Application of fucosan sulfate with low molecular weight in prevention and treatment of enteritis
A fucoidan sulfate, low-molecular-weight technology is applied in medical preparations containing active ingredients, medical raw materials derived from algae, organic active ingredients, etc., and can solve the problems of caution and uncertainty in immunodeficiency patients.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Extraction of kelp, Ascophyllum nodosum and other brown algae fucoidan sulfate: Take kelp, Ascophyllum nodosum and other brown algae, dry them in an oven, and then pulverize them. The pulverized brown algae powder is sieved with 20 meshes, soaked in 95% ethanol for 24 hours, During this period, change the ethanol and continue to decolorize 2-3 times until the poured ethanol is colorless. After drying at room temperature, shake and extract in hot water at 85°C for 3 hours, where the ratio of solid to liquid is 1:30 (g / ml), centrifuge to remove the supernatant, add 30 times the volume of water to the residue, and repeat the extraction 3 times. The three extracts were combined, distilled under reduced pressure, and the crude extract was added to absolute ethanol until the ethanol concentration was 50%. After shaking evenly, it was left standing overnight at 4°C, and the precipitate (algin, molecular weight about 7000kDa) was removed by centrifugation. Keep the supernatant....
Embodiment 2
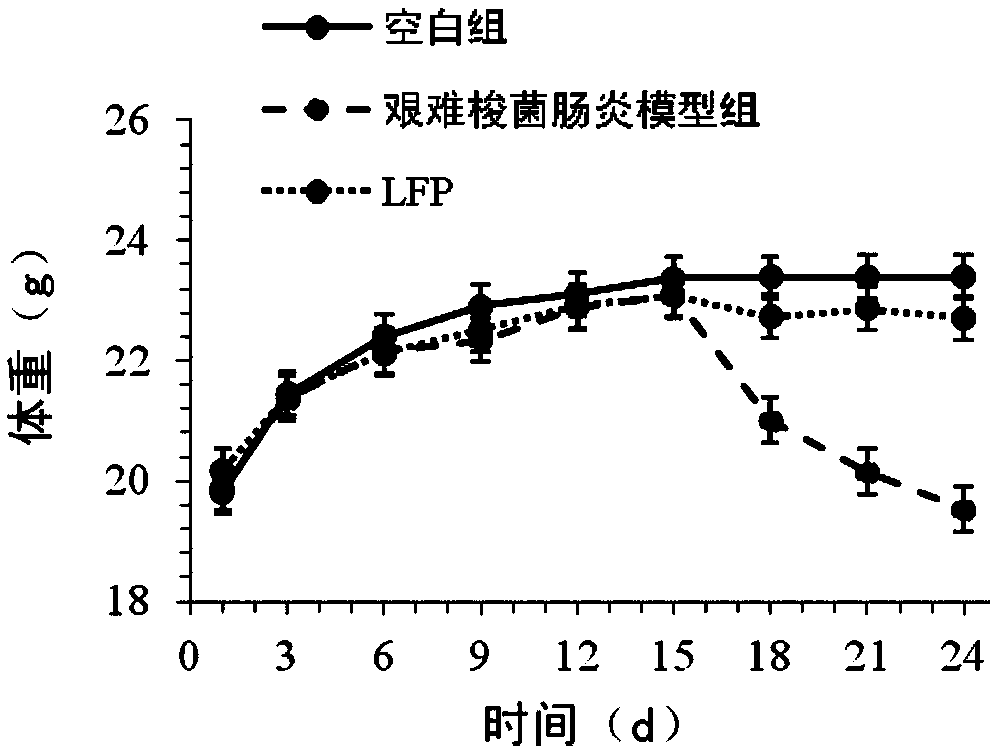
[0030] The preventive effect of the medium and low molecular weight fucoidan sulfate of the present invention on Clostridium difficile-associated intestinal infection and acquired diarrhea will be described below through specific animal experiments.
[0031] 1. Experimental conditions: third-level animal laboratory, temperature: 18-22 degrees Celsius, humidity: air conditioning.
[0032] 2. Experimental objects: 30 clean male BABL / c mice, weighing 19-20 grams, provided by Jinan Pengyue Experimental Animal Breeding Co., Ltd.
