefaciens PB6 for the prophylaxis or treatment of gastrointestinal and immuno-related diseases
InactiveUS20080057047A1BiocideBacteriaAntibiotic-associated diarrhoeaClostridium difficile (bacteria)
Bacteria of the sp. Bacillus that produce a lipopeptide are found to be effective in the treatment and prophylaxis of gastro-intestinal disease when administered as a probiotic. In particular, a strain of Bacillus bacteria identified as PB6 is useful for the treatment of Antibiotic Associated Diarrhea (AAD) or the more serious condition Clostridium difficile associated diarrhea (CDAD) when administered as a probiotic. Additionally, these bacteria have been found efficient for the treatment of immunorelated diseases such as Inflammatory Bowel Disease (IBD).
Owner:KEMIN IND INC
Macrolide polymorphs, compositions comprising such polymorphs, and methods of use and manufacture thereof
The invention relates to novel forms of compounds displaying broad spectrum antibiotic activity, especially crystalline polymorphic forms and amorphous forms of such compounds, compositions comprising such crystalline polymorphic forms and amorphous forms of such compounds, processes for manufacture and use thereof. The compounds and compositions of the invention are useful in the pharmaceutical industry, for example, in the treatment or prevention of diseases or disorders associated with the use of antibiotics, chemotherapies, or antiviral therapies, including, but not limited to, colitis, for example, pseudo-membranous colitis; antibiotic associated diarrhea; and infections due to Clostridium difficile (“C. difficile”), Clostridium perfringens (“C. perfringens”), Staphylococcus species, for example, methicillin-resistant Staphylococcus, or Enterococcus including Vancomycin-resistant enterococci.
Owner:MERCK SHARP & DOHME LLC
Macrocyclic Polymorphs, Compositions Comprising Such Polymorphs and Methods of Use and Manufacture Thereof
The invention relates to novel forms of compounds displaying broad spectrum antibiotic activity, especially crystalline polymorphic forms and amorphous forms of such compounds, compositions comprising such crystalline polymorphic forms and amorphous forms of such compounds, processes for manufacture and use thereof. The compounds and compositions of the invention are useful in the pharmaceutical industry, for example, in the treatment or prevention of diseases or disorders associated with the use of antibiotics, chemotherapies, or antiviral therapies, including, but not limited to, colitis, for example, pseudo-membranous colitis; antibiotic associated diarrhea; and infections due to Clostridium difficile (“C. difficile”), Clostridium perfringens (“C. perfringens”), Staphylococcus species, for example, methicillin-resistant Staphylococcus, or Enterococcus including Vancomycin-resistant enterococci.
Owner:MERCK SHARP & DOHME LLC
Antibiotic Formulations Providing Reduced Gastrointestinal Side Effects and Clostridium difficile Infection Relapse, and Related Methods
InactiveUS20110240512A1Antibacterial agentsSmall article dispensingClostridial infectionAntibiotic-associated diarrhoea
The invention includes a formulation comprising: (i) a therapeutically effective amount of at least one antibiotic; and (ii) a therapeutically effective amount of at least one probiotic material. The formulation is prepared in a dosage form for delivery to the gastrointestinal tract. The invention further includes a method of preventing or minimizing the proliferation of Clostridium difficile in the gastrointestinal tract of a mammal undergoing an antibiotic therapy comprising by administration of the formulation of the invention for the duration of the antibiotic therapy; methods of preventing or minimizing the occurrence of antibiotic-associated diarrhea in a mammal undergoing an antibiotic therapy that includes administering the formulation; and methods of preventing or minimizing the occurrence of antibiotic-associated colitis or pseudomembranous colitis in a patient undergoing an antibiotic therapy that includes administering the formulation.Also included are methods of reducing or preventing a failure of treatment for an antibiotic-treatable infection in a patient comprising orally administering the formulation.Described by the invention are formulations for the treatment of a C. difficile infection comprising: (i) a therapeutically effective amount of at least one antibiotic; and (ii) a therapeutically effective amount of at least one probiotic material. The antibiotic includes a non-systemic gram negative antibiotic (such as, for example, fodaximicin) and formulation is prepared in a dosage form for delivery to the gastrointestinal tract.Also included are methods of treatment of a C. difficile infection in mammal that include administering to the digestive tract of the mammal the above described formulation. Such methods provide that the likelihood that the mammal shall experience a reoccurrence of the C. difficile infection is 70% or less.
Owner:TECOPPA BIOPHARMA
Targeted gastrointestinal tract delivery of probiotic organisms and/or therapeutic agents
ActiveUS9907755B2Antibacterial agentsMetabolism disorderAntibiotic-associated diarrhoeaClostridium difficile infections
The present invention relates to the development of a targeted delivery system for the oral delivery of probiotics or therapeutic agent for various indications, including and not limited to active and prophylaxis treatment of Clostridium difficile infection, antibiotic associated diarrhea, irritable bowel syndrome, Crohn's disease, intestinal flora replacement, supplemental flora treatments for patients taking antibiotics, and for restoration of balance and signaling between the intestinal microbiome and the intestinal cells in patients under treatment of metabolic syndrome manifestations, specifically diabetes, insulin resistance, obesity, hyperlipidemia and hypertension.
