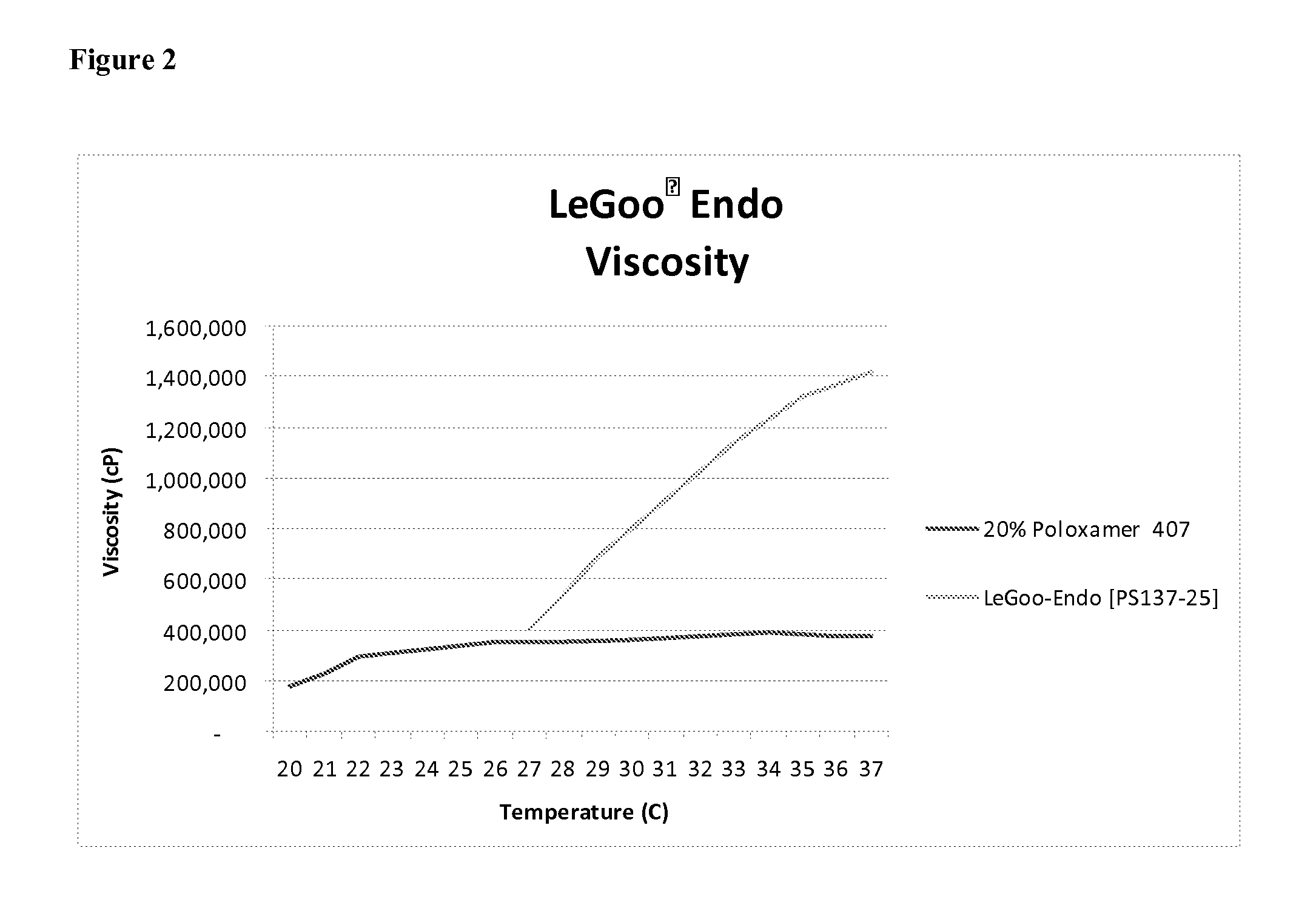
