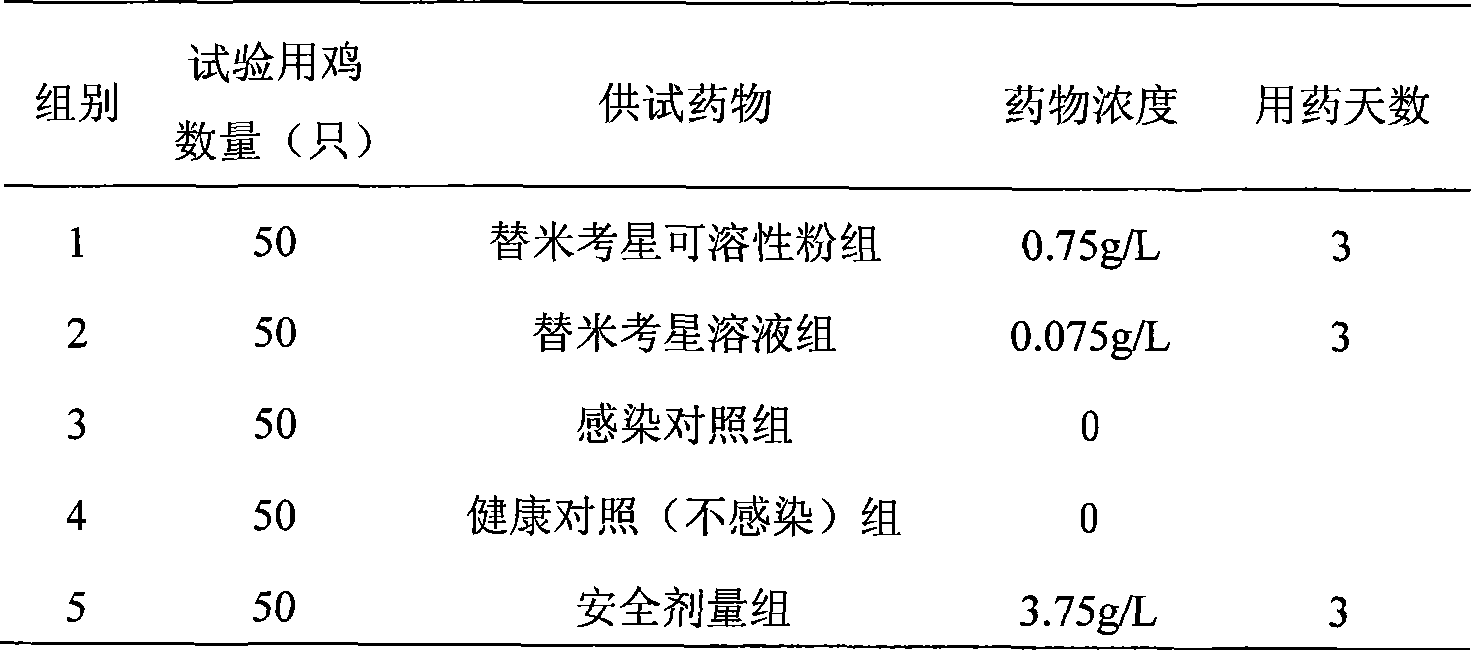
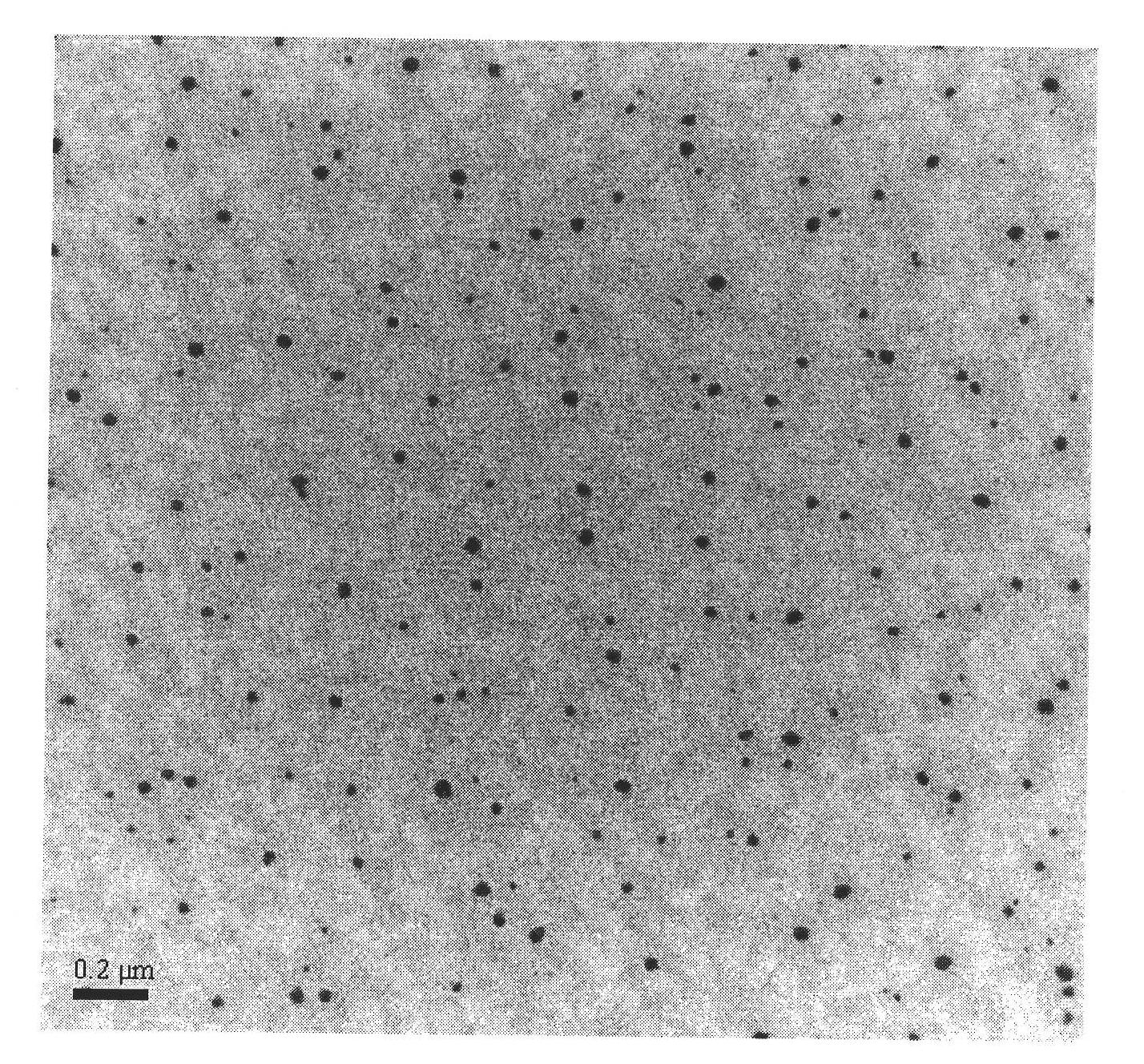
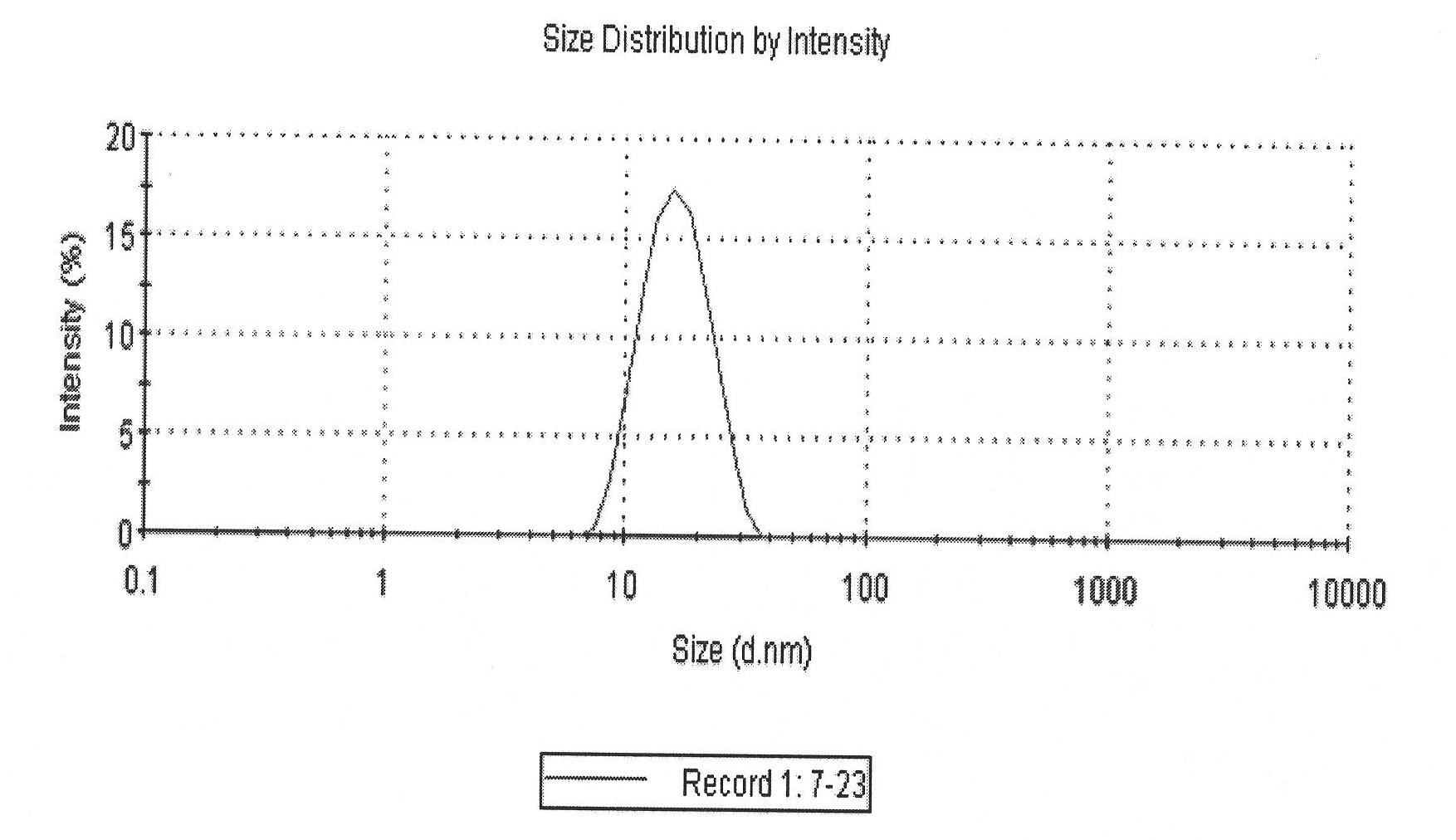
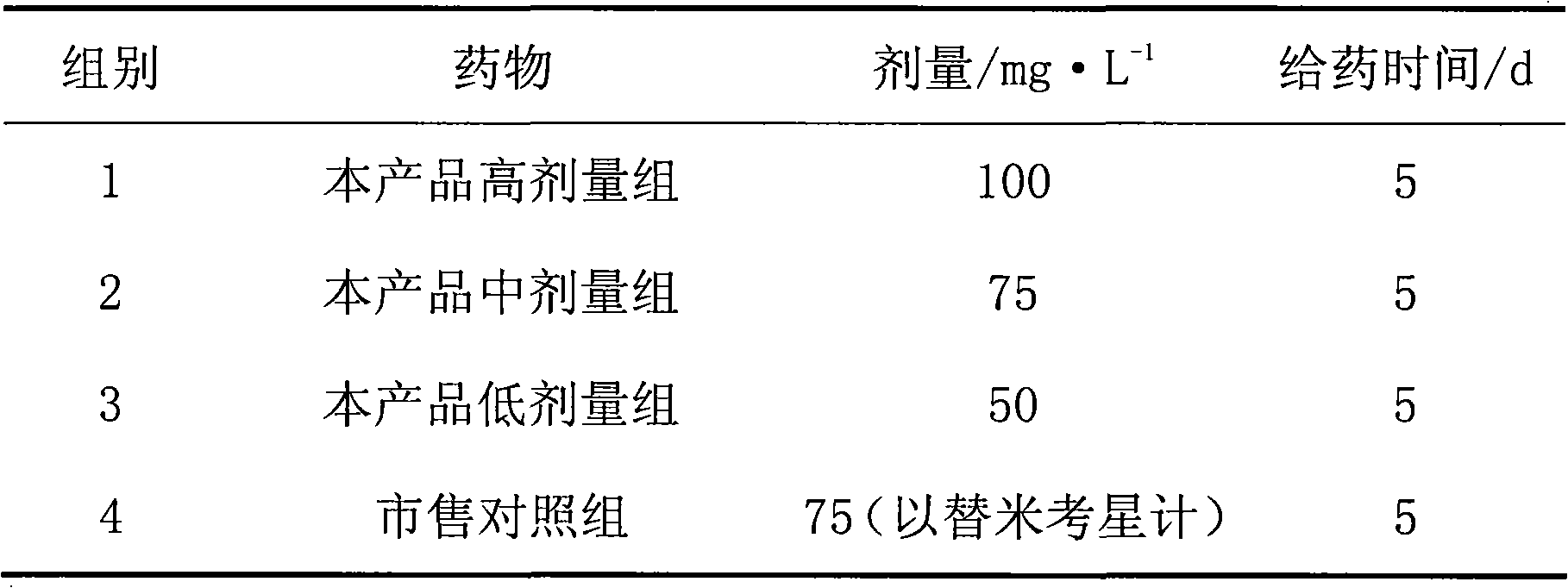
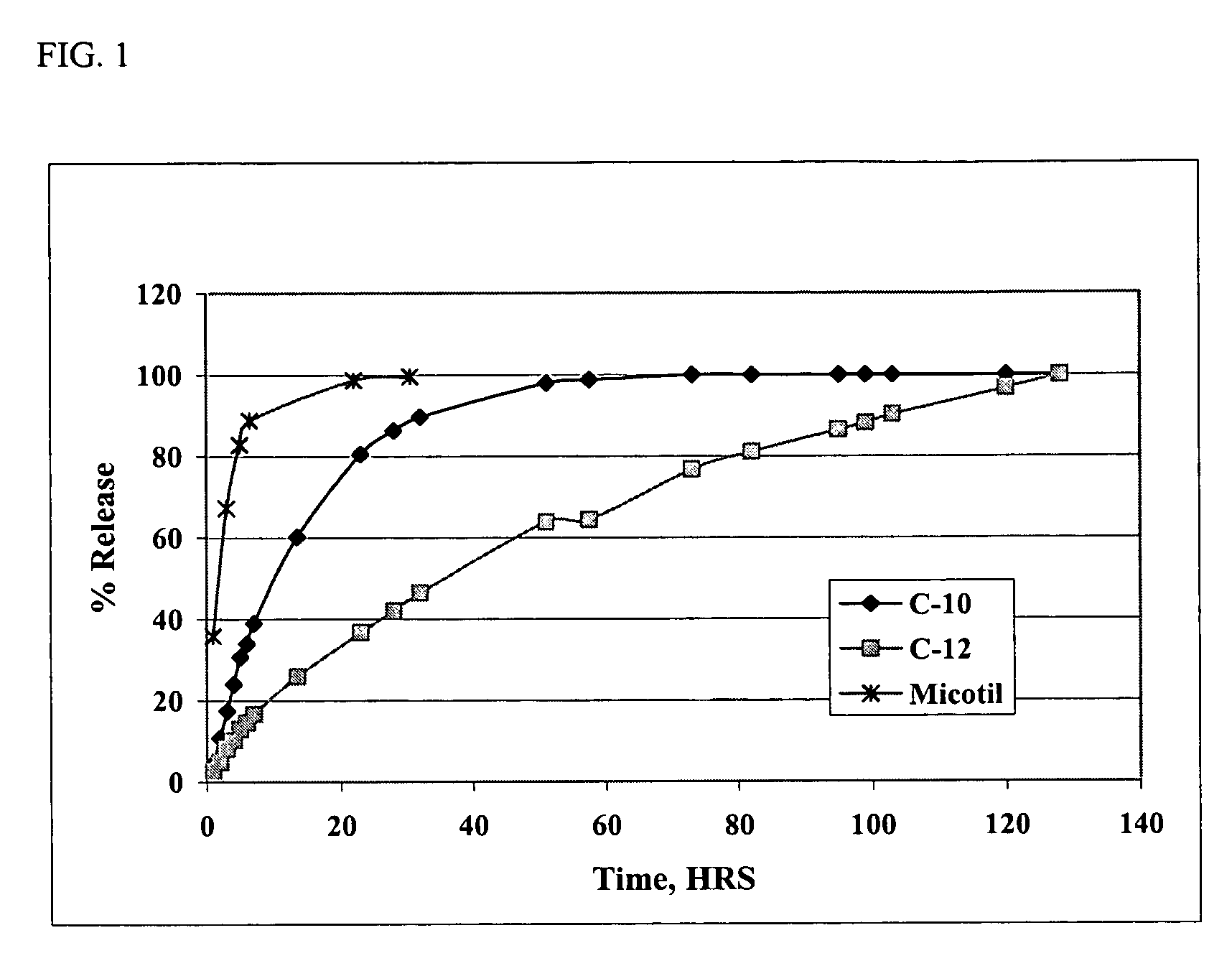
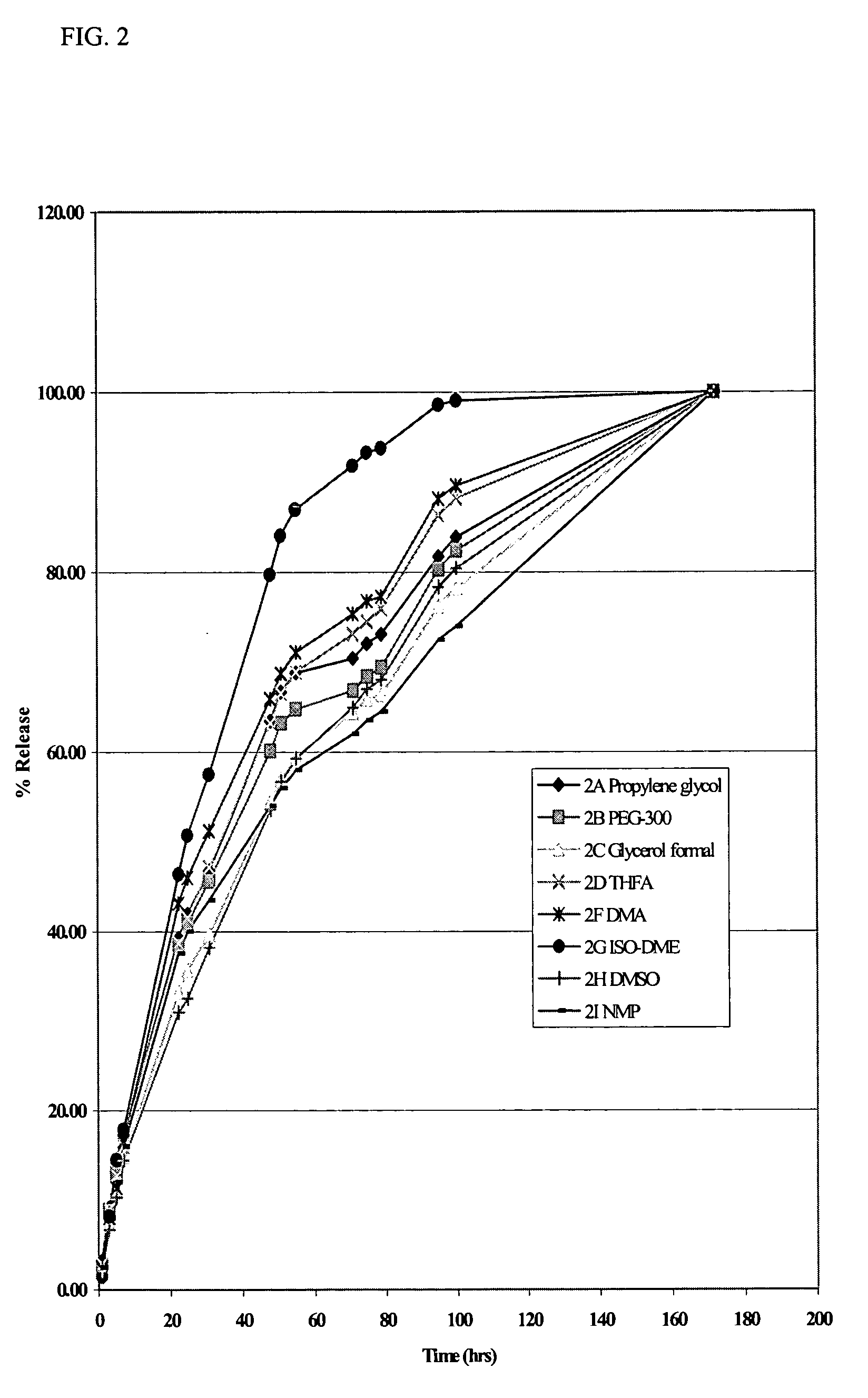
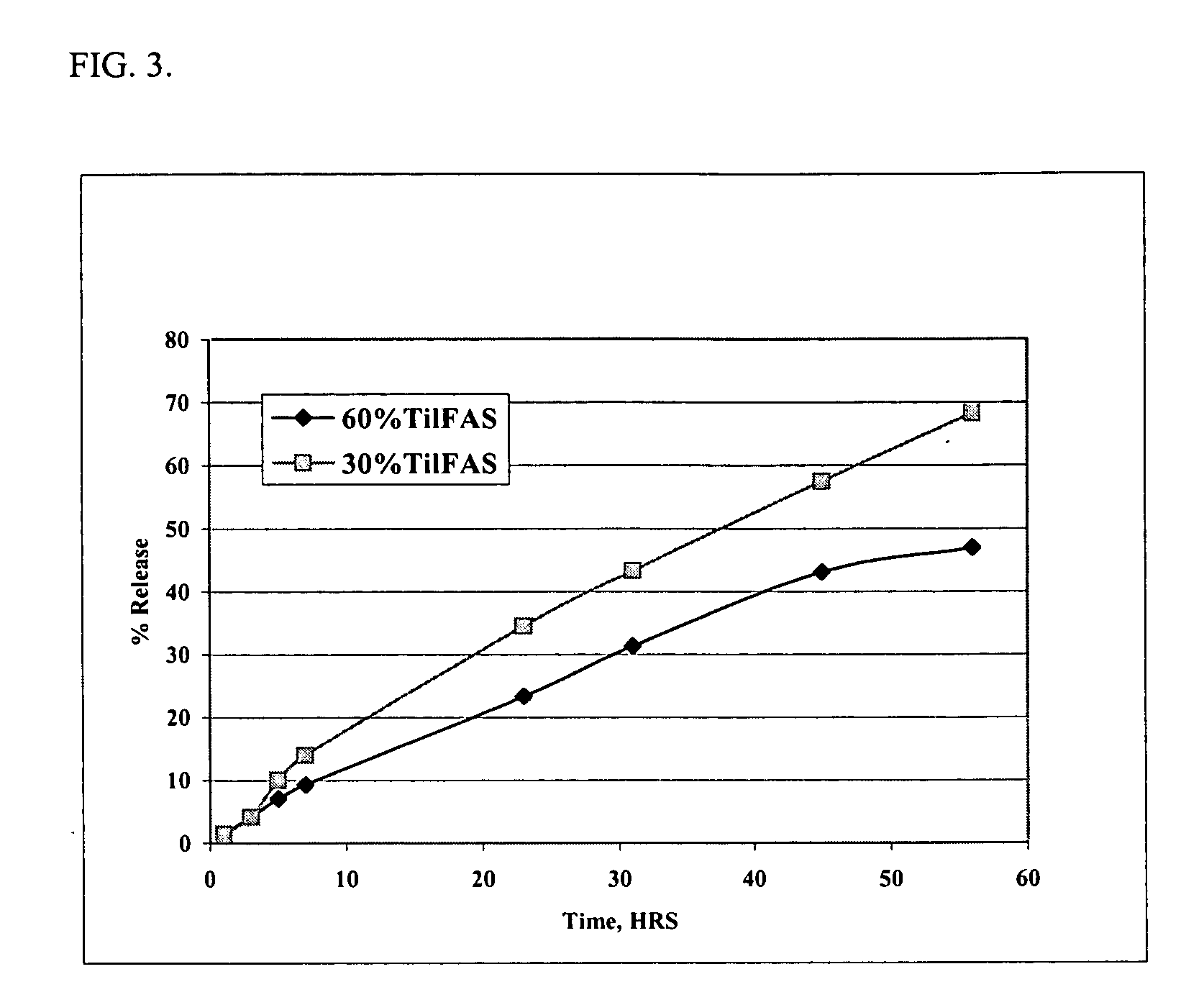
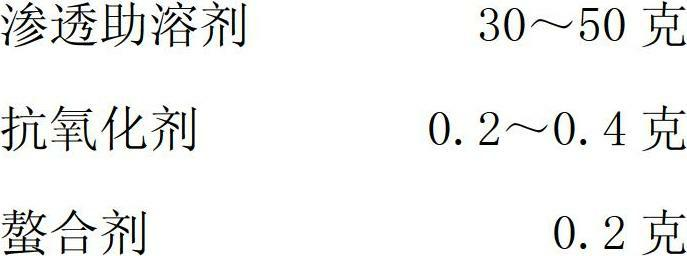Patents
Literature
223 results about "Tilmicosin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
