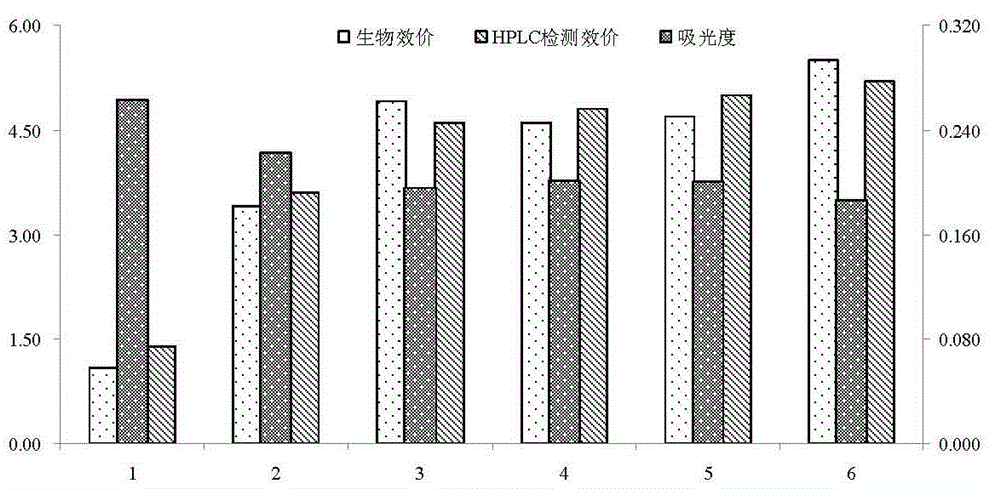
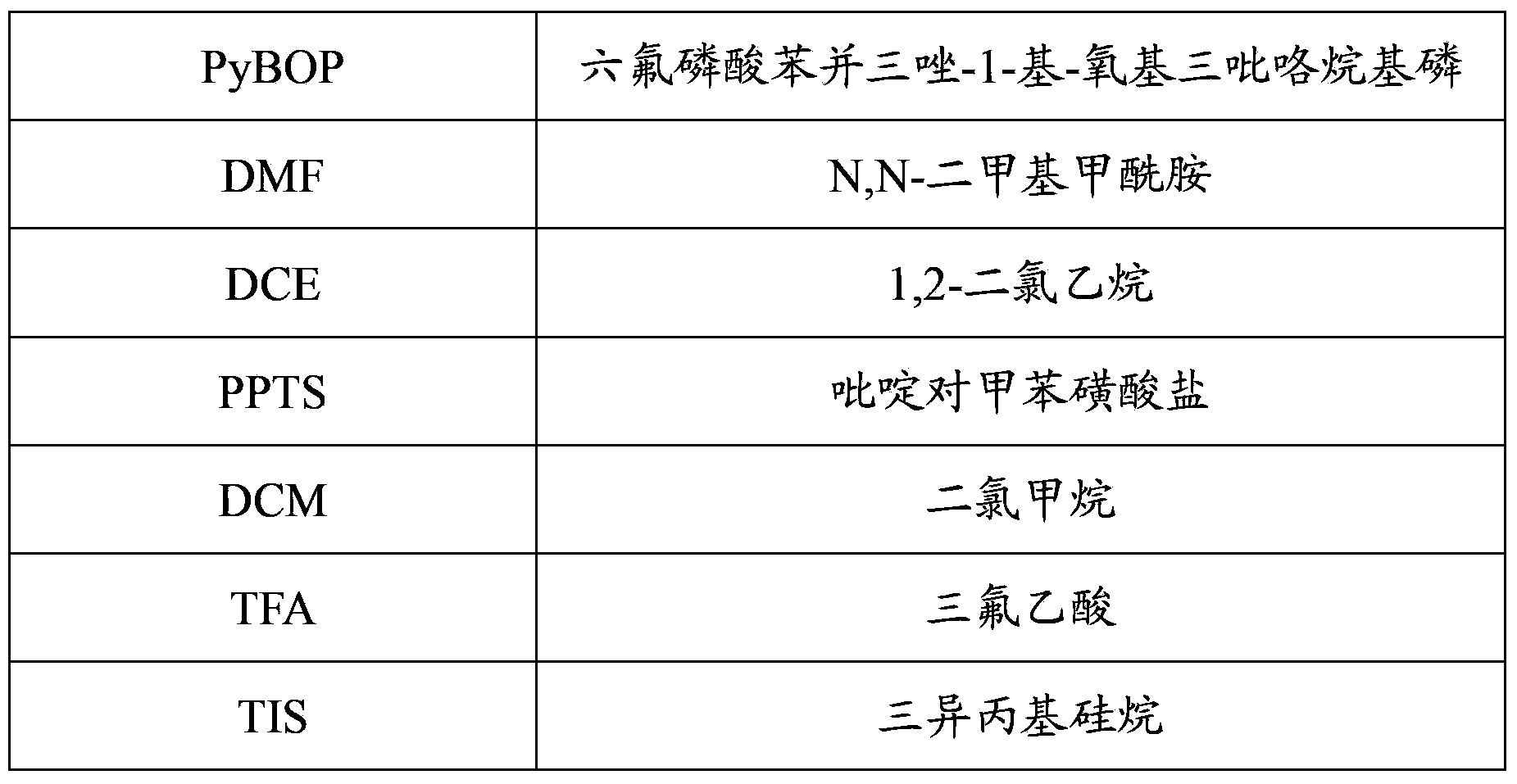
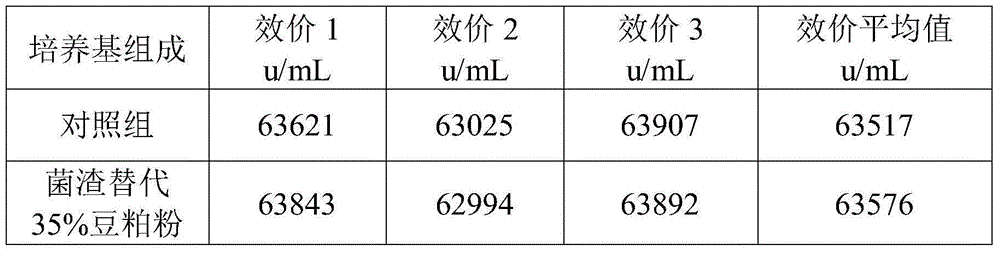
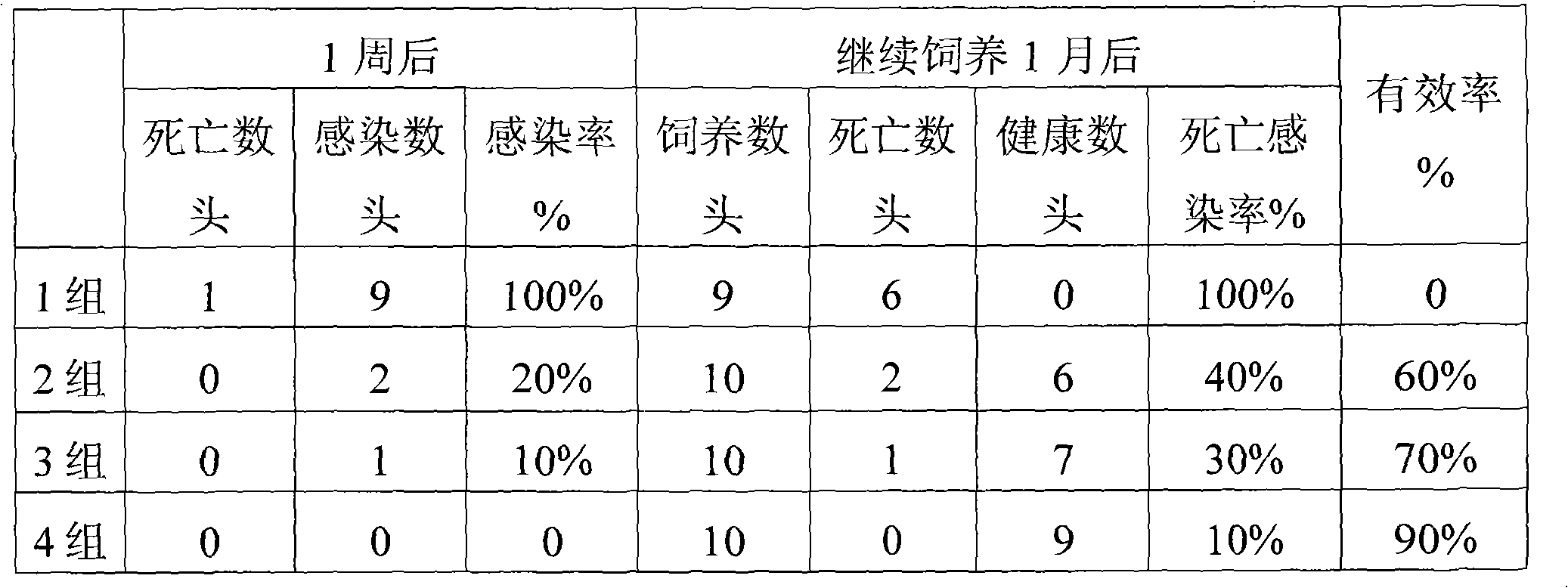
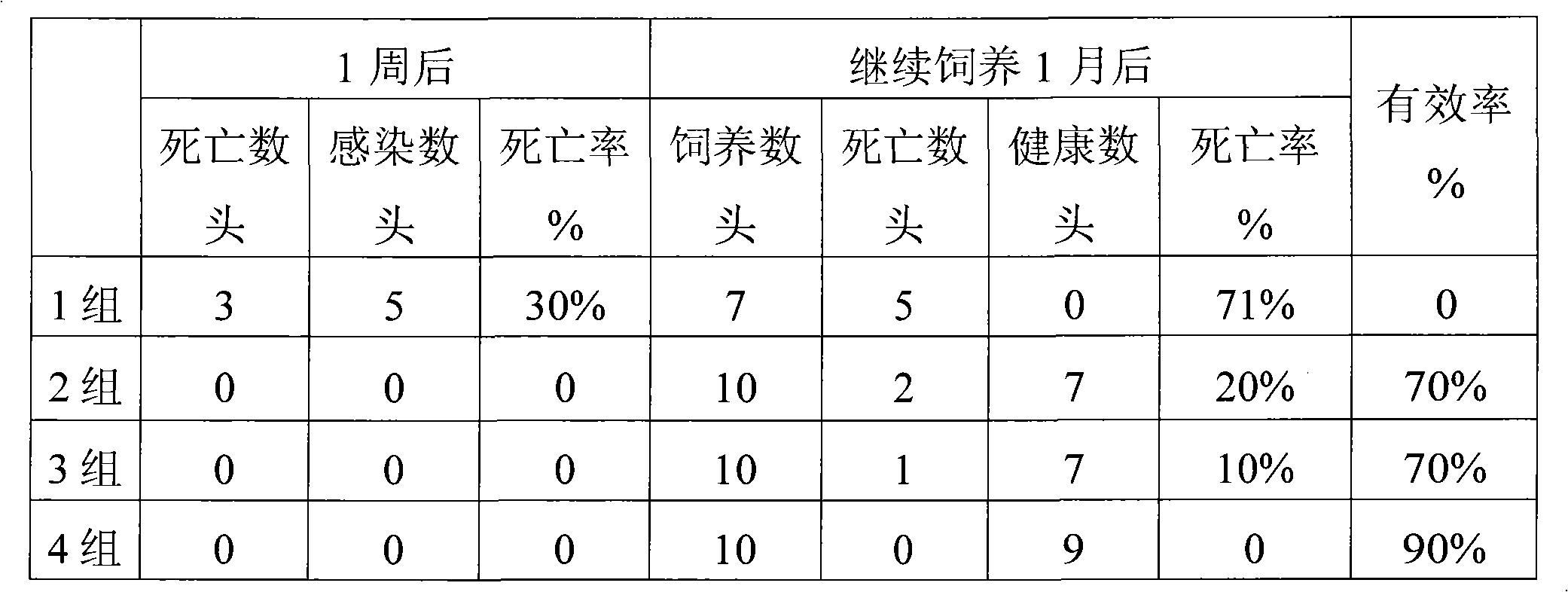
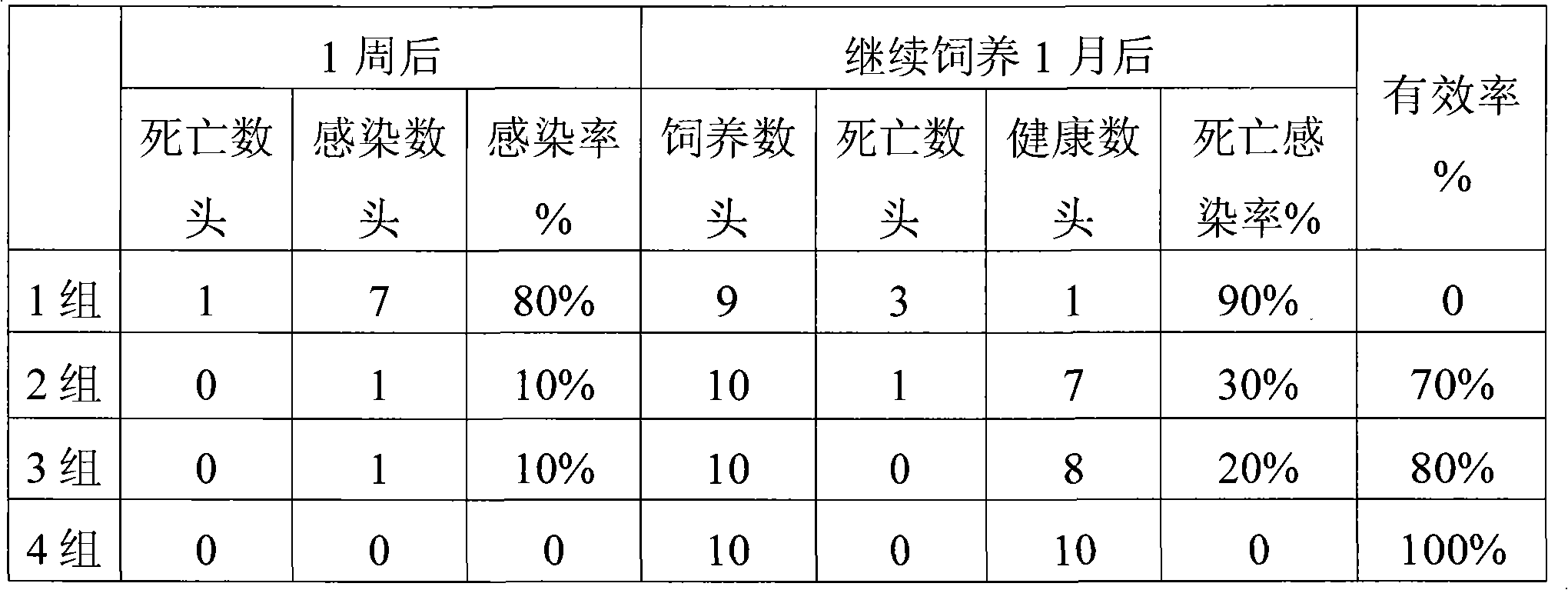
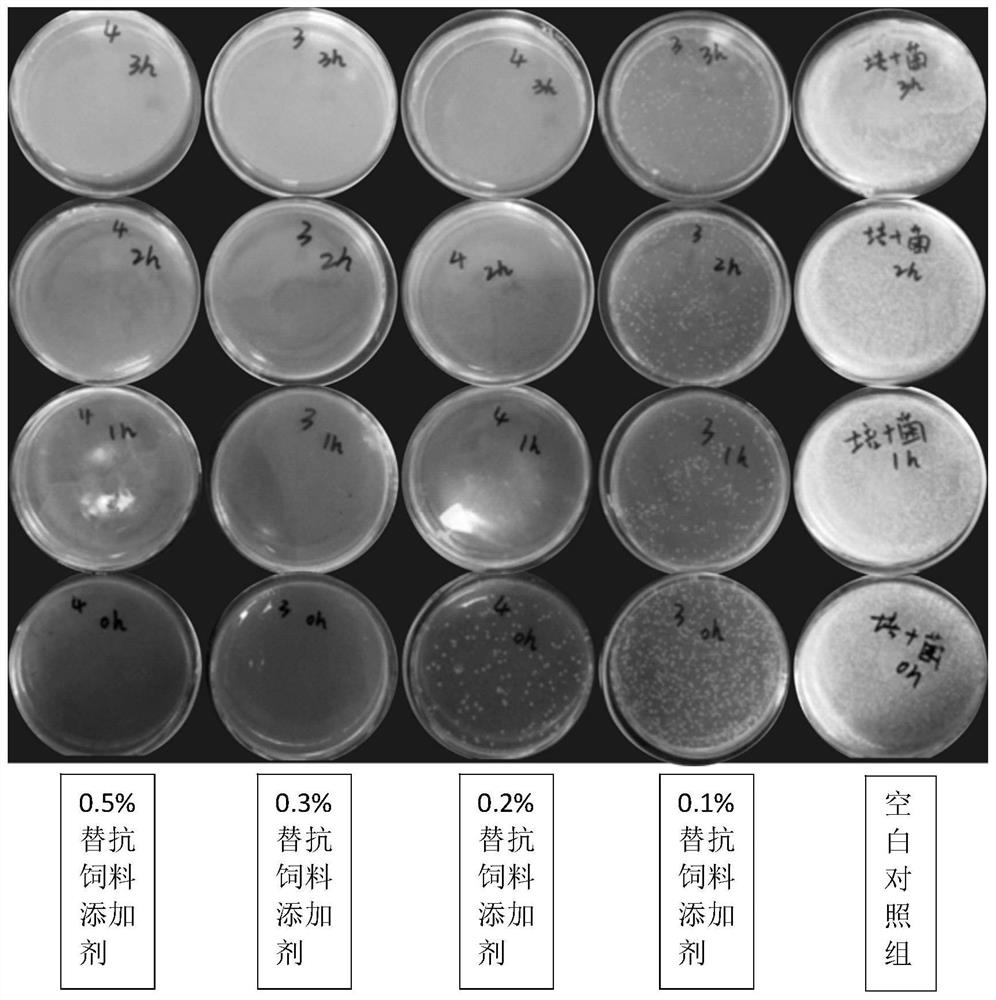
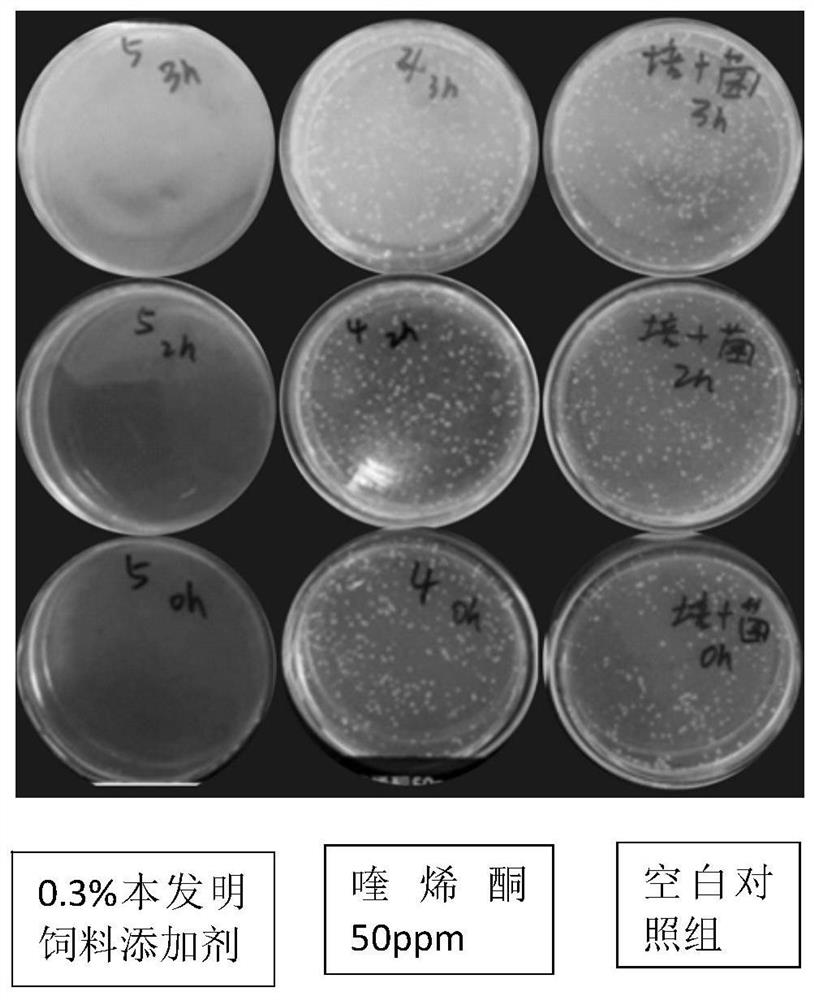
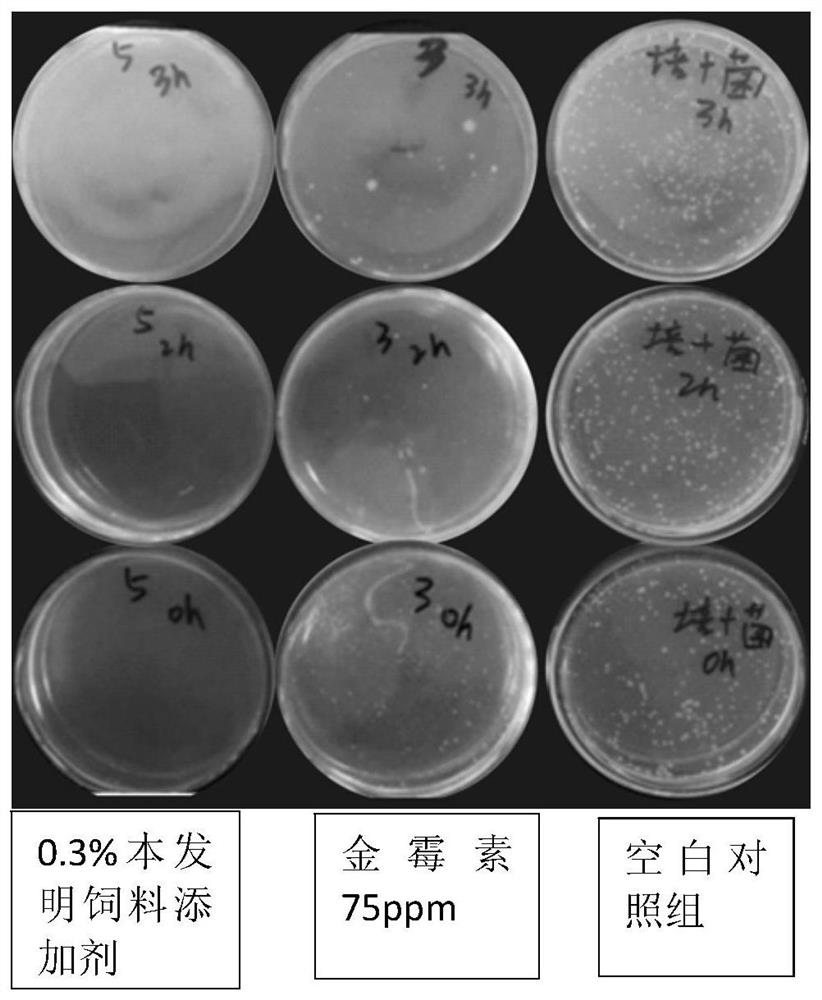
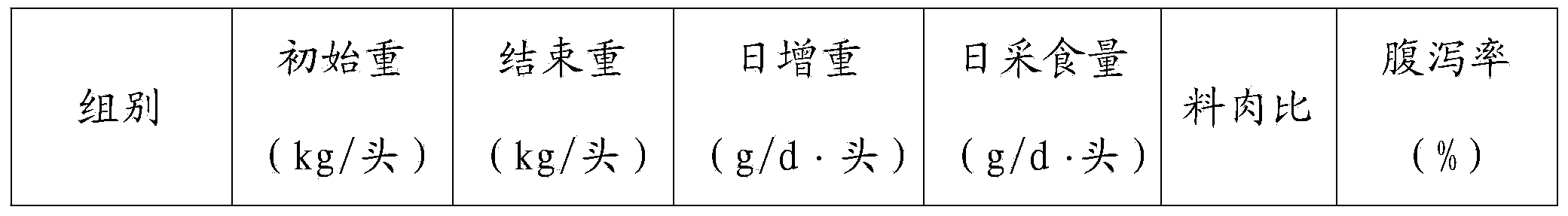
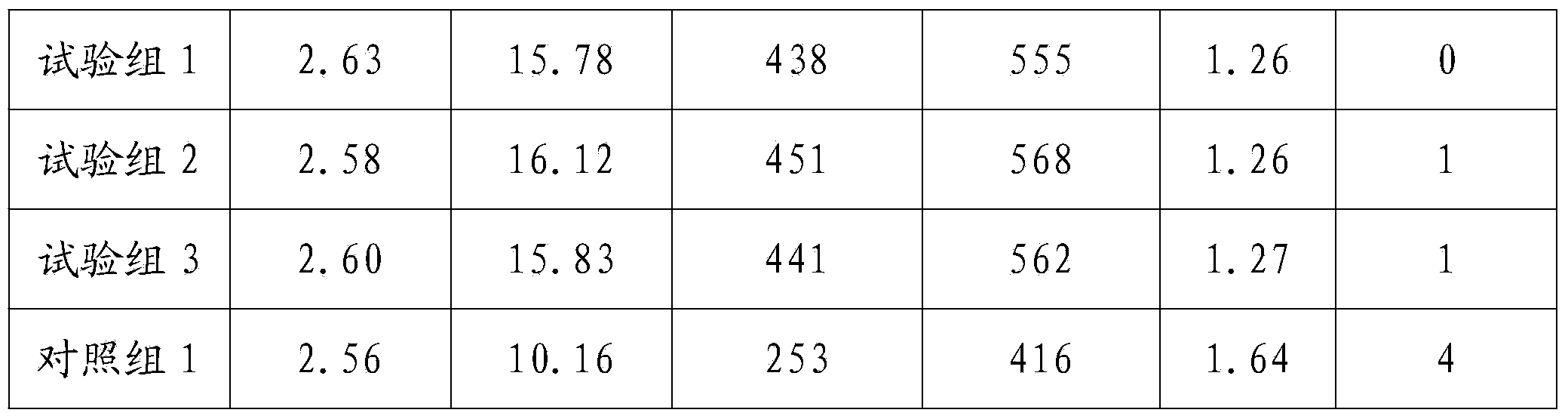
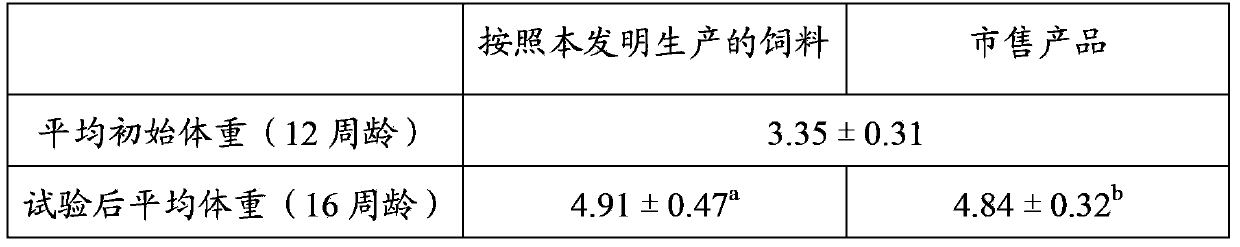
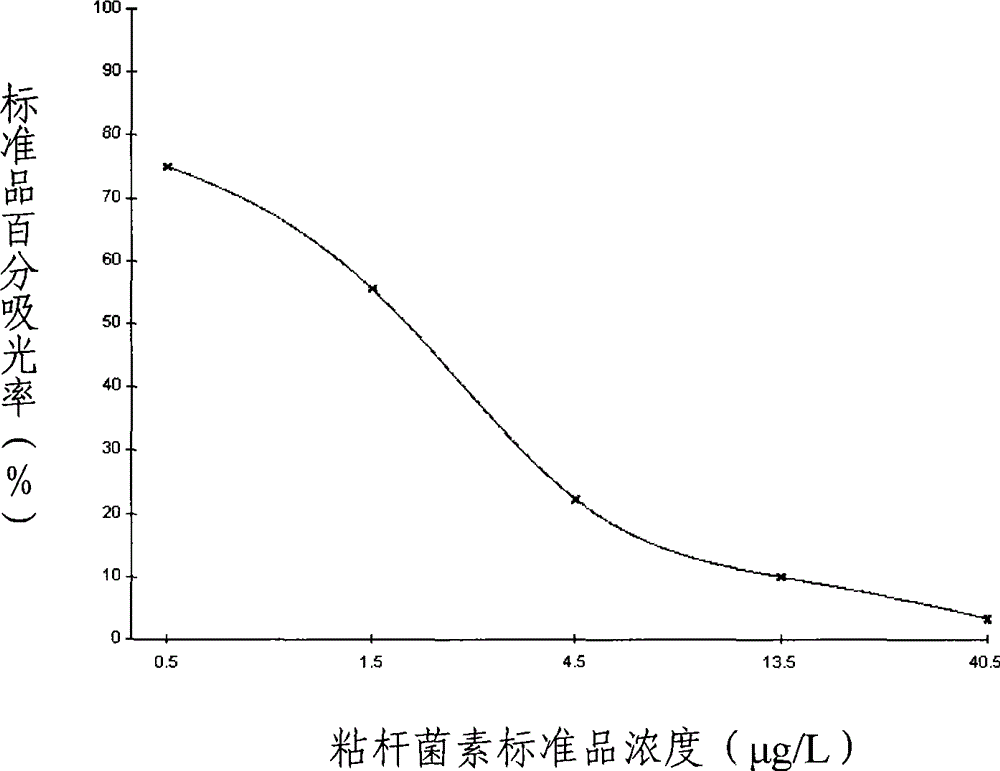
