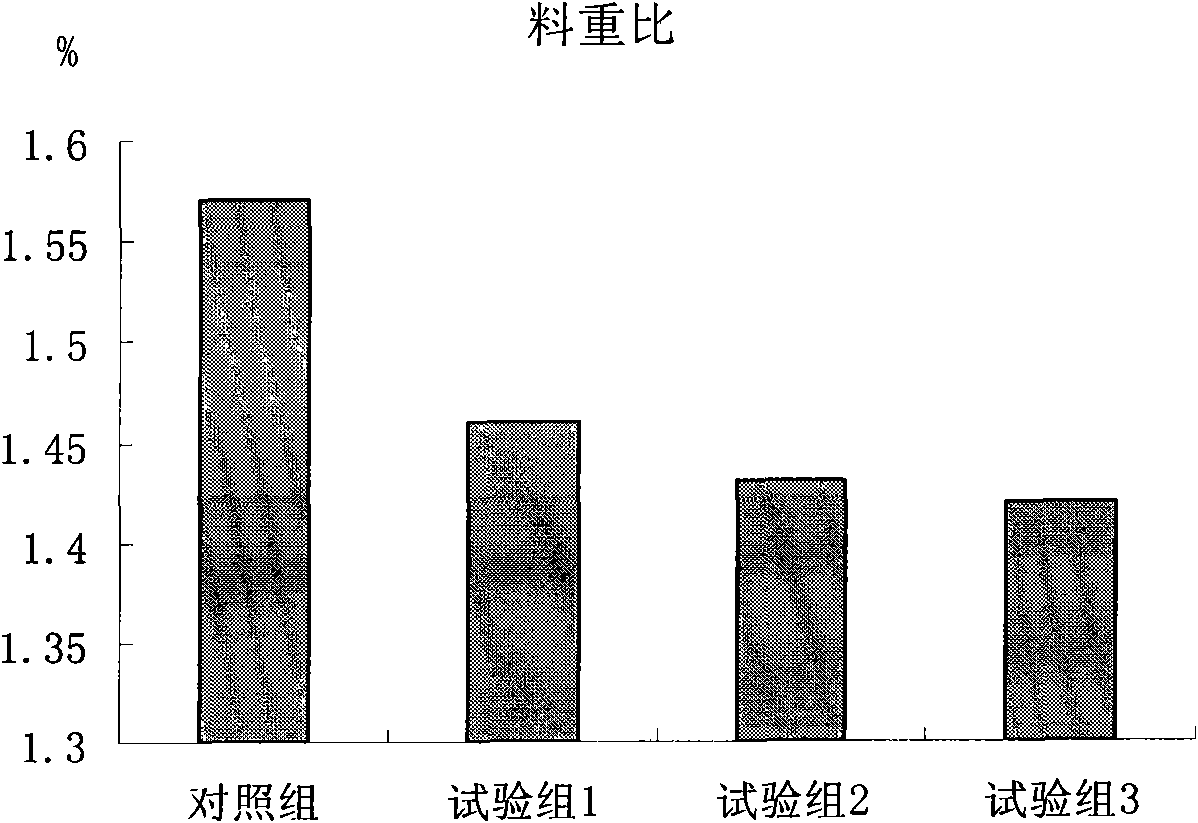
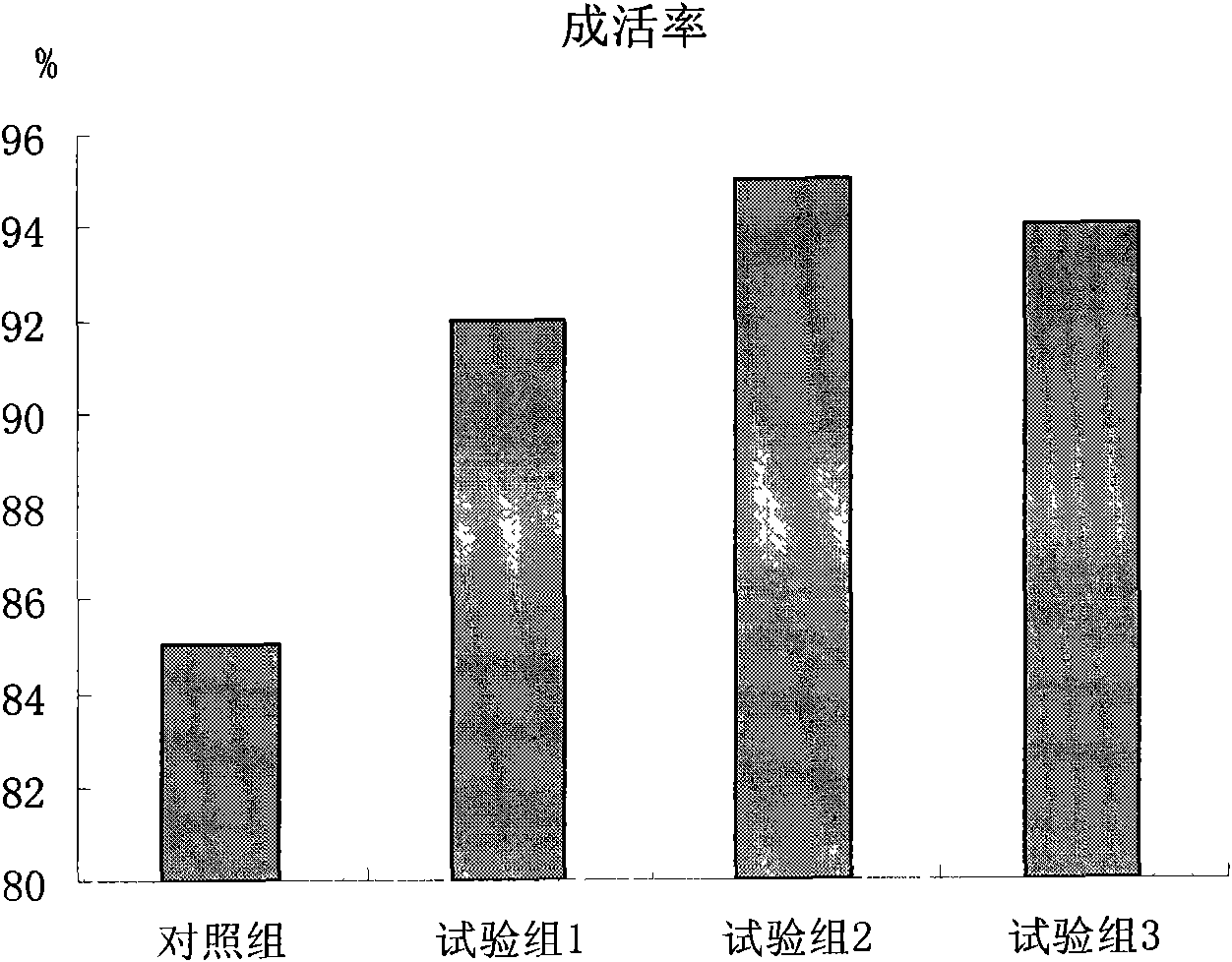
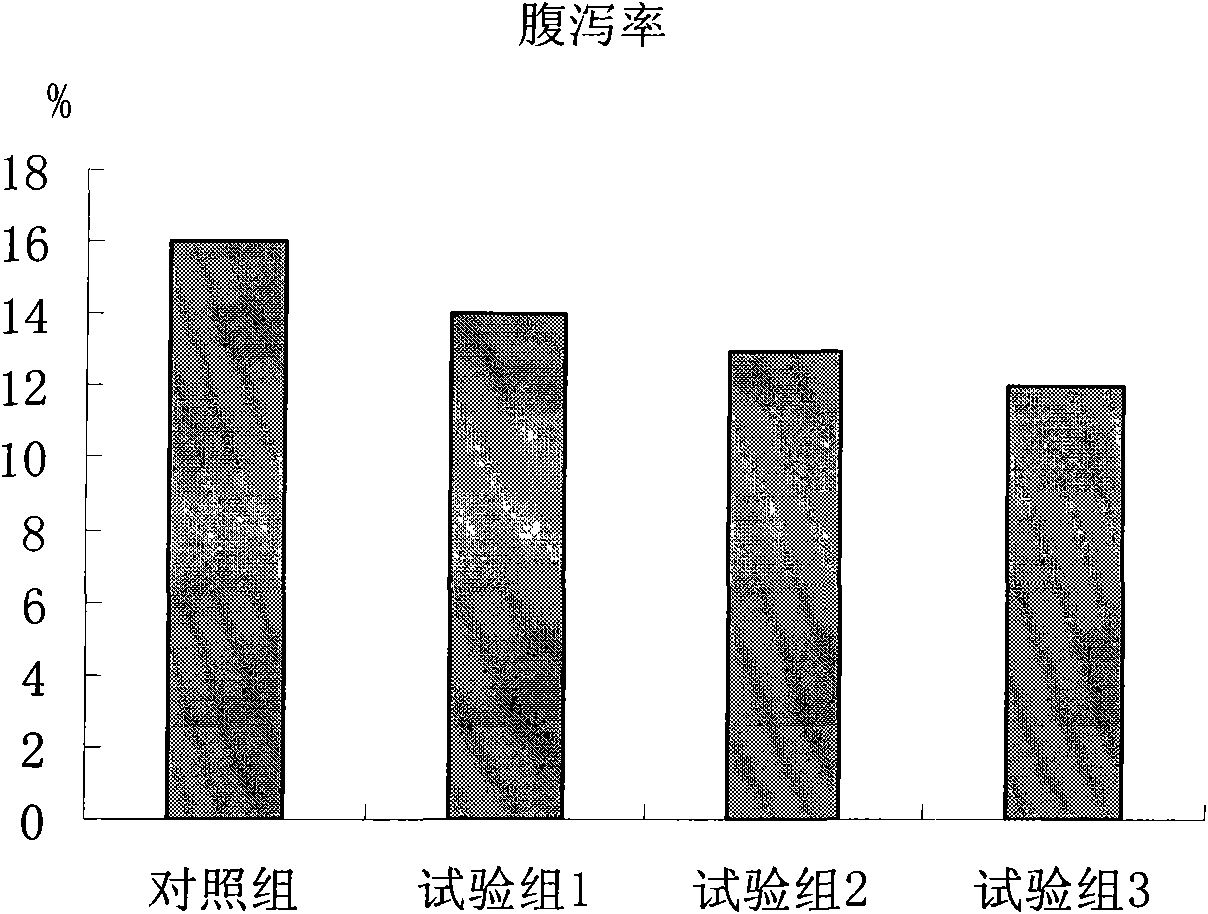
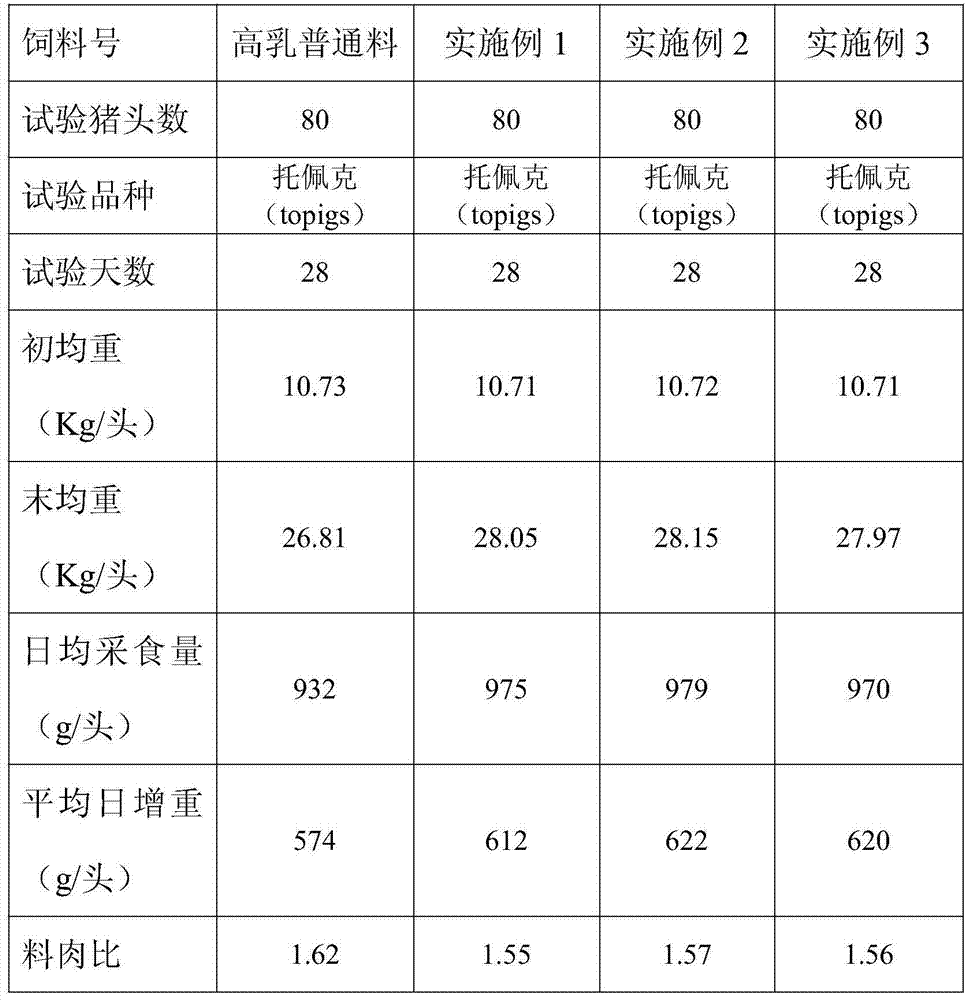
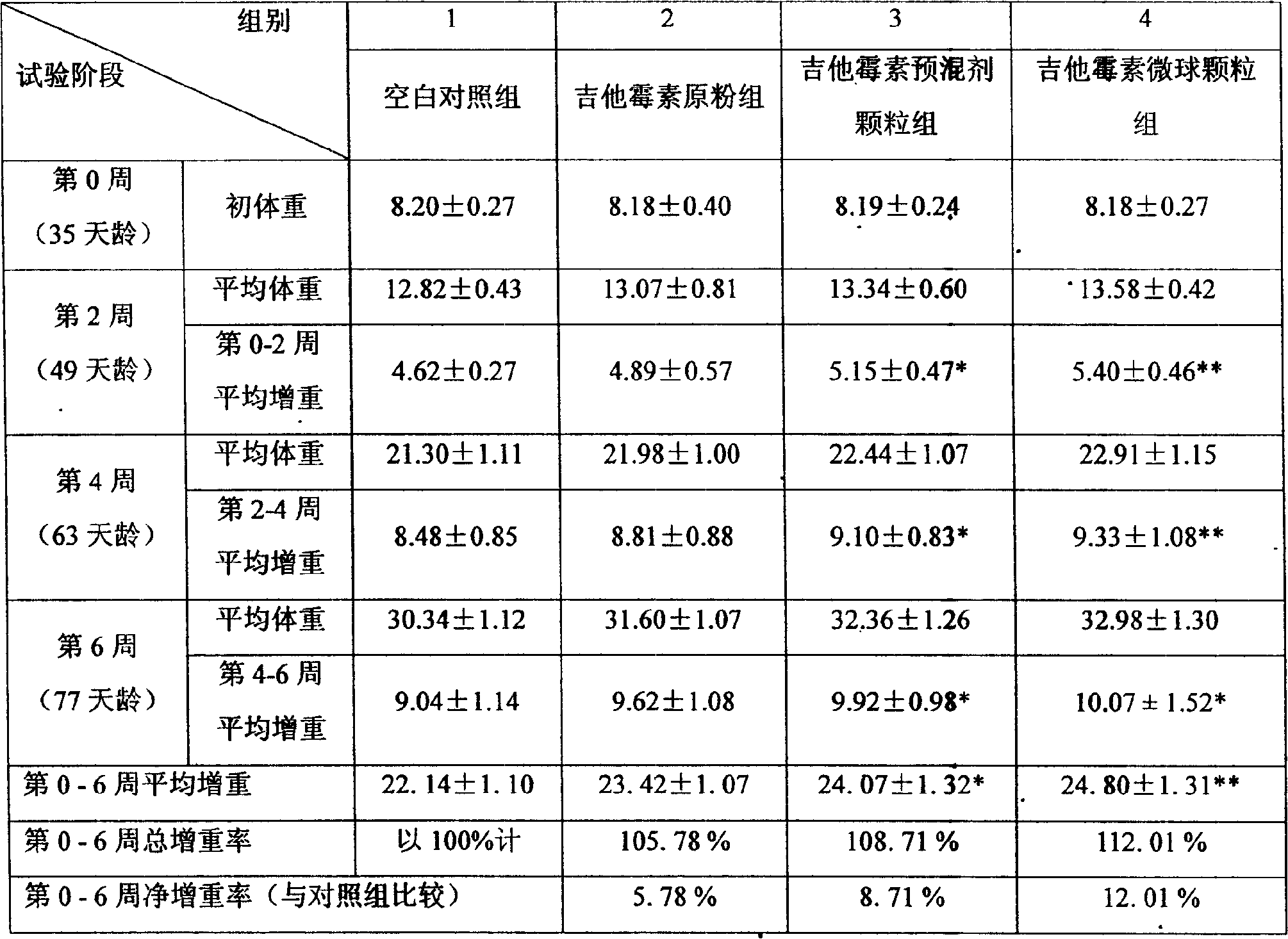
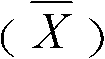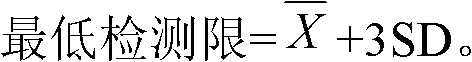Patents
Literature
57 results about "Kitasamycin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Kitasamycin (INN) is a macrolide antibiotic. It is produced by Streptomyces kitasatoensis. The drug has antimicrobial activity against a wide spectrum of pathogens. There are several generic names of this drug such as- •Kitasamycin (OS: BAN, JAN, USAN) •Kitasamycine (OS: DCF) •C 637 (IS) •Katasamycin (IS) •Leucomycin (IS) •Kitasamycin (PH: JP XV) •Kitasamycin Acetate (OS: JAN) •Leucomycin Acetate (IS) •Kitasamycin Acetate (PH: JP XV) •Kitasamycin Tartrate (OS: JAN) •Leucomycin Tartrate (IS) •Kitasamycin Tartrate (PH: JP XV)
Fodder for cultivating river crab
InactiveCN101233904APromote growthGrow without pollutionFood processingClimate change adaptationBiotechnologyAdditive ingredient
The invention relates to a feedstuff used for culturing fresh-water crabs. The composition of the feedstuff comprises import fishmeal powder, soybean meal, peanut meal, cotton meal, rapeseed meal, marine yeast, citric acid dregs, flour, soybean phospholipids, cuttle fish grease, bentonitic clay and primary calcium phosphate, and also comprises crabs-using multivitamin, crabs-using multiminerals, compound bacillus subtilis, microzyme, bdellovibrio, biological antibacterial peptide and microcapsule kitasamycin; Additive formulation mainly provides vitamin, mineral ion element, various enzymes, etc., for accelerating the growth of the fresh-water crabs. In addition, the additive formulation do not do harm to the growth of the fresh-water crabs. The additive also comprise antibiotics and various enzymes used for controlling the growth of hazardous enzymes in internal environment of the fresh-water crabs; therefore, after eating the feedstuff containing basic ingredients and the ingredients of the additive, the fresh-water crabs has remarkable growth effect without any side effects.