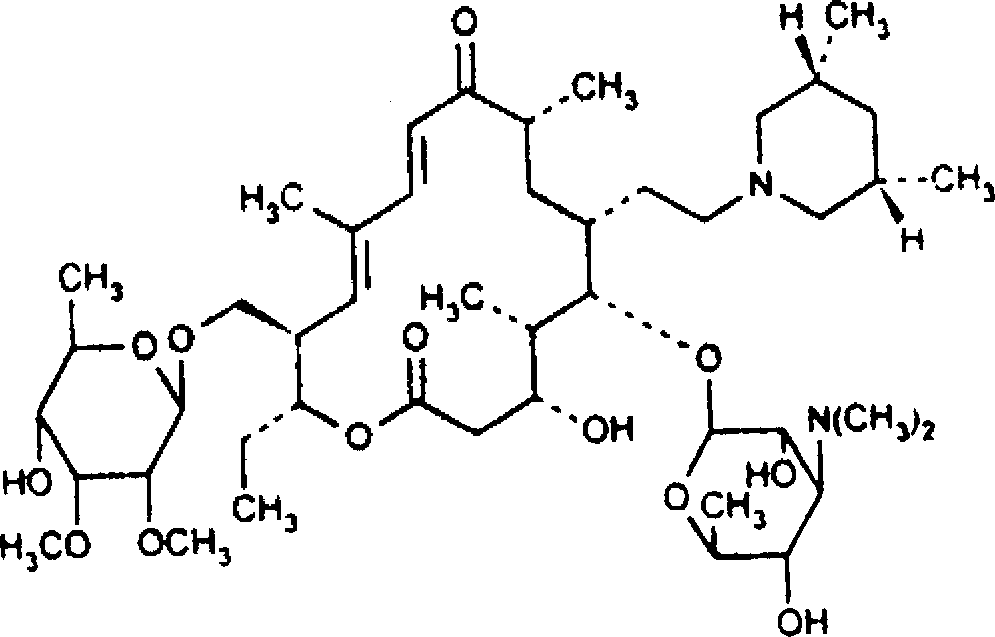
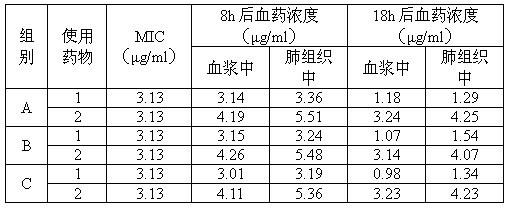
Tilmicosin is a macrolide antibiotic. It is used in veterinary medicine for the treatment of bovine respiratory disease and enzootic pneumonia caused by Mannheimia (Pasteurella) haemolytica in sheep. In humans, Tilmicosin causes fatal cardiotoxic effects at amounts greater than 1 milliliter when injected, something most commonly seen in veterinary personnel and farmers.