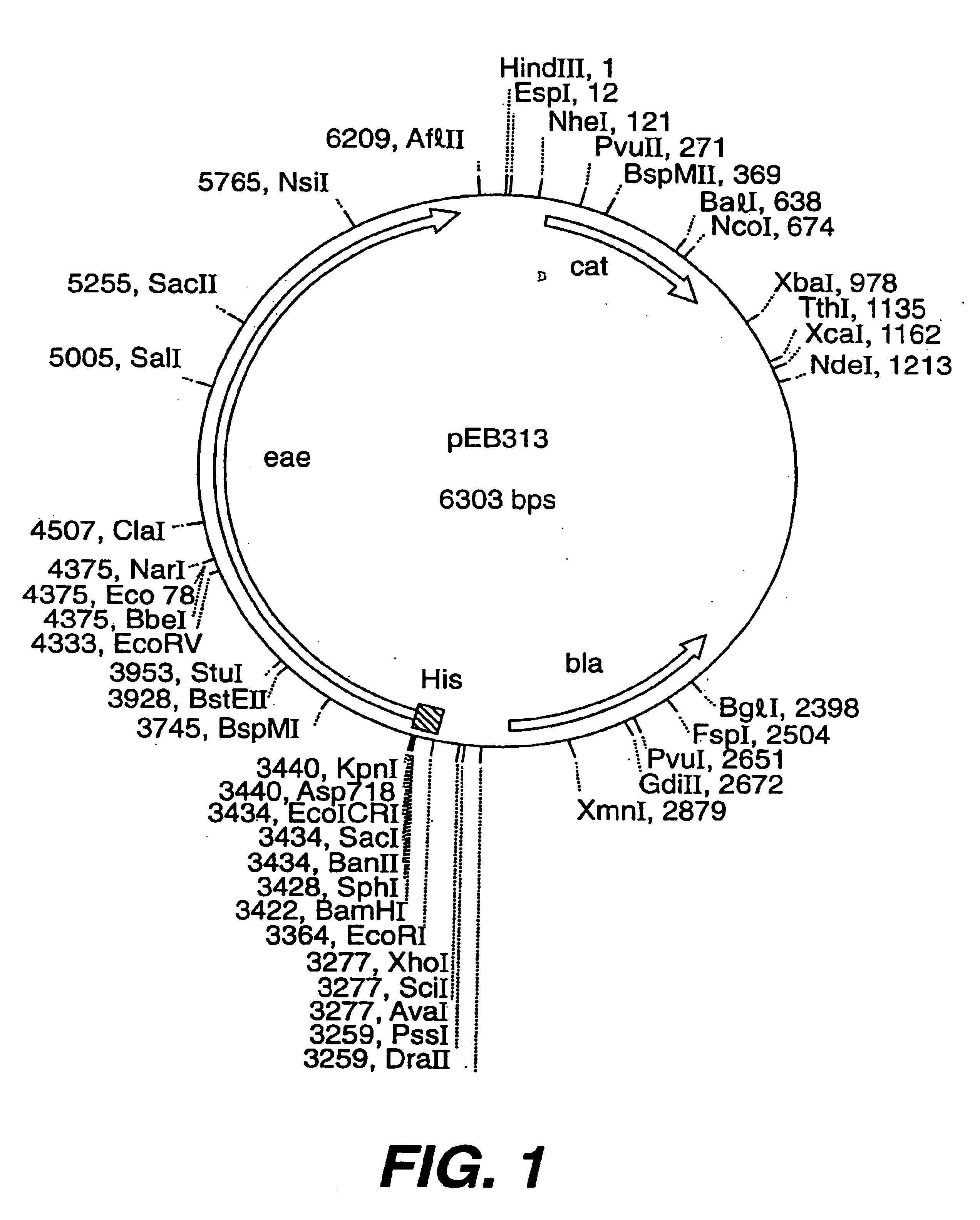