Owner:WUJIANG BANGNONG FEEDSTUFF
Compound feed premix for pigs and production method thereof
InactiveCN101982093AIncrease feed intakeImprove palatabilityAnimal feeding stuffAccessory food factorsTrace elementKitasamycin
The invention relates to a compound feed premix for pigs and a production method thereof. The formula of the compound feed premixing material comprises the following components of a compound enzyme preparation for the pigs, a compound vitamin premix for the pigs, a compound trace element premix for the pigs, a compound additive premix generated by lysine, an antioxidant, choline chloride and an acidulant, calcium hydrophosphate, rock flour, salt, luctarom, a kitasamycin micro-capsule preparation with the mass concentration of 50%, a colistin sulphate micro-capsule preparation with the mass concentration of 10% and zeolite powder. By adding the compound enzyme preparation, amino acid, compound trace elements and a feed sweetener to the compound feed premix, the utilization rate of the pigs on feed nutrients is enhanced, the production performance is improved, and the discharge of substances such as phosphorous, nitrogen and the like to natural environment through excrements of livestock and poultry is reduced; and meanwhile, the disease resistance of the pigs is strengthened, and the economic benefit of feeders is increased when the compound feed premix is applied to cultivation practice.
Owner:WUXI ZHENGDA POULTRY
Mixed feed for early weaned piglets
ActiveCN102771635AIncrease production capacityAvoid damageAnimal feeding stuffBiotechnologyIntestinal structure
The invention discloses a mixed feed for early weaned piglets. The mixed feed comprises the following components, by weight, 1.2*10<-7>-3*10<-7> parts of EGF, 20-30 parts of corn, 10-15 parts of soybean meal, 0.04-0.05 parts of a vitamin premix, 0.15-0.2 parts of choline chloride, 0.2-0.5 parts of an antibacterial peptide, 0.01-0.15 parts of a trace element premix, 0.3-0.5 parts of lysine, 0.2-0.3 parts of methionine, 0.3-1.0 part of a composite enzyme preparation, 0.1-0.15 parts of calcium hydrogen phosphate or / and stone flour, 1*10<-3>-5*10<-3> parts of colistin sulfate, and 0.1*10<-3>-2*10<-3> parts of kitasamycin. The EGF is added to the mixed feed in the invention, so the intestinal damage caused by weaning is avoided through the nutrition level and the reasonable price optimization, and the intestinal structure of the weaned piglets is capable of accepting more non-maternal feeds, thereby the production performance of the weaned piglets is improved.
Owner:SHENZHEN PREMIXINVE NUTRITION CO LTD
Kitasamycin microcapsule preparation and preparation and application thereof
InactiveCN101234097AAvoid instabilityReduce instabilityAntibacterial agentsOrganic active ingredientsMedicineDrug additive
Owner:GUANGDONG HINAPHARM PHARMA CO LTD
Liquid artificial milk feed, production method and feeding device
ActiveCN101984842AImprove palatabilityMeet the needs of rapid early growth and developmentAnimal feeding devicesAnimal feeding stuffBiotechnologyAnimal science
The invention relates to a liquid artificial milk feed, a production method and a feeding device, and the liquid artificial milk feed is characterized in that the formula comprises the following raw materials by weight percent: 4-6% of soy protein concentrate, 4-7% of beef extract, 1-3% of starch, 9-11% of whey powder, 4-7% of pure milk, 0.4-0.6% of calcium hydrogen phosphate, 0.2-0.4% of calciumcarbonate, 0.1-0.2% of common salt, 0.02-0.04% of compound vitamin, 0.1-0.3% of compound trace element, 0.01-0.03% of colistin, 0.01-0.02% of kitasamycin, 0.04-0.06% of bacillus subtilis and the balance of drinking water. The liquid artificial milk feed has the advantage of retarding a series of inadaptations of piglets in the aspects of emergency and a digestive system caused by direct transition from sow milk to powder material during delectation of the piglets. The product can not only meet the nutritional requirements for physiology, growth and development of the piglets, but also realize the scientific and safe transition from the liquid sow milk to the powder material.
Owner:沈阳科瑞思科技有限公司