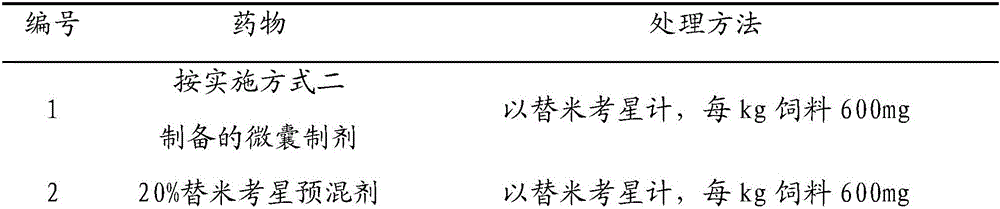
Injectable compositions for the controlled delivery of pharmacologically active compound
InactiveUS6887487B2Extend posting timeControl doseAntibacterial agentsBiocideRoxithromycinRelease time
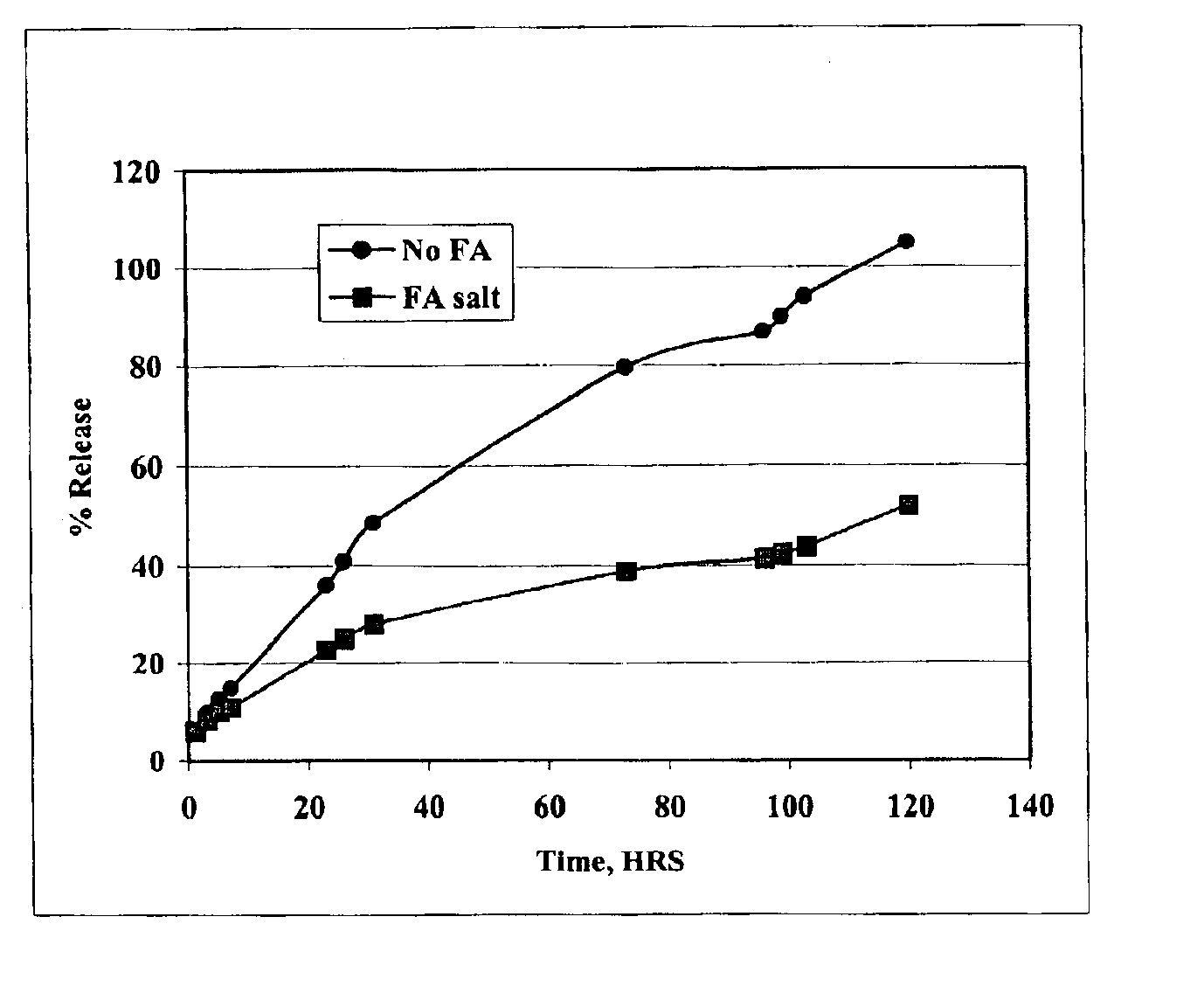
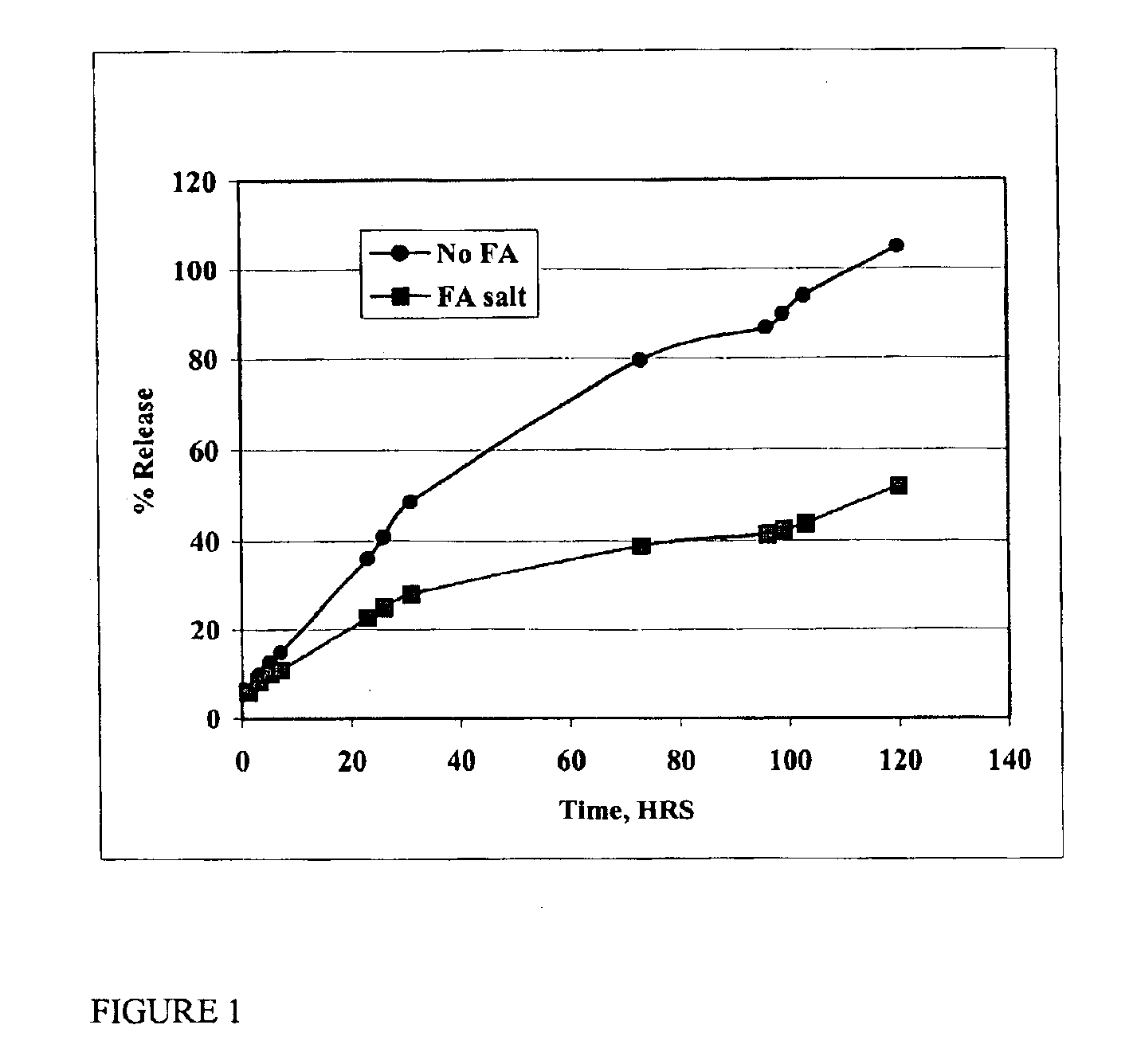
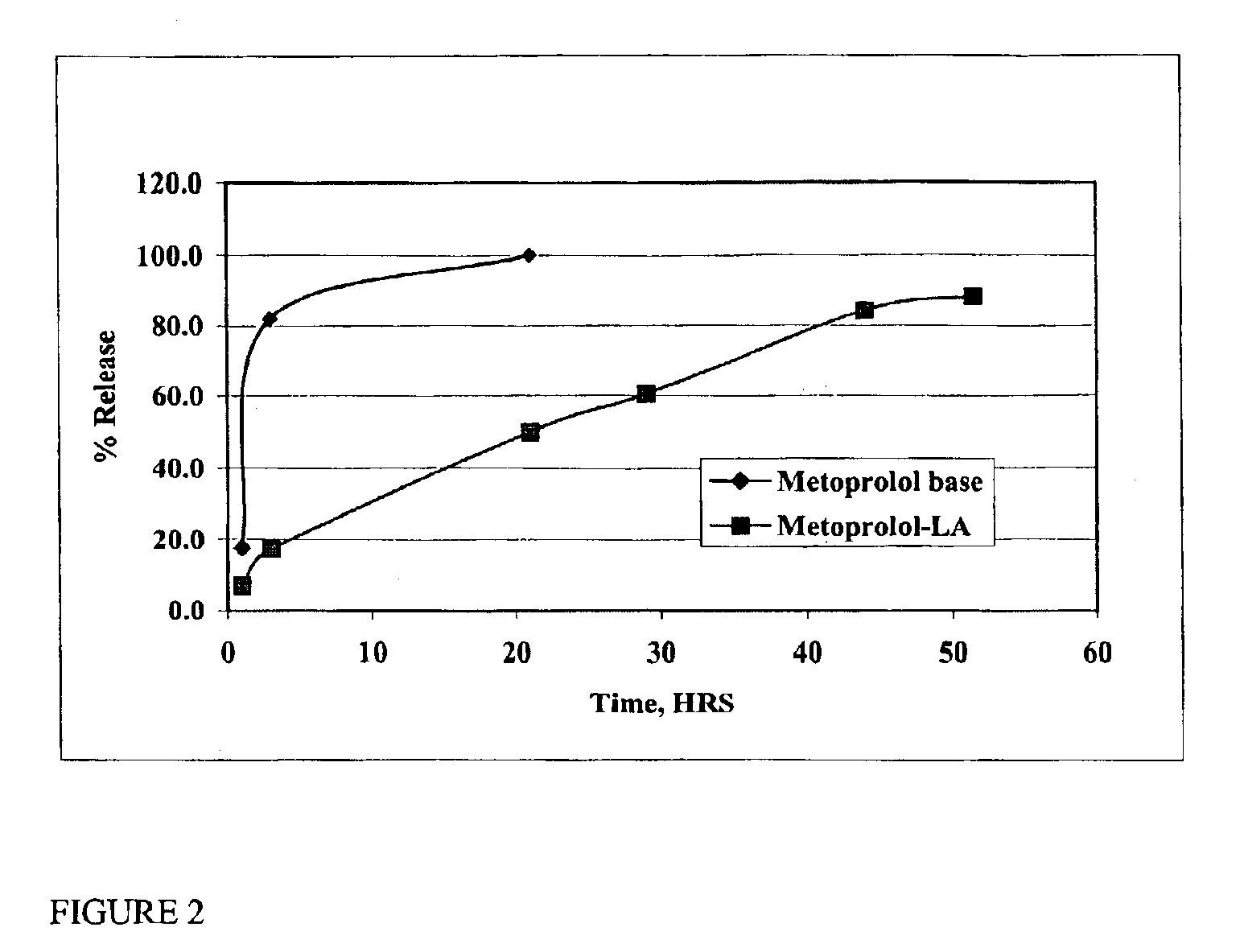
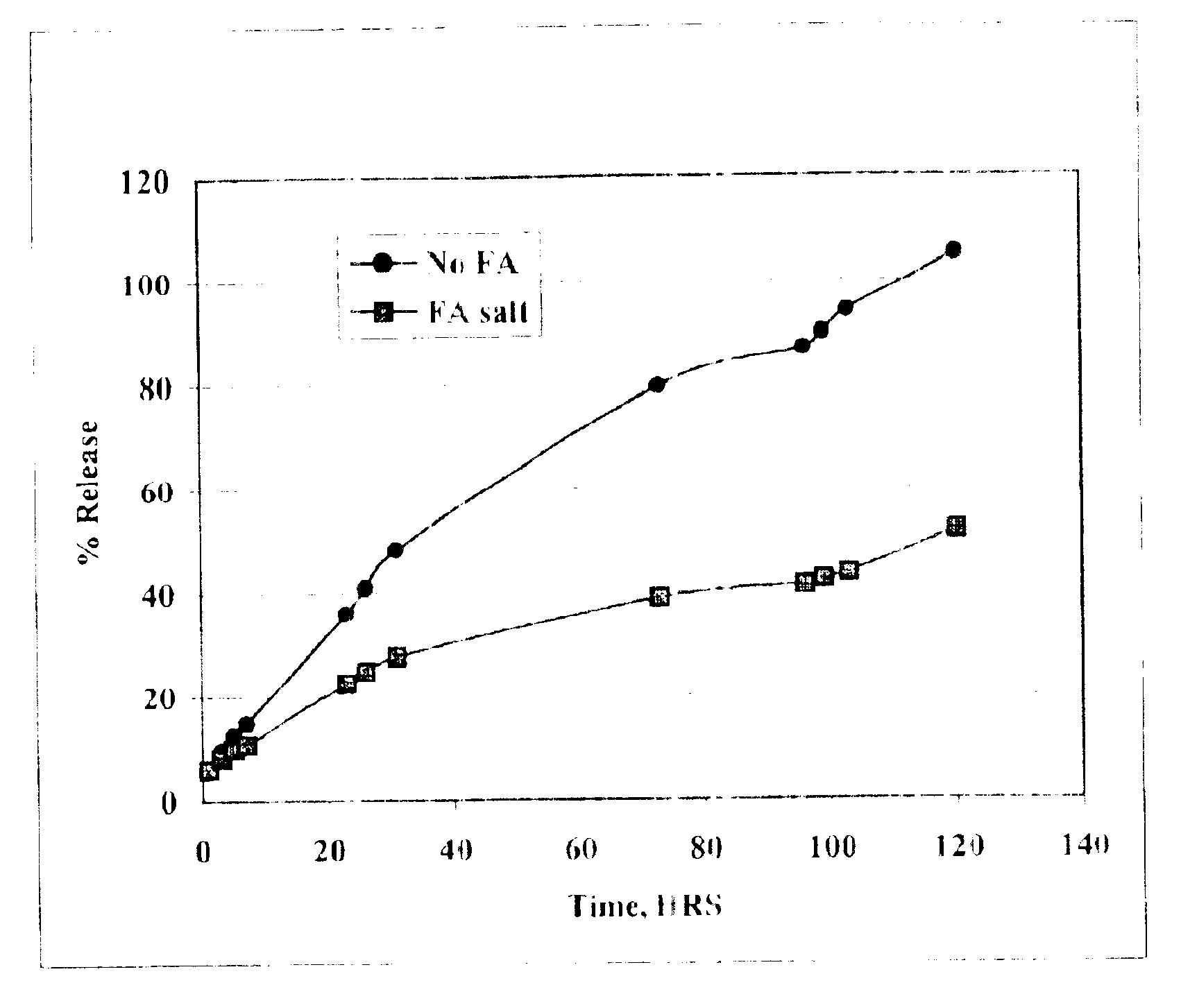
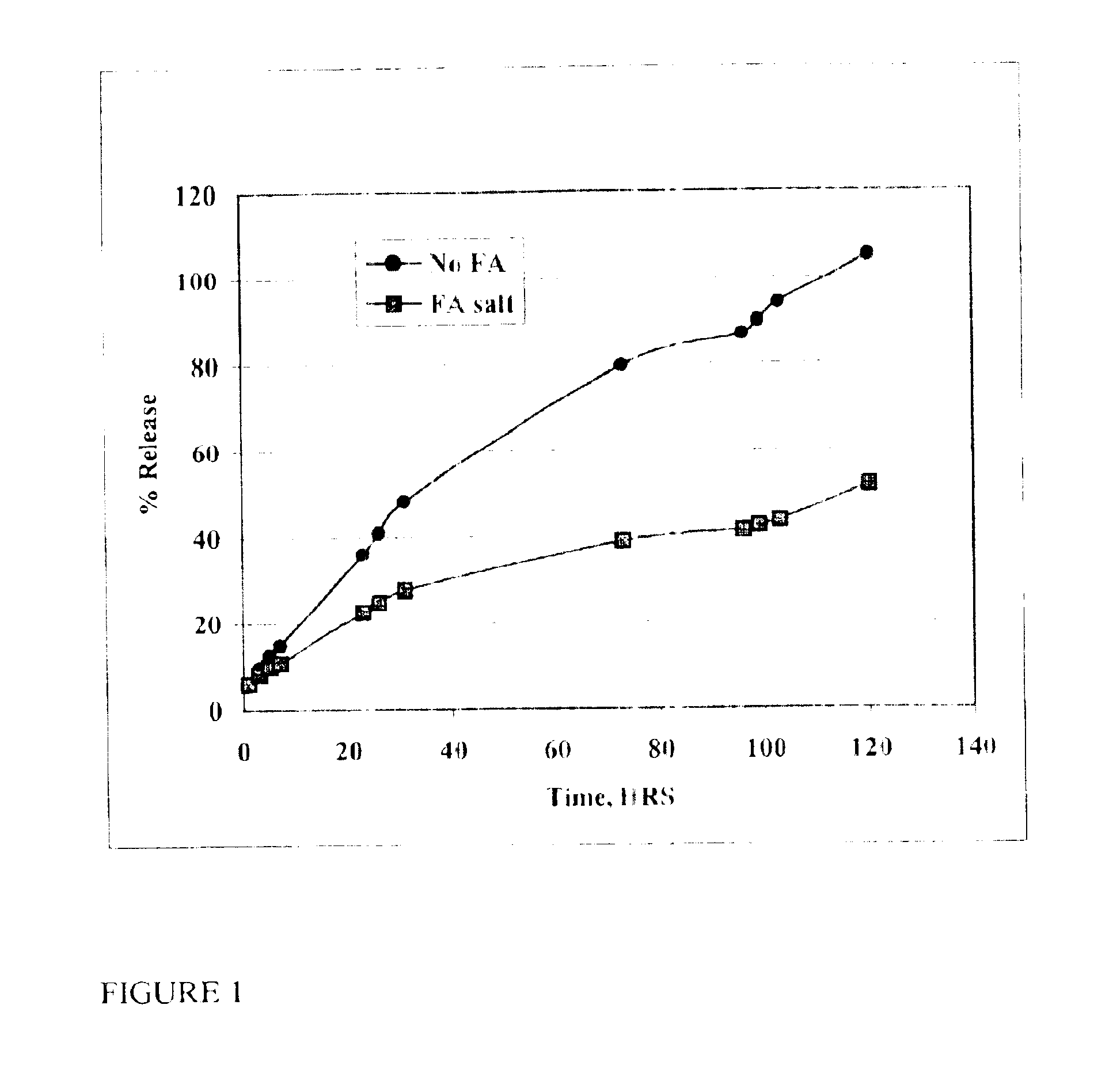
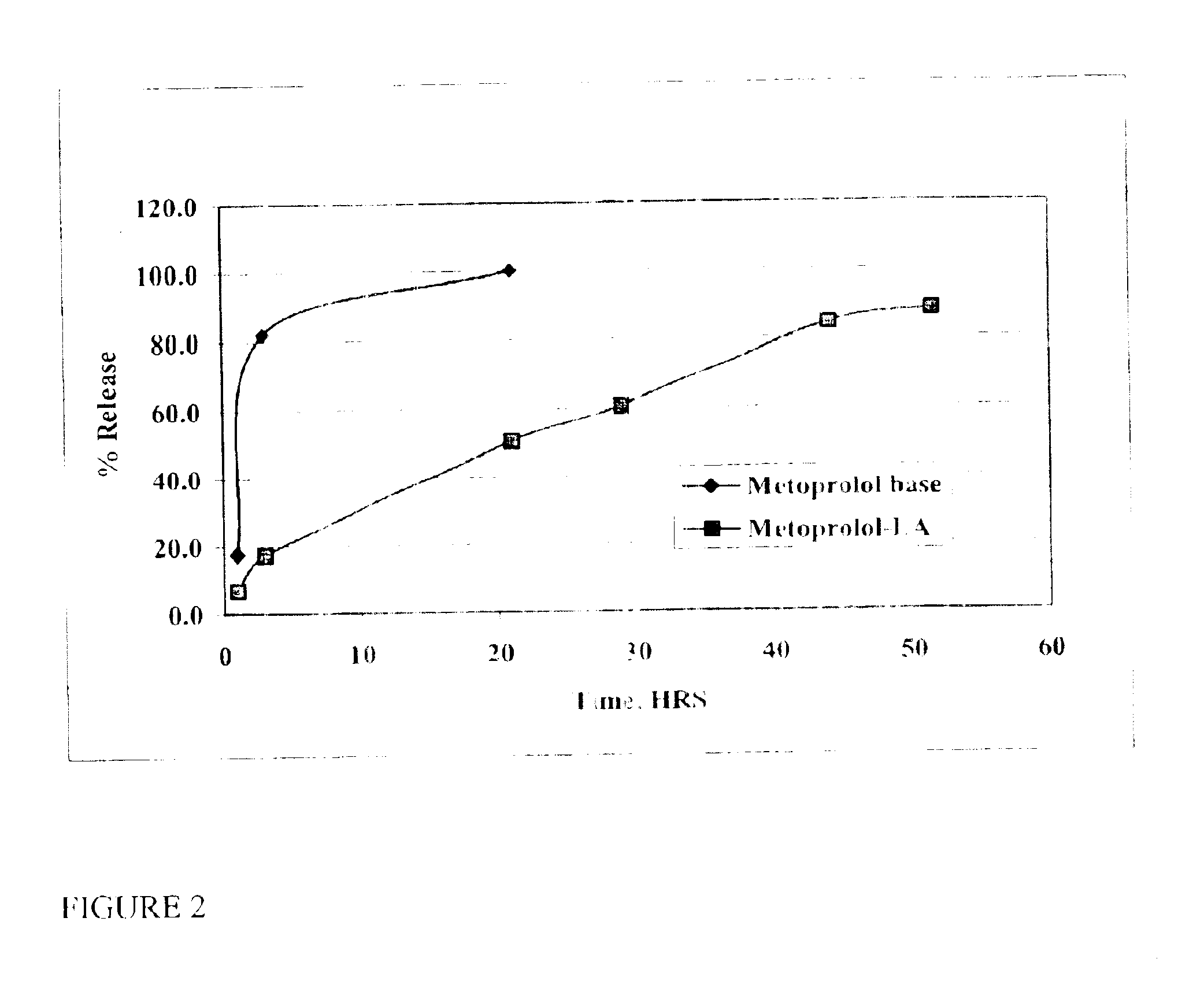
The present invention provides compositions and methods for extending the release times and lowering the toxicity of pharmacologically active compounds. The compounds comprise a salt of the pharmacologically active compound with a lipophilic counterion and a pharmaceutically acceptable water soluble solvent combined together to form an injectable composition. The lipophilic counterion may be a saturated or unsaturated C8-C22 fatty acid, and preferably may be a saturated or unsaturated C10-C18 fatty acid. When injected into a mammal, at least a portion of the composition precipitates and releases the active compound over time. Thus, the composition forms a slowly releasing drug depot of the active compound in the mammal. Therefore, the present invention enables one to provide a controlled dose administration of the active compound for a periods of up to 15 days or even longer. Many compounds can be administered according to the present invention including, but not limited to, tilmicosin, oxytetracycline, metoprolol, fluoxetine, roxithromycin, and turbinafine.
Owner:IDEXX LABORATORIES
Tilmicosin soluble powder and preparation method thereof
InactiveCN102670516AImprove palatabilityReduce moisture contentAntibacterial agentsPowder deliverySolubilitySolvent
The invention discloses tilmicosin soluble powder, which consists of the following components in parts by weight: 1-50 parts of tilmicosin, 1-50 parts of a solid dispersoid carrier auxiliary material and 0-70 parts of a medical filling auxiliary material. The invention further discloses a preparation method of the tilmicosin soluble powder. The method comprises the following steps of: (1) adding the solid dispersoid carrier auxiliary material into a solubilizing assistant for dissolving; (2) adding tilmicosin, stirring and dissolving; and (3) drying. The tilmicosin soluble powder disclosed by the invention has the advantages of low water content, high solubility, high bioavailability and convenience for administration, and the palatability of animals can be improved simultaneously.
Owner:GUANGDONG DAHUANONG ANIMAL HEALTH PRODS +1
Methods for the controlled delivery of pharmacologically active compounds
InactiveUS6946137B2Low toxicitySmall investmentBiocideTetracycline active ingredientsRoxithromycinRelease time
The present invention provides compositions and methods for extending the release times and lowering the toxicity of pharmacologically active compounds. The compounds comprise a salt of the pharmacologically active compound with a lipophilic counterion and a pharmaceutically acceptable water soluble solvent combined together to form an injectable composition. The lipophilic counterion may be a saturated or unsaturated C8-C22 fatty acid, and preferably may be a saturated or unsaturated C10-C18 fatty acid. The compounds precipitate in aqueous