Method for detecting residual quantity of multiple polypeptidepolypeptide veterinary drugs in animal-derived food
A technology for a variety of polypeptides and veterinary drug residues, applied in the fields of food safety and analytical chemistry, it can solve problems such as cumbersome processing methods, and achieve the effects of strong anti-interference ability, high recovery rate and less impurity dissolution.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
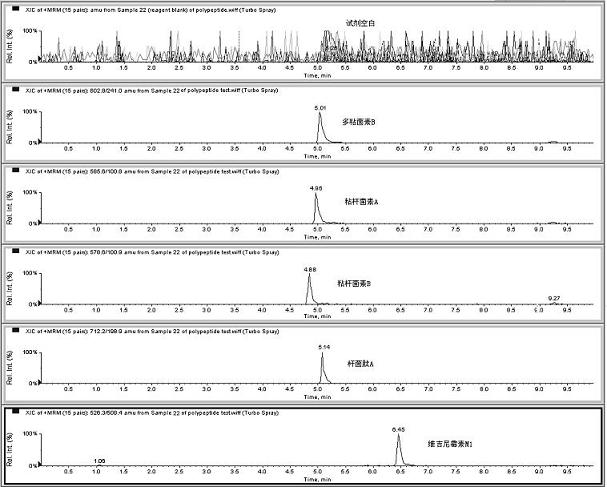
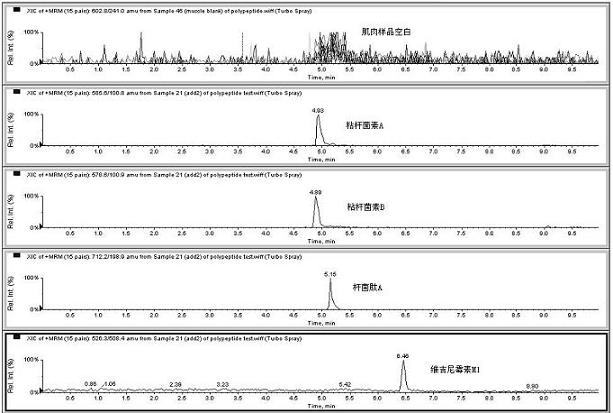
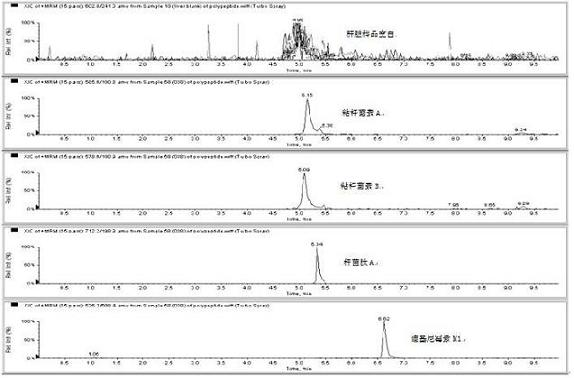
Image
Examples
Embodiment 1
[0059] 2. Degreasing the extract: Add 5-15 mL of n-hexane to the centrifuge tube of the extraction supernatant obtained in the previous step, vortex for 2 min, and centrifuge at 6 000 rpm for 5 min at 4°C. The upper layer (n-hexane layer) was removed, and the lower layer was purified by the solid phase extraction column.
[0060] 3. Solid-phase extraction column purification: Transfer the sample extract obtained above to the activated Oasis HLB purification column (activated with 6 mL of methanol and water), wash with 5 mL of water after loading the sample, and discard the effluent. Then elute with 5 mL of methanol, collect the eluate in a 10 mL graduated test tube, and control the flow rate during the entire solid phase extraction purification process to not exceed 2 mL / min. The eluent was blown dry with nitrogen on a water bath at 40°C, and the residue was dissolved in the standard sample solution to a volume of 1.0 mL, mixed evenly, and passed through a 0.22 μm microporous ...
Embodiment 2
[0102] 2, extract degreasing: with example 1.
[0103] 3. SPE column purification: same as example 1.
[0104] 4. Determination by liquid chromatography-mass spectrometry / mass spectrometer: same as example 1.
[0105] 5. Linear relationship test: Same as Example 1.
[0106] 6. Recovery rate and precision test: Accurately weigh milk samples that do not contain peptide veterinary drugs, add 3 different levels of peptide veterinary drug mixed standard solutions, the addition levels are shown in Table 5, and carry out 6 parallel samples for each concentration. Analysis was performed as described above in accordance with the above chromatographic and mass spectrometric conditions. The calculation results of recovery rate and coefficient of variation are shown in Table 7.
[0107] Table 7 Precision test results of peptide drugs added to milk (n=6)
[0108]
[0109] From the results in Table 7, it can be seen that the average recoveries of the three addition levels of polypept...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Ion source temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com