Production method of enteric-coated kitasamycin for feed
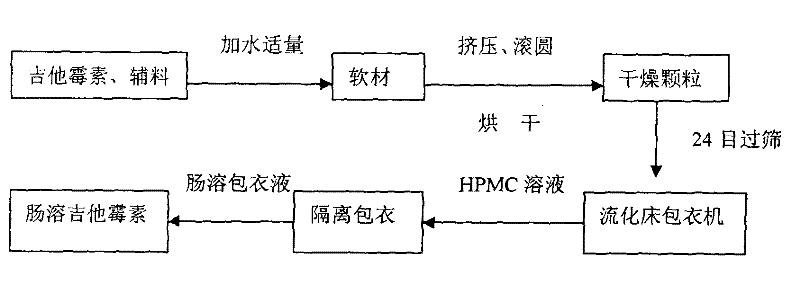
ActiveCN101611766AProtect weak alkaline antibioticsProlong the action timeAnimal feeding stuffAccessory food factorsAcrylic resinMicroparticle
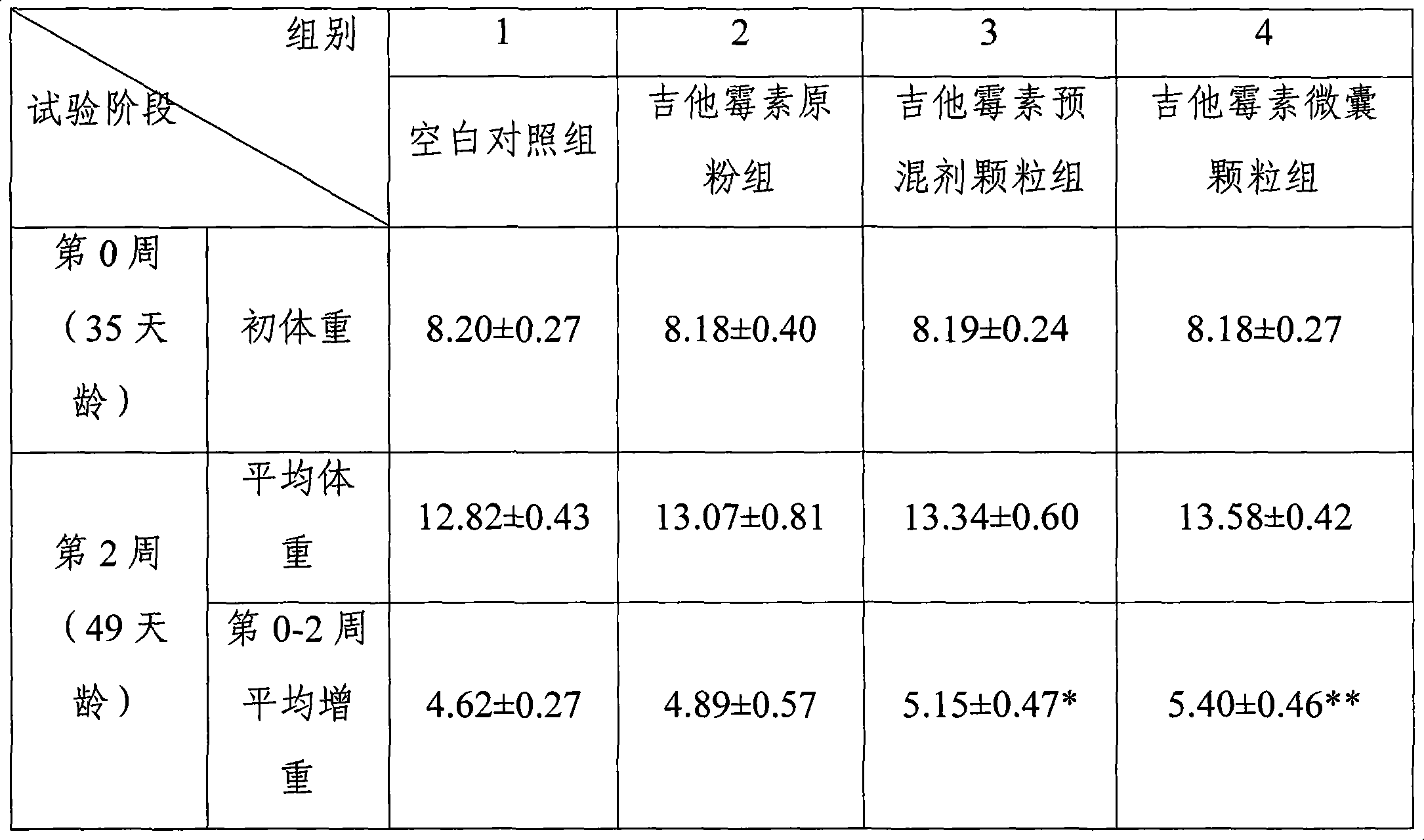
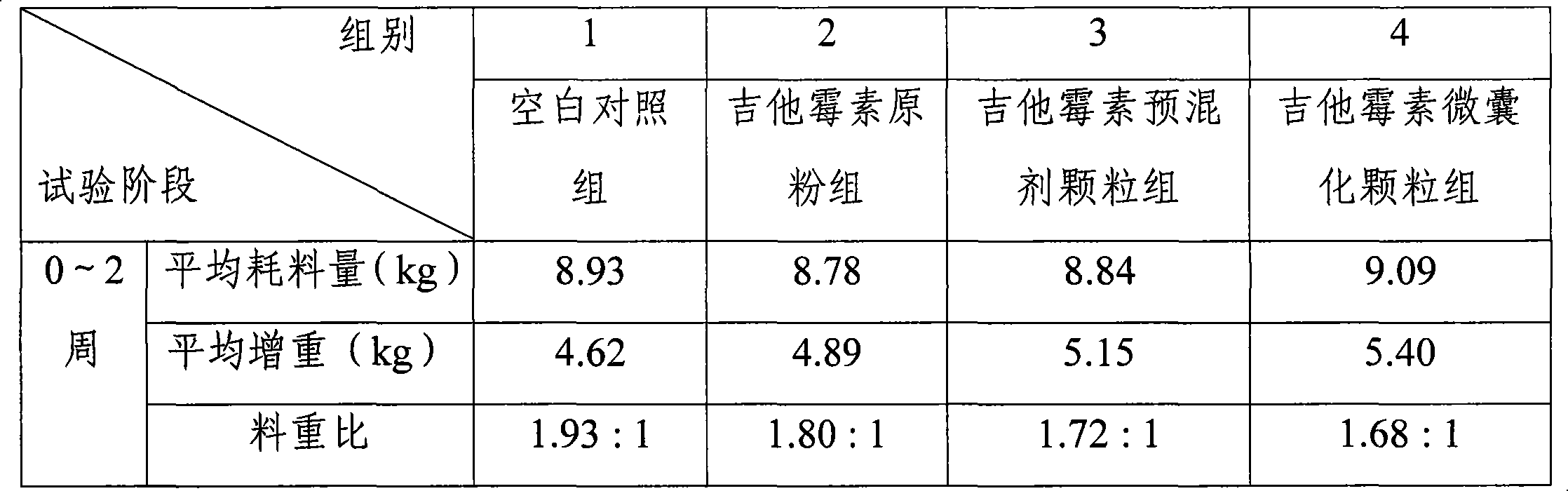
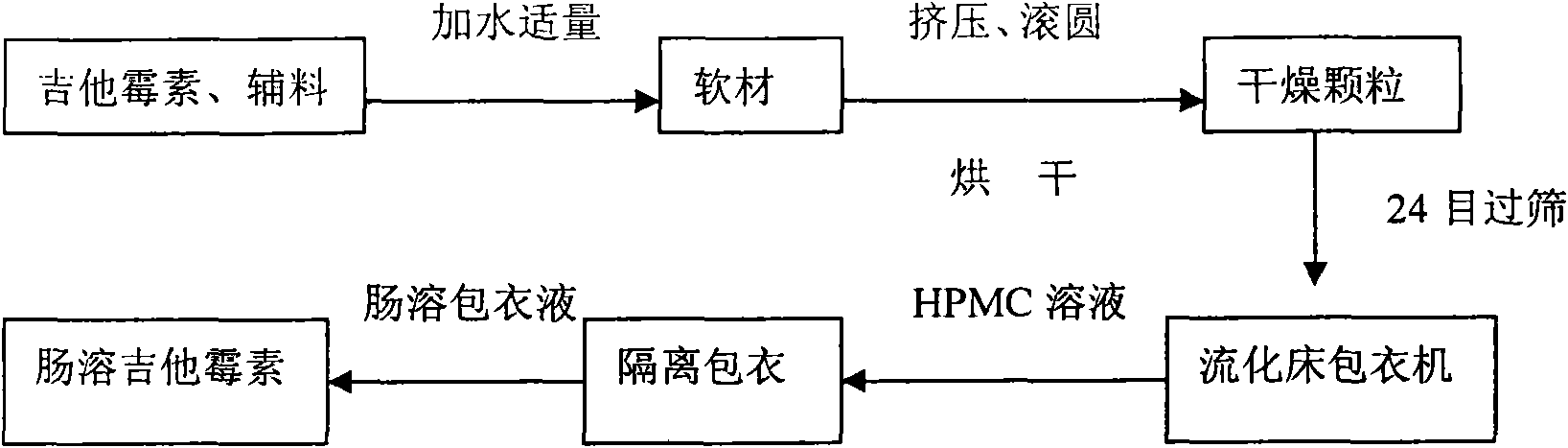
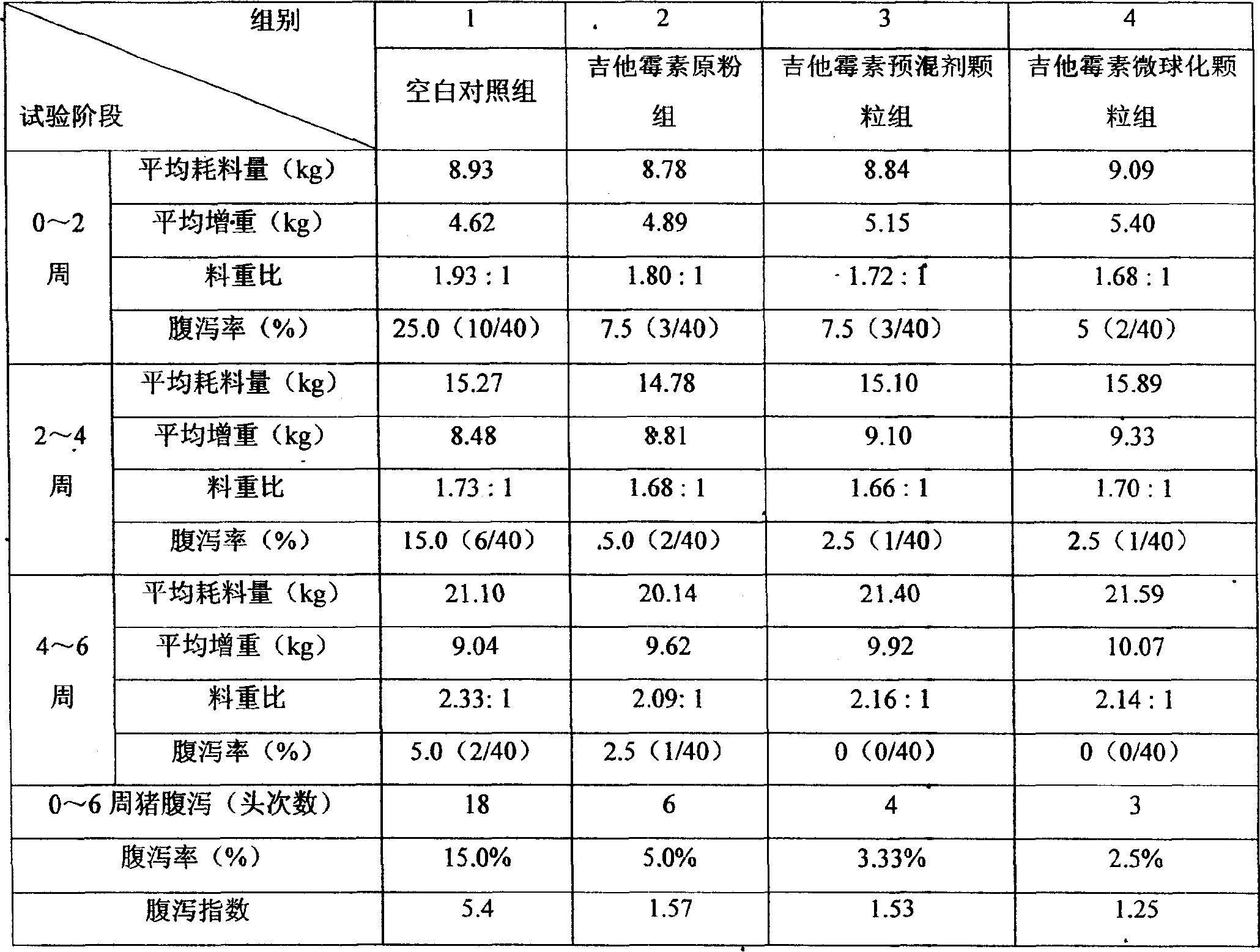
The invention discloses a production method of enteric-coated kitasamycin for feed, which comprises the following process steps: step one, the preparation of drug-loaded pellets; step two, inner layer sustained-release coating of the drug-loaded pellets, which prolongs the release and acting time of kitasamycin; and steps three, outer layer enteric coating of the drug-loaded pellets, which ensures the release in succus entericus and small or no release in gastric juice. The method has the advantages that the kitasamycin is subjected to pellets pelletizing and 99 percent of the prepared granulums can pass through 24 meshes, so the dust is greatly reduced and the fluidity is increased; the coating of the inner layer sustained-release agent (HPMC) prolongs the release and acting time of the kitasamycin, reduces medication times and reduces the medication cost; and a layer of enteric substance, namely acrylic resin-III is sprayed and coated on the outer layer of particles. The substance protects the kitasamycin which is a weakly alkaline antibiotic from being damaged by gastroc acid in stomach, quickly disintegrates after entering enteric canal and releases the kitasamycin; and then the kitasamycin is absorbed by gastrointestinal mucosa into blood drug to play a role of restraining the reproduction of pathogenic microorganism and preventing diarrhea. Insoluble in the stomach, kitasamycin coating formulations have no pessimal stimulation on the stomach, and cannot result in regurgitation and vomiting. In addition, the sustained-release formulation, namely the kitasamycin prolongs the acting time so the medication times is reduced, the medication cost of farmers is reduced, and the economic benefit is improved.
Owner:WUXI ZHENGDA POULTRY
Piglet early-stage care feed
PendingCN107095060AImprove immunityRaise the rate of looseningFood processingAnimal feeding stuffTrace element compositionPhytase
Piglet early-stage care feed is disclosed. Raw materials include corn, puffed corn, soybean meal, fermented soybean meal, bran, puffed soybean, soybean oil, sugar, glucose, fish meal, amino acids, organic trace elements, inorganic trace elements, choline chloride, betaine, sodium chloride, calcium carbonate, calcium hydrophosphate, composite vitamins, composite enzymes, phytase resistant to high temperature, quinocetone, calcium oxytetracycline, 4-6 parts of an acidifying agent and kitasamycin. The acidifying agent is used in the feed to adjust acidity-alkalinity balance in intestinal tracts. Antibiotics are combined to resist intestinal bacteria. The feed can also improve the digestion rate and piglet immunity. An amino acid composition is adopted to improve immunologic balance of piglets. An organic trace element composition and zinc oxide are used to improve body immunity and intestinal health of the piglets. A composition of the white sugar, the glucose, monosodium glutamate, and the like is adopted to improve immunity and palatability. Beneficial effects of the method are that palatability is improved, the feed intake is obviously increased, intestinal tract stress is reduced, the diarrhea rate is reduced by about 20%, and the survival rate is increased by about 30%.
Owner:重庆市万虹饲料有限公司
Monoclonal antibody for kitasamycin residual detection and preparation method and application thereof
InactiveCN103288963ASimple and fast operationImmunoglobulinsMaterial analysisMurine antibodyBovine serum albumin