environments. When injected into a mammal, at least a portion of the composition precipitates and releases the active compound over time. Thus, the composition forms a slowly releasing drug depot of the active compound in the mammal. Therefore, the present invention enables one to provide a controlled dose administration of the active compound for a period of up to 15 days or even longer. Many compounds can be administered according to the present invention including, but not limited to, tilmicosin, oxytetracycline, metoprolol, fluoxetine, roxithromycin, and turbinafine.
Owner:IDEXX LABORATORIES
Preparation method of tilmicosin
ActiveCN102382159ASimple manufacturing processReduce precipitationSugar derivativesSugar derivatives preparationAlcoholHydrogen
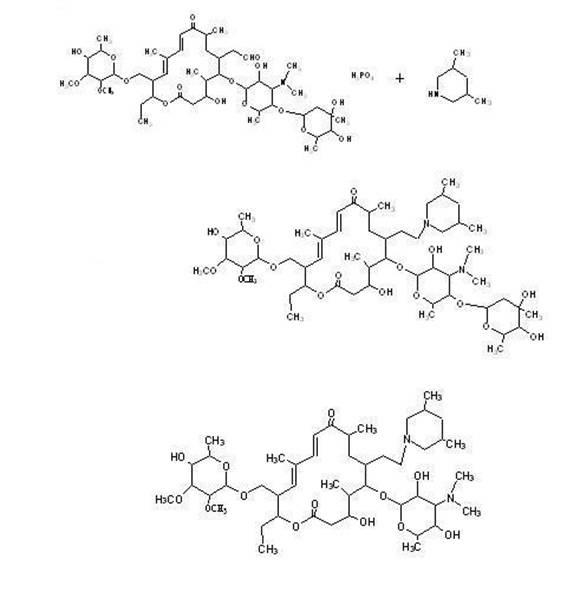
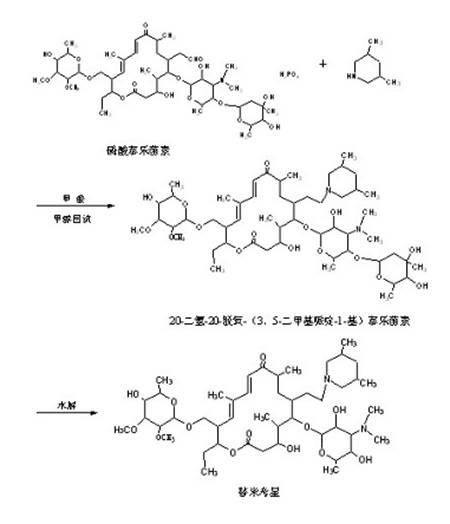
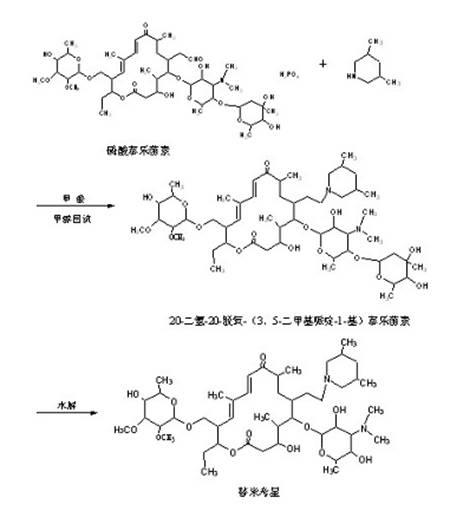
The invention discloses a preparation method of tilmicosin. The method comprises the following steps of: reacting tylosin phosphate serving as a raw material with 3,5-lupetidine by taking alcohol as a solvent and taking anhydrous formic acid as a catalyst at 65-97 DEG C to obtain 20-dihydro-20-deoxy-(3,5-lupetidine-1-radical)tylosin serving as an intermediate; concentrating the solvent; and adding residues into 0.1N sulfuric acid solution for hydrolyzing to obtain tilmicosin, wherein the mass volume ratio of the tylosin phosphate to the alcohol is 1:(2-10), the mass ratio of the tylosin phosphate to the 3,5-lupetidine to the anhydrous formic acid is 1:(0.11-0.17):(5.6-8), and the mass volume ratio of the tylosin phosphate to the 0.1N sulfuric acid solution is 1:(2-6). The reaction formula is shown in the specifications. By adopting the method, the defects of the prior art can be overcome. The method is easy to control, and has high yield and low production cost.
Owner:QILU ANIMAL HEALTH PROD +1
Tilmicosin solid dispersible granule as well as preparation method and application thereof
ActiveCN103830187ASimple processEasy to realize industryAntibacterial agentsOrganic active ingredientsParaffin waxFormulary
The invention discloses a tilmicosin solid dispersible granule as well as a preparation method and an application thereof. The tilmicosin solid dispersible granule consists of tilmicosin and a carrier accessory. The carrier accessory is one of glycerin monostearate, stearyl alcohol, saturated triglyceride, glycerinum simple lipid, paraffin wax, animal wax, vegetable wax or fatty powder or mixture thereof. The preparation method of the tilicosin solid dispersible granule comprises the following steps: weighing respective carrier accessories according to the formula proportion, heating, melting and mixing uniformly; adding the tilmicosin, stirring uniformly, cooling, pelleting through spraying of a fluidized bed, balling, cooling, sieving and collecting to obtain the tilmicosin solid dispersible granule. The tilmicosin solid dispersible granule solves the problem of medicine palatability, by feed mixing administration, the fluidity and dispersibility are good and medication is convenient; after animals take the granule, the medicine releases slowly, so that the safety is high; besides, the biological availability of tilmicosin medicine is enhanced, and the clinical using effect is remarkable.