Owner:THERABIOME
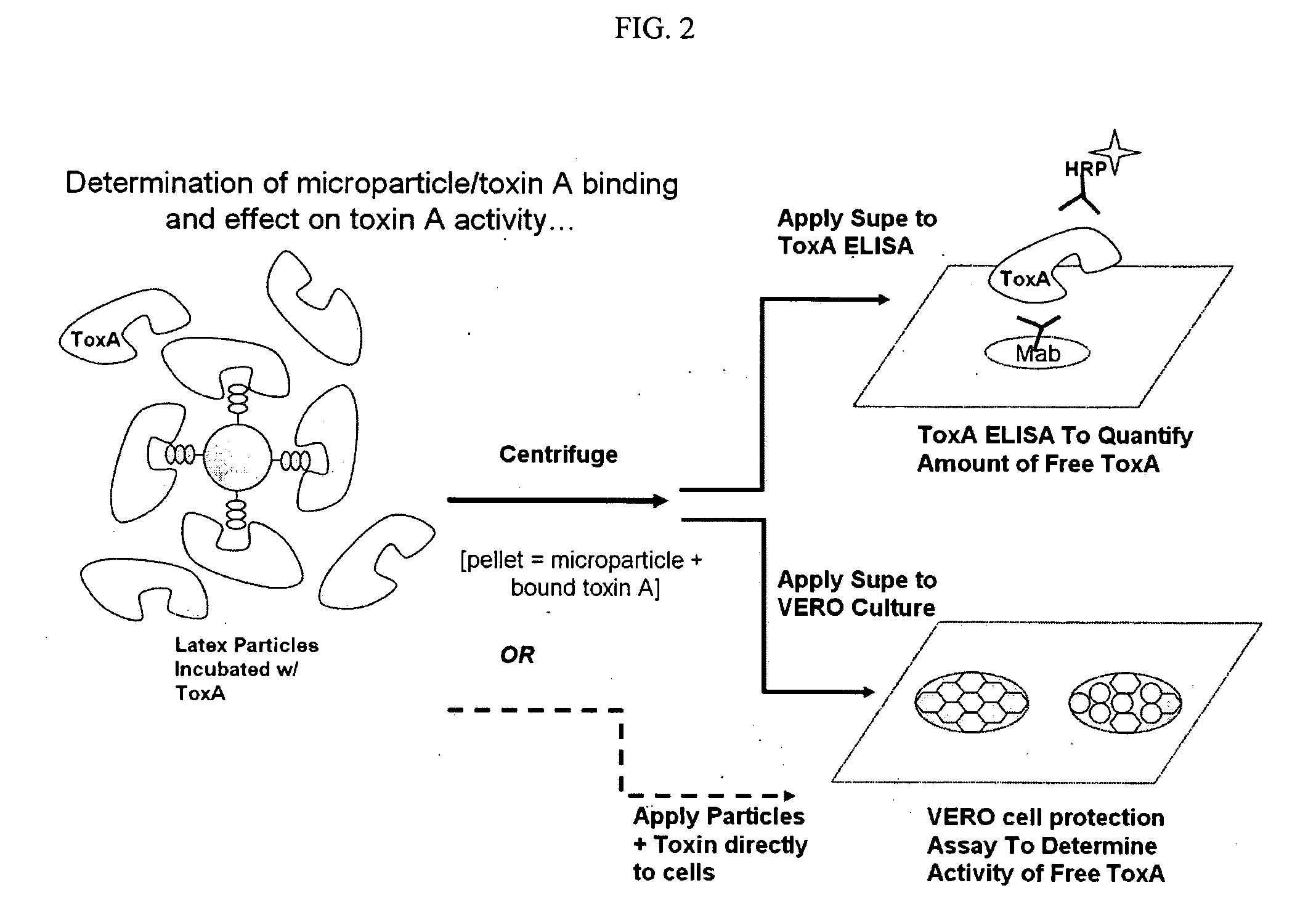
Toxin binding compositions
InactiveUS20060078534A1Powder deliveryOrganic active ingredientsAntibiotic-associated diarrhoeaClostridium difficile (bacteria)
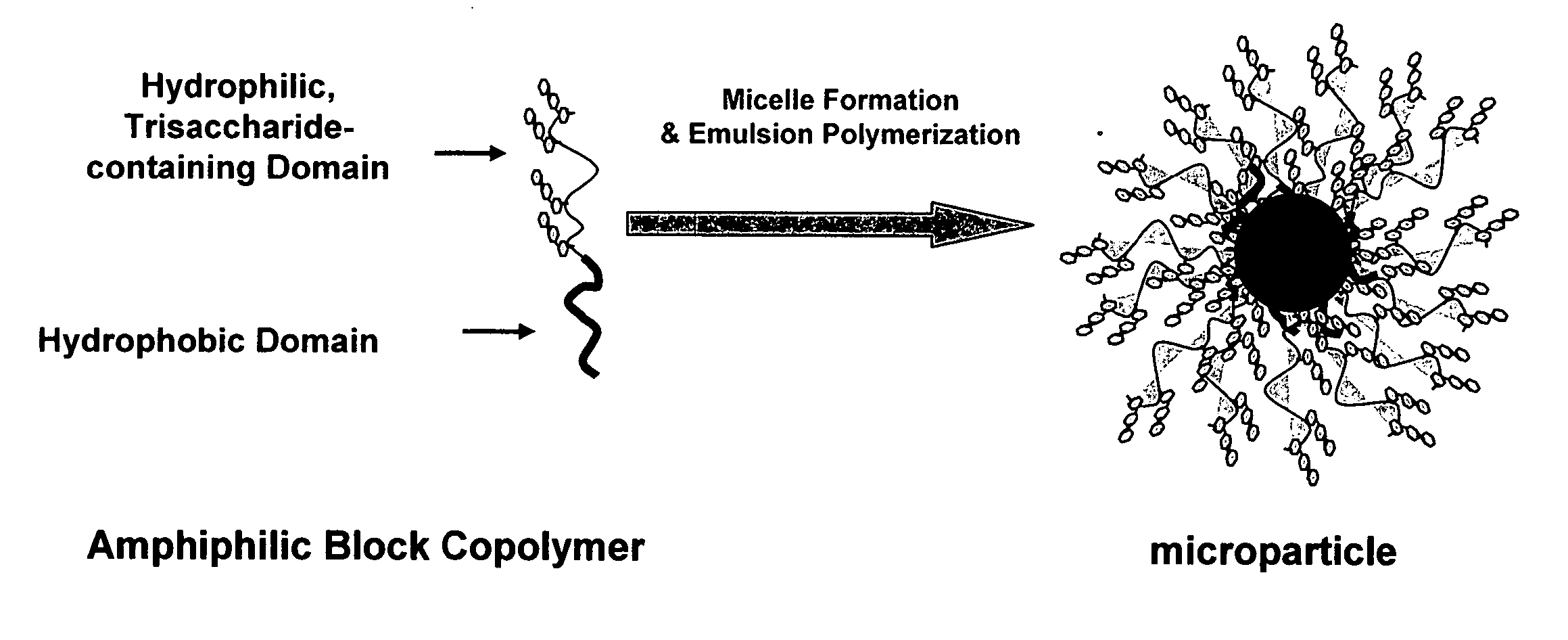
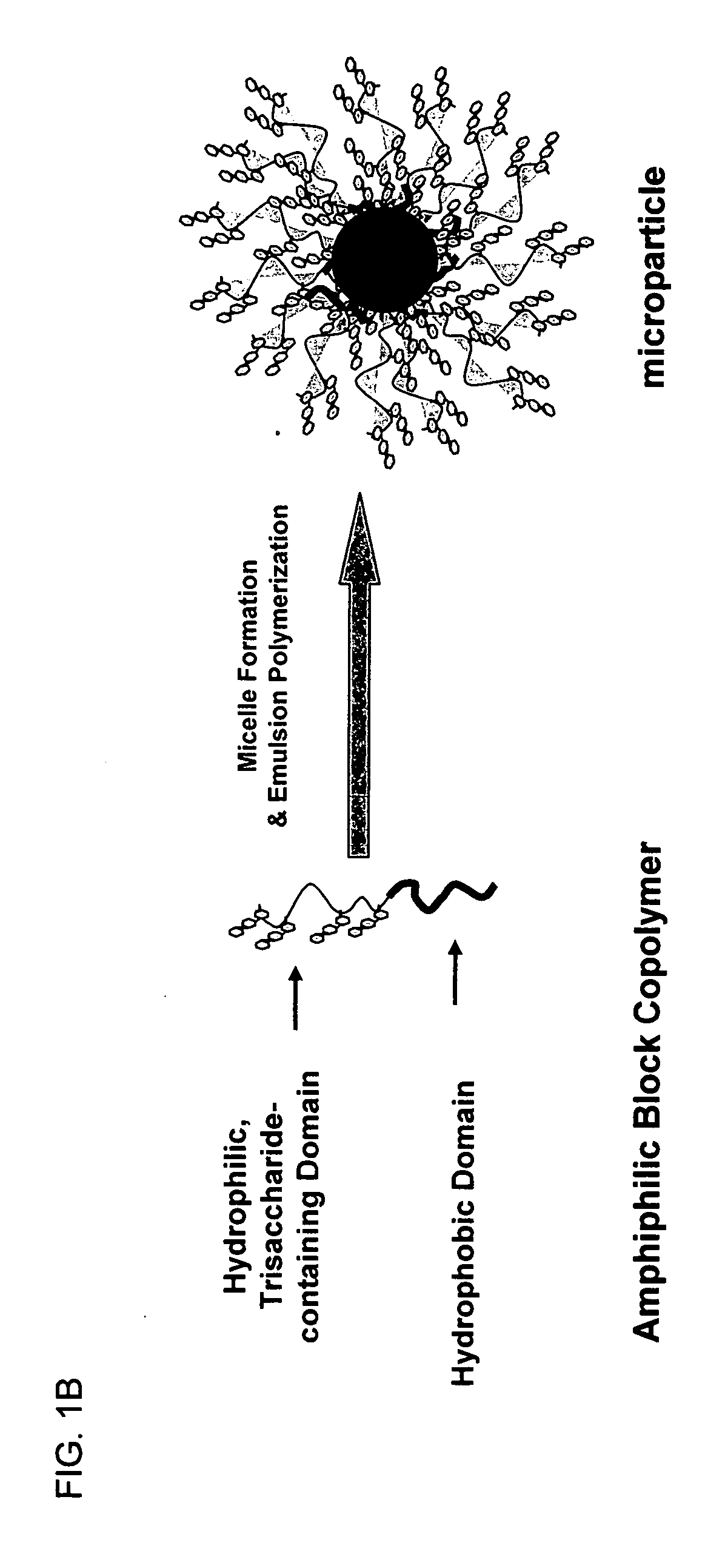
Methods and compositions for the treatment of toxin-mediated diseases are provided herein. One aspect of the invention is oligosaccharide-based therapeutics that interact with toxins and methods of uses thereof. In one embodiment the oligosaccharide-based therapeutics of the invention comprise polymeric particles with attached oligosaccharide binding moieties. The compositions of the invention can be used in the treatment of toxin-mediated diseases such as antibiotic-associated diarrhea and pseudomembranous colitis, including Clostridium difficile associated diarrhea.