The invention discloses a monoclonal antibody for kitasamycin residual detection and a preparation method and application thereof. The monoclonal antibody is secreted by a hybridoma KA / 2A9, which is collected by CCTCC (China Center for Type Culture Collection) and has the collection number of C201184. The preparation method comprises the steps of: (A) coupling hapten kitasamycin with bovine serum albumin so as to obtain an immunogen; (B) coupling hapten kitasamycin with ovalbumin so as to obtain a coating antigen; (C) preparing the monoclonal antibody secreted by the hybridoma KA / 2A9 by using the immunogen; (D) coating a solid phase carrier by using the coating antigen; (E) extracting a sample to be detected by using metaphosphoric acid in the presence of ethanol, carrying out back extraction by using ethyl acetate, blow-drying by using nitrogen gas, and diluting the sample so as to obtain a substance to be detected; and (F) carrying out enzyme-linked immunoassay on the substance to be detected. A kit for kitasamycin residual detection comprises an ELISA (Enzyme-Linked Immunosorbent Assay) plate coated by the coating antigen kitasamycin-AOOA-OVA, a kitasamycin standard solution, a goat anti-mouse IgG (Immunoglobulin G) antibody working solution labeled by a horseradish peroxidase, a concentrated phosphate buffer, a concentrated washing solution, a substrate mixture and a stop solution. The method has the characteristics of simplicity, convenience, rapidness, sensitiveness and accuracy.
Owner:HUAZHONG AGRI UNIV
Preparation method for kitasamycin industrial production strains
InactiveCN105420148AGrowth synchronizationAvoid saving time slotsBacteriaMicroorganism based processesBiotechnologySporeling
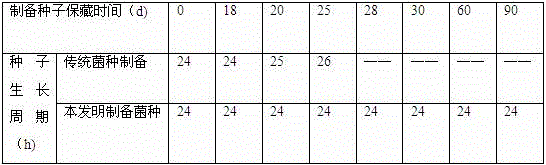
The invention discloses a preparation method for kitasamycin industrial production strains, which comprises the main steps: performing plate streaking on an original strain to prepare a single colony; transferring the single colony onto an inclined plane, culturing same for a proper time; then scraping spores on the inclined plane with an inoculation loop, and preparing a freeze-dried strain tube with a vacuum freeze-drying method; preparing a spore suspension by using the freeze-dried strain tube, coating the oblique plane with the spore suspension, culturing the spore suspension for a proper time; then scraping spores on the oblique plane with the inoculation loop to prepare a spore and milk suspension; subpackaging the spore and milk suspension and performing freezing preservation so as to prepare a spore and milk freezing tube; melting the spore and milk freezing tube, and transferring same to a seed culture medium, performing shake culture by bottle shaking for a proper time, and then transferring to a seed tank. The method overcome the defects of poor synchronization of inoculation production of inclined strain production, nonuniform growth cycle of seed bottles and frequency of batch number change. The preparation method disclosed by the invention is simple and convenient to operate, the inoculum age of strains and consistency of quality of seeds are improved, the batch number change of seeds is reduced, and the preparation method is very suitable for kitasamycin industrial production.
Owner:TOPFOND PHARMA CO LTD
Kitasamycin fermenting culture medium
The invention relates to a Kitasamycin fermenting culture medium and belongs to the field of production of kitasamycin. The fermenting culture medium comprises the components in percentages by weight: 1.5-4.5% of starch, 0.1-0.3% of glucose, 2.0-4.0% of soybean cake powder, 0.5-1.5% of fibroin powder, 0.2-0.5% of calcium carbonate, 0.006-0.015% of amylase, 2.0-3.5% of soya-bean oil, 0.01-0.06% of GPE, 0.05-0.1% of potassium dihydrogen phosphate, 0.15-0.55% of ammonium sulfate, 0.04-0.06% of manganese chloride, 0.005-0.007% of zinc sulfate as well as an emulsifier which is tween 80. The tween 80 is uniformly mixed with the soy-bean oil and is then mixed with other comments; the pH of the fermenting culture medium is 6.3-7.5. By using the culture medium, the fermenting level of kitasamycin can be improved. Moreover, after fermentation of the culture medium, oil in fermenting liquor is fully used, so that the use level of the soy-bean oil is reduced, and the production cost is lowered; generation of emulsification in the extraction process is further reduced to facilitate extraction of a product in a purifying section, so that the extraction yield is improved, and the product quality is improved to a great extent.