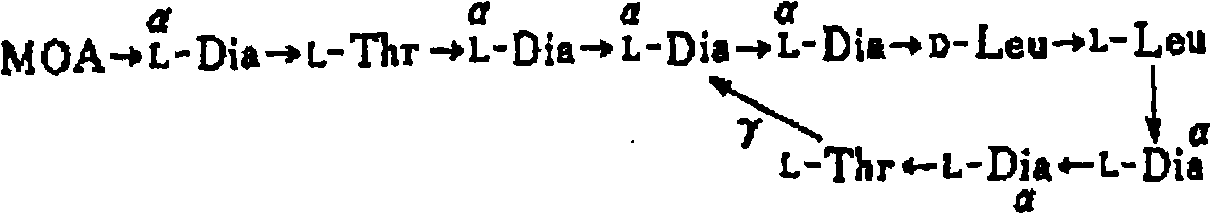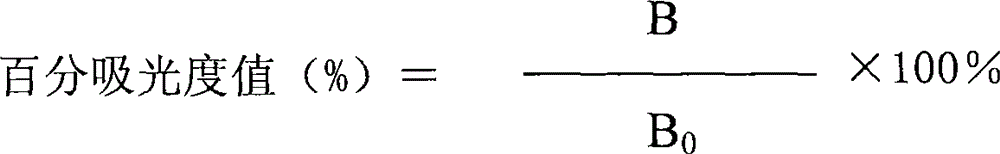Patents
Literature
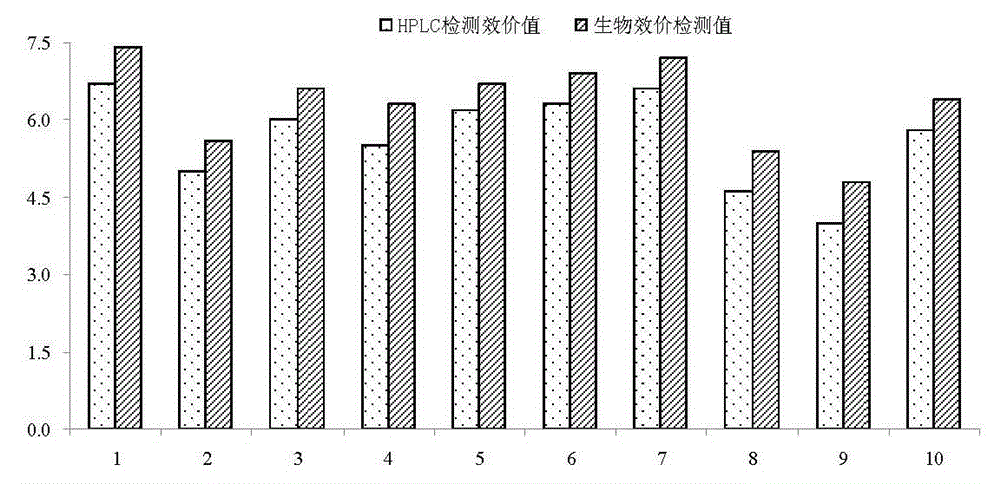
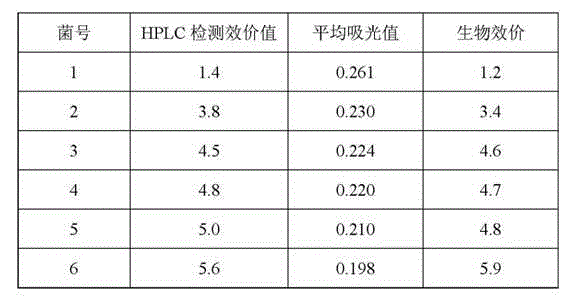
65 results about "Colistin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Colistin, also known as polymyxin E, is an antibiotic produced by certain strains of the bacteria Paenibacillus polymyxa. Colistin is a mixture of the cyclic polypeptides colistin A and B and belongs to the class of polypeptide antibiotics known as polymyxins. Colistin is effective against most Gram-negative bacilli.