Owner:ILYPSA
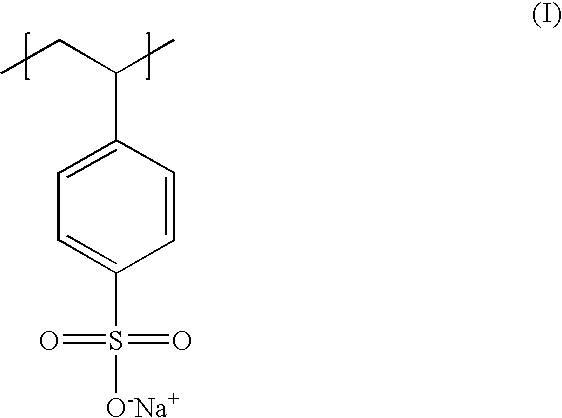
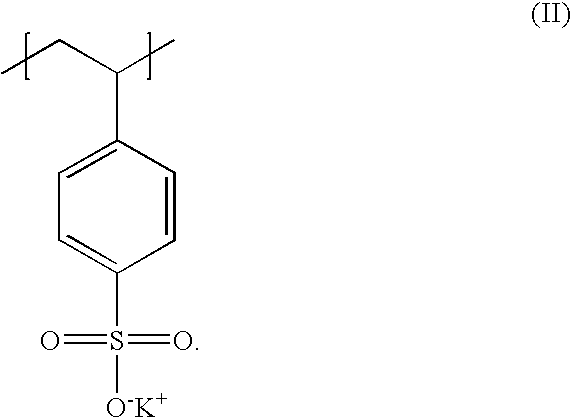
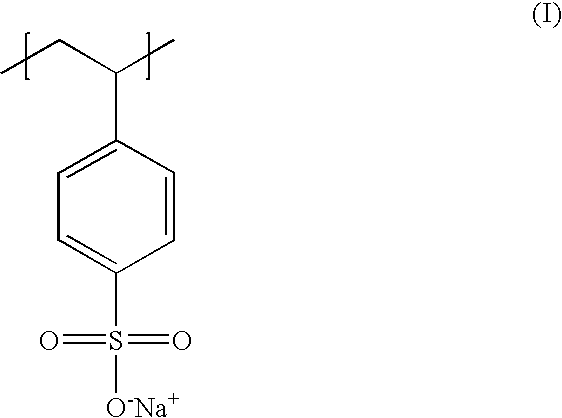
Poly(potassium and sodium styrene sulfonate), its manufacture and its uses
InactiveUS20050214246A1Easy to prepareInhibit and prevent relapseAntibacterial agentsDigestive systemAntibiotic-associated diarrhoeaSulfonate
Antibiotic-associated diarrhea, such as that caused by Clostridium difficile, represents a serious medical complication that can result from administering a broad-spectrum antibiotic to a subject. Such diarrhea leads to significant potassium loss from the subject. The present invention discloses a polymeric therapeutic agent that treats antibiotic-associated diarrhea and is physiologically potassium neutral. This polymer contains polystyrene sodium sulfonate and polystyrene potassium sulfonate repeat units.