Owner:SOUTH CHINA AGRI UNIV
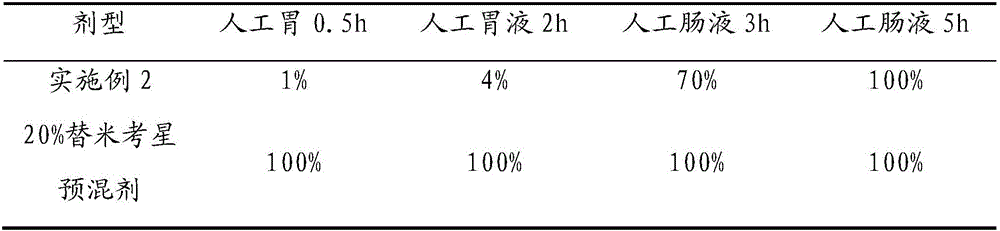
Enteric-coated tilmicosin slow-release micro-capsule preparation and preparation method thereof
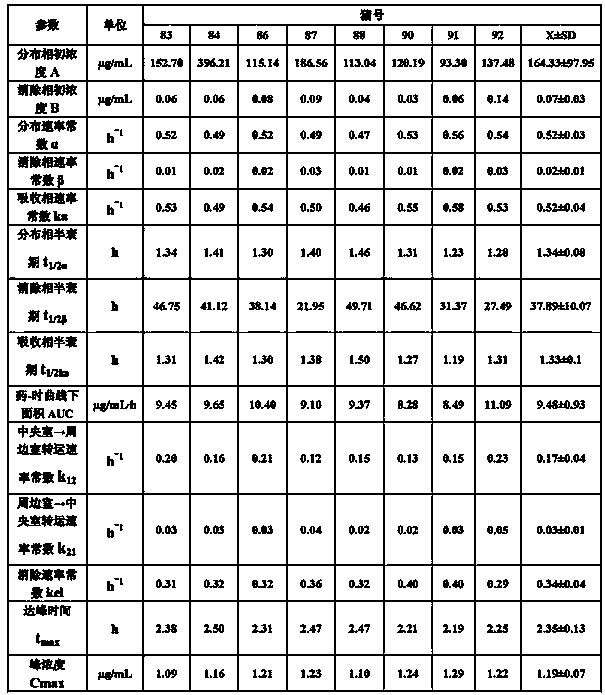
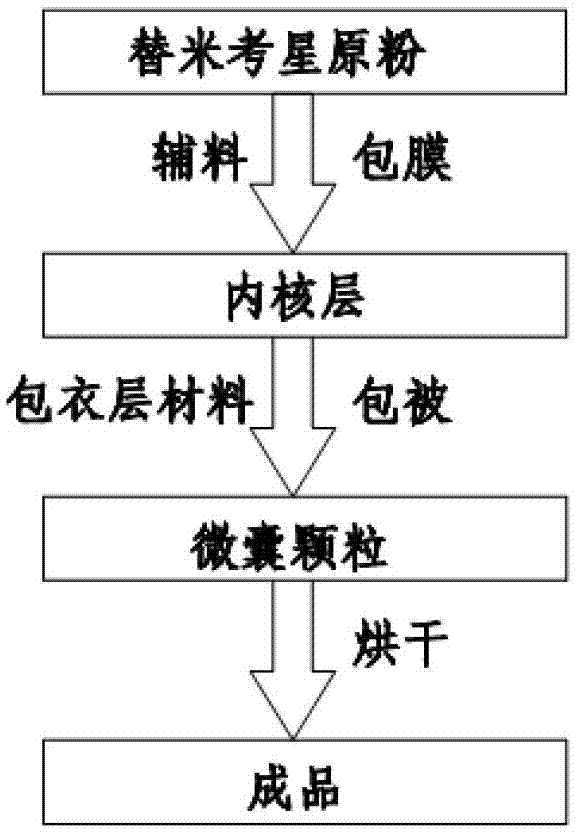
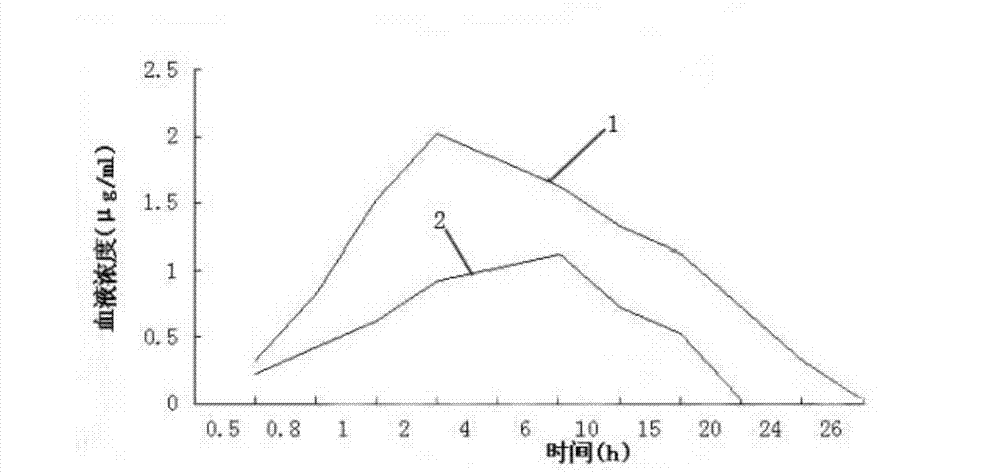
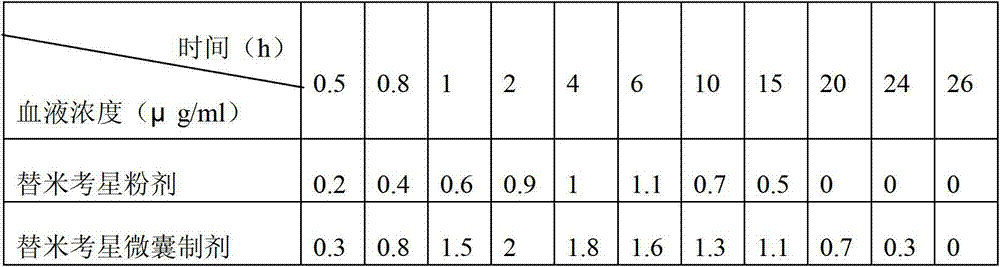
InactiveCN103083281AProlong the action timeTo achieve the purpose of sustained releaseAntibacterial agentsOrganic active ingredientsMonoglycerideAcrylic resin
The invention relates to an enteric-coated tilmicosin slow-release micro-capsule preparation and a preparation method thereof and belongs to the field of tilmicosin preparations. The enteric-coated tilmicosin slow-release micro-capsule preparation provided by the invention comprises an inner core layer and a coating layer, wherein the inner core layer comprises tilmicosin raw powder and an auxiliary material; the auxiliary material comprises one or more than one of stearic acid, glycerin monostearate, stearyl alcohol, saturated triglyceride, monoglyceride and paraffin; and the coating layer is made from one or more than one of cellulose acetate phthalate, hydroxypropyl methyl cellulose phthalate, acrylic resin, polyvinyl acetate phthalate and acetic hydroxypropyl methylcellulose succinate. The preparation method comprises the following steps of: carrying out primary coating on the tilmicosin raw powder and the auxiliary material, carrying out secondary coating by using the materials of the coating layer, and drying to obtain the finished product. According to the invention, the tilmicosin is coated by using high polymer materials and the coated tilmicosin micro-capsule is undissolved in acid environment and slowly dissolved in alkaline environment of enteric canal, so that the purpose of slow release is achieved and the action time of the tilmicosin is prolonged.
Owner:GUANGZHOU GREAT BIOLOGICAL TECH
Tilmicosin dry suspension, method for preparing dry suspension and uses thereof
ActiveCN103054808AGood dispersionImprove palatabilityAntibacterial agentsPowder deliveryExcipientTilmicosin
The present invention provides a tilmicosin dry suspension. The invention also provides a method for preparing the dry suspension and uses thereof. According to the tilmicosin dry suspension of the present invention, via reasonable compatibility of excipients, dispersion of tilmicosin in water is significantly improved, so the tilmicosin is enabled to be evenly distributed in the water in a long time, convenient for clinical administration of medicine by drinking water. Meanwhile, the dry suspension has greatly improved the palatability of the tilmicosin, so the dry suspension can be used not only for animal groups with underdeveloped taste such as chickens, but also for other animal groups with sensitive taste such as pigs, thus expanding the scope of application of the product.
Owner:CHENGDU QIANKUN VETERINARY PHARMA
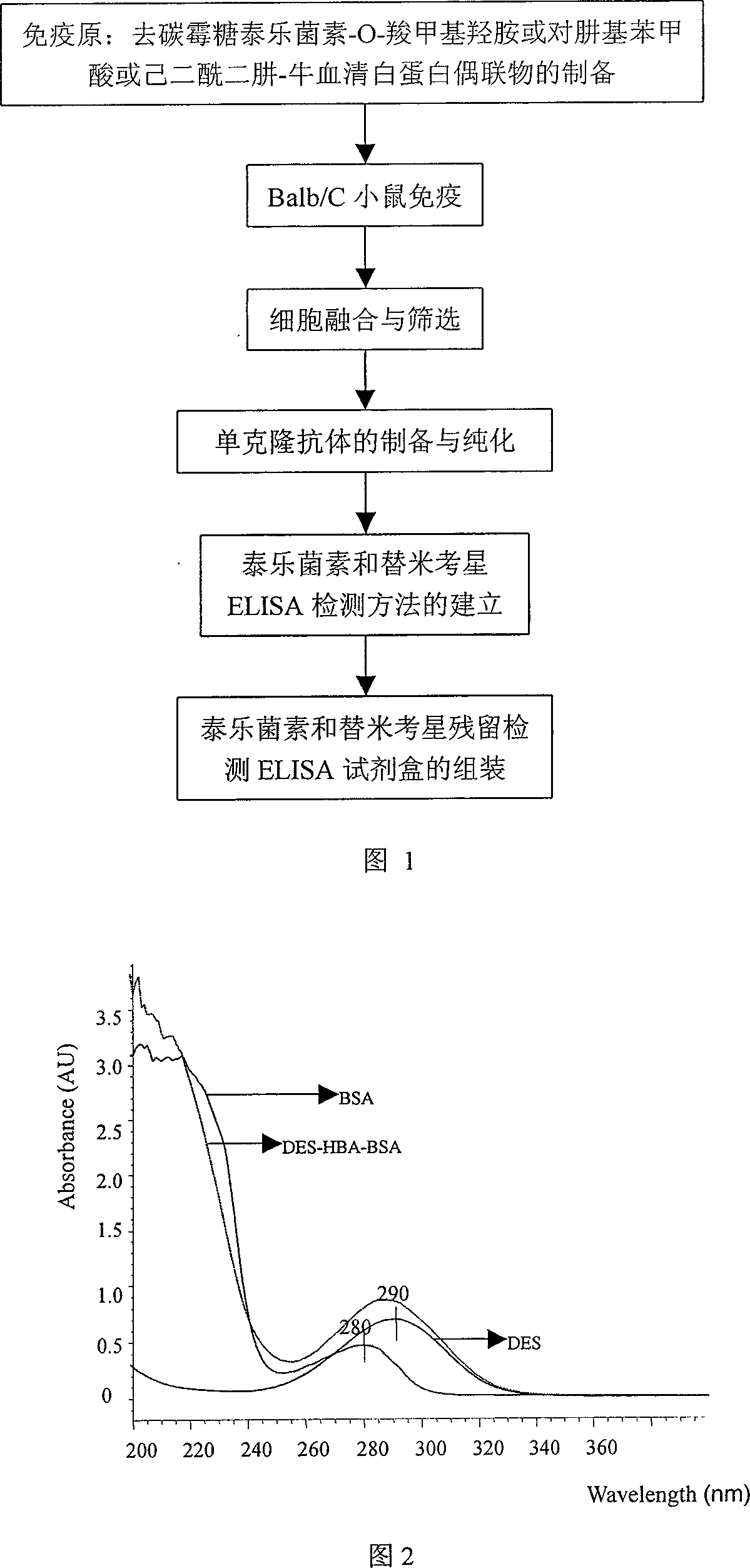
Monoclonal antibody and enzyme-linked immunoassay method and reagent kit for detecting tylosin and tilmicosin residue
InactiveCN101105492AIncrease typeSave analysis timesFused cellsBiological testingEnzyme linked immunoassayHybridoma cell
The invention belongs to the veterinary medicine residual analysis and immunity analysis technique field and specifically relates to an enzyme-linked immunity method and the reagent kit thereof which can discern specificity monoclonal antibodies of tylosin and tilmicosin and detect the residuals of tylosin and tilmicosin at the same time; the monoclonal antibodies in the invention is secreted from hybridoma cell strain P3C4 established by the applicant; the hybridoma cell strain is preserved in China Center of Type Culture Collection; the number of preservation of the hybridoma cell strain is CCTCC No: C200719; the invention discloses the preparation method and enzyme-linked immunity detection method of the monoclonal antibody, coating antigen and immunogen. Compared with the prior art, the monoclonal antibody prepared in the invention can discern tylosin and tilmicosin at the same time and add the detecting object in the prior art. The reagent kit and the method in the invention has the advantages of simple, convenience, swiftness, sensitivity and accuracy; furthermore, the reagent kit and method in the invention can detect the residuals of tylosin and tilmicosin in an animal edibility tissue at the same time.
Owner:HUAZHONG AGRI UNIV
Tilmicosin micro-capsule preparation and preparation method thereof
InactiveCN102688220AMask bitternessProlong the action timeAntibacterial agentsOrganic active ingredientsWaxAcrylic resin
The invention relates to a tilmicosin micro-capsule preparation, and in particular relates to a tilmicosin micro-capsule preparation and a preparation method of the tilmicosin micro-capsule preparation, belonging to the technical field of veterinary medicine. The tilmicosin micro-capsule preparation comprises an inner core layer and a coating layer, wherein the inner core layer comprises a tilmicosin raw material and macromolecule auxiliary materials, the macromolecule auxiliary materials are selected from one or more than one of 12-carbon fatty acid-18-carbon fatty acid, paraffins and vegetable wax; and the coating layer comprises an inner coating layer and an outer coating layer, wherein the material of the inner coating layer is one or more than one of starch, calcium carbonate and calcium hydrophosphate, and the material of the outer coating layer is acrylic resin. The tilmicosin micro-capsule preparation disclosed by the invention is tasteless, enteric, and good in palatability. According to the invention, the tilmicosin is packed by the macromolecule auxiliary materials, a layer of enteric coating layer is sprayed on the surfaces of the tilmicosin grains in the process of rolling circle when the materials are prepared, and the strong bitter taste and odor of the tilmicosin can be completely covered due to the double-layer package, and the preparation method is simple in technology, and low in cost.
Owner:HUZHOU AIBAOLAI ANIMAL PHARMA
Taste-masking tilmicosin gastric-soluble particle preparation
The invention relates to a taste-masking tilmicosin gastric-soluble particle preparation, in particular to a gastric-soluble particle preparation consisting of a gastric-soluble coating material, a high-polymer carrier, a disintegrating agent and the like and aims to solve the problem of poor palatability of tilmicosin.
Owner:烟台爱士津动物保健品有限公司
Enteric-coated tilmicosin sustained release microcapsule and preparation method thereof
InactiveCN106176680AProlong the action timeTo achieve the purpose of sustained releaseAntibacterial agentsOrganic active ingredientsDispersityAcrylic resin
The invention discloses an enteric-coated tilmicosin sustained release microcapsule and a preparation method thereof, and belongs to the field of tilmicosin preparations. The enteric-coated tilmicosin sustained release microcapsule is prepared from 10wt%-50wt% of raw tilmicosin powder, 40wt%-88wt% of an auxiliary fatty powder material and 2wt%-10wt% of an enteric coating material, wherein the enteric coating material is prepared from one or more of cellulose acetate phthalate, hydroxypropyl methylcellulose phthalate, L-type acrylic resin, S-type acrylic resin, polyvinyl acetate phthalate and hydroxypropyl methylcellulose acetate succinate; the diameter of the prepared microcapsule is 50-200 mu m. The preparation method comprises the steps as follows: the raw tilmicosin powder and the auxiliary material are subjected to primary coating, are subjected to secondary coating with the enteric coating material and then are dried, and a finished product is obtained. According to the enteric-coated tilmicosin sustained release microcapsule and the preparation method thereof, the sustained release purpose is achieved, the acting time of tilmicosin is prolonged, the fluidity and the dispersity of a drug are improved, the pharmacodynamical function is remarkably improved, and the dosage of the drug is reduced.