Method for detecting residual quantity of multiple polypeptidepolypeptide veterinary drugs in animal-derived food
InactiveCN102236005AAchieving Simultaneous DetectionReduce dissolutionComponent separationVirginiamycinVeterinary Drugs
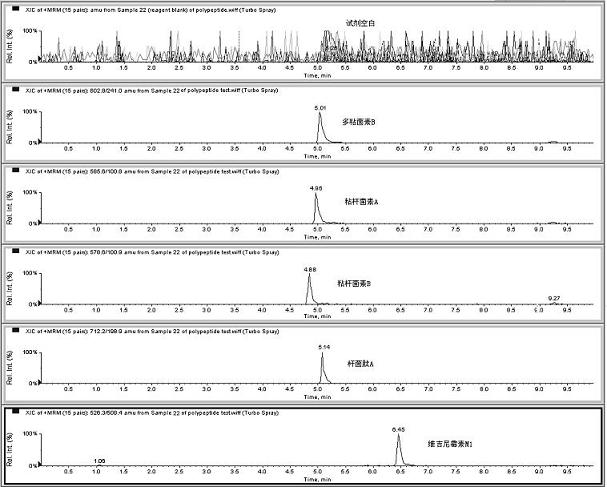
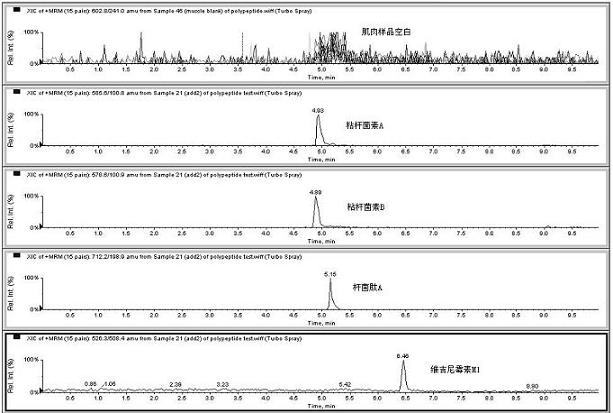
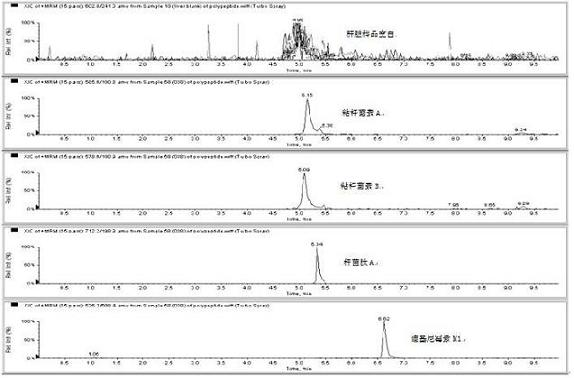
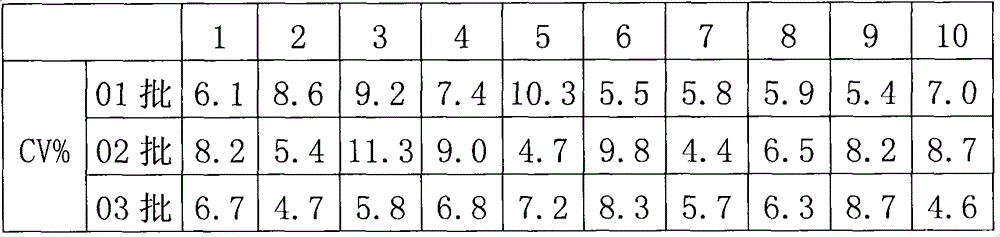
The invention relates to the fields of analytical chemistry and food safety, particular to a method for detecting the residual quantity of multiple polypeptide veterinary drugs in animal-derived foods. The method comprises the following steps of: processing a sample with a TCA(trichloroacetic acid) and acetonitrile system for depositing proteins; extracting with a carbinol and 0.1% formic acid aqueous solution system; purifying with a Oasis HLB solid phase extraction column; performing gradient elution with an Eclipse XDB-C18 analytical column in the presence of an acetonitrile and 0.1% formic acid aqueous solution used as a mobile phase; and then performing electrospray and positive ion scanning mode separation for finally detecting four polypeptides. The limits of the four polypeptides, namely colistin, bacitracin A, polymyxin B and virginiamycin M, are 25 micrograms / kilogram, 100 micrograms / kilogram, 250 micrograms / kilogram and 120 micrograms / kilogram respectively; the recovery rates of colistin, bacitracin A, polymyxin B and virginiamycin M are 74.9-88.1%, 76.2-89.0%, 76.6-81.2% and 77.3-86.9% respectively; and the coefficients of variation (CV%) of colistin, bacitracin A, polymyxin B and virginiamycin M are 5.7-15.1%, 7.2-15.7%, 6.0-8.0% and 9.5-18.6% respectively. The detection limit, the recovery rate, the accuracy and other technical indexes all meet related detection requirements at home and aboard.
Owner:林维宣
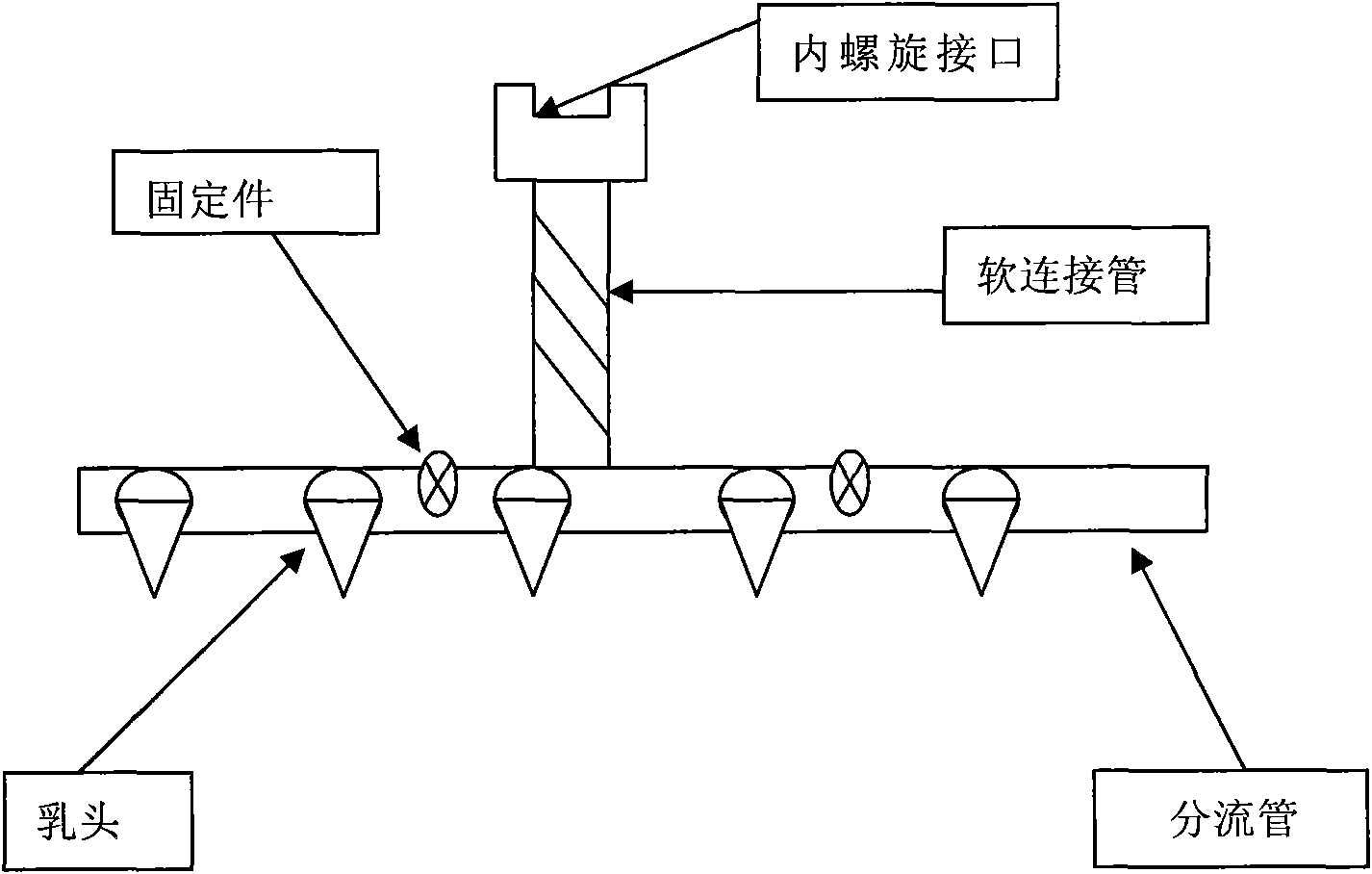
Liquid artificial milk feed, production method and feeding device
ActiveCN101984842AImprove palatabilityMeet the needs of rapid early growth and developmentAnimal feeding devicesAnimal feeding stuffBiotechnologyAnimal science
The invention relates to a liquid artificial milk feed, a production method and a feeding device, and the liquid artificial milk feed is characterized in that the formula comprises the following raw materials by weight percent: 4-6% of soy protein concentrate, 4-7% of beef extract, 1-3% of starch, 9-11% of whey powder, 4-7% of pure milk, 0.4-0.6% of calcium hydrogen phosphate, 0.2-0.4% of calciumcarbonate, 0.1-0.2% of common salt, 0.02-0.04% of compound vitamin, 0.1-0.3% of compound trace element, 0.01-0.03% of colistin, 0.01-0.02% of kitasamycin, 0.04-0.06% of bacillus subtilis and the balance of drinking water. The liquid artificial milk feed has the advantage of retarding a series of inadaptations of piglets in the aspects of emergency and a digestive system caused by direct transition from sow milk to powder material during delectation of the piglets. The product can not only meet the nutritional requirements for physiology, growth and development of the piglets, but also realize the scientific and safe transition from the liquid sow milk to the powder material.
Owner:沈阳科瑞思科技有限公司
Colistin colloidal gold detection kit and application thereof
The invention discloses a colistin colloidal gold detection kit. The kit mainly comprises a colistin colloidal gold detection test strip, a lyophilized colloidal gold marked colistin specific monoclonal antibody microporous reagent, a microporous panel, a quantitative pipette and a sample diluting liquid, wherein the detection test strip is formed by a PVC back board, a water absorption pad, a sample pad, and a nitrocellulose film (NC film) which is sprayed with a detected antigen coated detection line (T line) and a goat-anti-mouse-anti antibody coated quality control line (C line). The kit is used for detecting colistin residue in eggs or milk without expensive equipment, is simple and convenient to operate, does not need special training, has high detection sensitivity, and is suitablefor wide application in the fields of local laboratories, field detection and the like.
Owner:北京纳百生物科技有限公司
Screening method of colistin strains
InactiveCN103060227ASpeed up screeningImprove screening efficiencyBacteriaMicrobiological testing/measurementIndicator bacteriaCulture mediums
The invention discloses a screening method of colistin strains. The screening method comprises the following steps of: adding Bacilluscolistinus into a solid culture medium containing L-asparaginic acid, transferring a single colony to an inclined plane for preservation after the single colony is mature, simultaneously inoculating into a fermentation culture medium, culturing till maturation, then centrifuging, taking supernatant liquid, adding into a bacterial solution of indicator bacteria for culture to prepare a detected sample, detecting the absorbance of the detected sample, and selecting the strains with low absorbance for preservation and later use. According to the method provided by the invention, the screening speed of polymyxin strains is greatly increased; and the screening period is shortened from original 3-4 weeks to one week, and the screening efficiency is improved by above 70%.