Owner:SHANDONG DONGYAO PHARMACEUTICAL CO LTD
High-ration fiber piglet compound feed
InactiveCN104719667AImprove developmentIncrease the absorption surfaceAnimal feeding stuffORIGANUM OILStress syndrome
The invention relates to high-ration fiber piglet compound feed. The high-ration fiber piglet compound feed consists of the following ingredients in parts by weight: 250 to 400 parts of corns, 250 to 350 parts of wheat, 80 to 150 parts of extracted soybean meal, 50 to 75 parts of fermented soybean meal, 50 to 100 parts of extruded soybeans, 30 to 100 parts of brown rice, 30 to 60 parts of 65% fish meal, 30 to 70 parts of whey powder, 20 to 80 parts of flour, 10 to 20 parts of soybean oil, 5 to 10 parts of calcium hydrogen phosphate, 5 to 10 parts of stone powder, 4 to 5 parts of piglet micro-mineral premixed material, 5 to 10 parts of citric acid, 3 to 6 parts of 99% lysine, 3 to 5 parts of sodium chloride, 1 to 2 parts of 50% choline chloride, 1 to 2 parts of methionine, 1 to 2 parts of threonine, 4 to 5 parts of self-compounding multi-dimensional premixed material, 0.3 to 0.6 part of tryptophan, 0.3 to 0.5 part of an antioxidant, 0.5 to 0.7 part of origanum oil, 0.05 to 0.1 part of 50% kitasamycin and 0.15 to 0.2 part of colistin sulfate. The ratio of soluble ration fiber to insoluble fiber in the ration is adjusted, so that the weanling stress syndrome of the piglet can be controlled, the intestine health of the piglet can be improved, the diarrhea is reduced, the dependence of the piglet on the antibiotics and the pharmaceutical dose zinc oxide can be alleviated, and the effect is remarkable.
Owner:HUNAN LIUYANGHE FEED
Immunocolloidal-gold detection card for kitasamycin and preparation method thereof
The invention provides an immunocolloidal-gold detection card for kitasamycin and a preparation method thereof, belonging to the technical field of detection of antibiotics. A test strip in the shell of the detection card is composed of a PVC rubber sheet, a sample pad, a colloidal-gold binding pad, a coating film and a water-absorbing pad, wherein a colloidal-gold film is a glass cellulose film containing monoclonal antibody against kitasamycin; the coating film is a cellulose nitrate film which is provided with a T line and C line; the T line is coated with a kitasamycin protein conjugate; and the C line is coated with a goat anti-mounse antibody. The immunocolloidal-gold detection card is used for rapid detection of kitasamycin, is convenient and fast and produces accurate results.
Owner:ZHENJIANG YITE BIOTECH DEV CO LTD
Gitamycin slow-release micro-ball preparation and its preparing process
InactiveCN101045064AReduce flyingReduce lossAntibacterial agentsOrganic active ingredientsDiseaseMicrosphere
A slow-release kitasamycin microball as a veterinary medicine with high effect to prevent and treat animal' s diseases, and its preparing process with high productivity are disclosed.
Owner:GUANGDONG HINAPHARM PHARMA CO LTD
Production method for kitasamycin solid micro-capsules for feed
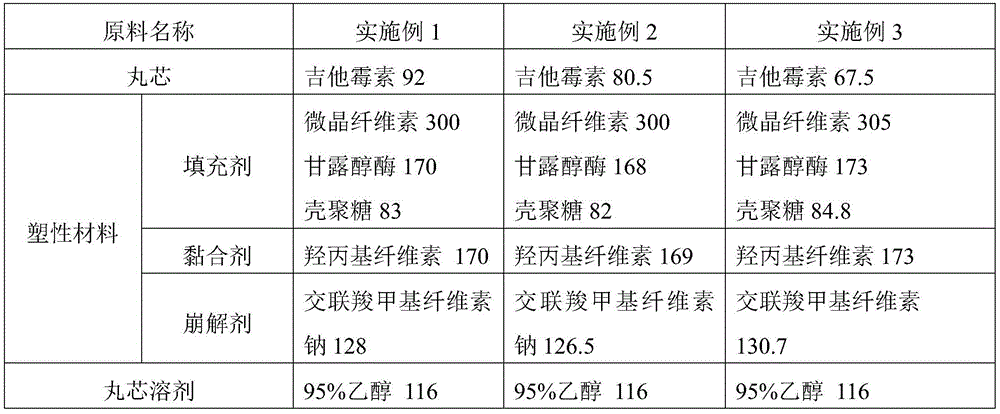
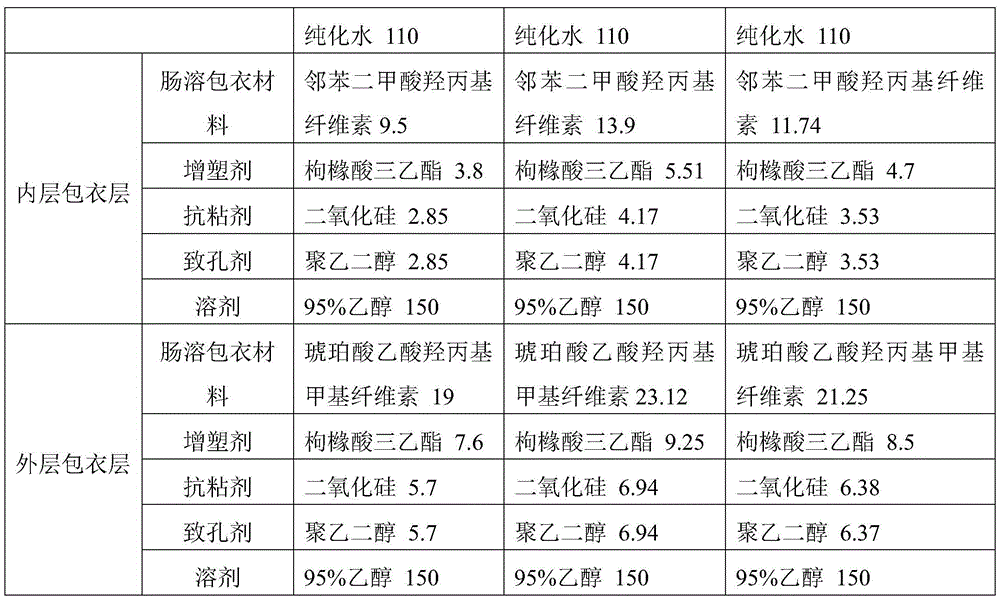
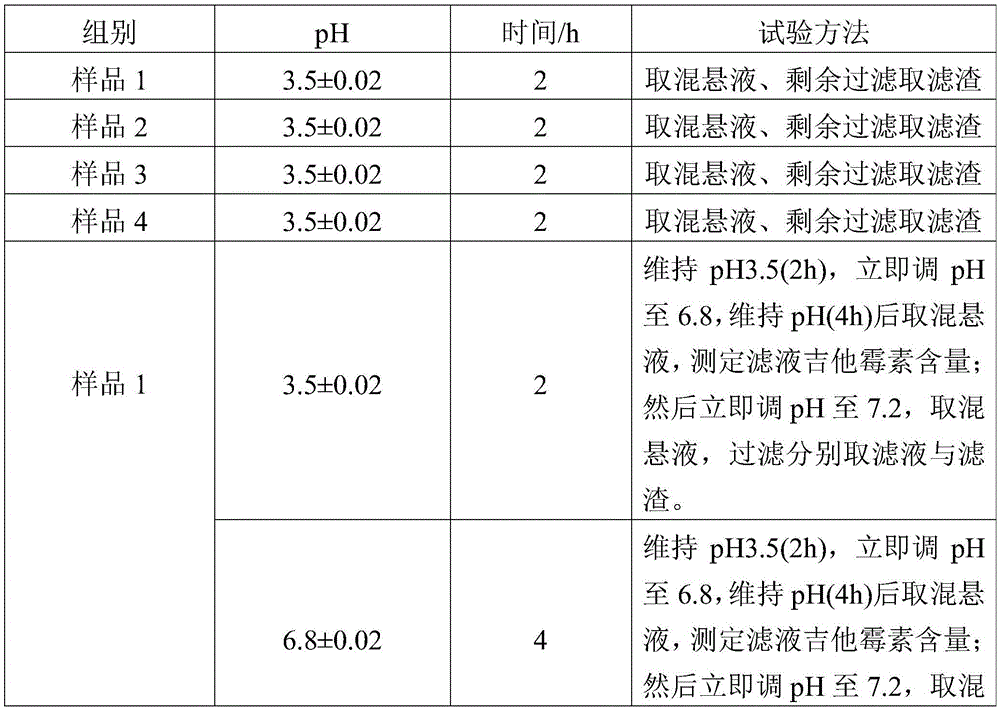
InactiveCN105534950APromote dissolutionImprove stabilityAntibacterial agentsOrganic active ingredientsPlastic materialsKitasamycin
The invention discloses a production method for kitasamycin solid micro-capsules for feed. The method comprises the following steps that 1, kitasamycin, plastic materials and a solvent are mixed to form pill cores; 2, the pill cores are wrapped by an inner enteric coating layer and an outer enteric coating layer; the inner enteric coating layer and the outer enteric coating layer are formed by mixing inner enteric coating layer materials with the solvent and mixing outer enteric coating layer materials with the solvent respectively, and then the pill cores are sequentially wrapped to form granules. According to the method, the phenomenon that pessimal stimulation is caused to the stomach by unprocessed kitasamycin which has the bitter taste can be avoided, meanwhile, it can be ensured that effective ingredients can completely and accurately reach the intestinal tract, the dissolution rate of intestinal drugs is improved, the concentration of the intestinal drugs and the drug effect intensity are greatly improved, and therefore the using performance of the kitasamycin is improved.