Owner:GENZYME CORP
Composition containing L. acidophilus 1.1878 and Chinese medicaments and application thereof
InactiveCN101953916AOrganic active ingredientsBacteria material medical ingredientsMedicinal herbsDrug product
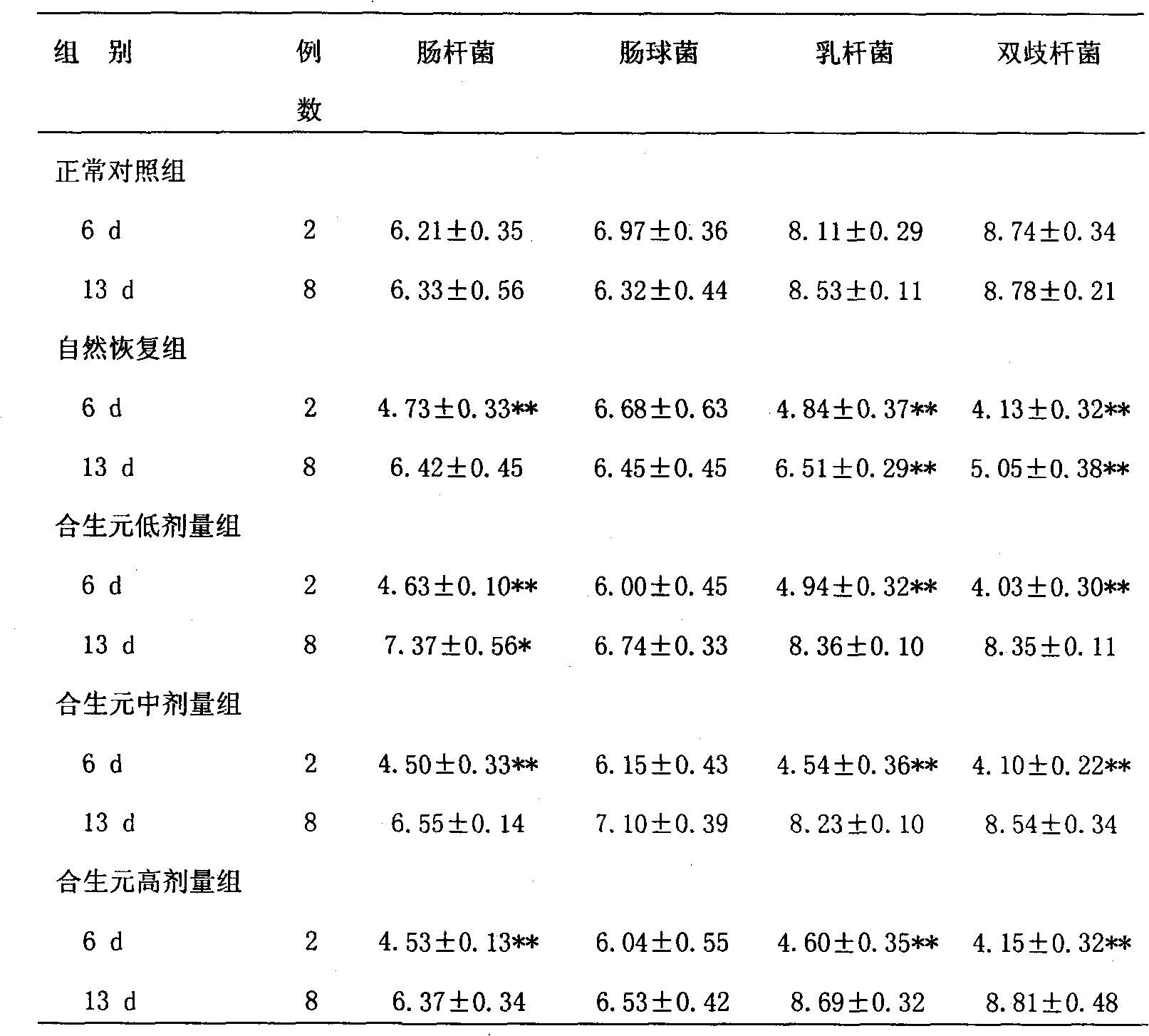
The invention relates to a composition containing L. acidophilus 1.1878 and Chinese medicaments and application thereof. The L. acidophilus 1.1878 has high acid resistance and bile salt resistance and good adhesion. The composition contains the L. acidophilus 1.1878, and also contains oligosaccharide, Chinese medicinal herbs and the like. The composition can be used as food, health-care products or medicaments, is suitable for eating for a long term, and is mainly used for preventing and treating antibiotic associated diarrhea, regulating intestinal flora, protecting intestinal mucosa and enhancing immunity.
Owner:杜丹
Application of fucosan sulfate with low molecular weight in prevention and treatment of enteritis
InactiveCN108904524ANo side effectsInhibition of toxin productionAntibacterial agentsOrganic active ingredientsMedicineSulfate
The invention provides application of fucosan sulfate with low molecular weight in treatment of enteritis. According to the application, antibiotic-associated diarrhea or clostridium difficile associated enteric infection and acquired diarrhea are prevented and treated through offering an effective amount of fucosan sulfate with low molecular weight, so that the shortage that preparations for preventing and treating clostridium difficile enteritis are scarce currently is made up.