Owner:HEBEI SHENGXUE DACHENG PHARMA
Antibodies for detection of colistin-resistance
ActiveUS20190322727A1High of confidenceHigh degree of sensitivityImmunoglobulins against bacteriaPolymyxinsMonoclonal antibodyColistin resistance
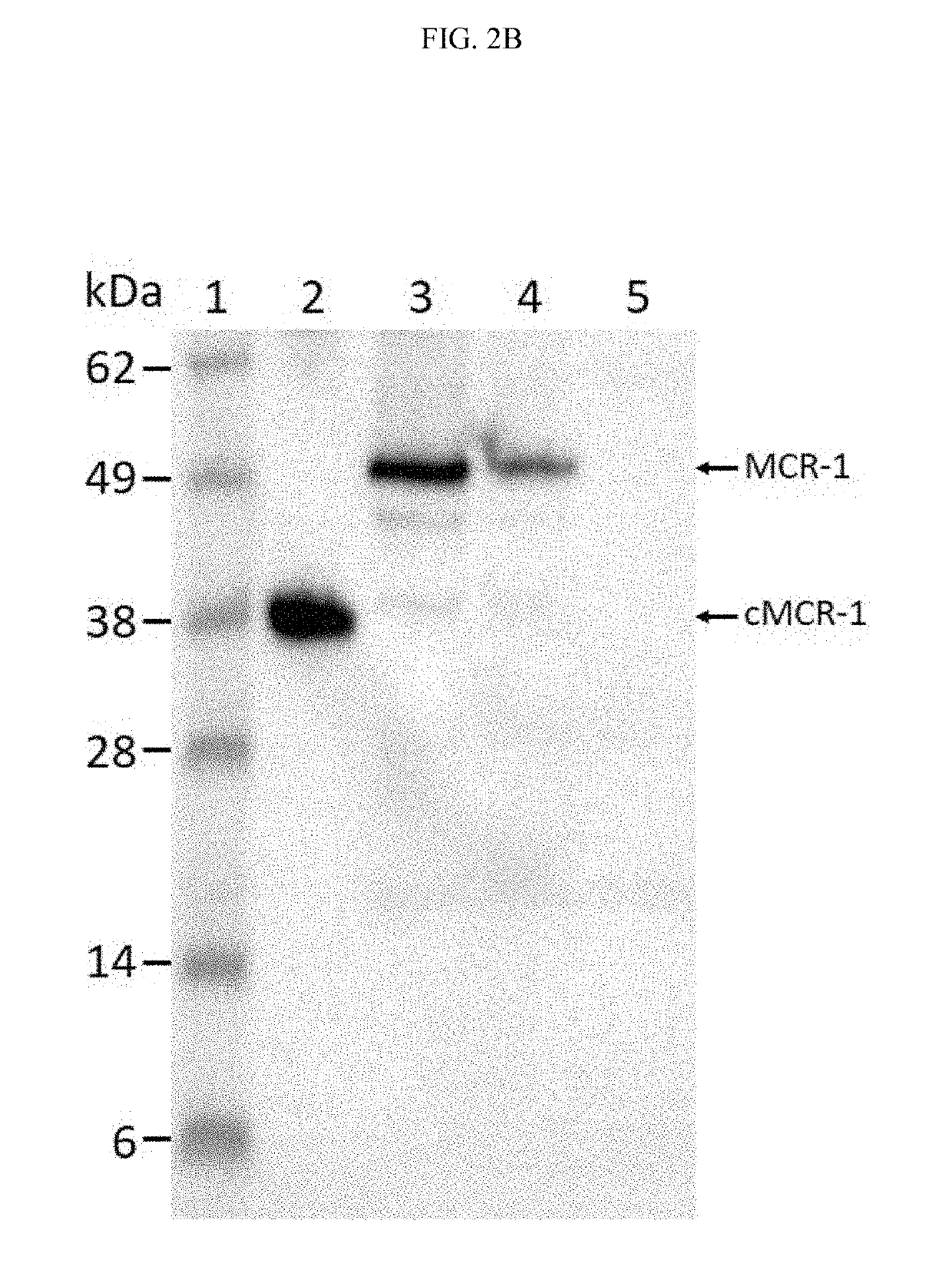
Monoclonal antibodies against bacterial proteins that confer resistance to the antibiotic colistin and hybridomas that produce such monoclonal antibodies are described. The monoclonal antibodies have a strong affinity for MCR proteins and may be used in detection methods and kits to detect the presence of MCR proteins including variants thereof in a sample.
Owner:UNITED STATES OF AMERICA
Preparation method for compound tilmicosin enteric-coated granules
ActiveCN104586875AIncrease payEffective infectionAntibacterial agentsOrganic active ingredientsOral glucoseDisease
The invention belongs to the technical field of veterinary medicines, and specifically relates to a preparation method for compound tilmicosin enteric-coated granules. The preparation method sequentially comprises the steps of: preparing materials, mixing the materials, granulating, preparing a coating solution, coating and the like, wherein the raw materials of the granules are tilmicosin, colistin, gatifloxacin, a sweetening agent, a flavouring agent, bromhexine hydrochloride, oral glucose, corn starch, saccharose powder, dextrin, talcum powder and starch slurry; the raw materials of the coating solution are HPMC, Tween-80, polyethylene glycol 6000, talcum powder and ethanol. The method disclosed by the invention enables the tilmicosin effective ingredient not to be broken in gastric acid, and to safely arrive at intestinal tracts to be absorbed; the obtained compound tilmicosin enteric-coated granules are capable of preventing and treating respiratory diseases such as mycoplasma diseases, pleuropneumonia and swine plague, capable of reducing the occurrences of bacterial diseases such as diarrhoea and salmonellosis, and capable of increasing the feed conversion.
Owner:ZHENGZHOU DOURIN VETERINARY TECH
Oil-in-water type compound colistin nanoemulsion
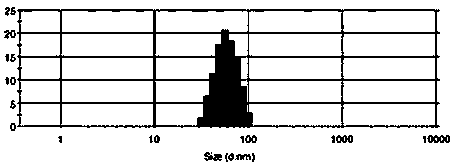
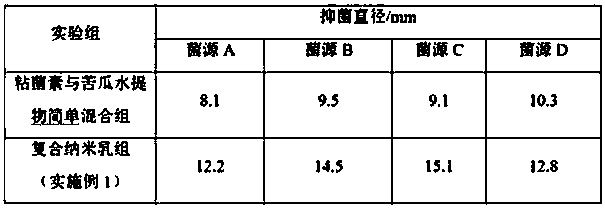
ActiveCN104274826ANarrow particle size distributionSystem transparencyAntibacterial agentsCyclic peptide ingredientsBitter gourdActive agent
The invention relates to an oil-in-water type compound colistin nanoemulsion and belongs to the technical field of medicines. The oil-in-water type compound colistin nanoemulsion comprises the following components in parts by weight: 1-18 parts of bitter gourd aqueous extract, 15-40 parts of surfactant, 0-20 parts of cosurfactant, 1-20 parts of colistin, 1-20 parts of oil and 20-70 parts of water. The compound colistin nanoemulsion is narrow in particle size distribution, transparent in system, good in stability, relatively low in surface tension, good in liquidity and easy to take, and the nanoemulsion preparation is used for improving dissolvability and permeability of colistin, so that bioavailability of the medicine is improved; a bitter gourd aqueous extract and the colistin are combined, so that the antibacterial activities of the bitter gourd aqueous extract and the colistin are mutually promoted and enhanced, and the antibacterial effect is more stable.
Owner:HENAN SOAR VETERINARY PHARMA
Culture medium supplementary method for producing colomycin by fermentation of panebacillus polymyxa
The invention relates to a culture medium and supplementary method for producing colomycin by fermentation of panebacillus polymyxa. By arranging a formula of a seed medium and a fermentation medium which are suitable for producing colomycin by the fermentation of panebacillus polymyxa, and a fermentation supplementary process, fermentation unit of colomycin is increased; a fermentation period is shortened; fermentation cost is reduced; influences of the source of raw materials and auxiliary materials on fermentation titer is reduced at the maximum degree; the source of the raw materials and auxiliary materials is not influenced by an environment; sufficient supply is guaranteed; and stable and efficient production of colomycin can be realized.
Owner:宁夏泰瑞制药股份有限公司
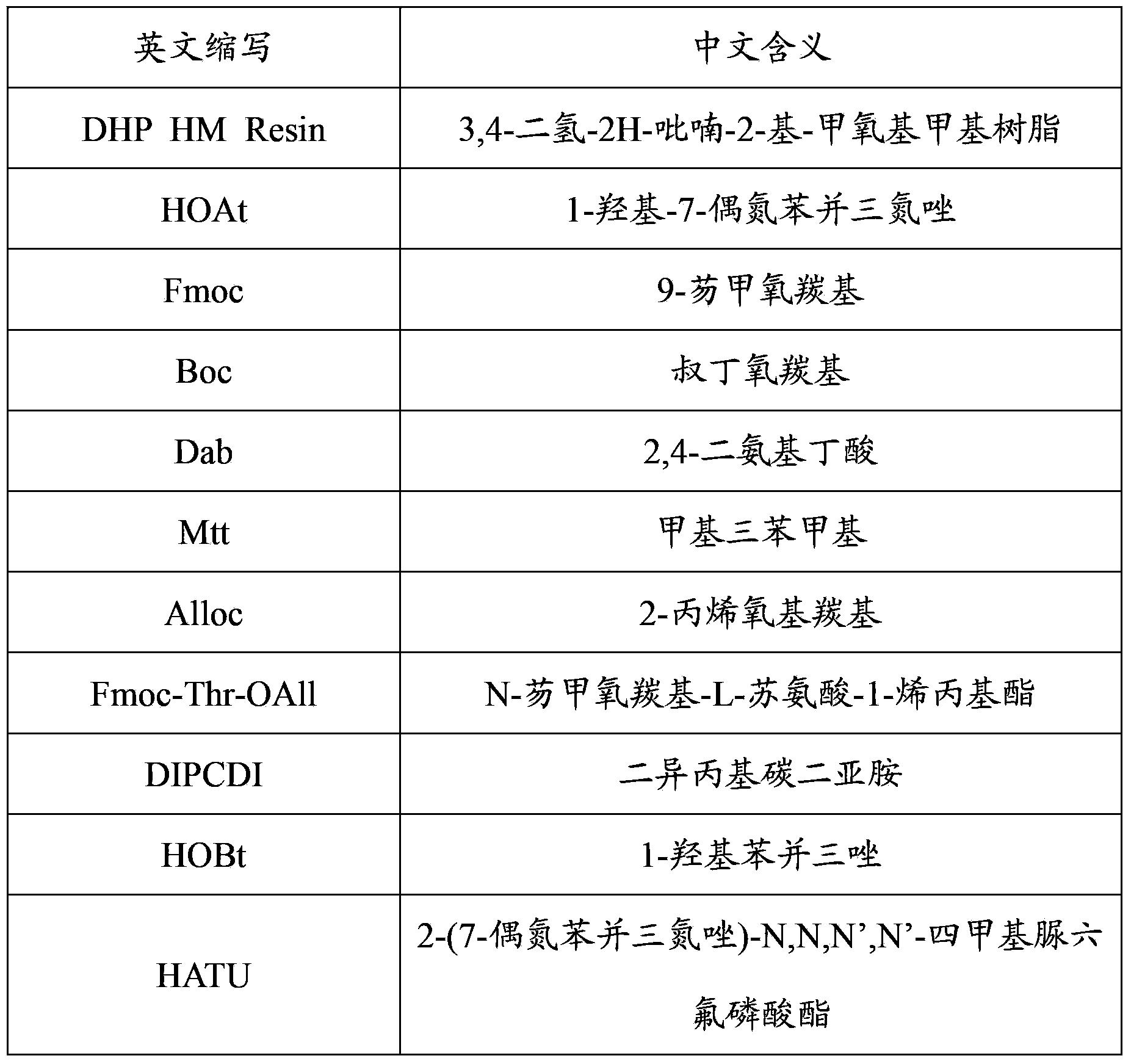
Pure-solid-phase synthesis method of polypeptide antibiotic Colistin
InactiveCN103396475ASimplify the synthesis processSimple stepsPolymyxinsPeptide preparation methodsDrugs synthesisSolvent
The invention belongs to the technical field of medicine synthesis, and discloses a method of pure-solid-phase synthesis of polypeptide antibiotic Colistin. By use of the method, the synthetic process and procedure of the Colistin are simplified, the synthesis time is shortened, and the use amount of solvents is reduced.
Owner:HYBIO PHARMA
Oral administration composition of colistin
InactiveCN105617353APromote absorptionLower doseAntibacterial agentsPowder deliveryOral medicationNeurotoxicity
The invention discloses an oral administration composition of colistin and belongs to the technical field of medicines. The oral administration composition of the colistin is prepared from the following components in parts by weight: 1 part of colistin, 0.01 to 10 parts of absorption promoter and 0 to 100 parts of pharmaceutically acceptable auxiliaries and excipient. In addition, antibacterial drugs are added into a formula, and the addition amount of the antibacterial drugs is one third of therapeutically effective amount. According to the composition, the absorbance of the colistin is greatly improved, the possibility of oral administration treatment for systemic bacterial infection by the colistin is realized; the oral administration composition can replace an injection, so the prejudice of the prior art is overcome. Besides, when the composition contains other antibacterial drugs, a good synergic effect in vivo is realized, and when two thirds of dose of the colistin is reduced, an ideal bacterial infection resisting effect can be still maintained, so that the renal toxicity and the neurotoxicity of the colistin are greatly reduced.
Owner:李志海 +1
Circulation harmlessness treatment method of colistin fermentation bacterial residue
InactiveCN106434800AChange physical propertiesEmission reductionMicroorganism based processesFermentationMeal powderNitrogen source
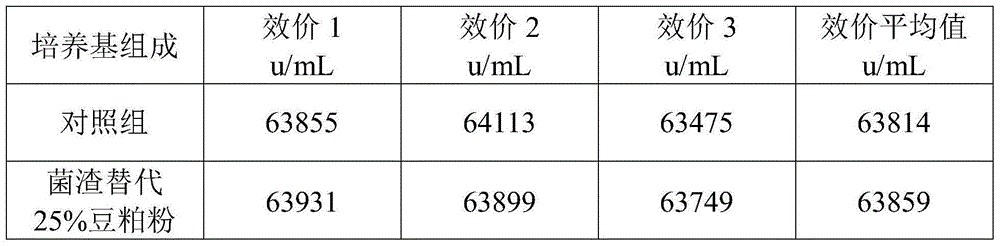
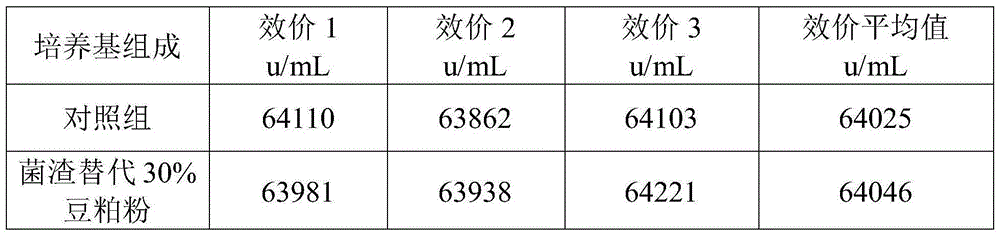
The present invention discloses a circulation harmlessness treatment method of colistin fermentation bacterial residue, and particularly relates to the field of antibiotic fermentation bacterial residue circulation utilization. The circulation harmlessness treatment method comprises: a, adding the mixture of an organic nitrogen source and an organic carbon source to colistin fermentation bacterial residue, uniformly mixing, and pressing to obtain a bacterial residue premix; b, carrying out solid-state fermentation on the bacterial residue premix in the step a; c, drying the bacterial residue solid-state fermentation product in the step b at a temperature of 85-95 DEG C, crushing, and screening with a 60 mesh sieve to obtain bacterial residue powder; and d, replacing 25-45% of bean meal powder in a colistin fermentation culture medium by using the bacterial residue powder obtained in the step c so as to recycle. According to the present invention, the circulation utilization of the colistin fermentation bacterial residue is achieved through the bacterial residue modification method, such that the partial raw material is saved, the difficult problem that the colistin residue is not easily treated is solved, and the advantages of simple and feasible process, low cost and no influence on the colistin fermentation effect and the like are provided.
Owner:HEBEI SHENGXUE DACHENG PHARMA
Pig feed
InactiveCN106107143AGain weight fastImprove palatabilityFood processingAnimal feeding stuffAnimal scienceAntioxidant
The invention provides a pig feed, and relates to the technical field of agricultural breeding. The pig feed is prepared from the following raw material components by a ratio: 35-40% of fermented material, 15-20% of dried mulberry leaf powder, 15-20% of dried potato vine powder, 15-20% of corn flour, 10-15% of rice bran, 2-3% of table salt, 1.5-2% of citric acid, 0.05-0.1% of colistin and 0.01-0.05% of a mouldproof antioxidant. The pig feed is good in palatability and rapid in weight increment of pigs, and the pork of the pigs fed with the pig feed is environmental-friendly and high in protein content.
Owner:滁州朝昱农业科技有限公司
Virosis vaccine oral liquid for livestock, poultry and marine lives animal and preparation thereof
InactiveCN101264318AImprove the body's immunityImprove disease resistanceAntiviralsUnknown materialsSide effectAquatic animal
The invention relates to a composition and the preparation method for livestock, poultry and aquatic animals. The virus disease vaccine oral liquid comprises the following materials according to the weight account: loosestrife 12 to 25 parts, humifuse euphorbia 15 to 25 parts, tuber of dwarf lilyturf 10 to 20 parts, phyllanfhus 5 to 20 parts, bitter almond 10 to 25 parts, prunella vulgaris 15 to 25 parts, balloonflower 10 to 20 parts, snake gall 2 to 5 parts, ox gall 10 to 20 parts, aloe 40 to 60 parts, crystal sugar 25 to 40 parts, rimantadine 5 to 10 parts, ribavirin 10 to 15 parts, and colistin 10 to 20 parts. The composition has the advantages of ability to reinforce antibody immunity, to relieve mutual interference among vaccines, and to reinforce the immune effect of the vaccine, and convenient drinking water and administration, high absorptivity, wide distribution, no drug residue or poison or side effect, and wide safety range.