Owner:无锡华诺威动物保健品有限公司
New process for preparing meleumycin
InactiveCN103103236AFast growthIncrease unit fermentation volumeSugar derivativesMicroorganism based processesStreptomycesFermentation
The invention discloses a method for preparing meleumycin through fermentation of streptomyces mycarofaciens. The method comprises the following steps of: fermenting streptomyces mycarofaciens through a new fermenting base formula, to improve contents of medemycin A1 and kitasamycin back A6; after fermentation, through an extraction and a back extraction, extracting by a new mixing extracting agent, to improve extracting rate; and automatically crystallizing, to separate out the meleumycin. The process disclosed by the invention, which produces the meleumycin through a new fermenting base formula, simplifies meleumycin extraction process, and has characteristics of simple and convenient operation, energy conservation and the like; and the meleumycin produced by the process disclosed by the invention is accordant with related requirements of Chinese Pharmacopoeia 2010 version, and is applicable to industrial mass production.
Owner:贯虹科技有限公司
Pig respiratory tract diseases prevention and treatment drug composition, premix and matching material thereof
InactiveCN104784411AReasonable compositionFormulation ScienceAnimal feeding stuffRespiratory disorderDiseaseRespiratory tract disease
The present invention relates to a pig respiratory tract diseases prevention and treatment drug composition, a premix and a matching material thereof, and belongs to the field of veterinary drugs and premixes. The drug composition of the present invention comprises, by weight, 40-95 parts of a traditional Chinese medicine component, and 10-60 parts of a chemical drug component, wherein the traditional Chinese medicine component is prepared from the fructus arctii, roripa montana and common yam rhizome, and the chemical drug component is selected from florfenicol, tilmicosin, tiamulin, valnemulin, acetylisovaleryltylosin, tylosin, kitasamycin and a salt thereof. The drug compositions of the present invention is suitable for pig respiratory tract diseases prevention and treatment, has characteristics of significant treatment effect, high safety, stable formulation, controllable quality, simple preparation process and the like, is suitable for industrial production, and can further be added to the feed so as to be used for preparation of pig growth promoting feed such as premixes, matching materials and the like.
Owner:BEIJING DABEINONG ANIMAL HEALTH TECH +2
Kitasamycin magnetic immunochemiluminescence detection kit
The invention relates to a high sensitivity, high accuracy and high accuracy kitasamycin magnetic immunochemiluminescence detection kit. The kit comprises a horse radish peroxidase-labeled kitasamycinhapten label, an enzyme-labeled antigen diluent, a magnetic label antibody, a magnetic label antibody diluent, a series of kitasamycin standard solutions, a concentrated compound solution, a concentrated cleaning solution and solutions A and B of chemiluminescent substrates. A lot of tests screen specific monoclonal antibodies with excellent properties and determine appropriate concentrations ofcomponents in the reaction solution. The kitasamycin magnetic immunochemiluminescence detection kit can fast and accurately detect low content kitasamycin and is suitable for detection of kitasamycinresidue content of pig or poultry animal foods.
Owner:QINGDAO UNIV
Clean kitasamycin extraction process
ActiveCN106188186AEmission reductionOvercoming technical bias in direct emissionsSugar derivativesSugar derivatives preparationWater basedFiltration
The invention belongs to an antibiotic production process and particularly relates to a clean kitasamycin extraction process. The clean kitasamycin extraction process comprises the steps of fermentation broth acidification, filtration, extraction, raffinate recovery and the like. In an existing production process, extracted raffinate is subjected to harmless treatment and then is always and directly discharged. According to the clean kitasamycin extraction process, it is considered that the main component of the raffinate is water based on relevant production technology study, the raffinate can be reused for the production process after being treated properly, the sewage treatment cost can be better reduced while water resources are saved in use, and accordingly production benefits are improved. The process overcomes the technical prejudice on direct discharge of production wastewater to some degree. Compared with existing production processes, the clean kitasamycin extraction process is lower in improvement cost and is simple. Due to the fact that the wastewater discharge proportion can be obviously reduced, the production cost can be reduced and the environmental protection benefits and production benefits are more remarkable, and the antibiotic production process has better practicability and a popularization and application value.
Owner:TOPFOND PHARMA CO LTD
Method for preparing kitasamycin tartrate injection
InactiveCN1820760ASimple processQuality is easy to controlAntibacterial agentsOrganic active ingredientsChemical structureKitasamycin
The present invention solves the difficult problem of microlide medicine kitasamycin tartrate in unstable structure, and provides preparation process of kitasamycin tartrate injection. The kitasamycin tartrate injection consists of kitasamycin tartrate or kitasamycin as main medicine component, solvent for injection, pH stabilizer, chemical structure stabilizer, metal ion complexing agent, isosmotic regulator and other auxiliary components. The kitasamycin tartrate injection has high stability, high safety and effectiveness, simplified clinical use and other advantages.
Owner:刘小清
Ultraviolet spectrophotometric detection method for kitasamycin content
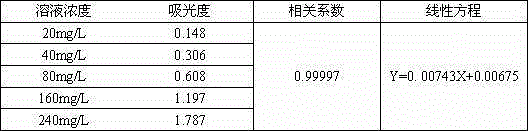
InactiveCN106596440AThe detection process is fastImprove accuracyColor/spectral properties measurementsUltravioletKitasamycin
Belonging to the technical field of kitasamycin content detection, the invention discloses an ultraviolet spectrophotometric detection method for kitasamycin content. The method includes preparation of a standard solution, determination of a regression equation, sample detection and other processes. The method has short detection time, the detection process can be restored, and the detection process only uses water as the solvent, therefore the method has the characteristics of green and environmental protection, safety and no toxicity.
Owner:JIANGSU WISE SCI & TECH DEV
Buffer liquid system and application thereof to extraction of kitasamycin
ActiveCN101845068AAvoid pollutionWide variety of sourcesSugar derivativesSugar derivatives preparationO-Phosphoric AcidKitasamycin
The invention belongs to the field of antibiotic medicaments, and provides a new buffer liquid system for extraction of kitasamycin. The buffer liquid system is solution mainly containing unitary or polybasic carboxylic acid having less than 10 carbon atoms and soluble salt thereof, wherein pH ranges from 1.5 to 4.2. The buffer liquid system can effectively solve the problem that a large amount of phosphorus element is brought into production to cause over high phosphorus content in waste water because phosphoric acid buffer liquid is generally used for back extraction of the kitasamycin in aconventional process. The system greatly increases the problem of pressure on environment-friendly treatment and can effectively prevent the phosphorus element from being brought into the production to relieve the pressure on environment-friendly dephosphorization.
Owner:浙江普洛生物科技有限公司 +1
Method for raising n-propanol tolerance of kitasamycin producing strain
InactiveCN105349522AImprove toleranceIncrease productionMutant preparationElectrical/wave energy microorganism treatmentBiotechnologyPropanol
The invention discloses a method for raising n-propanol tolerance of kitasamycin producing strain. The method comprises the following steps: using the kitasamycin producing strain as an original strain, adding 1.5% of n-propanol into a spore suspension, performing microwave mutagenesis, and performing preliminary screening and secondary screening to obtain a strain with strong n-propanol tolerance. The method has advantages of simple equipment, convenience in operation and slight radiation damage. The method is safe and can be adopted to stably raise the level of fermentation production.
Owner:SHANDONG FANGMING PHARMACEUTICAL CO LTD
Ureaplasma urealyticum/mycoplasma hominis combined rapid culture and drug sensitivity detection kit
InactiveCN104988206AResolve detectionResolve accuracyMicrobiological testing/measurementMicroorganism based processesPenicillinArginine
Owner:姜洪波 +1
A kitasamycin antimicrobial nanoemulsion drug and a preparation method thereof
InactiveCN103284949AGood dispersionAppearance is clear and transparentAntibacterial agentsOrganic active ingredientsBiotechnologyActive agent
A kitasamycin antimicrobial nanoemulsion drug and a preparation method thereof. The kitasamycin antimicrobial nanoemulsion drug is a composition having a particle size range of between 1nm to 100nm and comprising 0.1%-5% by weight of kitasamycin, 0.1%-8% by weight of an oil phase, 25%-40% by weight of a surfactant, and 4%-25% by weight of a cosurfactant, and the balance water. Combining the composition with the preparation method, the kitasamycin antimicrobial nanoemulsion drug with high bioavailability, low toxic and side effects, good stability, and good palatability is obtained.