Owner:OCEAN UNIV OF CHINA
Macrocyclic polymorphs, compositions comprising such polymorphs and methods of use and manufacture thereof
The invention relates to novel forms of compounds displaying broad spectrum antibiotic activity, especially crystalline polymorphic forms and amorphous forms of such compounds, compositions comprising such crystalline polymorphic forms and amorphous forms of such compounds, processes for manufacture and use thereof. The compounds and compositions of the invention are useful in the pharmaceutical industry, for example, in the treatment or prevention of diseases or disorders associated with the use of antibiotics, chemotherapies, or antiviral therapies, including, but not limited to, colitis, for example, pseudo-membranous colitis; antibiotic associated diarrhea; and infections due to Clostridium difficile (“C. difficile”), Clostridium perfringens (“C. perfringens”), Staphylococcus species, for example, methicillin-resistant Staphylococcus, or Enterococcus including Vancomycin-resistant enterococci.
Owner:MERCK SHARP & DOHME LLC
The use of bacillus PB6 for the prophylaxis or treatment of gastrointestinal and immuno-related diseases
Bacteria of the sp. Bacillus that produce a lipopeptide are found to be effective in the treatment and prophylaxis of gastro-intestinal disease when administered as a probiotic. In particular, a strain of Bacillus bacteria identified as PB6 is useful for the treatment of Antibiotic Associated Diarrhea (AAD) or the more serious condition Clostridium difficile associated diarrhea (CDAD) when administered as a probiotic. Additionally, these bacteria have been found efficient for the treatment of immunorelated diseases such as Inflammatory Bowel Disease (IBD).
Owner:KEMIN IND INC
Probiotic compositions and methods of use thereof
InactiveUS20190262407A1Digestive systemUnknown materialsAntibiotic-associated diarrheaCrohn's disease
Various probiotic therapies including butyrogenic bacteria are provided for the treatment of gastrointestinal diseases caused by microbial dysbiosis. Butyrogenic bacteria produce the short chain fatty acid, butyric acid. Gastrointestinal disorders that are associated with microbial dysbiosis include but are not limited to, inflammatory bowel disease, such as Crohn's disease and ulcerative colitis, irritable bowel syndrome, recurrent Clostridium difficile infection, and antibiotic-associated diarrhea.
Owner:LIFEBRIDGE HEALTH INC
Toxin binding compositions
Methods and compositions for the treatment of toxin-mediated diseases are provided herein. One aspect of the invention is oligosaccharide-based therapeutics that interact with toxins and methods of uses thereof. In one embodiment the oligosaccharide-based therapeutics of the invention comprise polymeric particles with attached oligosaccharide binding moieties. The compositions of the invention can be used in the treatment of toxin-mediated diseases such as antibiotic-associated diarrhea and pseudomembranous colitis, including Clostridium difficile associated diarrhea.
Owner:ILYPSA
Targeted synbiotic therapy for dysbiosis-related intestinal and extra-intestinal disorders
InactiveUS20180110800A1Increase productionOrganic active ingredientsDigestive systemAntibiotic-associated diarrhoeaSynbiotics