Owner:雷清莲 +1
Feed additive replacing antibiotics and preparation method of feed additive
PendingCN111903849AImprove stabilityImprove palatabilityAnimal feeding stuffAccessory food factorsEscherichia coliBenzoic acid
The invention discloses a feed additive capable of replacing antibiotics. The feed additive comprises plant essential oil, medium-chain fatty acid, organic acid and a carrier, the medium-chain fatty acid is fatty acid which can be melted at a relatively high temperature and form a solid at a normal temperature so as to form an enveloped film of plant essential oil droplets, the carrier is a porousinorganic carrier which can be used in an animal feed, and the droplets of plant essential oil can be accommodated in holes of the carrier; and the organic acid comprises benzoic acid and / or fumaric.According to the invention, through a cold spraying or hot spraying processing technology, the essential oil and the medium-chain fatty acid coating are fused together, so that the stability of the plant essential oil is improved, and the palatability of the feed additive is also improved. The feed additive disclosed by the invention can completely replace conventional feed medicine additives including quinocetone, chlorotetracycline and colistin, and can achieve the purpose of inhibiting and killing escherichia coli.
Owner:广州市正百饲料科技有限公司
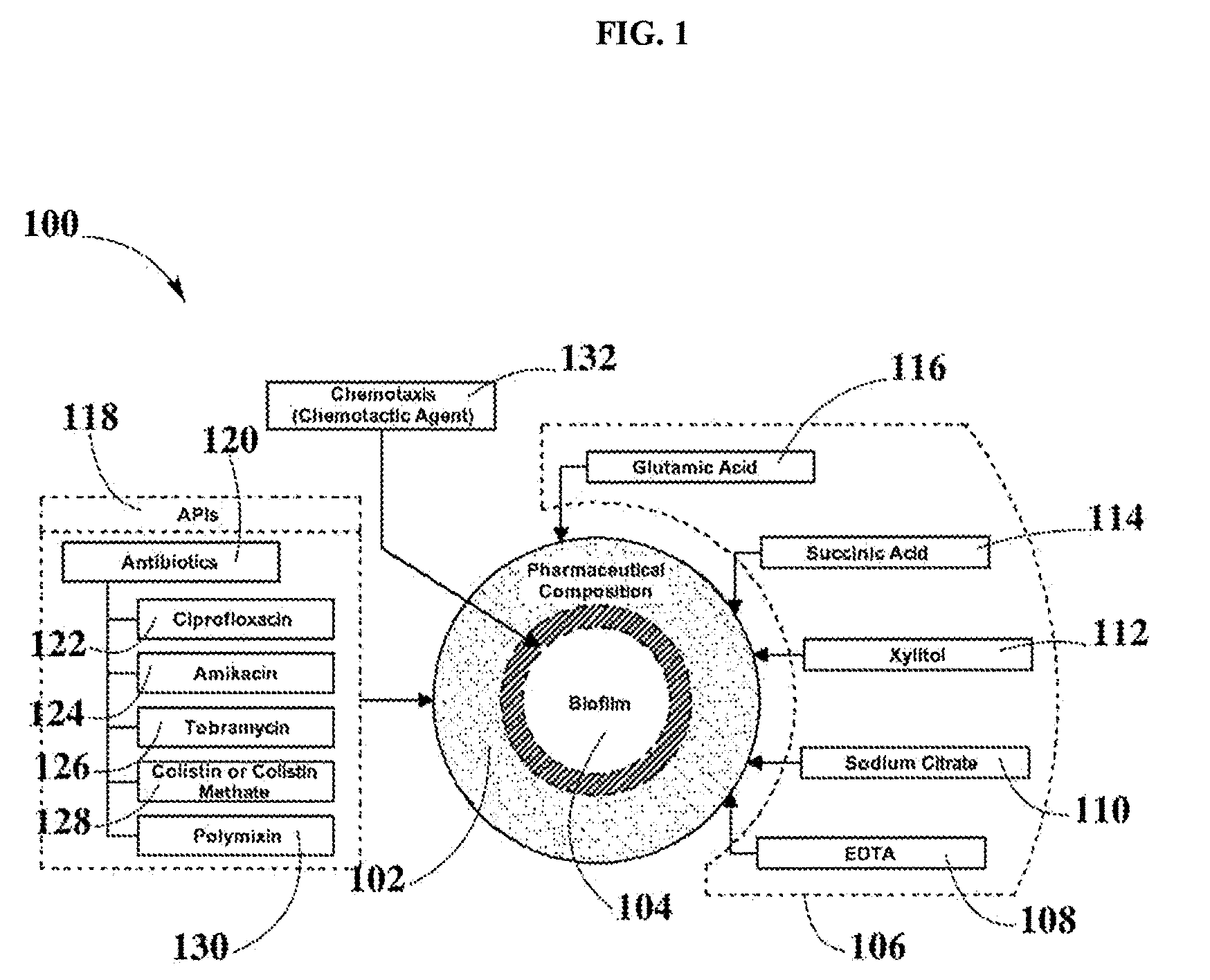
Antibiotic composition comprising a chemotactic agent and a nutrient dispersion
ActiveUS20160279191A1Improve antibiotic activityOrganic active ingredientsTripeptide ingredientsBacteroidesEscherichia coli
Compositions and methods for treating infectious diseases produced by biofilms are disclosed. More specifically, the present disclosure refers to a pharmaceutical composition which may be used for treating biofilm infections, specifically, biofilms formed by bacteria such as Pseudomonas, E. coli, Klebsiella, and other human pathogens. Pharmaceutical compositions may include a nutrient dispersion that can include sodium citrate, succinic acid, xylitol, glutamic acid, and ethylenediaminetetraacetic acid (EDTA), among others. Additionally disclosed pharmaceutical composition may include API such as antibiotics. Subsequently, the antibiotics agent may be ciprofloxacin, amikacin, tobramycin, colistin methate, or polymixin, among others. Pharmaceutical compositions disclosed may employ chemotactic agents in order to disrupt biofilms and therefore enhance the antibiotic response. Pharmaceutical compositions disclosed may include suitable vehicles which may depend on the dosage form.
Owner:PROFESSIONAL COMPOUNDING CENTS OF AMERICA PCCA
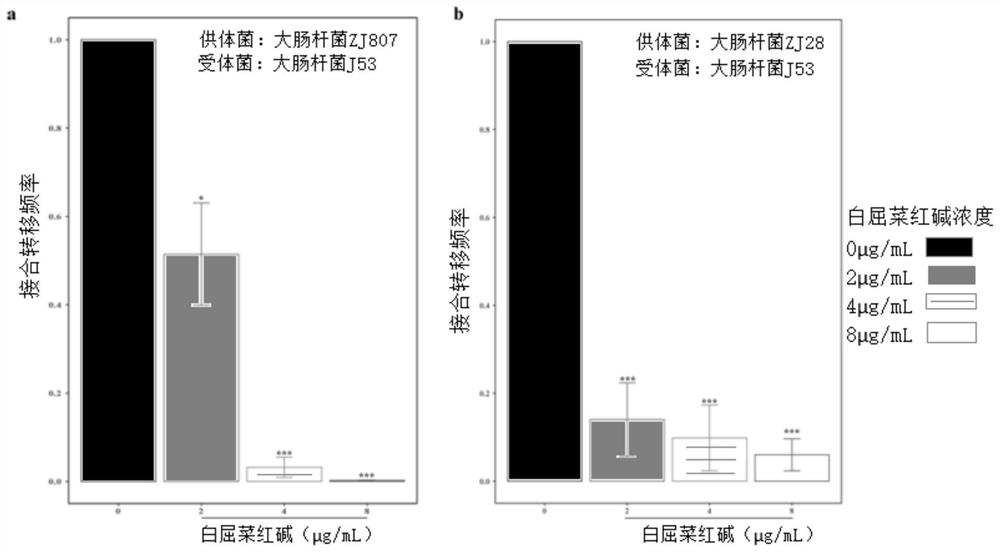
Application of chelerythrine in inhibition of colistin drug resistance gene transfer
PendingCN114774449AInhibit transferInhibited DiffusionBacteriaMicroorganism based processesBiotechnologyEscherichia coli
The invention discloses an application of chelerythrine in inhibition of colistin drug resistance gene transfer. A conjugational transfer test proves that chelerythrine can obviously inhibit the transfer of plasmids carrying a colistin drug-resistant gene mcr-1 between escherichia coli. Therefore, the chelerythrine can inhibit propagation of colistin drug-resistant plasmids, provides a new thought and source for control of colistin drug resistance, and has wide application value in the fields of medicine, food, veterinary public health and the like.
Owner:CHINA AGRI UNIV
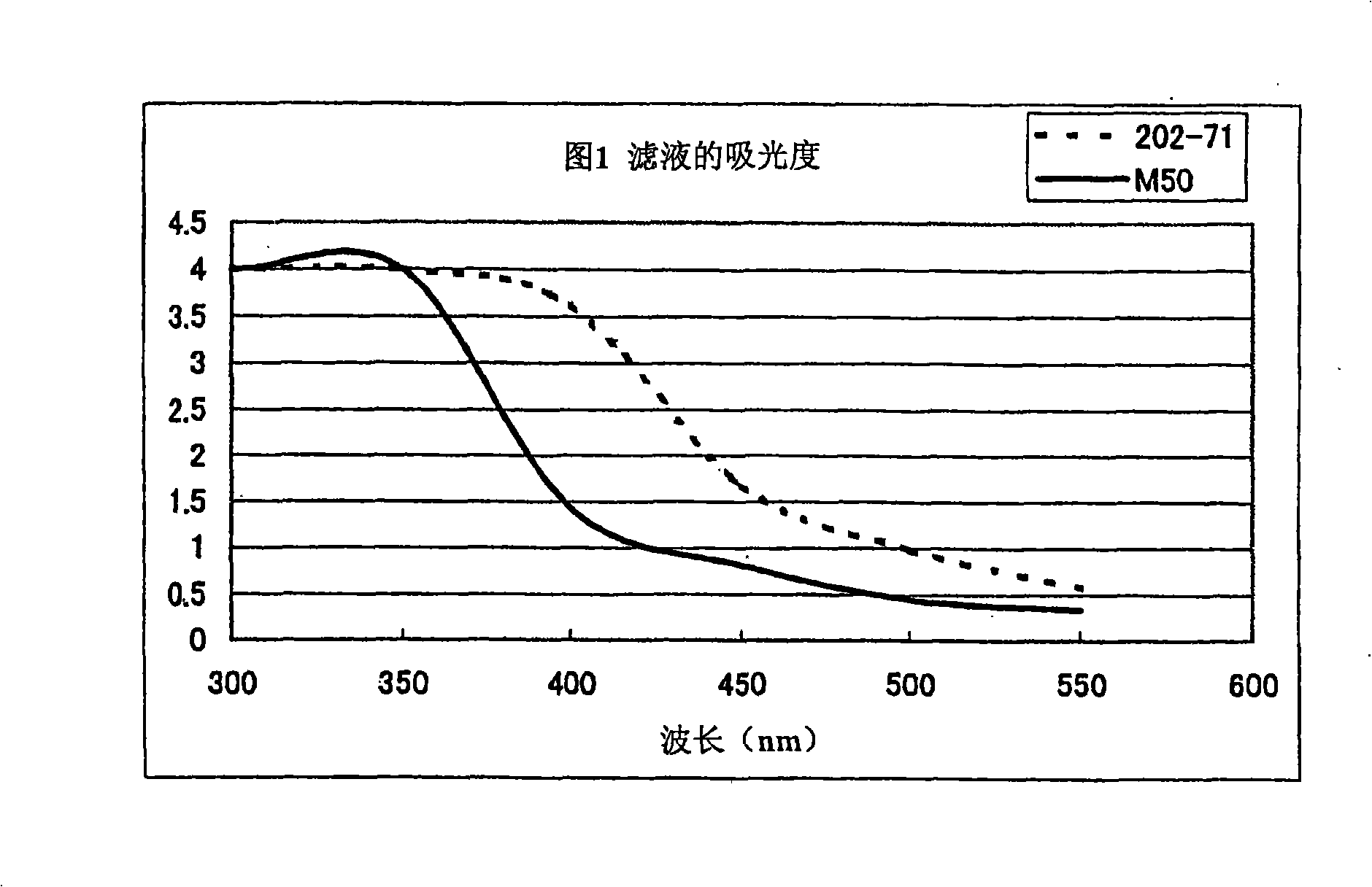
Production method of colistin rough material powder
It is intended to provide a method of producing a colistin bulk powder without resorting to a decolorizing step whereby problems occurring in the existing production methods (for example, a prolonged production time due to the decolorizing step, an increase in the cost due to a lowering in the yield of the main colistin component and an environmental burden caused by the evolution of a carbon waste) can be overcome. Namely, Bacillus polymyxa var. colistinus which is capable of producing a colistin bulk powder having a high whiteness; a colistin bulk powder having a high whiteness; and a method of producing a colistin bulk powder having a high whiteness.
Owner:核芯医药(山东)有限公司
Feed for suckling pigs
InactiveCN104222627AFast absorptionHigh biological potencyFood processingAnimal feeding stuffNutritive valuesAntioxidant
The invention discloses a feed for suckling pigs, and relates to the technical field of animal feed. The feed for the suckling pigs is prepared by mixing a base feed and a feed additive, wherein the weight ratio of the base feed to the feed additive is (8-10) to (1-1.5); the feed additive comprises composite multivitamin for suckling pigs, composite multi-minerals for pigs, plant polysaccharide, zinc oxide, lysine, corn flour, baking soda, choline, colistin, ferrous fumarate, an acidifying agent, copper sulfate, fenbendazole, a sweetening agent, an antioxidant, vitamin C, aureomycin, a mould inhibitor and a carrier; the base feed comprises corn, bean pulp, a mixture of rice chaff and husk, cured corn, cured soybeans and fish meal. The feed for the suckling pigs is comprehensive in nutritive value, the palatability of the feed is improved, the feed utilization rate is high, the problems that the suckling pigs are poor in body temperature adjustment capability, are lack of innate immunity, have underdeveloped digestive organs, are not perfect enough in development of cerebral cortex when being born, and the like can be solved, the immunity of the suckling pigs is improved, and the disease resistance of the suckling pigs can be improved.
Owner:GUANGXI FULAIKANG FEED
Feed for blue foxes in the growing period as well as preparation method and application of feed
The invention discloses a feed for blue foxes in the growing period as well as a preparation method and application of the feed. According to the invention, the nutrition requirements of the blue foxes in the growing period are analyzed comprehensively, on the basis of nutrient collocation, sodium humate, aureomycin, colistin and other active biological substances are used to scientifically and reasonably prepare a mixed feed capable of satisfying the nutrition requirements of the blue foxes in the growing period. The feed comprises the following raw materials: swelled maize meal, bean pulp, meat and bone meal, feather meal, blood meal, high-fat DDGS, the sodium humate, stone powder, corn germ meal, lard, phospholipid oil, dairy salt, compound polymineral, methionine, lysine, compound vitamin, choline chloride, the aureomycin and the colistin. Experimental results show that compared with a conventional product, the feed provided by the invention has the advantages of enhancing the immunity of the blue foxes, increasing the conversion rate, preventing the blue foxes from diarrhea, lowering the feeding cost of the blue foxes, and the like.