Owner:SHAANXI SHENGAO ANIMALS PHARMA
Kitasamycin monoclonal antibody hybridoma cell strain SML and application thereof
InactiveCN110257342AImprove featuresHigh detection sensitivitySugar derivativesSerum albuminBALB/cEnzyme linked immunoassay
The invention relates to a Kitasamycin monoclonal antibody hybridoma cell strain SML and an application thereof, and belongs to the field of food safety immune detection. The hybridoma cell line SML is deposited in the general microbiology center of the Council for the preservation and administration of microbial strains in China, with the preservation number of CGMCC No. 17392. The complete antigen of kitasamycin and equivalent freund's adjuvant are mixed and emulsified to immunize BALB / c rats. Complete freund's adjuvant is used for the first immunization, incomplete freund's adjuvant is used for several times to strengthen immunization, and finally, kitasamycin complete antigen is used for sprint immunization. Splenic cells of rats with high titer and low IC50 are fused with myeloma cells of rats by a PEG method, cultured and screened; then the cells are screened by indirect competitive enzyme-linked immunosorbent assay and subcloned three times to finally obtain a monoclonal antibody hybridoma cell strain. The monoclonal antibody secreted by the cell strain has good specificity and detection sensitivity to kitasamycin, can realize the detection of kitasamycin residues in aquatic products, and has practical application value.
Owner:JIANGNAN UNIV
Preparation method of kitasamycin microcapsules
InactiveCN108685871AHigh embedding rateImprove stabilityAntibacterial agentsOrganic active ingredientsEmulsionKitasamycin
The invention discloses a preparation method of kitasamycin microcapsules. The method includes the steps of firstly, evenly stirring and mixing liquid grease and an oleophylic emulsifier to obtain first mixed liquid, adding a kitasamycin solution into the first mixed liquid, and evenly stirring and mixing to obtain a primary emulsion; secondly, evenly stirring and mixing a sodium alginate solutionand a hydrophilic emulsifier to obtain second mixed liquid, adding the primary emulsion into the second mixed liquid, and evenly stirring and mixing to obtain a compound emulsion; thirdly, dropwise adding the compound emulsion into a calcium chloride solution to perform calcification, and filtering to obtain the kitasamycin microcapsules. The method has the advantages that a water-oil-water emulsification method is used to wrap kitasamycin inside sodium alginate, calcium alginate is formed through the calcification reaction to tightly wrap the emulsification system inside, the kitasamycin ishigh in encapsulation rate, and good stability is achieved; the kitasamycin microcapsules can increase the bioavailability of the kitasamycin, and the kitasamycin is not released in the stomach, doesnot irritate the stomach and is only released and absorbed in the intestinal tract.
Owner:QINGDAO BRIGHT MOON SEAWEED GROUP
Fermentation medium and fed batch method for improving fermentation level of meleumycin
ActiveCN108060192AReduce riskQuality improvementBacteriaMicroorganism based processesPhosphateKitasamycin
The invention provides a fermentation medium for improving the fermentation level of meleummycin. The fermentation medium includes 1.5-3.0% of glucose, 2-5% of soybean powder, 0.1-0.3% of yeast powder, 0.1-0.5% of fish meal, 0.05-0.2% of potassium dihydrogen phosphate, 0.05-0.2% of magnesium sulfate and 1-10% of oil. The invention also provides a method for improving the fermentation level of meleomycin. The medium is used, Streptomyces mycarofaciens is used as a fermentation strain, and a glucose solution is added when the pH of a fermentation broth rebounds to 6.0 from the lowest point in order to improve the fermentation level of meleomycin and the proportion of kitasamycin. The method is simple and is easy to implement, and when the method is used for the fermentative production of meleummycin, the content of the meleummycin can reach 3400-4000 mg / L, the weight proportion of medemycin A1 is about 58.38-59.75%, and the weight proportion of kitasamycin A6 can reach 24.72-29.72%; andthe tank yield of meleomycin after fermentation is about 1.2 times more than that of a control group without a supplemental glucose solution, and the A6 component content is increased by about 25% ormore.
Owner:PEKING UNIV FOUNDER GRP CO LTD +2
Production method of enteric-coated kitasamycin for feed
ActiveCN101611766BProtect weak alkaline antibioticsProlong the action timeAnimal feeding stuffAccessory food factorsAcrylic resinGastric fluid
The invention discloses a production method of enteric-coated kitasamycin for feed, which comprises the following process steps: step one, the preparation of drug-loaded pellets; step two, inner layer sustained-release coating of the drug-loaded pellets, which prolongs the release and acting time of kitasamycin; and steps three, outer layer enteric coating of the drug-loaded pellets, which ensures the release in succus entericus and small or no release in gastric juice. The method has the advantages that the kitasamycin is subjected to pellets pelletizing and 99 percent of the prepared granulums can pass through 24 meshes, so the dust is greatly reduced and the fluidity is increased; the coating of the inner layer sustained-release agent (HPMC) prolongs the release and acting time of the kitasamycin, reduces medication times and reduces the medication cost; and a layer of enteric substance, namely acrylic resin-III is sprayed and coated on the outer layer of particles. The substance protects the kitasamycin which is a weakly alkaline antibiotic from being damaged by gastroc acid in stomach, quickly disintegrates after entering enteric canal and releases the kitasamycin; and then the kitasamycin is absorbed by gastrointestinal mucosa into blood drug to play a role of restraining the reproduction of pathogenic microorganism and preventing diarrhea. Insoluble in the stomach, kitasamycin coating formulations have no pessimal stimulation on the stomach, and cannot result in regurgitation and vomiting. In addition, the sustained-release formulation, namely the kitasamycin prolongs the acting time so the medication times is reduced, the medication cost of farmers is reduced, and the economic benefit is improved.
Owner:WUXI ZHENGDA POULTRY
Feed for benefiting spleen, removing dampness and promoting growth of growing-finishing pig
The invention discloses a feed for benefiting the spleen, removing dampness and promoting growth of a growing-finishing pig. The feed comprises the following raw materials in percentage by weight: 8%-15% of coix seed bran and 85%-92% of a general feed. The feed is prepared by the following steps: performing superfine grinding on the coix seed bran through a 100-mesh sieve, heating to 90 DEG C, curing at the high temperature, adding the general feed according to the proportion, and uniformly mixing. According to the feed, the coix seed byproduct is added into the general feed, so that the effects of benefiting the spleen, removing dampness and promoting growth of the growing-finishing pig are improved and the disease resistance of the growing-finishing pig can be enhanced; the coix seed bran is used for replacing healthcare drugs and antibiotics in the pig feed, and the effect of the coix seed bran is remarkably superior to that of the antibiotics such as kitasamycin and enramycin; wastes are turned into wealth, the resources are saved, the cost is reduced, and great economic benefits are brought to regions abounding in coix seeds.
Owner:GUIZHOU QIANNONG AGRI TECH
4% healthcare premix of middle pigs
InactiveCN103931890AEnsure nutritional needsImprove immunityFood processingAnimal feeding stuffDiseasePhytase
The invention discloses a 4% healthcare premix of middle pigs, which relates to the technical field of animal feeds and is made from the following raw materials in parts by weight: 26 parts of middle pig multi-minerals, 1.5 parts of phytase, 2.5 parts of middle pig multi-vitamins, 2 parts of arsanilic acid, 1.3 part of kitasamycin, 2 parts of specific section enzyme, 2 parts of probiotics, 2 parts of organic chromium, 1.5 parts of a flavoring agent, 2 parts of a sweetening agent, 1 part of an antioxidant, 2.5 parts of red skin element, 28.8 parts of choline chloride, 14 parts of 98.5% lysine, 75 parts of calcium hydrophosphate, 45 parts of salt, 150 parts of rock flour, 108 parts of zeolite, 53.5 parts of a mixture of rice chaff and husk, 16 parts of mulberry leaf powder, 20 parts of sweet potato powder, 15 parts of sweet potato leaf powder, 20 parts of peanut shell powder, 10 parts of bean cake, 10 parts of rapeseed oil dreg cake, 15 parts of sesame oil dreg cake and 6 parts of traditional Chinese medicine additives. The premix provided by the invention contains the traditional Chinese medicine ingredients, so that the normal nutrition demand of the middle pigs is ensured, diseases can be prevented and cured, the immunity of the animals can be improved, and the growth of the animals can be promoted; no toxic and side effects are caused, no drug resistance is generated, the environment is not polluted, the body immunity can be increased, the effect is rapid, the cost is low and the advantages of popularization and application are provided.
Owner:ANHUI ZHUANGDA FEED
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com