Various types of synbiotic therapies are provided for the treatment of a variety of gastrointestinal and other disorders. The combination of prebiotics to probiotics is defined as a synbiotic therapy. The principal GI disorders associated with dysbiosis that can be treated from such a therapeutic intervention include but are not limited to: inflammatory bowel disease (IBD) (Crohn's disease and ulcerative colitis), irritable bowel syndrome (IBS), antibiotic-associated diarrheas such as recurrent Clostridium difficile infection, and possibly variants of Celiac disease. Other disorders that may also be ameliorated by the proposed synbiotic therapy include metabolic syndromes and central nervous system disorders. The disclosed methods and compositions were developed to improve upon currently available probiotics through consideration of the human intestinal microbiota, and its relationship to various intestinal metabolic and neuropsychiatric disorders. In one embodiment, the disclosed synbiotic compositions include prebiotics and a targeted delivery system, which altogether promote the survival, growth, and attachment of probiotic microbiota.
Owner:LIFEBRIDGE HEALTH INC
Use of Bifidobacterium animalis A6 in the preparation of medicaments
InactiveCN109200064ACompounds screening/testingOrganic active ingredientsAntibiotic-associated diarrhoeaMedicine
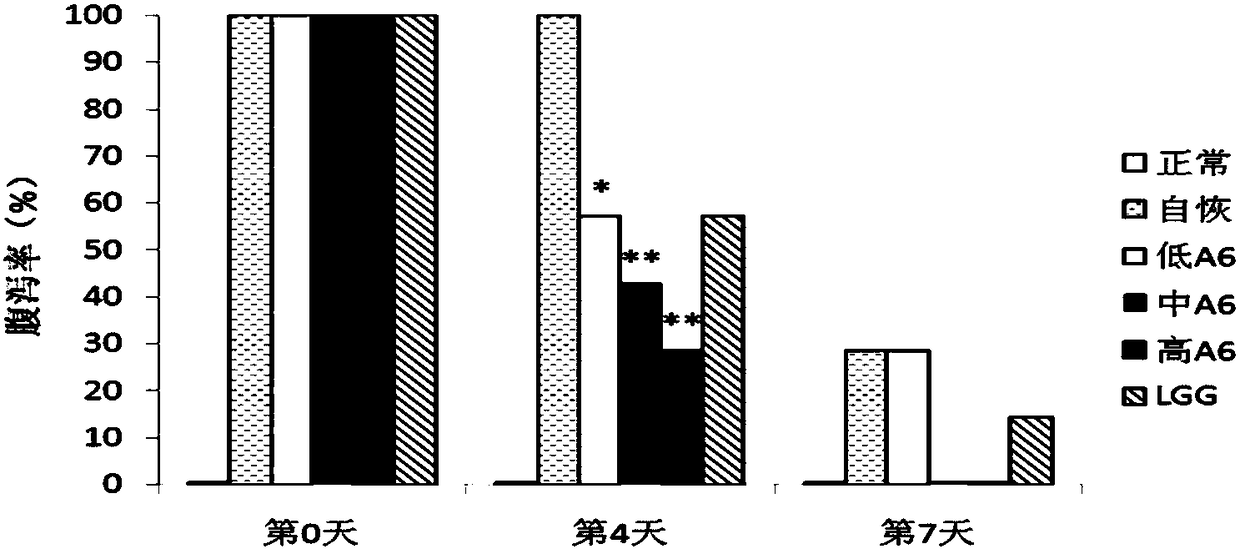
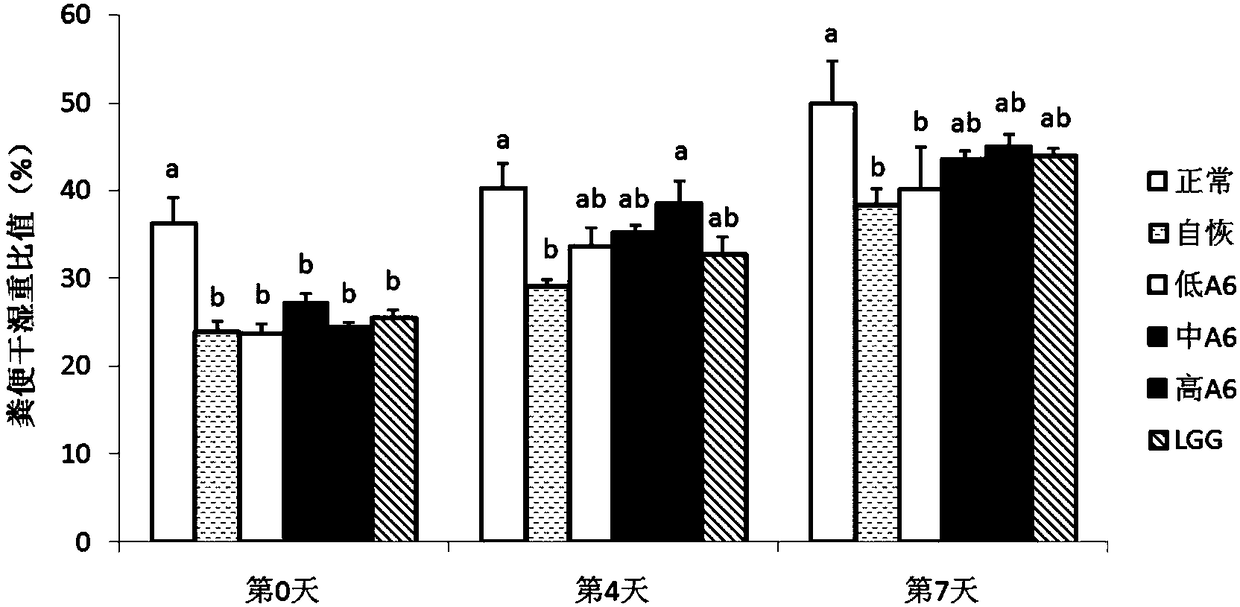
The present invention proposes the use of Bifidobacterium animalis A6 in the preparation of medicaments for the treatment or prevention of antibiotic-associated diarrhea. The inventors found through experiments that Bifidobacterium animalis A6 can effectively alleviate antibiotic-associated diarrhea by improving intestinal function of mice.
Owner:CHINA AGRI UNIV
Solid drink capable of improving stomach health problems
InactiveCN107996936AGood for healthSymptoms improvedAntipyreticDigestive systemSide effectLactobacillus rhamnosus
The invention discloses a solid drink capable of improving stomach health problems. The solid drink contains fermented soybean meal, calcium carbonate and probiotics, the probiotics comprise lactobacillus helveticus and lactobacillus rhamnosus, the solid drink also contains xylitol and maltodextrin, and the fermented soybean meal is prepared through fermentation of soybeans with lactobacillus delbruckii subsp. Rosell-187. The solid drink is powdery and is directly poured into the mouth to be taken with water during usage. Raw materials are all food materials which are safe and effective and have no side effects. The solid drink can relieve stomach health problems such as stomachache, hyperacidity and heartburn caused by ulcer, improve balance of microbial florae of the human body and relieve side effects caused by use of bactericidal drugs or antibiotics, such as antibiotic associated diarrhea.
Owner:XUZHOU YITONG FOOD IND CO LTD
Lactic Bacteria and Their Use in the Prevention of Diarrhea
InactiveUS20070248582A1Non invasiveAvoid it happening againAntibacterial agentsBiocideLactobacillusAntibiotic-associated diarrhoea
The present invention concerns a lactic composition useful for the prevention or treatment of diarrhea such as antibiotic associated diarrhea or “tourista”. The composition according to the invention contains at least a bacterial strain selected from the group consisting of Lactobacillus acidophilus, Lactobacillus acidophilus I-1492 Lactobacillus casei and a mixture of thereof.
Owner:BIO K PLUS INT
Application of stachyose in preparing medicine for preventing and curing diarrhea
InactiveCN101810631AOrganic active ingredientsDigestive systemAntibiotic-associated diarrhoeaStachyose
The invention discloses application of stachyose in preparing a medicine for preventing and curing diarrhea. Experiments prove that the stachyose has certain alleviating and curing effects for intestinal infection diarrhea, antibiotic-associated diarrhea, bacillary dysentery diarrhea, rotavirus infection diarrhea, non-specific diarrhea and the like.