Owner:NORTHEAST FORESTRY UNIVERSITY
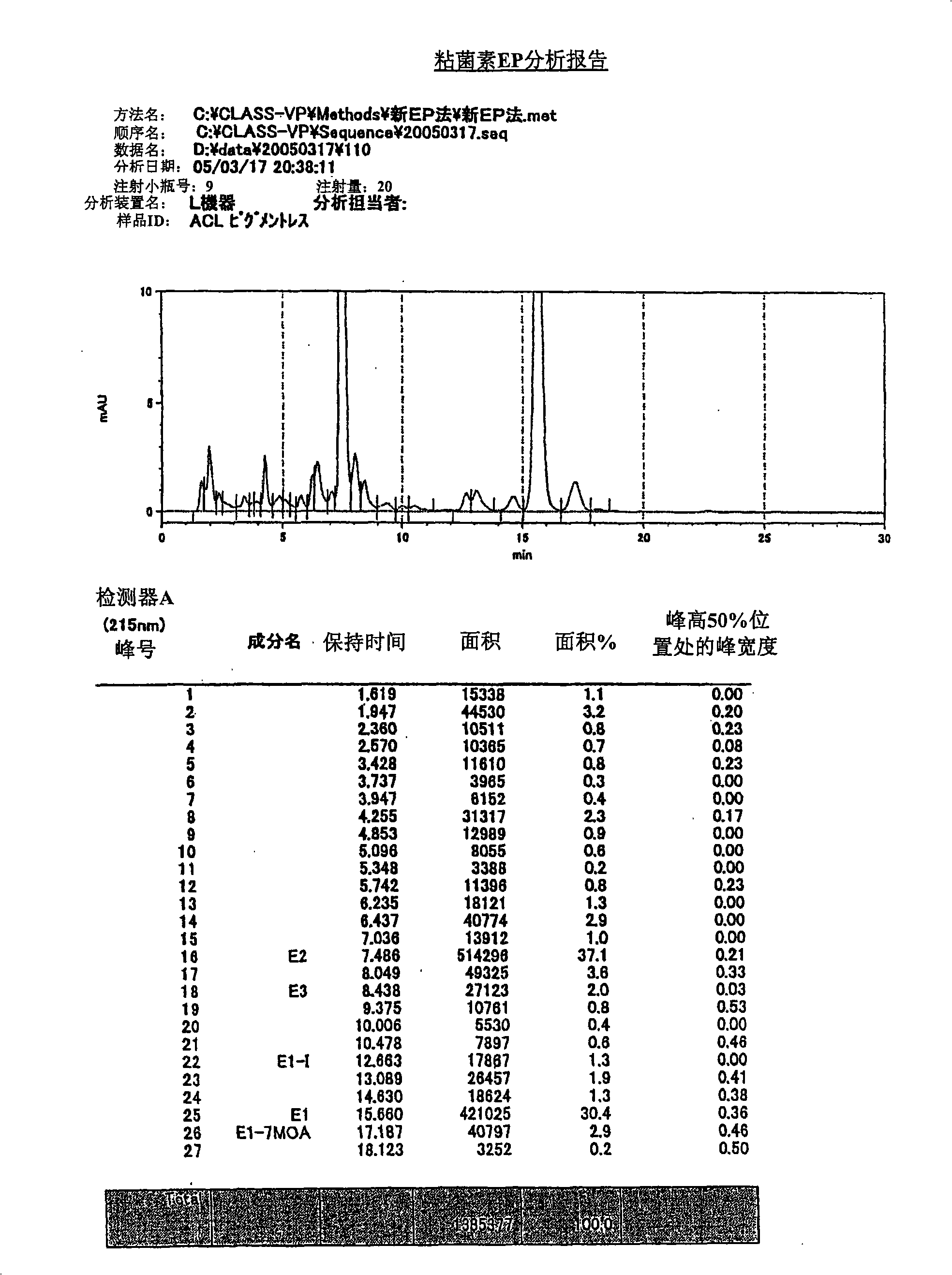
Method for detecting colimycin and special ELISA kit thereof
InactiveCN101358966BStrong specificityHigh sensitivityFused cellsBiological testingElisa kitMonoclonal antibody
The present invention discloses a method of detecting colistin, and a special enzyme linked immunosorbent kit thereof. The enzyme linked immunosorbent kit comprises the colistin and specific antibodies of the colistin; and the specific antibodies are polyclonal antibodies or monoclonal antibodies of the colistin. The kit adopts the high specificity monoclonal antibodies of the colistin, which guarantees the reliability of detection results, and the experimental results show that the kit has the characteristics of high specificity, high sensitivity, high precision, high accuracy and the like; and the main reagents of the kit all adopt the form of working liquid, the operation is convenient, and the cost is low. The operation of the method which adopts the kit to detect the colistin is simple, the detection time is shortened, the operation have low requirements for the pretreatment of the sample, and the method can be used for simultaneously and rapidly detecting the samples in bulk. Therefore, the method which adopts the enzyme linked immunosorbent kit for detection can play a significant role in the detection of the colistin.
Owner:BEIJING WANGER BIOTECH
Poultry and livestock compound medicine containing cinnamon, cherokee rose fruit and colimycin
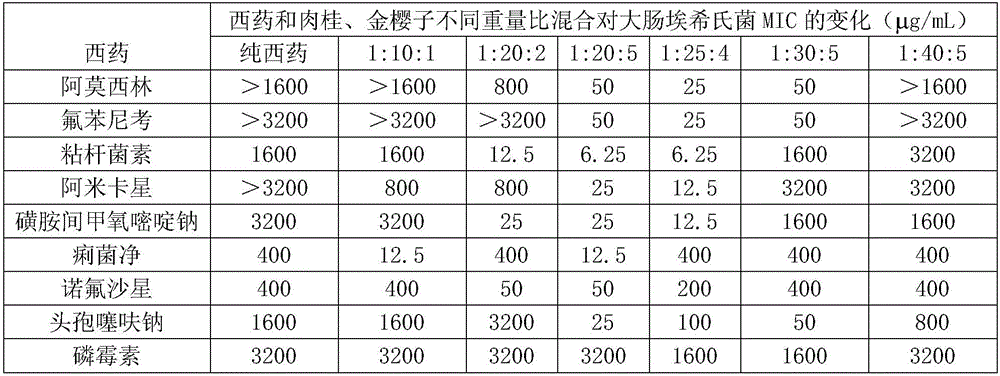
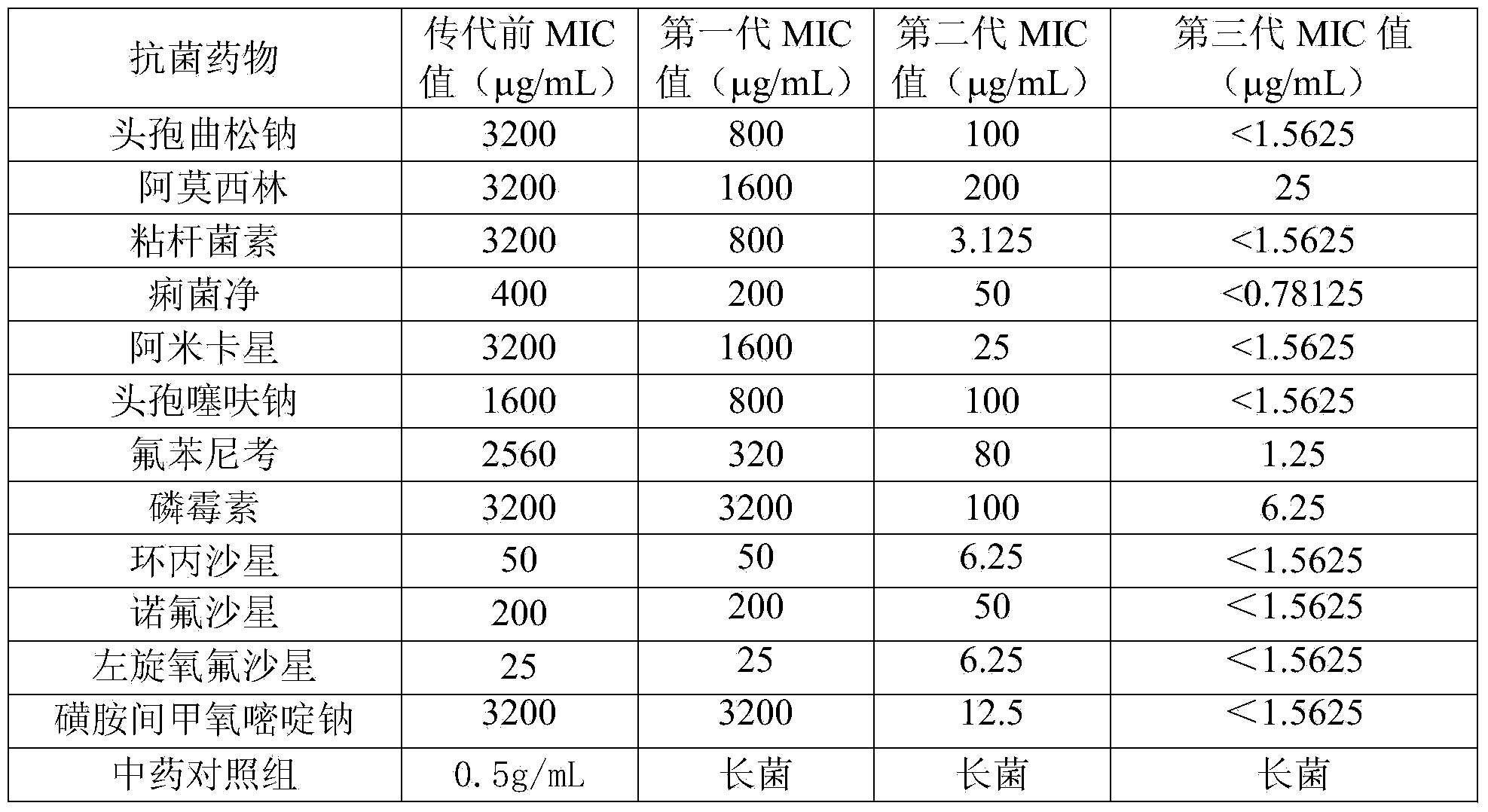
InactiveCN106139121AGood synergyGood curative effectAntibacterial agentsCyclic peptide ingredientsTreatment effectCurative effect
The invention relates to a poultry and livestock compound medicine containing cinnamon, cherokee rose fruit and colimycin. The poultry and livestock compound medicine comprises cinnamon, cherokee rose fruit and colimycin according to the weight ratio of 1:(20-25):(2-5). The invention also relates to a compound preparation prepared by the poultry and livestock compound medicine. The poultry and livestock compound medicine has the beneficial effects that after proofing by test and study, the cinnamon, cherokee rose fruit and colimycin are jointly used according to special weight ratio; the advantages of obvious synergistic function, quick therapy effect, low cost and the like are realized.
Owner:GUANGXI UNIV
Acalypha australis L. and colistin-containing compound medicine for livestock and poultry
InactiveCN104162145AReverse drug resistanceGood treatment effectAntibacterial agentsCyclic peptide ingredientsTreatment effectFood safety
Owner:GUANGXI UNIV
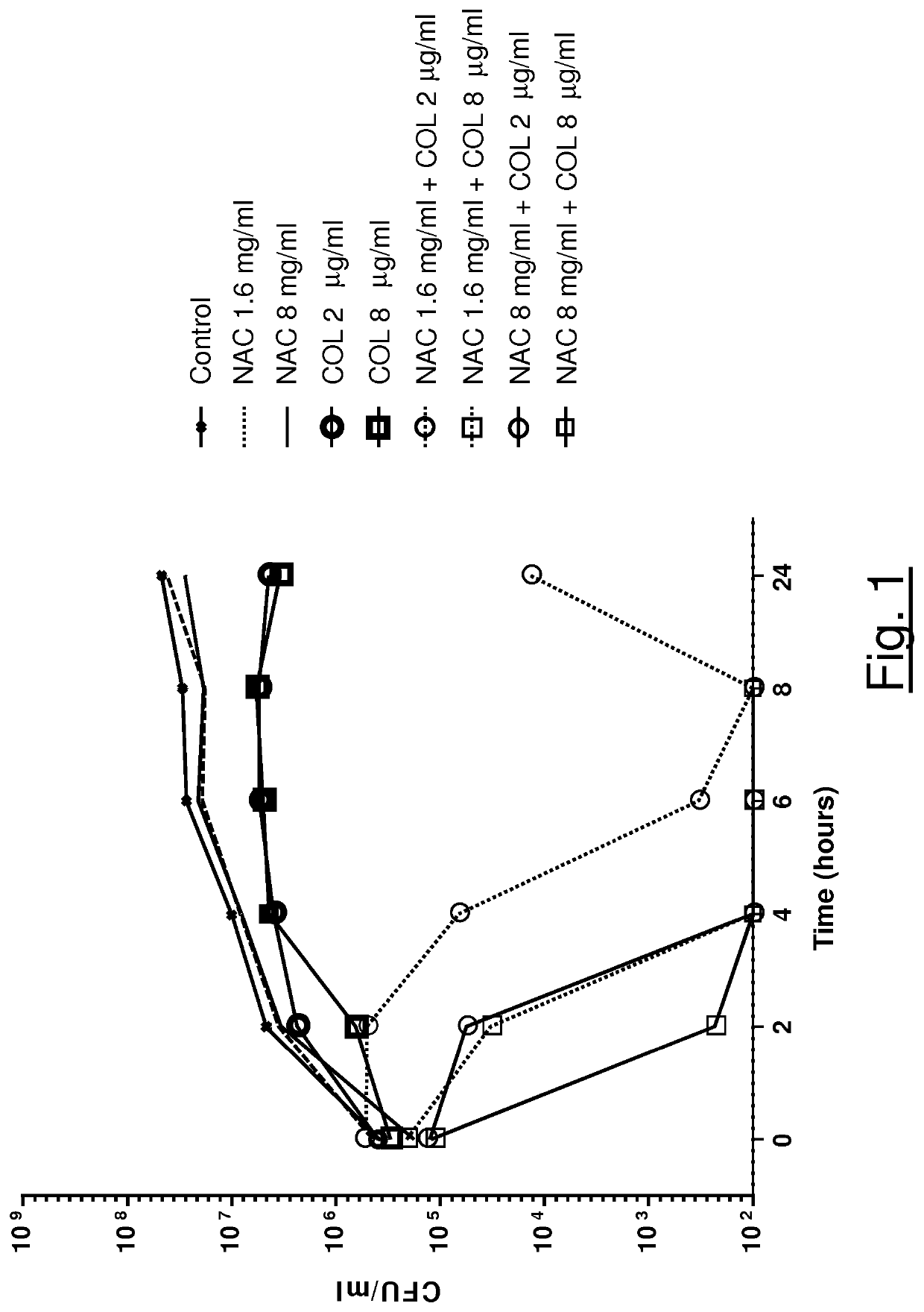
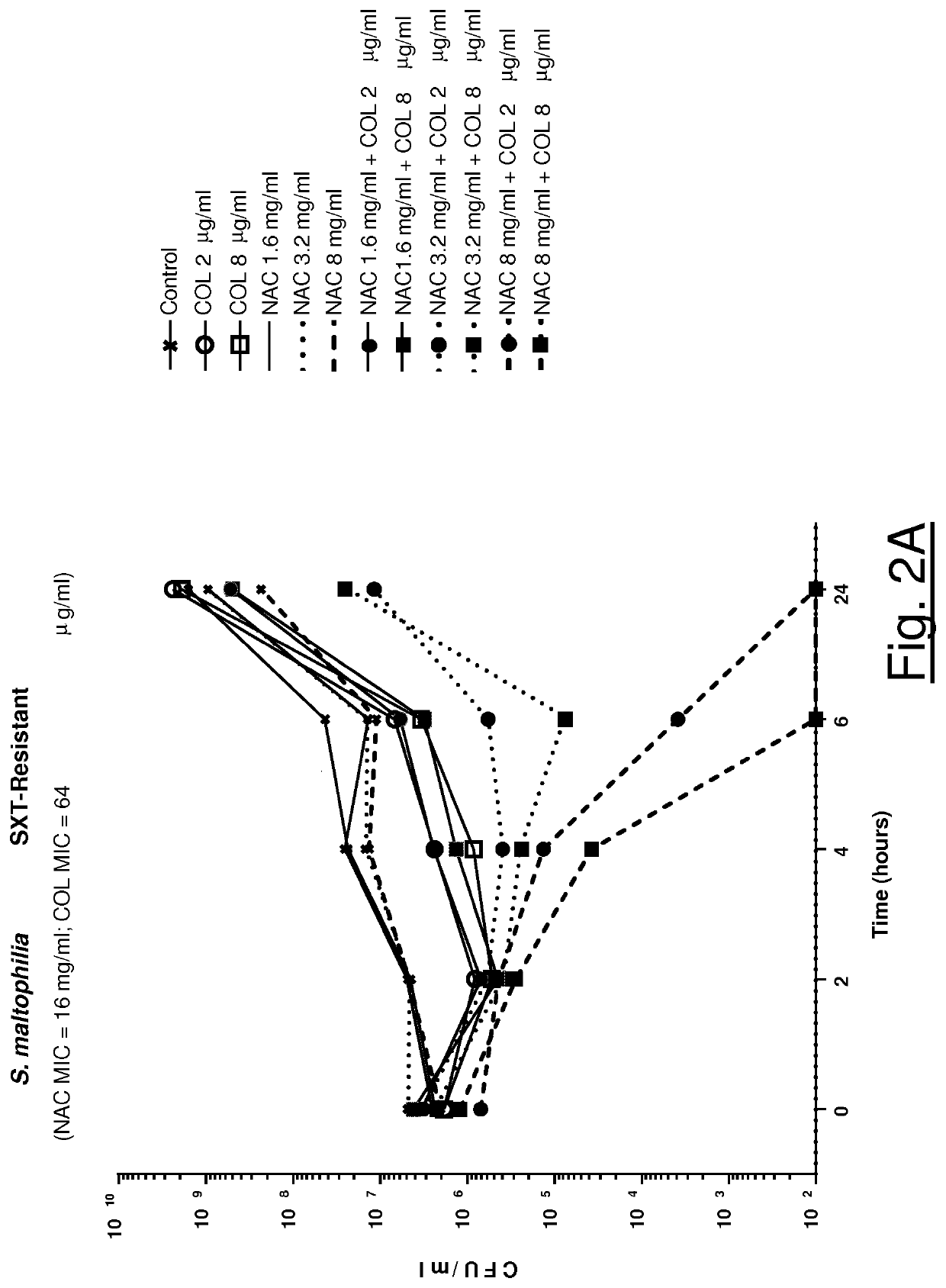
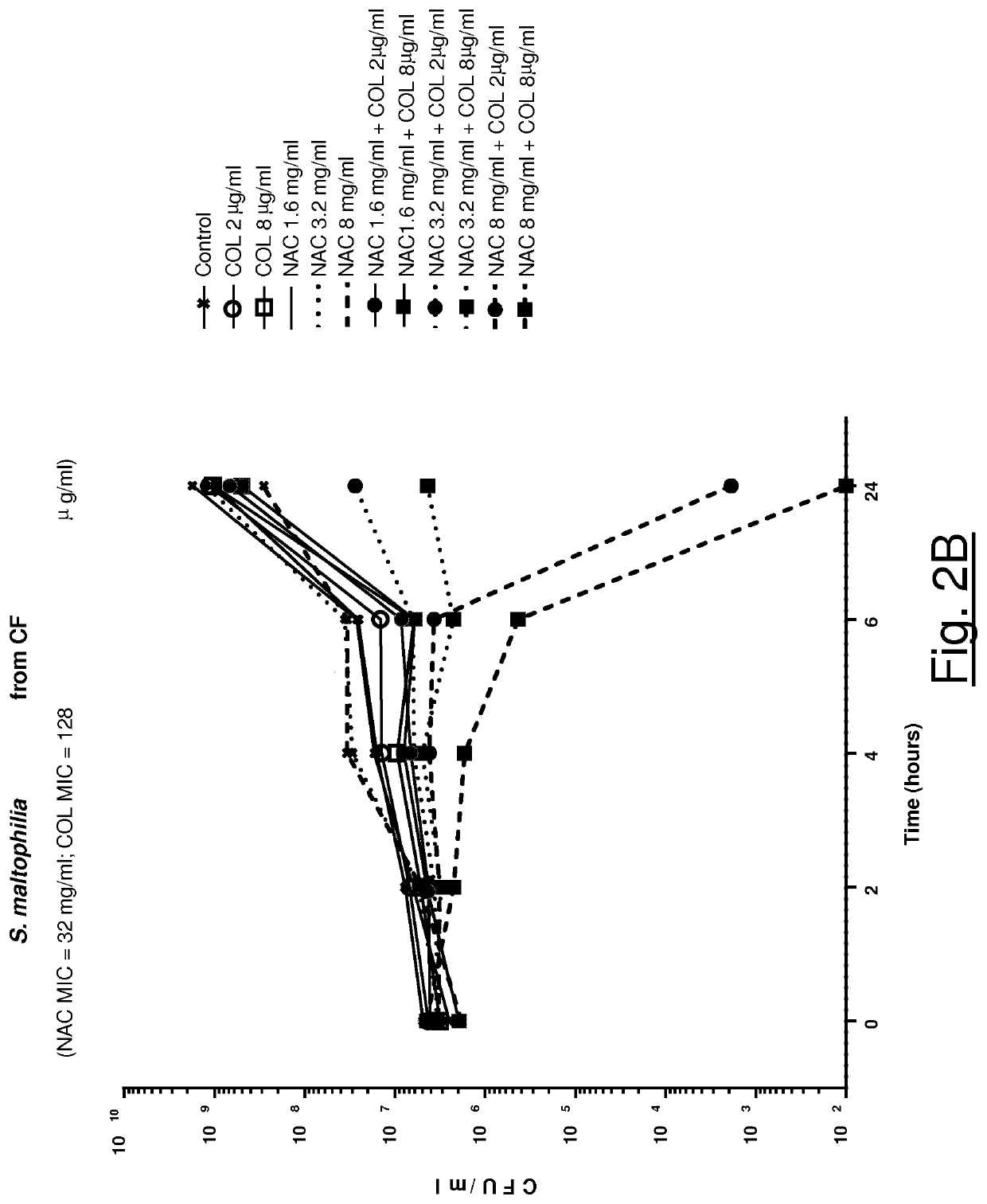
Association of n-acetylcysteine and colistin for use in bacterial infections
ActiveUS20210128678A1Synergistic effectAntibacterial agentsOrganic active ingredientsDiseaseRespiratory tract disease
The present invention relates to a synergistic pharmacological association of NAC and colistin for use in the treatment of a bacterial infection caused by one or more pathogens selected from S. maltophilia and A. baumannii strains, in particular a bacterial infection associated with a respiratory tract disease, such as a chronic respiratory tract disease comprising CF, bronchiectasis non CF and COPD.
Owner:ZAMBON SPA
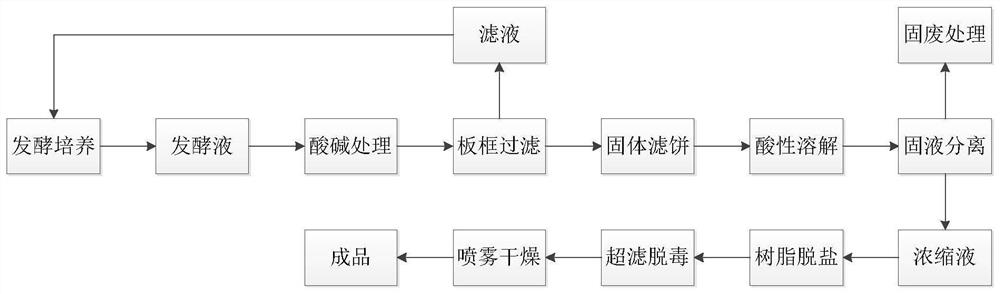
Green colistin extraction method
InactiveCN112250736AReduce processing costsReduce generationPolymyxinsPeptide preparation methodsActivated carbonFiltration
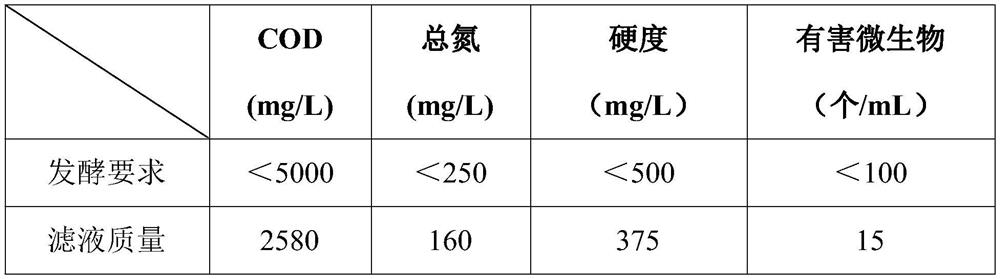
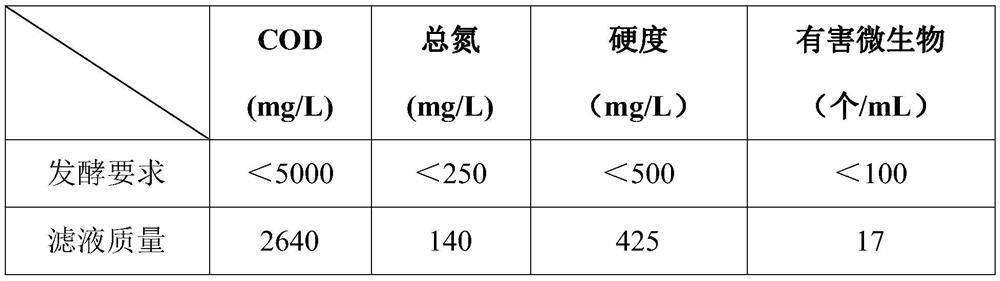
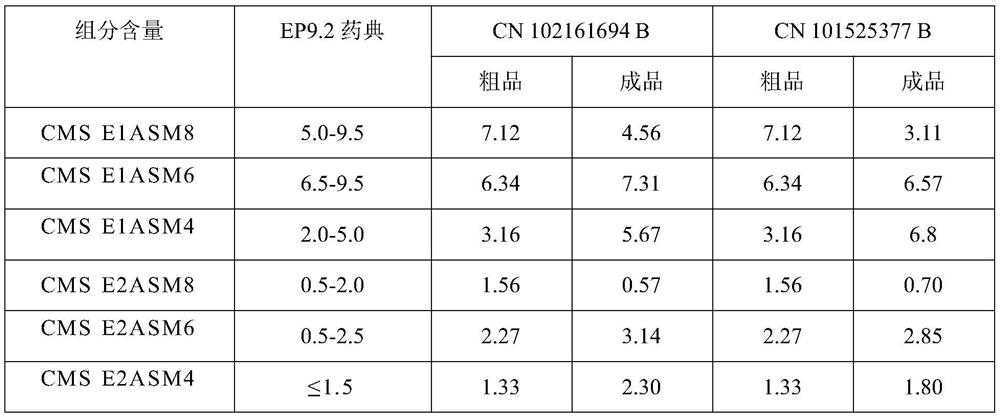