Owner:GUANGZHOU GREAT BIOLOGICAL TECH
Soluble and stable tilmicosin composition
ActiveCN101496811AStable in natureSolve the problem of water solubilityAntibacterial agentsOrganic active ingredientsEscherichia coliDisease
The invention relates to a soluble and stable Tilmicosin composition. The soluble preparation consists of the following components: Tilmicosin, a latent solvent, a pH value stabilizing agent and proper auxiliary materials. The Tilmicosin obtained by the invention can be quickly dissolved into water and the effective components cannot be degraded with the prolonging of the placing time of an aqueous solution. The preparation composition can be combined with other substances for use so as to strengthen the drug effect. The composition is mainly used for treating respiratory tract infection, mixed infection of mycoplasma and Escherichia coli, other various pneumonia and pulmonary infectious diseases of livestock and poultry caused by Pasteurella, the mycoplasma, and the like.
Owner:RINGPU TIANJIN BIOLOGICAL PHARMA
Compound Tilmicosin nanoemulsion antibacterial agent and preparation method thereof
InactiveCN101983632AReduce energy consumptionImprove securityAntibacterial agentsOrganic active ingredientsDistilled waterPhysical chemistry
The invention discloses a compound Tilmicosin nanoemulsion antibacterial medicament with a particle size of 1-100 nm, which comprises, by mass, 0.01%-15.2% of Tilmicosin, 0.001%-2.12% of Florfenicol, 13.38%-36% of surfactant, 0%-26.5% of cosurfactant, 2.43%-5.32% of oil, 0.004%-2.12% of Florfenicol solubilizer, 0%-2.07% of Tilmicosin solubilizer and 14.89%-84.17% of distilled water. The sum of the mass percentages of the above components is 100%. The medicament is primrose, clear and transparent liquid in appearance and is characterized by low viscidity, good stability, high dispersibility, quick absorption and the like. The medicament has the advantages of higher bioavailability, longer half-life in a body, lower toxicity and sustained releasing and targeting effect.
Owner:NORTHWEST A & F UNIV
Tilmicosin smell masking preparation and preparing method thereof
InactiveCN104958764AMaintain the efficacy of the drugReduce exposureAntibacterial agentsPowder deliverySolubilityGastric fluid
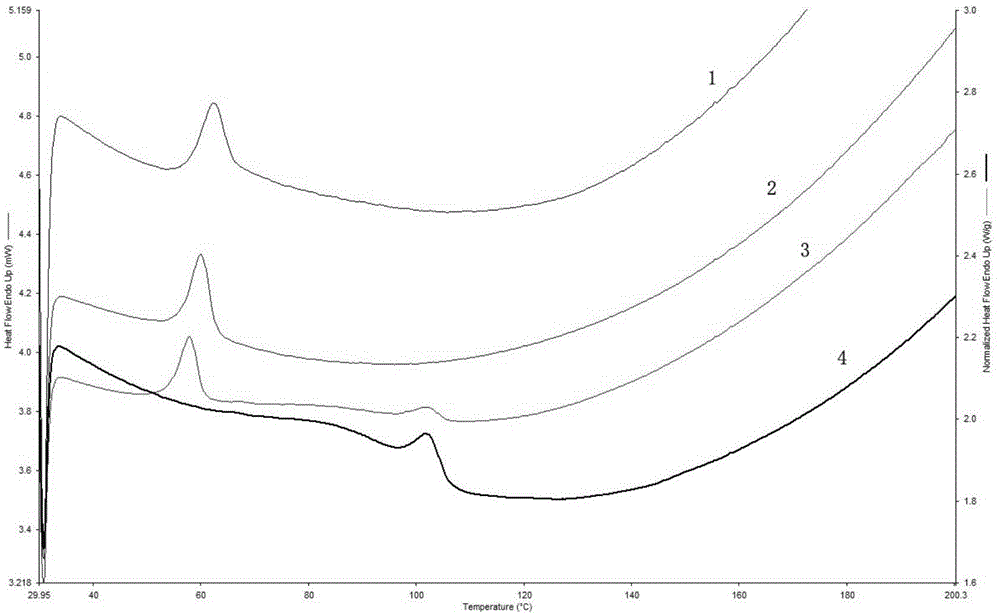
The invention discloses a tilmicosin smell masking preparation and a preparing method thereof, and belongs to the field of antibiotic preparations for livestock. According to the smell masking preparation, tilmicosin and gastric solubility high polymer materials serve as raw materials; and the smell masking preparation is prepared through the steps of fusion through an extruding machine, shearing and conveying. Compared with the prior art, digestion of the tilmicosin smell masking preparation in artificial saliva is slow, so that the effect of stopping releasing of the medicine in the oral cavity is achieved; contact between the medicine and the taste bud of the tongue is less, so that the bitter and hemp tastes can be obviously reduced, and the masking effect is achieved; and the medicine is rapidly released in a 0.01 mol / L hydrochloric acid solution (simulated gastric fluid), and the medicine keeps to play the medicine effect. In addition, the tilmicosin smell masking preparation is prepared with the hot melting extrusion technology, the technology is advanced, the process is simple, organic solvents are not used, and therefore the tilmicosin smell masking preparation is safe and free of pollution; mixing is carried out without a dead corner, the dispersion effect is good, and the medicine losses are fewer; and operation of multiple units is integrated together, so that space is saved, the cost is reduced, and the tilmicosin smell masking preparation is suitable for industrial large-scale production.
Owner:GUANGXI UNIV
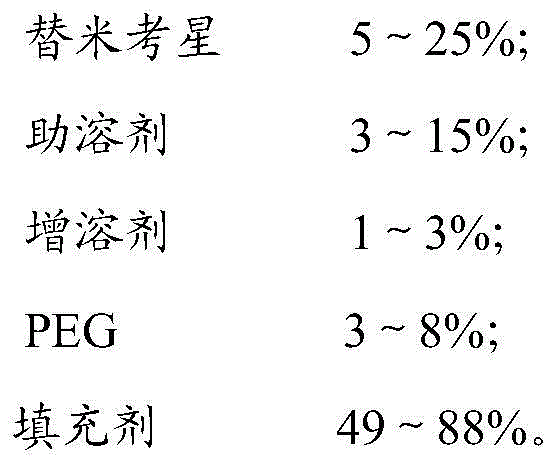
Tilmicosin premix and preparation method thereof
ActiveCN104940147AGood curative effectReduce dosageOrganic active ingredientsPharmaceutical non-active ingredientsAntioxidantBioavailability
The invention relates to the field of medicines for livestock and poultry, and particularly provides a tilmicosin premix and a preparation method thereof. The tilmicosin premix is prepared from the following raw materials in percentage by mass: 5%-50% of tilmicosin, 0.5%-20% of an adhesive and 0.05%-2% of an antioxidant. According to the tilmicosin premix provided by the invention, the medicine odor of the tilmicosin is covered; ingestion of animals is not affected; the tilmicosin premix is wide in raw and supplemental material source, low in cost, and relatively high in absorption speed in an animal body; the bioavailability is greatly improved, so that the dosage of the tilmicosin is reduced; the medicine effect is improved; and the cost is also reduced.
Owner:湖南慧谷农业生态研究院有限公司
Process for preparing tilmicosin pellets
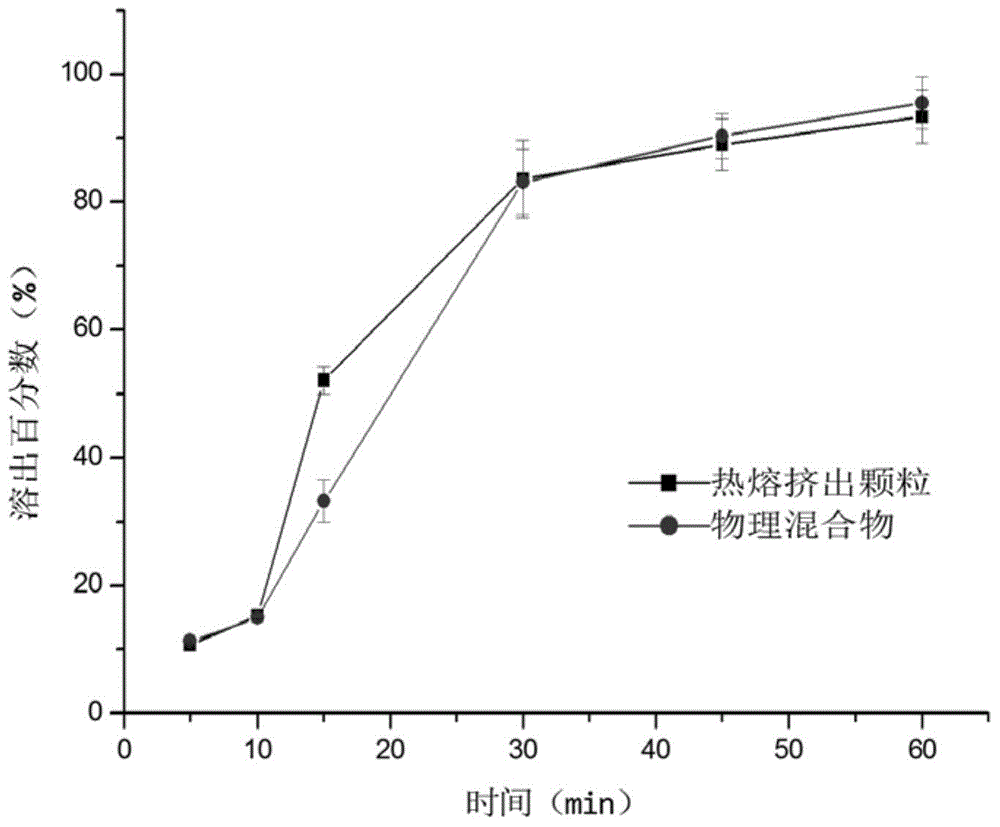
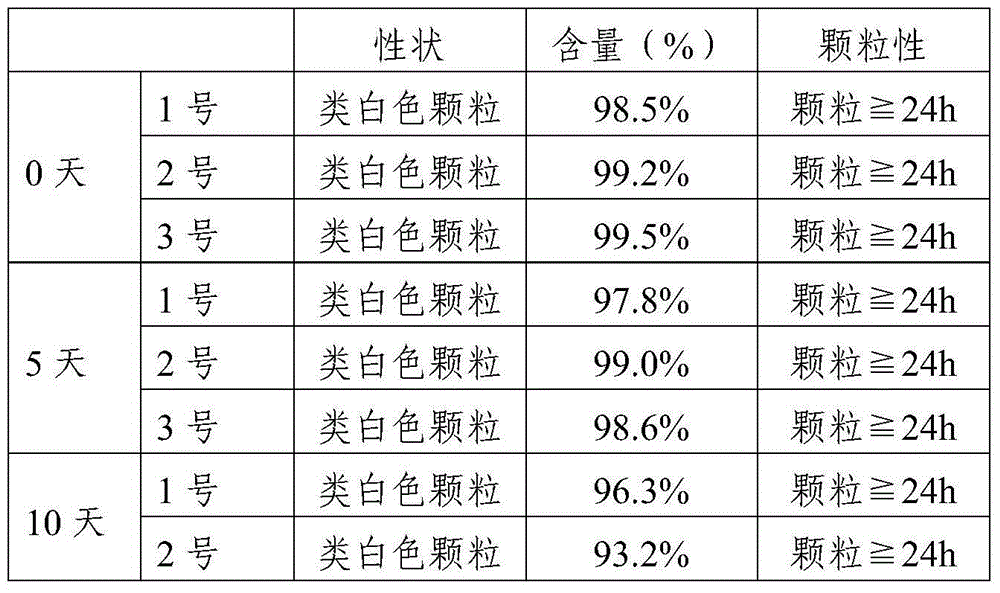
ActiveCN104586774AEasy to useIncrease blood concentrationAntibacterial agentsOrganic active ingredientsSustained release pelletsFluidized bed
The invention provides a process for preparing tilmicosin pellets. The process comprises the following steps: a, uniformly mixing tilmicosin with a diluting agent and a disintegrating agent, adding ethanol, wetting, screening, rounding the particles in a rounding machine, and drying to obtain matrix type sustained-release pellets I; b, dissolving adhesive and carbomer in a 80% ethanol solution, adding tilmicosin into the ethanol solution to constantly stir to form a uniform suspension, adding the pellets I which are prepared in the step a and serve as pill cores into a fluidized bed turntable, spraying the suspension on the surfaces of the pill cores, drying, and collecting the dried pellets II; c, dissolving a coating agent in 80% ethanol, coating the pellets II, and drying to obtain the tilmicosin pellets. The tilmicosin pellets are stable and convenient to use, the bitter taste can be covered, and the pellets can be pressed into pellet tables for pets. The process is suitable for industrial production and popularization application.
Owner:CHENGDU QIANKUN VETERINARY PHARMA
Enteric targeted tilmicosin particles and preparation method thereof
InactiveCN102068412AAvoid destructionSolve the problem of difficult drug administrationAntibacterial agentsOrganic active ingredientsIrritationMicroparticle
The invention discloses enteric targeted tilmicosin particles and a preparation method thereof, which relate to the enteric targeted particles and the preparation method thereof and aim to solve the problems of difficulties in administration in an administration process. The enteric targeted tilmicosin particles consist of tilmicosin, a bonding agent and a coating fluid. The preparation method for the enteric targeted tilmicosin particles is implemented by the following steps of: 1, preparing the bonding agent; 2, preparing particles; 3, preparing the coating fluid; 4, coating the particles; and 5, drying the particles. In the invention, enteric coating technology is adopted, the tilmicosin is prepared into the enteric targeted particles, and the intrinsic bitterness and irritation of the medicaments are hidden, thereby solving the problems of difficulties in the administration. The preparation method is particularly applied in the field of preparation of the enteric targeted particles.
Owner:HEILONGJIANG UNIV
Tilmicosin enteric micro pellet
InactiveCN104606141AImprove stabilitySolve the problem that the masking effect is limited and it is difficult to really mask the bitternessAntibacterial agentsOrganic active ingredientsMedicineTilmicosin
The invention relates to a tilmicosin enteric micro pellet. The tilmicosin enteric micro pellet is composed of tilmicosin, a high molecular carrier, a binder, and a coating liquid. Problems of tilmicosin, such as poor palatability and instability under acidic conditions, are solved, and clinical using amount is increased.