Owner:CHINA NAT RES INST OF FOOD & FERMENTATION IND CO LTD
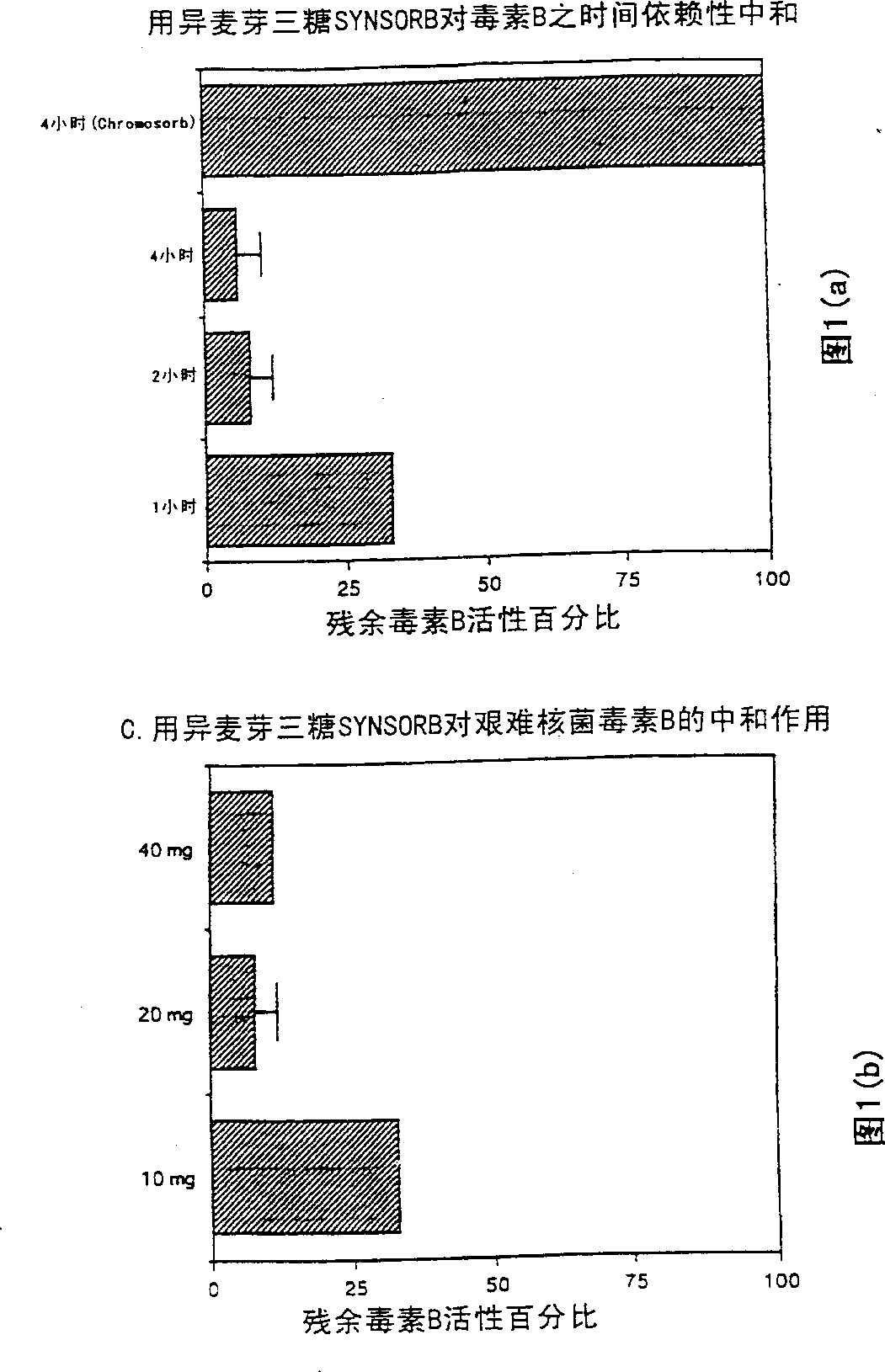
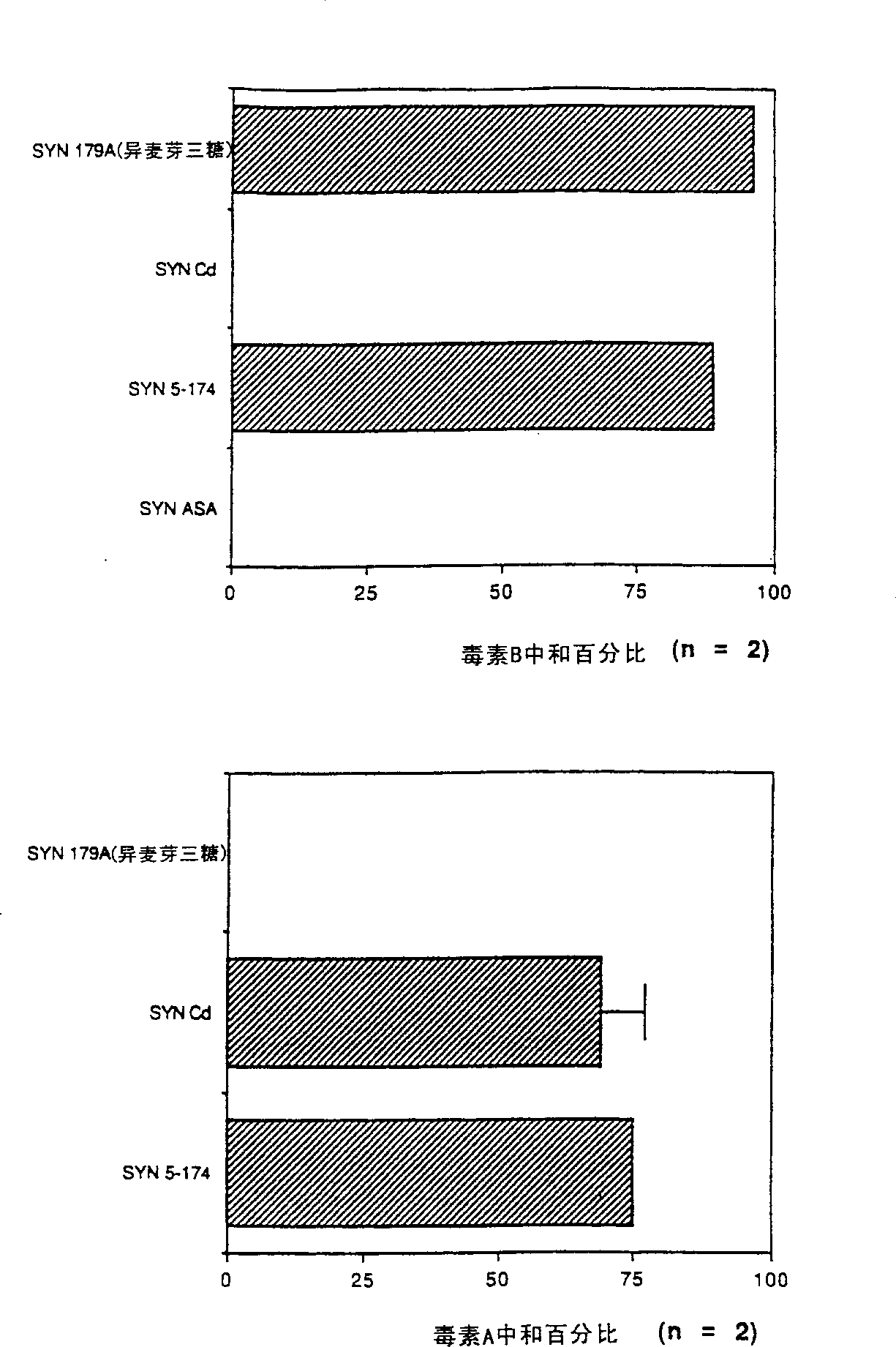
Treatment of i (C. Difficile) toxin B associated conditions
This invention relates to prevention and / or treatment of antibiotic associated diarrhea, including Clostridium difficile associated diarrhea (CDAD), pseudomembranous colitis (PMC) and other conditions associated with C. difficile infection, using oligosaccharide compositions which bind C. difficile toxin B, More specifically, the invention concerns neutralization of C. difficile toxin B associated with such conditions.