The invention belongs to the technical field of medicines, and particularly relates to a green colistin extraction method. The key is that the method comprises the following steps of (a) heating colistin fermentation liquor; (b) adjusting the pH value to 1.5-2.0 by using an acidic substance, and then performing stirring for 30 minutes; (c) adjusting the pH value to 9.5-11.5 by using a divalent alkaline substance, and then performing stirring for 30 minutes; (d) performing plate frame filtration, collecting solids, neutralizing a filtrate, and taking the neutralized filtrate as water for fermentation of the next batch of colistin; (e) dissolving the solids obtained by the plate frame filtration with an acidic solution; (f) adding activated carbon and a purifying agent into an acidic dissolving solution, and performing plate frame filtration to obtain a colistin concentrated solution; (g) carrying out resin desalination and ultrafiltration on the concentrated solution to remove endotoxin, and finally carrying out spray drying to obtain a finished product; and (h) applying a trapped fluid of an ultrafiltration membrane to the next batch of colistin fermentation liquor for dilution. The extraction method has the advantages of short process, low sewage treatment cost and less environmental pollution.
Owner:HEBEI SHENGXUE DACHENG PHARMA
Composition containing epimedium herb and colistin and used for livestock and poultry, and preparation method thereof
InactiveCN104840942AReverse drug resistanceGood treatment effectAntibacterial agentsCyclic peptide ingredientsEpimediumFood safety
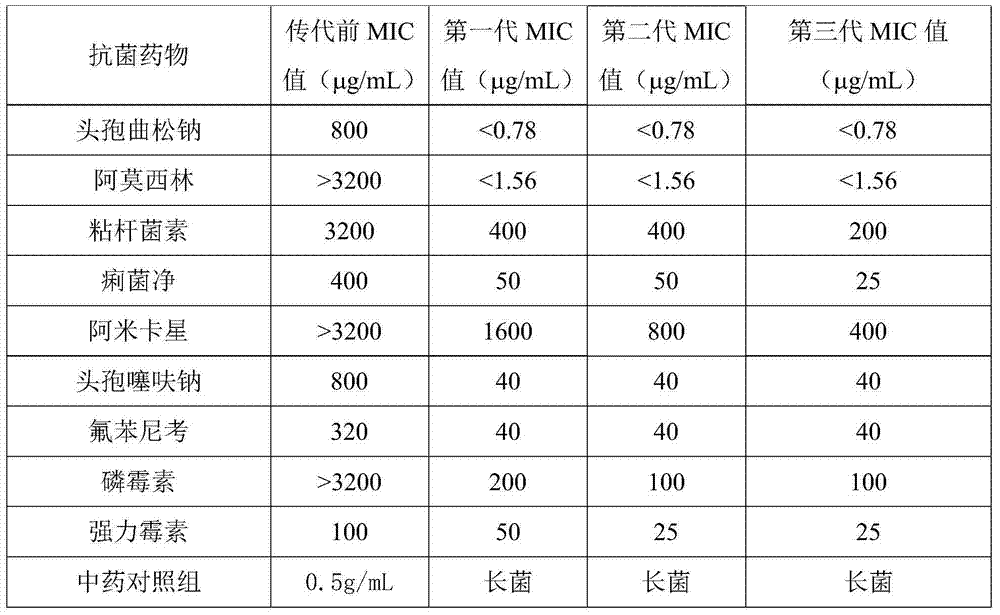
The invention discloses a composition containing epimedium herb and colistin and used for livestock and poultry, and a preparation method thereof. The composition comprises the following raw materials by weight: 5 to 200 parts of epimedium herb and 3 to 30 parts of colistin. The raw materials are uniformly mixed and then a powder, tablet, oral liquid or granule is prepared. 0.1 to 7 g of the composition used for livestock and poultry is added into each kg of a feed for livestock and poultry, and livestock and poultry are fed with the feed; so resistance of bacteria to colistin can be reversed, which enables treatment effect of colistin to be improved, drug cost and drug residual can be greatly reduced, higher economic benefits are created for farmers, and food safety is guaranteed at the same time.
Owner:广西南宁市桃源兽药厂
Method for preparing polymyxin sodium methanesulfonate by crystallization process
ActiveCN113121646AAvoid hydrolysisSolve the problem of changing componentsPolymyxinsPeptide preparation methodsBiotechnologySodium Medronate
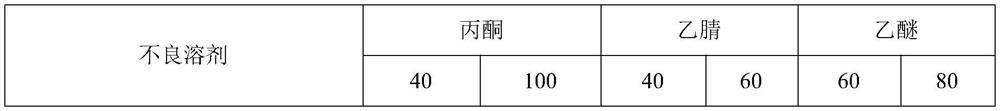
The invention belongs to the technical field of synthetic antibiotic extraction and purification, and particularly relates to a method for preparing polymyxin sodium methanesulfonate by a crystallization process, which comprises the following steps: (1) reacting colistin, formaldehyde and sodium hydrogen sulfite under certain conditions to obtain a polymyxin sodium methanesulfonate solution, and drying to obtain a polymyxin sodium methanesulfonate crude product; (2) stirring and dissolving the polymyxin sodium methanesulfonate crude product in a solvent A at 30-45 DEG C for 0.5-5 hours according to a mass volume ratio of the polymyxin sodium methanesulfonate crude product to the solvent A of 1: (3-10), preserving heat and filtering to obtain insoluble substance sodium salt, thereby obtaining filtrate; (3) slowly cooling the filtrate to 0-20 DEG C, then adding a poor solvent B with a volume of 20-50% of the filtrate volume, and carrying out stirring crystallization at the rotating speed of 20-60 r / min for 0.5-3 h; and (4) filtering, and drying a filter cake to obtain the polymyxin sodium methanesulfonate. The method has the advantages of short steps, simple operation and high yield, and is suitable for commercial popularization and production.
