Enteric-coated tilmicosin slow-release micro-capsule preparation and preparation method thereof
A tilmicosin and slow-release technology, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problem of affecting animal feed intake, no slow-release function, antibacterial and growth-promoting Poor efficacy and other problems, to achieve the effect of avoiding the first pass effect of the liver, good palatability, and improving drug efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
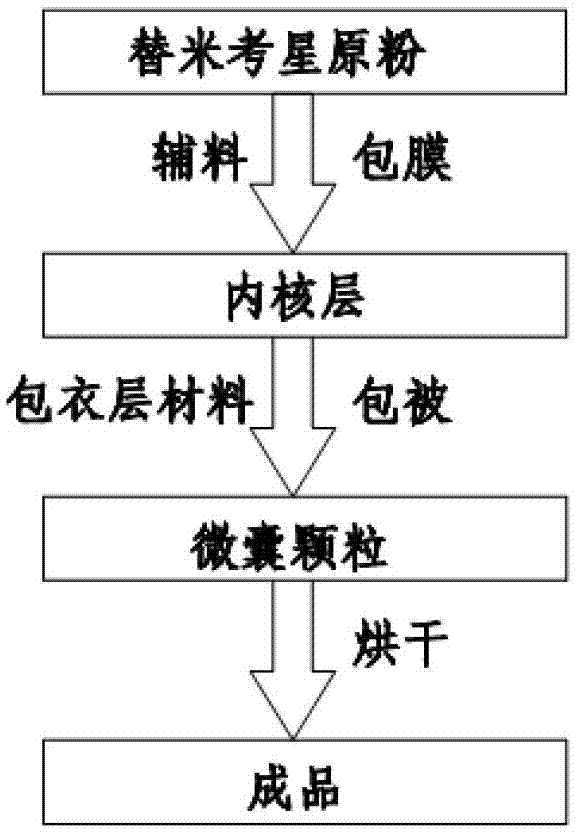
[0028] A preparation method of enteric-coated tilmicosin sustained-release microcapsule preparation, comprising the following steps:
[0029] Step 1. Add tilmicosin raw powder to the auxiliary material at a temperature of 70°C to 100°C, and coat it to form an inner core layer;
[0030] Step 2. Spray coating coating layer material on the surface of the inner core layer in the step 1 to obtain approximately spherical microcapsule particles with a diameter of 250 μm to 800 μm;
[0031] Step 3, drying the microcapsule particles prepared in step 2 to obtain the finished product.
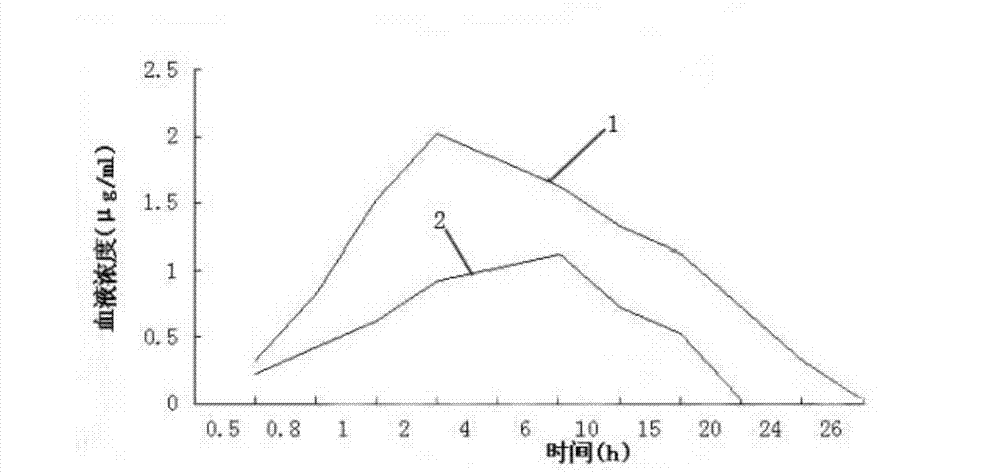
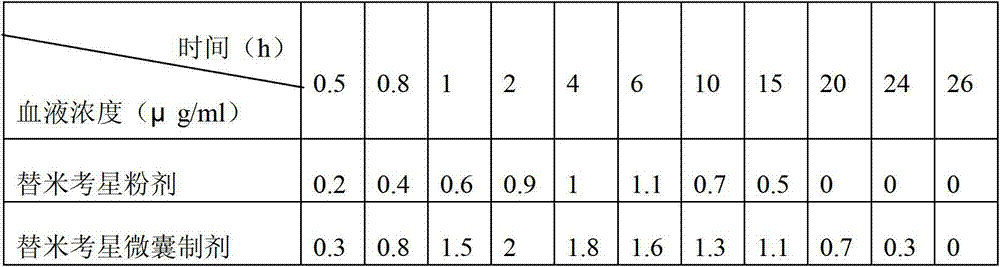
[0032] In the present invention, the mass percentage of tilmicosin raw powder is 10% to 50%, and in this embodiment, the mass percentage of tilmicosin is preferably 10%.
[0033] Commonly used auxiliary materials are stearic acid, glyceryl monostearate, stearyl alcohol, saturated triglycerides, monoglycerides, fat powder and paraffin, in the present invention auxiliary materials are stearic acid, glycery...
Embodiment 2
[0042] Same as Example 1, the difference is:
[0043] The mass percentage of tilmicosin is 20%, and the auxiliary material is a mixture of glyceryl monostearate and fat powder, wherein the mass percentage of glyceryl monostearate is 50%, and the mass percentage of fat powder is 25%. The layer material is hypromellose phthalate, its mass percentage is 5%, and its melting temperature is 95°C.
Embodiment 3
[0045] Same as Example 1, the difference is:
[0046]The mass percentage of tilmicosin is 30%, and the auxiliary material is a mixture of glyceryl monostearate and fat powder, wherein the mass percentage of glyceryl monostearate is 40%, and the mass percentage of fat powder is 20%. The layer material is hypromellose phthalate, its mass percentage is 10%, and its melting temperature is 90°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com