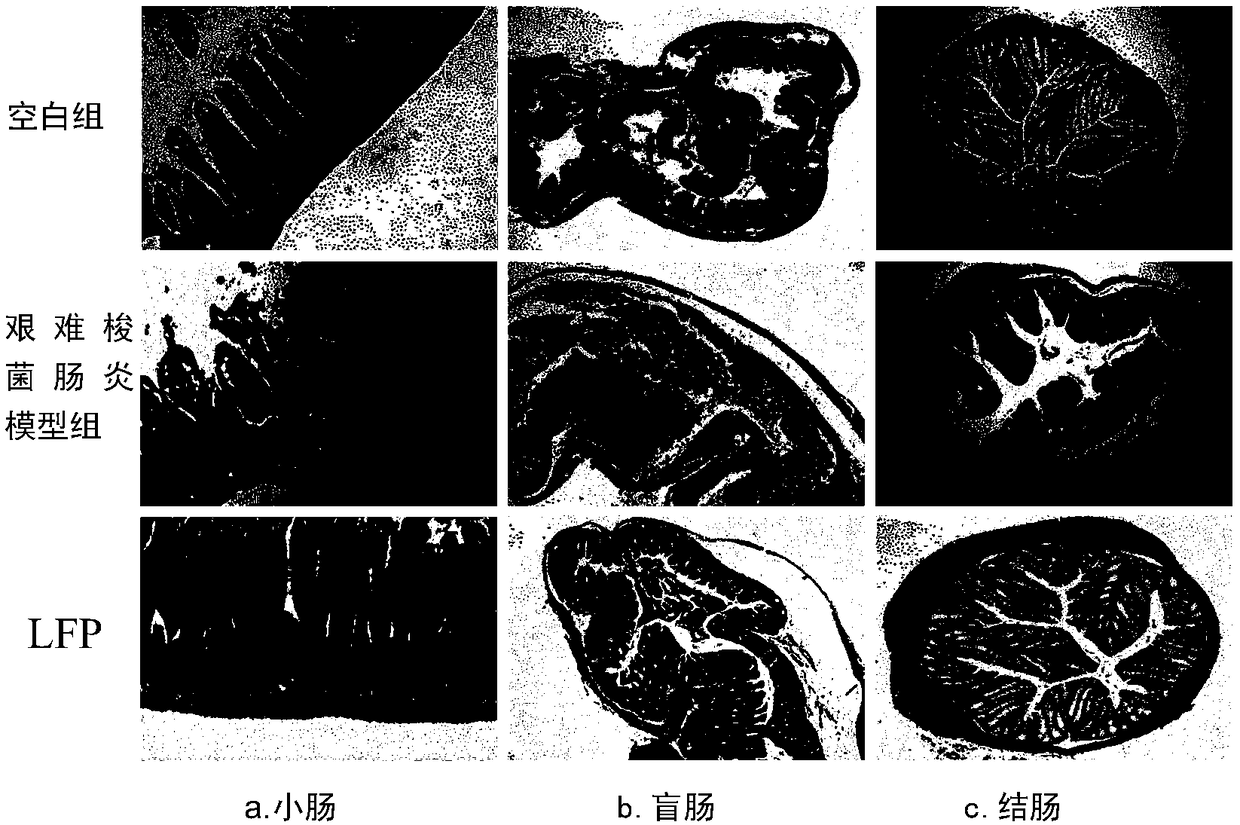
[0033] 3. Experimental method: the mice were randomly divided into three groups: blank group, Clostridium difficile enteritis model group, and low molecular weight fucoidan sulfate prevention group (LFP), with 10 experimental mice in each group. The blank group was given normal saline intragastrically from day 1 to day 24. On days 1-12, the Clostridium difficile enteritis model group was administered with normal saline, and the low molecular weight fucoi...
Embodiment 3
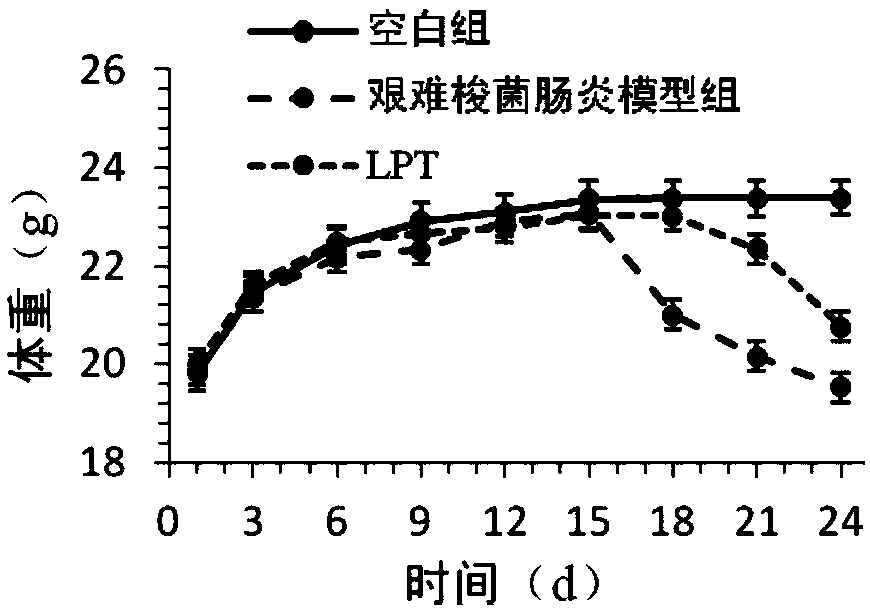
[0056] The following specific animal experiments illustrate the therapeutic effect of the medium and low molecular weight fucoidan sulfate of the present invention on Clostridium difficile-associated intestinal infection and acquired diarrhea.
[0057] 1. Experimental conditions: third-level animal laboratory, temperature: 18-22 degrees Celsius, humidity: air conditioning.
[0058] 2. Experimental objects: 30 clean male BABL / c mice, weighing 19-20 grams, provided by Jinan Pengyue Experimental Animal Breeding Co., Ltd.
[0059] 3. Experimental method: the mice were randomly divided into three groups: blank group, Clostridium difficile enteritis model group, and low molecular weight fucoidan sulfate treatment group (LFT), with 10 experimental mice in each group. On days 1-12, all animals were given normal saline. On the 13th-14th day, except the blank group, all animals were given 0.2mL mixed antibiotics (kanamycin 0.4mg / mL, gentamicin 0.035mg / mL, colistin 850U / mL, metronidazol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com