Owner:HEBEI SHENGXUE DACHENG PHARMA
Traditional Chinese medicine active ingredient targeted screening method and kit
ActiveCN111983215ASolve consumptionAchieving one-step targeted immobilizationCompound screeningApoptosis detectionBULK ACTIVE INGREDIENTColistin
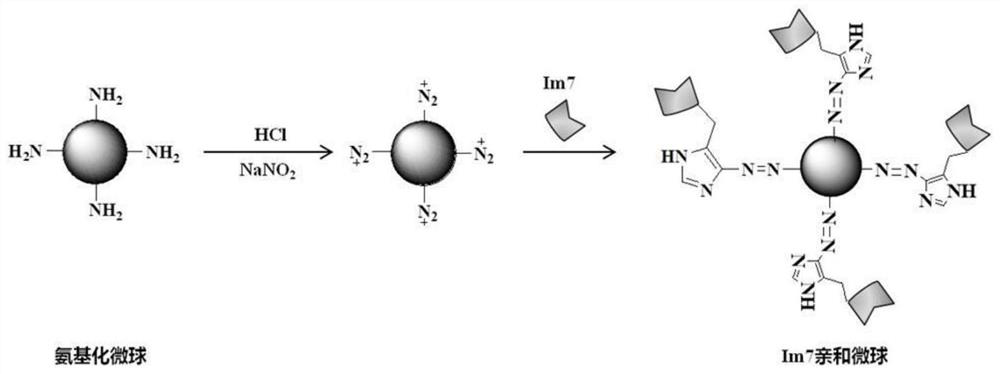
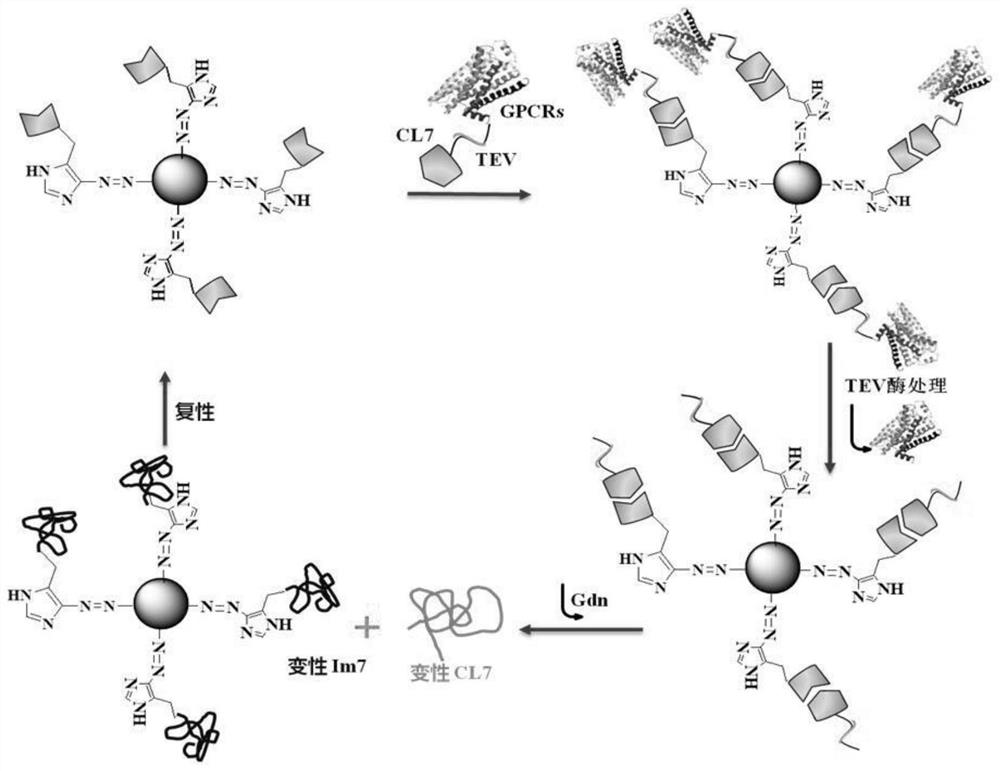
The invention discloses a traditional Chinese medicine active ingredient targeted screening method and a kit. According to the targeted screening method, a traditional Chinese medicine active ingredient targeted screening medium is based on ultrahigh affinity acting force between an inactive mutant CL7 of colistin E7 DNA enzyme and homologous immune protein Im7 of the inactive mutant CL7; therefore, one-step reversible immobilization of fusion of the CL7 label in the cell lysis solution and the GPCRs is realized, and thus the targeted screening method integrates ultrahigh affinity between Im7and CL7 and high specificity of receptor recognition drugs, and high-specificity targeted rapid screening of GPCRs targeted traditional Chinese medicine active ingredients can be realized.
Owner:NORTHWEST UNIV
Production method of colistin rough material powder
It is intended to provide a method of producing a colistin bulk powder without resorting to a decolorizing step whereby problems occurring in the existing production methods (for example, a prolonged production time due to the decolorizing step, an increase in the cost due to a lowering in the yield of the main colistin component and an environmental burden caused by the evolution of a carbon waste) can be overcome. Namely, Bacillus polymyxa var. colistinus which is capable of producing a colistin bulk powder having a high whiteness; a colistin bulk powder having a high whiteness; and a method of producing a colistin bulk powder having a high whiteness.
Owner:核芯医药(山东)有限公司
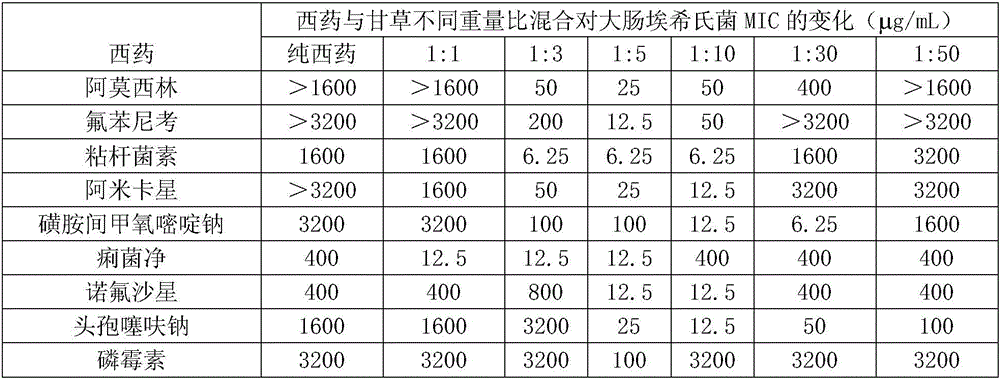
Compound medicine containing licorice roots and colistin for livestock and poultry
InactiveCN106177905AGood synergyGood curative effectAntibacterial agentsCyclic peptide ingredientsCurative effectLicorice roots
The invention relates to a compound medicine containing licorice roots and colistin for livestock and poultry. The compound medicine is composed of licorice roots and colistin, and the weight ratio of licorice roots to colistin ranges from 1:3 to 1:10. The invention is further required to protect a compound preparation prepared through the compound medicine for the livestock and poultry. The compound medicine has the advantages of being obvious in synergistic effect, quick in curative effect, low in cost and the like by using colistin and licorice roots according to the specific weight ratio in a combined mode through an experimental study.
Owner:GUANGXI UNIV
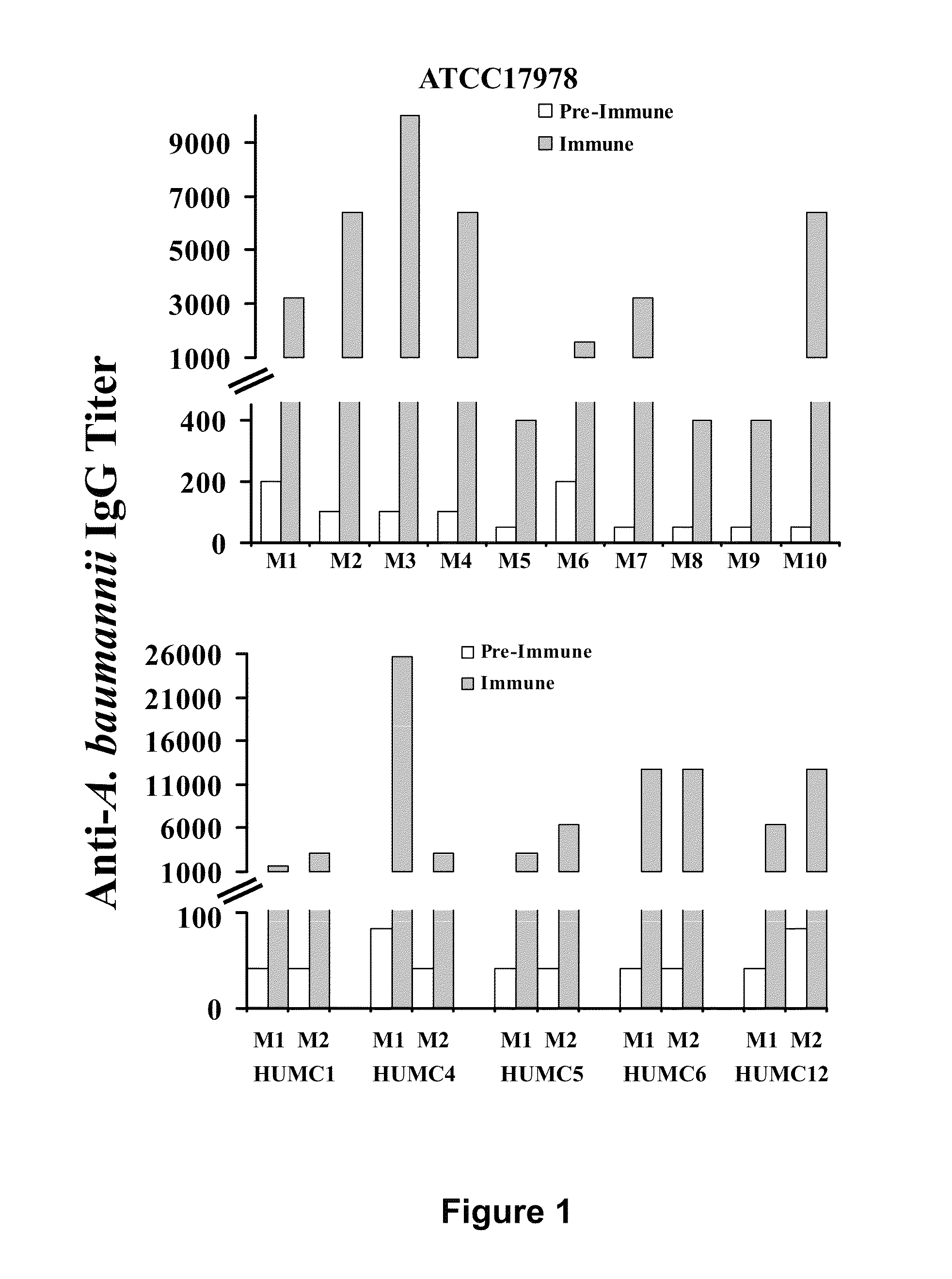
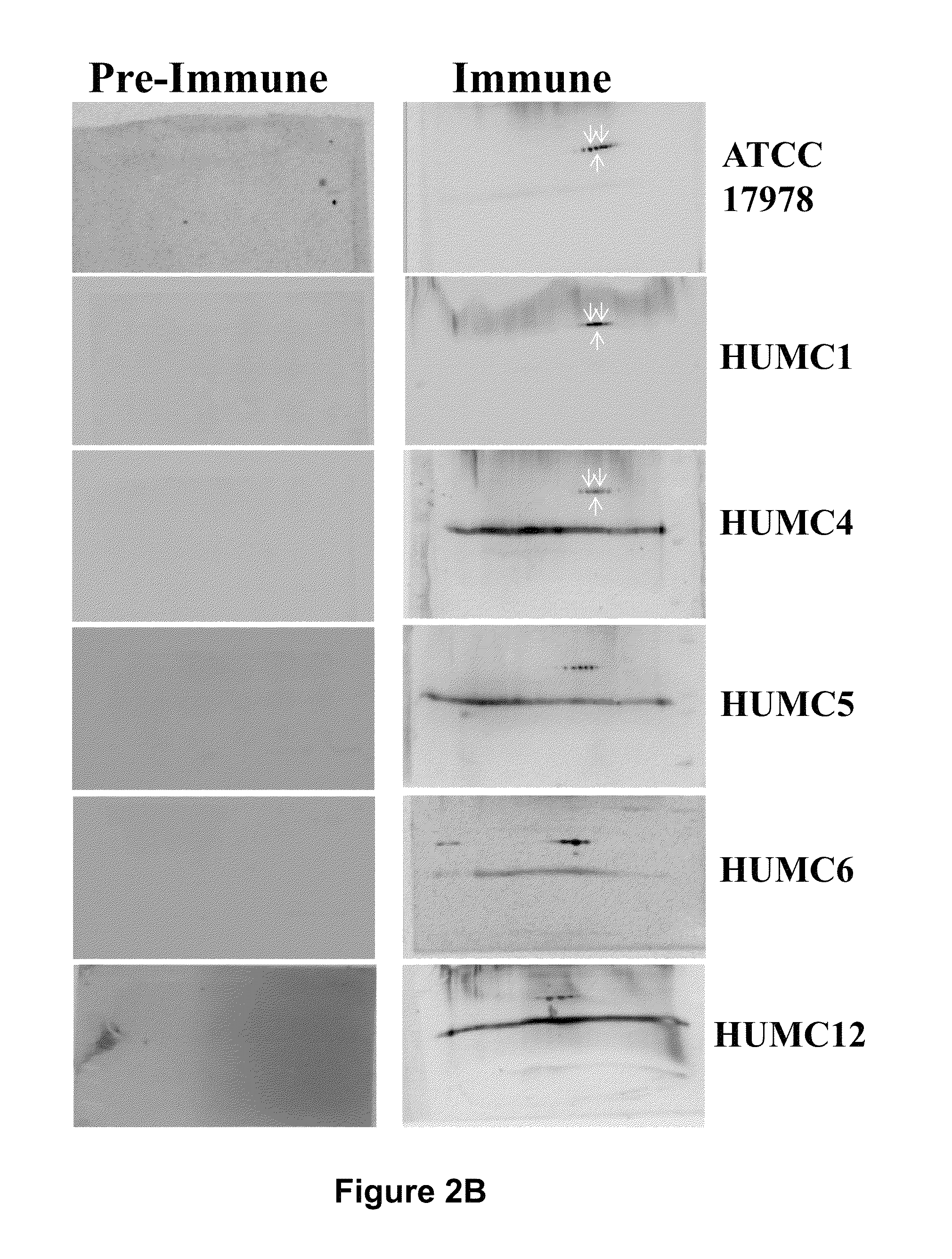
Compositions and methods for immunization against drug resistant Acinetobacter baumannii
InactiveUS8747846B2Antibacterial agentsTripeptide ingredientsPassive ImmunizationsPan drug resistant
Owner:LOS ANGELES BIOMEDICAL RES INST AT HARBOR UCLA MEDICAL CENT
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com