Owner:SYNSORB BIOTECH INC
Synbiotic compositions for restoration and reconstitution of gut microbiota
The present invention relates to synbiotic compositions comprising probiotic bacterial strains and prebiotic substances that, when combined exhibit synergistic behavior. The synergetic compositions will stimulate the indigenous microflora to restore and reconstitute in vivo gut like conditions after antibiotic associated diarrhea (AAD), and / or other gut infections caused by gastrointestinal pathogens, and relapses thereof, as well as the prevention of said disorders.
Owner:LAVIVO
Synbiotic compositions for restoration and reconstitution of gut microbiota
ActiveUS8927252B2Promote growthAntibacterial agentsBiocideAntibiotic-associated diarrhoeaIntestinal microorganisms
The present invention relates to synbiotic compositions comprising probiotic bacterial strains and prebiotic substances that, when combined exhibit synergistic behavior. The synergetic compositions will stimulate the indigenous microflora to restore and reconstitute in vivo gut like conditions after antibiotic associated diarrhea (AAD), and / or other gut infections caused by gastrointestinal pathogens, and relapses thereof, as well as the prevention of said disorders.
Owner:SYNBIOTICS CORP
Medicine for treating antibacterial drugs associated enteritis and preparation method thereof
ActiveCN101190299AClearing damp heatFunctionalDigestive systemPill deliveryAntibiotic-associated diarrhoeaGut flora
The invention discloses a medicine for treating antibiotic-associated diarrhea. The effective components contained in the invention are prepared by rhizoma coptidis, radix scutellariae, radix codonopsitis, rhizoma atractylodis alba, Indian buead, liquoric root, rhizoma alismatis and costus root according to a certain proportion by weight. The effective components can be prepared into any common oral formulation. The invention has the functions of clearing heat and dampness, reinforcing the spleen, replenishing qi, alleviating the symptoms of diarrhea and bellyache and facilitating the recovery of alteration of intestinal flora, and can treat the antibiotic-associated diarrhea or intestinal flora alterative enteritis. The invention further discloses the preparation method of the medicine.
Owner:BEIJING ZHONGYAN TONGRENTANG CHINESE MEDICINE R & D
Application of aucklandia-coptis pill extract in preparing drugs for treating intestinal flora alternation diseases
InactiveCN110237138AImprove diarrhea symptomsSymptoms improvedDigestive systemPlant ingredientsAlcoholIntestinal microorganisms
The invention relates to application of an aucklandia-coptis pill extract in preparing drugs for treating intestinal flora alternation diseases, especially for intestinal microflora alternation caused by antibiotics, and belongs to the field of application of traditional compound Chinese medicine preparations. Firstly, an ethyl alcohol extracting solution and residues are extracted by adopting ethyl alcohol refluxing from a raw aucklandia-coptis pill, then the residues are extracted with water to obtain an aqueous extracting solution, and finally, the ethyl alcohol extracting solution and the aqueous extracting solution are combined to obtain the aucklandia-coptis pill extract. The aucklandia-coptis pill extract as a drug for treating the intestinal flora alternation diseases has a good curative effect, and can significantly ameliorate the symptoms of antibiotic-associated diarrhea while significantly increasing intestinal beneficial microbiota and improving the intestinal microenvironment.
Owner:SOUTHWEST UNIVERSITY
Lactic Bacteria and Their Use in the Prevention of Diarrhea
InactiveUS20120107289A1Toxic preventionToxic treatmentAntibacterial agentsBiocideLactobacillusBacterial strain
The present invention concerns a lactic composition useful for the prevention or treatment of diarrhea such as antibiotic associated diarrhea or “tourista.” The composition according to the invention contains at least a bacterial strain selected from the group consisting of Lactobacillus acidophilus, Lactobacillus acidophilus I-1492,Lactobacillus casei and a mixture of thereof.
Owner:BIO K PLUS INT
Compositions and methods for treating clostridium infection and preventing recurrence of infection
ActiveUS20150133366A1High IgG levelReduce productionBiocideSaccharide peptide ingredientsAntibiotic-associated diarrhoeaRegimen
C. difficile infection (CDI) is the most common cause of antibiotic-associated diarrhea. Unfortunately, antibiotic therapy remains as the standard treatment for this antibiotic-induced disease and relapses are common. Antibiotic treatment typically is given for 10 to 14 days for initial or second episode of CDI. For recurrent episodes, more prolonged courses are recommended. It is disclosed herein that lower dose or shorter course of the antimicrobial treatment is sufficient to treat the disease and prevent recurrent disease by enabling a good immunologic response to infection, and perhaps also by better preserving normal flora, thus protecting against relapses or reinfection.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com