Method for preparing polymyxin sodium methanesulfonate by crystallization process
A technology of sodium methanesulfonate and polymyxin, which is applied in the direction of preparation methods of polymyxin and peptides, chemical instruments and methods, etc., can solve problems such as environmental burden, long operation cycle, and affecting the quality of finished products, so as to avoid The effect of hydrolysis, simple operation, and short steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Study on the Stability of Components in the Extraction Process of Polymyxin Sodium Methanesulfonate Aqueous Solution
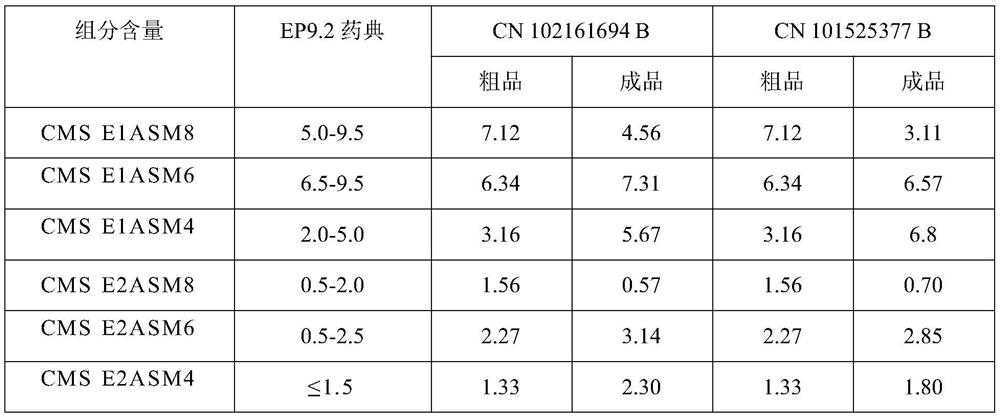
[0024] Colistin, formaldehyde and sodium bisulfite are reacted under certain conditions to obtain a crude product solution of sodium polymyxin methanesulfonate, and then refer to the technology of patent CN 102161694 B and CN 101525377 B to obtain polymyxin methanesulfonic acid Sodium finished product, each component of contrast crude product solution and finished product (the component that EP9.2 Pharmacopoeia stipulates) sees Table 1.
[0025] Table 1. Polymyxin sodium methanesulfonate crude product solution and finished product component content contrast (%)
[0026]
[0027] As can be seen from the above table, the conventional extraction process of the aqueous phase will lead to changes in the content of each component of polymyxin sodium methanesulfonate. Therefore study the crystallization process, shorten the time of polymyxin sodium methane...
Embodiment 2
[0029] Study on Solubility of Sodium Polymyxin Methanesulfonate
[0030] Solubility studies were performed using polymyxin sodium mesylate powder to provide data for determining the crystallization mode of polymyxin sodium mesylate.
[0031] Experiment 1: Investigate the solubility of polymyxin sodium methanesulfonate at room temperature. 100mL of water can dissolve more than 70g of polymyxin sodium methanesulfonate under stirring at 20°C, indicating that evaporation crystallization and cooling crystallization are not applicable.
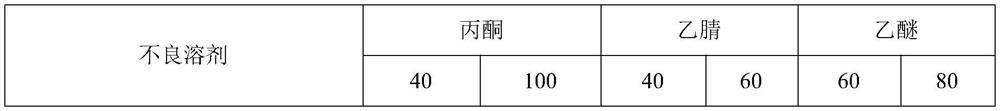
[0032] Experiment 2: To investigate the solubility of polymyxin sodium methanesulfonate in common solvents. At normal temperature (20°C), methanol and ethanol are slightly soluble, and 0.5-1g can be dissolved in 100mL of water; Butyl esters, dichloromethane, and n-butanol are all insoluble. The experiment obtained the poor solvent of polymyxin sodium methanesulfonate.
Embodiment 3
[0034] Selection of crystallization mode of polymyxin sodium methanesulfonate
[0035] According to the results of Experiment 1 and Experiment 2, the possibility of elution crystallization was investigated.
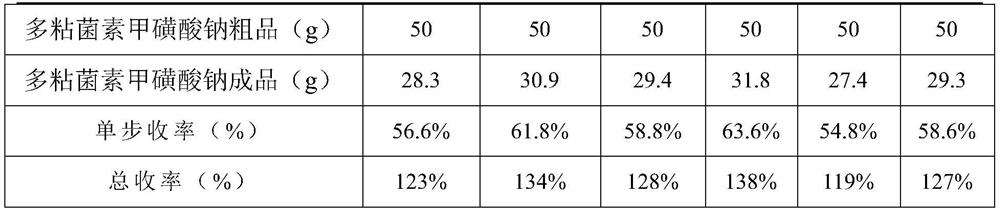
[0036] Experiment 3: Investigate the possibility of elution crystallization. Prepare the solution: Dissolve 50 g of polymyxin sodium methanesulfonate in 100 mL of water. Then add a poor solvent miscible with water: 500 mL each of methanol, ethanol, acetone, acetonitrile, propanol, and isopropanol. There is no obvious solid precipitation, and this plan is not feasible. Analysis of experimental phenomena: ① acetone, acetonitrile, propanol, isopropanol and polymyxin sodium methanesulfonate aqueous solution are mixed unevenly, the next time it is light yellow, and last time it is white; ③ methanol and ethanol can mix with polymyxin A Sodium sulfonate is miscible in aqueous solution.
[0037] After analyzing the phenomenon of experiment 3, it is considered that the solubili...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com