Owner:RINGPU TIANJIN BIOLOGICAL PHARMA
Process for preparing tilmicosin enteric-coated pellet by centrifuge method
ActiveCN104784123ASave spaceSimple process equipmentAntibacterial agentsOrganic active ingredientsAdhesiveBitter taste
The invention discloses a process for preparing a tilmicosin enteric-coated pellet by a centrifuge method. The tilmicosin enteric-coated pellet is prepared from the following raw materials in percentage by mass: 21-26% of tilmicosin, 10-20% of an adhesive, 23-30% of microcrystalline cellulose and 10-20% of a coating material, and is prepared through the following steps: performing centrifugal pelleting on the raw materials, and coating the pellet obtained through centrifugal pelleting. The tilmicosin enteric-coated pellet is high in encapsulation efficiency, can mask the bitter taste of tilmicosin well, is dissolved out in an intestinal environment, can be used as an enteric-coated sustained-release reagent, solves the adverse reaction caused after the tilmicosin enteric-coated pellet is absorbed in stomach, is simple in preparation process, and is suitable for industrial large-scale production, thereby having a very wide application prospect; besides, the raw materials are cheap and easy to obtain.
Owner:WEIDA HUNAN TECH
Preparation method of tilmicosin liposomes
InactiveCN101647778AImprove stabilityNo side effectsAntibacterial agentsOrganic active ingredientsLipid formationYolk
The invention relates to a preparation method of tilmicosin liposomes. In the technical scheme, every 3g of tilmicosin raw powder is processed into tilmicosin liposomes by the following steps: (1) preparing a PBS buffer solution with the pH value of 6.5 to 7.5; (2) mixing egg yolk lecithin, cholesterol, octadecylamine, polysorbate-80 and ether in the proportion into a solution; (3) mixing the tilmicosin raw powder with the PBS buffer solution obtained in the step (1), and then pouring the mixture into the solution prepared in the step (2) and uniformly stirring; pulverizing the mixture by ultrasound into a suspension which does not delaminate within at least 5min; (4) transferring the suspension in the step (3) into a rotary evaporator to perform pressure-reduction rotary evaporation so that the suspension is condensed on the wall of the rotary evaporator to form lipid membranes; and (5) washing off the lipid membranes completely with the PBS solution obtained in the step (1), and thencontinuing the pressure-reduction evaporation until the ether is drained entirely to obtain tilmicosin liposome crude products. The method has the advantages of simple steps and strong operability. The prepared tilmicosin liposomes can be easily absorbed after being injected into livestock and poultry organisms; and the phenomenon of local swelling can not be caused.
Owner:ZHENGZHOU HOUYI PHARMA
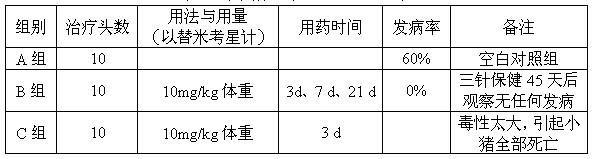
Method for treating bacterial infections in horses or pigs with tilmicosin
InactiveUS20060094669A1Low toxicitySmall investment in timeAntibacterial agentsBiocideSolventTilmicosin
The invention relates to methods of treating a bacterial infection in a horse or a pig. The method involves administering to the horse or pig a composition comprising (i) a salt made from tilmicosin and a lipophilic acid and (ii) a pharmaceutically acceptable solvent combined together to form an injectable composition that precipitates when injected into water
Owner:IDEXX LABORATORIES
Tilmicosin controlled-release microcapsule preparation containing plant essential oil and preparation method thereof
InactiveCN106727436AEffective controlIncrease production capacityAntibacterial agentsOrganic active ingredientsWaxTilmicosin
The invention relates to the technical field of veterinary medicines, and discloses a tilmicosin controlled-release microcapsule preparation containing plant essential oil and a preparation method thereof. The tilmicosin controlled-release microcapsule preparation containing the plant essential oil is prepared from raw materials with the mass percentage of 10% to 50% and the balance of an auxiliary material by adopting mist spraying condensation granulation, wherein the raw materials comprise tilmicosin and the plant essential oil; and the auxiliary material is the mixture of one or more of fatty powder, hydrogenated vegetable oil, stearic acid, stearyl alcohol, glycerin monostearate, monoglyceride and wax. The tilmicosin and the plant essential oil are simultaneously contained in the tilmicosin controlled-release microcapsule preparation containing the plant essential oil, the animal diseases are prevented and controlled, the using amount of antibiotics is reduced, and the pollution caused by the antibiotics is reduced; the bitter taste of the tilmicosin is covered up, the ingestion of animals is induced, and the preparation can be dissolved until reaching intestinal tracts, so that the holding time of effective blood concentration of medicine is prolonged, and the cross contamination caused in the feed mixing process is eliminated.
Owner:FOSHAN STANDARD BIO TECH
Tilmicosin soluble powder and preparation method thereof
ActiveCN104473876AImprove performanceComplianceAntibacterial agentsOrganic active ingredientsAdditive ingredientCombinatorial chemistry
Owner:SHANGHAI TONGREN PHARM CO LTD
Tilmicosin liposome preparation and preparation method thereof
InactiveCN101422435AHigh content of tilmicosinHigh encapsulation efficiencyAntibacterial agentsOrganic active ingredientsCirculatory timeCurative effect
The invention relates to a Tilmicosin lipidosome preparation and a preparation method. The Tilmicosin lipidosome preparation consists of the Tilmicosin lipidosome and the acceptable vector of a medicament; wherein, the Tilmicosin lipidosome contains Tilmicosin, phospholipid and cholesterin with the weight ratio of 1 : 2 to100 : 1 to 15; the acceptable vector of the medicament comprises an internal buffer system, alkali used for regulating pH, and a frozen-dried supporting agent; the adding amount of the frozen-dried supporting agent calculated according to the weight ratio of the phospholipid is as follows: 0.2 to 4 portions of the frozen-dried supporting agent is added into one portion of the phospholipid; the adding amount of the internal buffer system is 5 to 15mL. The invention has the advantages that: the Tilmicosin content in the unit volume of the Tilmicosin lipidosome preparation is high; the envelop rate is high; the stability is good; moreover, the medicament-loading rate is stable. The medicament in the lipidosome is continuously released, thus remarkably improving the concentration of blood medicament and prolonging the circulating time of the medicament in the blood. The Tilmicosin lipidosome preparation improves the curative effect of the medicament and enhances the clinic usability of the medicament.
Owner:ZHEJIANG KING TECHINA TECH
Tilmicosin liposome injection and preparation method thereof
ActiveCN102327225ASmall toxicityImprove immunityAntibacterial agentsOrganic active ingredientsSide effectMedicine
The invention belongs to the technical field of veterinary drugs, and particularly relates to a tilmicosin liposome injection and a preparation method thereof. The particle diameter of the tilmicosin liposome injection is 7-10mu m, and every 100ml of injection contains the following components: 5-10g of tilmicosin, 3-5g of soya bean lecithin, 1.5-2.5g of cholesterol, 2g of non-ionic surfactant, 20ml of solvent and the balance of water for injection. The tilmicosin liposome injection provided by the invention greatly reduces the toxic and side effects of the tilmicosin, and can improve the body immunity in use.
Owner:河南省帝一方生物制药有限公司
Natural compound feed additive for removing veterinary drug residues
InactiveCN104543404AAchieve productionGuarantee normal productionAnimal feeding stuffAstaxanthinVeterinary Drugs
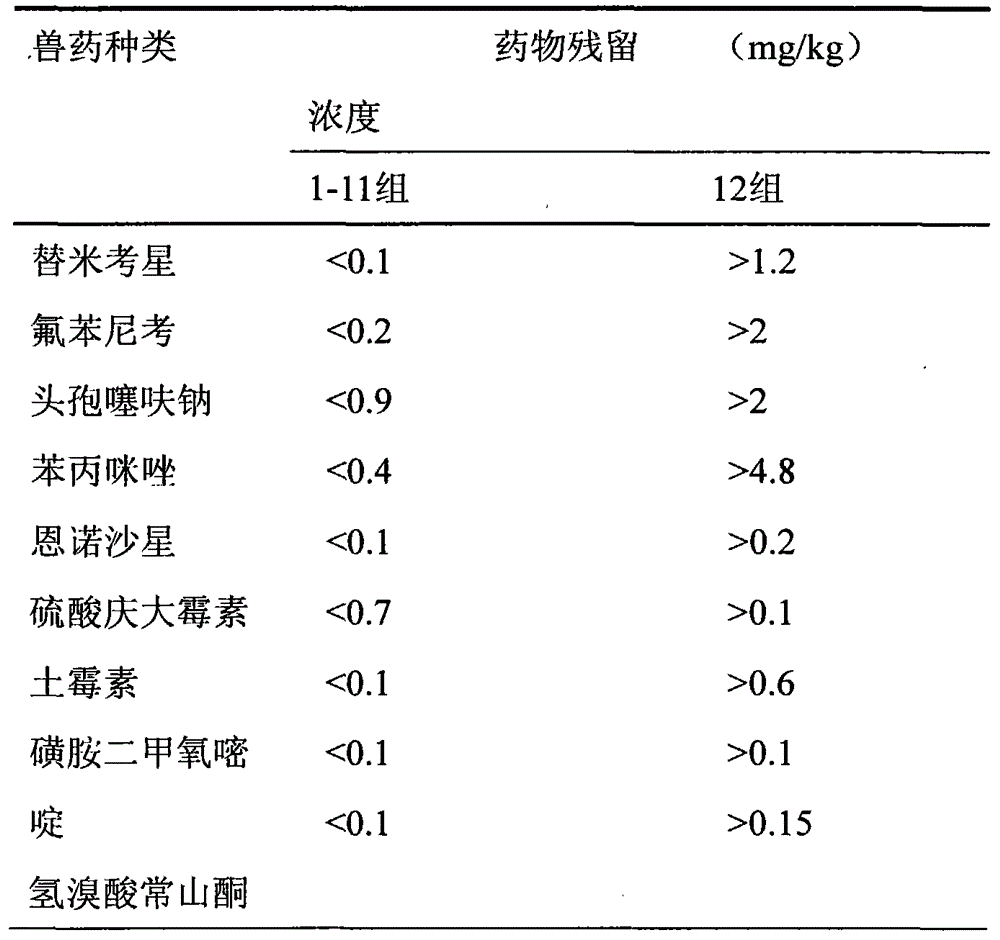
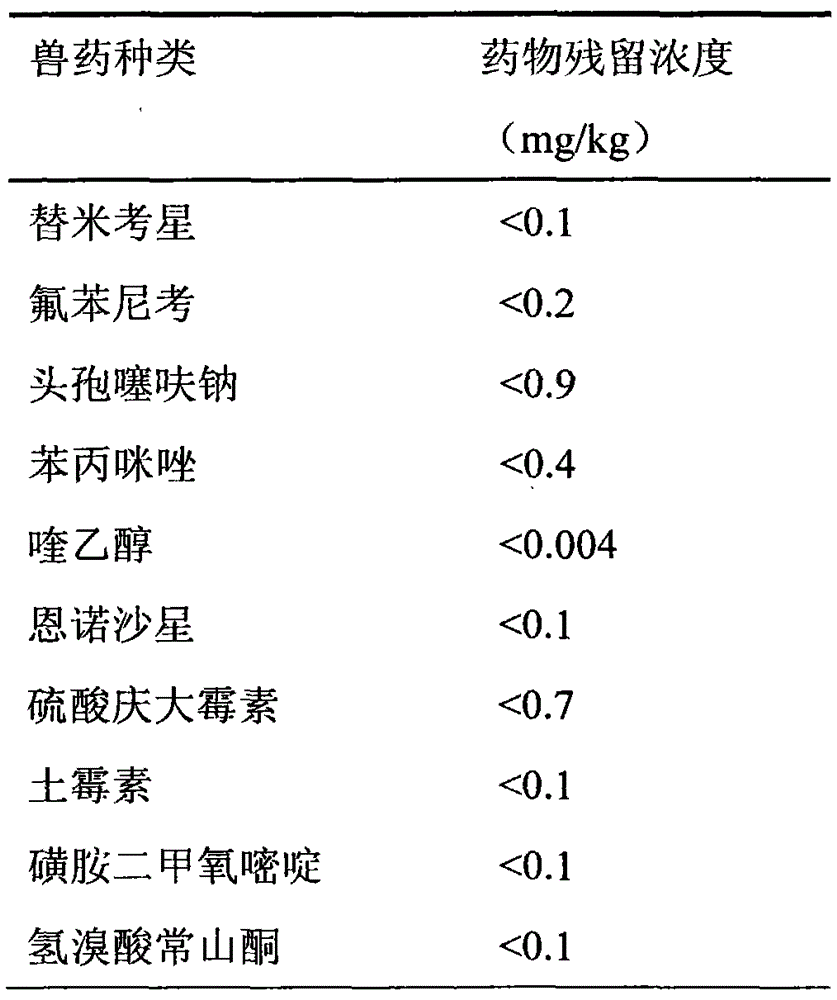
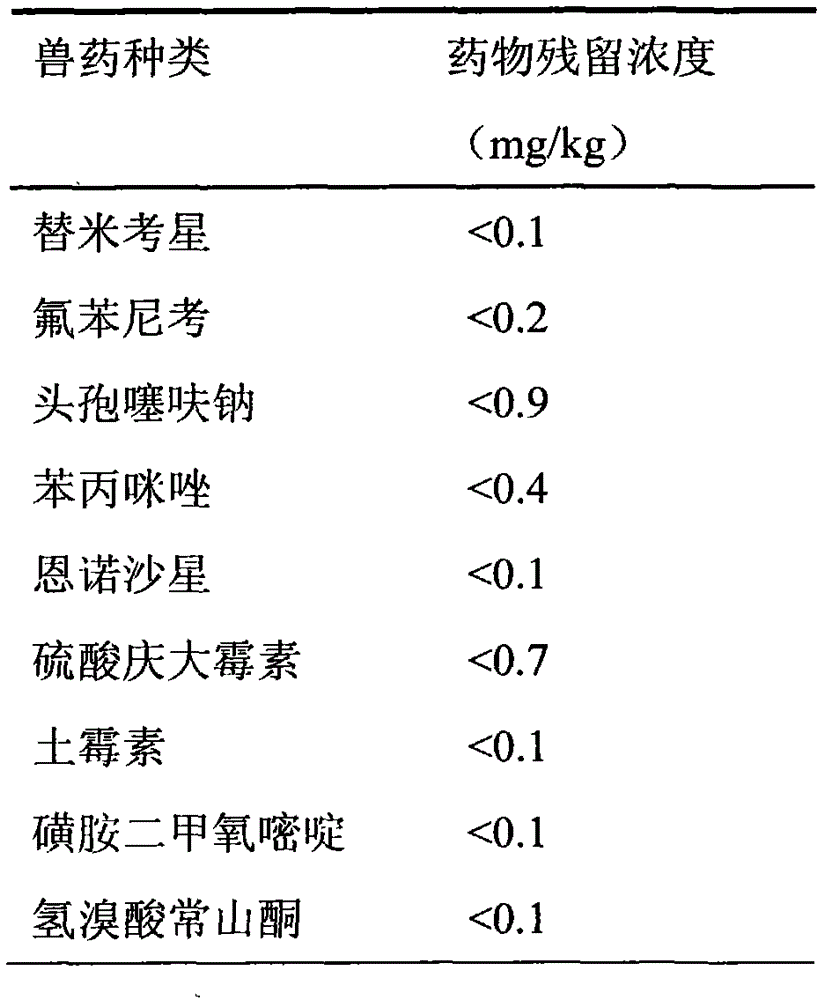
The invention discloses a natural compound feed additive for removing veterinary drug residues. The natural compound feed additive is prepared from the following components in parts by weight: 1 part of procyanidine, 1 part of astaxanthin, 1 part of resveratrol, 1 part of curcumin, 1 part of allicin, 1 part of porphyra polysaccharide, 1 part of porphyra polyphenol, 1 part of phycobiliprotein, 1 part of tea polyphenol, 1 part of quercetin, 1 part of chitosan and 1 part of tangeretin; and the ratio of the feed additive to the feed is (1:100) to (1:500) in use. According to the method, the residues of tilmicosin, florfenicol, ceftiofur sodium, thiabendazole, enrofloxacin, gentamicin sulfate, oxytetracycline, sulfadimethoxine and halofuginone hydrobromide in livestock or poultry bodies can be effectively reduced; the natural compound feed additive has the characteristics of being free of pollution and free of public hazard, and can be applied to removal of veterinary drug residues from food animals of which the veterinary drug residues exceed the standard due the factors such as veterinary drug application and environmental pollution, so as to ensure the hygiene and safety of animal food.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Tilmicosin stabilizing agent and preparation method thereof
ActiveCN102657607ANo adverse reactionGood curative effectAntibacterial agentsOrganic active ingredientsHydrogenBlood concentration
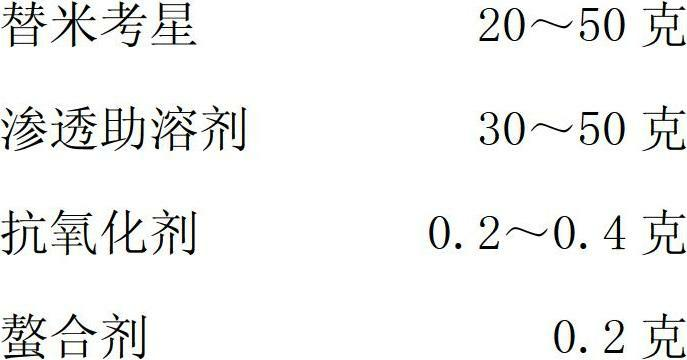
The invention relates to a tilmicosin stabilizing preparation. The preparation is neutral and comprises the following components in each 100 ml: 20-50 g of tilmicosin, 30-50 g of penetration cosolvent, 0.2-0.4 g of antioxidant and 0.2 g of chelating agent. According to the invention, the tilmicosin, the penetration cosolvent, the antioxidant and the chelating agent are integrated together to form a faint yellow homogeneous solution, and the pH (Potential of Hydrogen) is 7; when the tilmicosin stabilizing preparation is stored, the character is stable; when the tilmicosin stabilizing preparation is used, an animal organism does not have adverse reactions; the time of reaching a blood concentration peak is much shorter than that of a traditional injection solution, and a tissue seepage force is 4-5 times as much as that of the traditional injection solution, so that the tilmicosin stabilizing preparation is one stable preparation with good curative effects.
Owner:DINGZHENG XINXING BIOTECH TIANJIN
Novel preparation of tilmicosin and salt of same for livestock and preparation method thereof
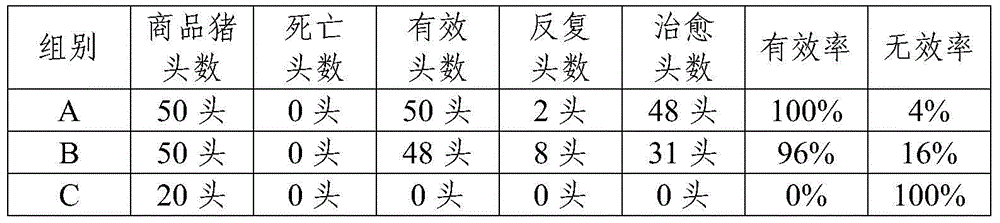
InactiveCN101590009AIncrease drug concentrationSignificant effectPowder deliveryOrganic active ingredientsGelatin microspheresSide effect
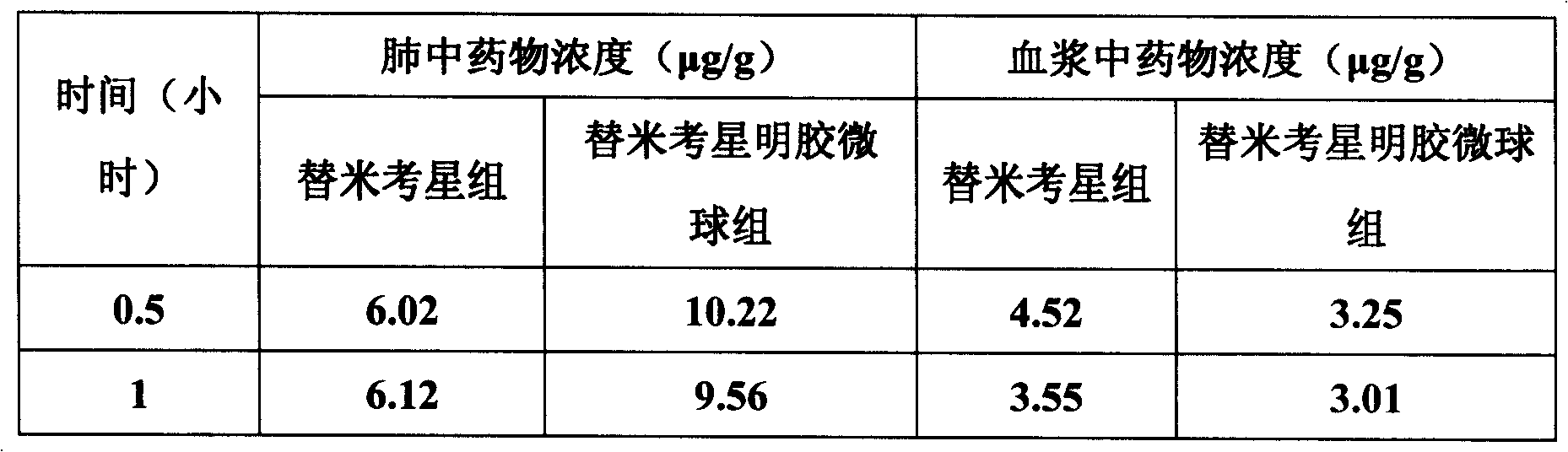
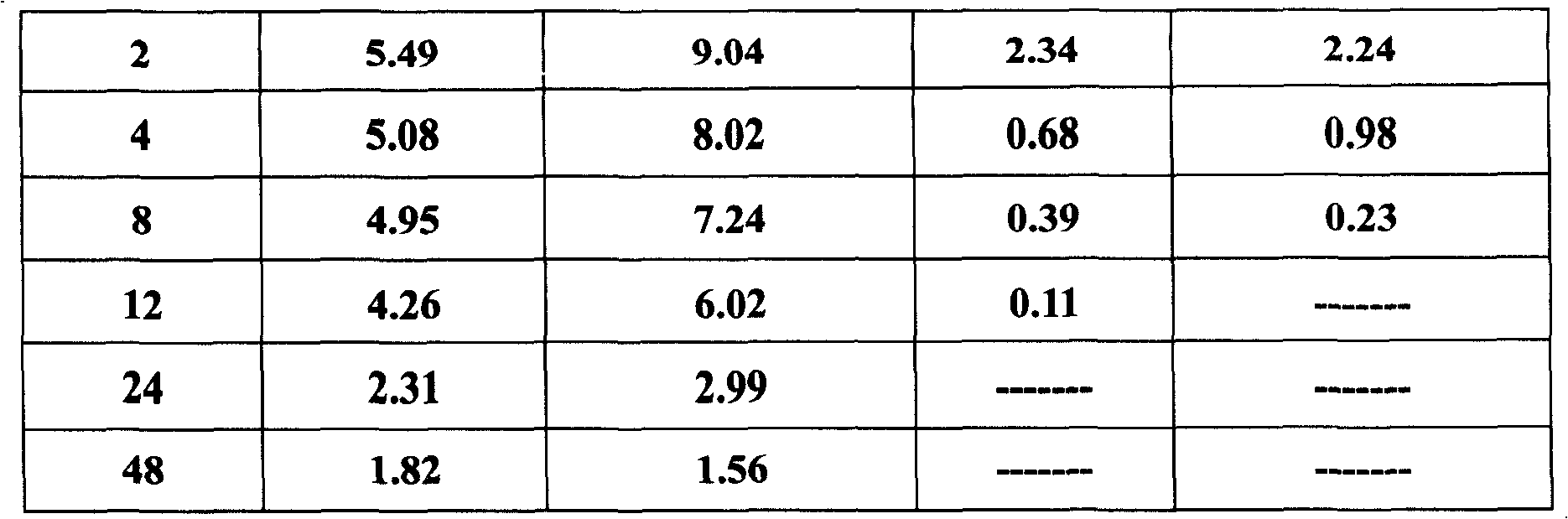
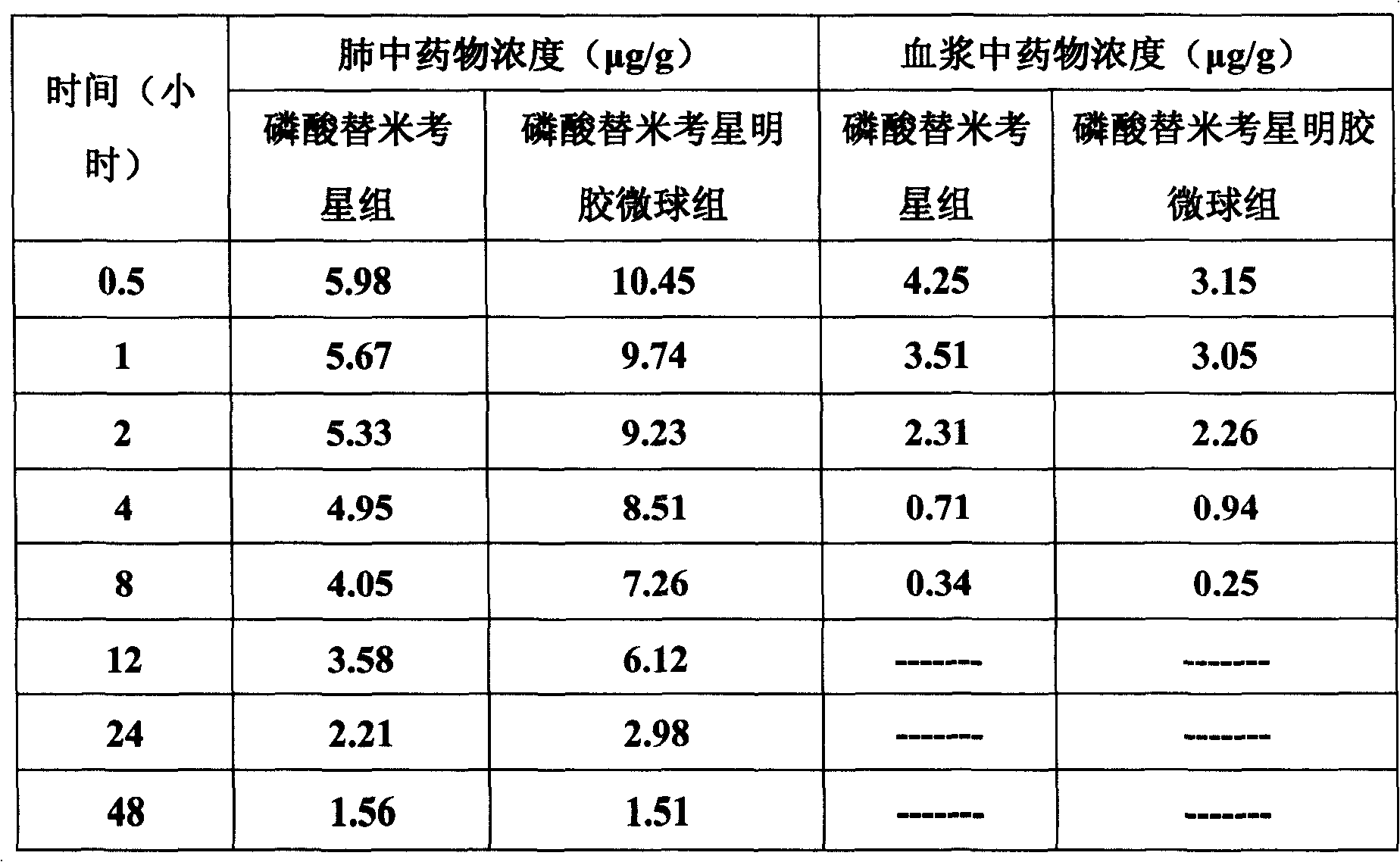
The invention belongs to the veterinary antibiotics preparation field and relates to a novel preparation of tilmicosin and salt of same for livestock and a preparation method thereof. The preparation is of lung targeting gelatin microsphere preparation by using tilmicosin or tilmicosin salt as principal components. Clinical drug experiments show that compared with conventional preparations the lung targeting gelatin microsphere of tilmicosin and tilmicosin salt of the invention has better organization selectivity, the drug concentration of lung tissue is higher, the duration is longer, the drug effect is longer and more effective and the side effect is low.
Owner:BEIJING DABEINONG ANIMAL HEALTH TECH +1
Soluble tilmicosin premix and preparation method thereof
ActiveCN104171433AImprove solubilityImprove bioavailabilityAntibacterial agentsOrganic active ingredientsAnimal sciencePharmaceutical drug
The invention relates to a premix of a veterinary drug for livestock and a preparation method of the premix, and particularly relates to a soluble tilmicosin premix and a preparation method thereof, belonging to the field of veterinary drug processing, and aiming at overcoming the technical defects of low feeding rate of tilmicosin series of products and low utilization rate of tilmicosin in the feed in the prior art. The bad smell and the irritation of tilmicosin are covered in the premix, so that the stability of the drug is greatly improved, and the irritation to the stomach is reduced, the dissolution concentration of tilmicosin is improved, and the bioavailability of organism is greatly increased.
Owner:GUANGDONG GALLOPER VETERINARY PHARMA
Tilmicosin micelle preparation and preparation method thereof
InactiveCN102397237AStrong retentionHigh encapsulation efficiencyAntibacterial agentsOrganic active ingredientsPharmaceutical formulationSuccinates
The invention belongs to the technical field of medicinal preparations and relates to a tilmicosin micelle preparation and a preparation method thereof. The tilmicosin micelle preparation comprises the following components in part by weight: 3 to 5 parts of tilmicosin, 5.0 to 15.5 parts of pharmaceutically acceptable carrier materials, 52 to 68 parts of poly(butylene succinate) (PBS ), 0 to 8 parts of additive and a pH modifier, wherein the pH value of the pH modifier is 5 to 7. In the tilmicosin micelle preparation, poloxamer 188 serves as the main micelle component. The poloxamer 188 is an asymmetric amphiphilic block copolymer and can automatically form the micelle in aqueous solution, wherein the hydrophobic block of the micelle gathers inwards and the hydrophilic block gathers outwards. Because the formed micelle is relatively smaller low in particle size, the micelle is higher in retention capability to an inflammatory part and stable onto heat and is not easy to oxidize. The entrapment rate and the drug loading capacity of the tilmicosin micelle preparation are high.
Owner:HENAN SOAR VETERINARY PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com