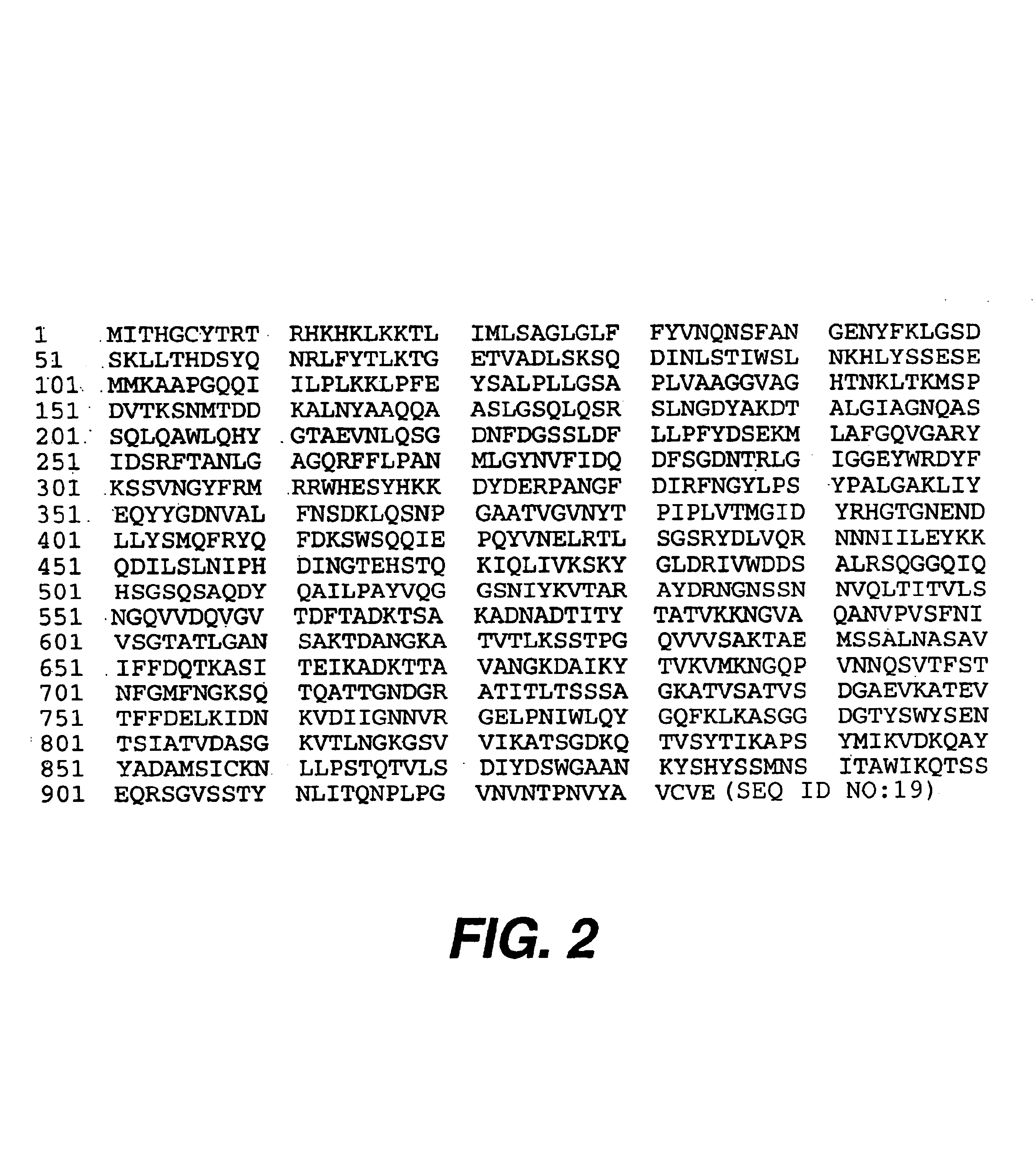
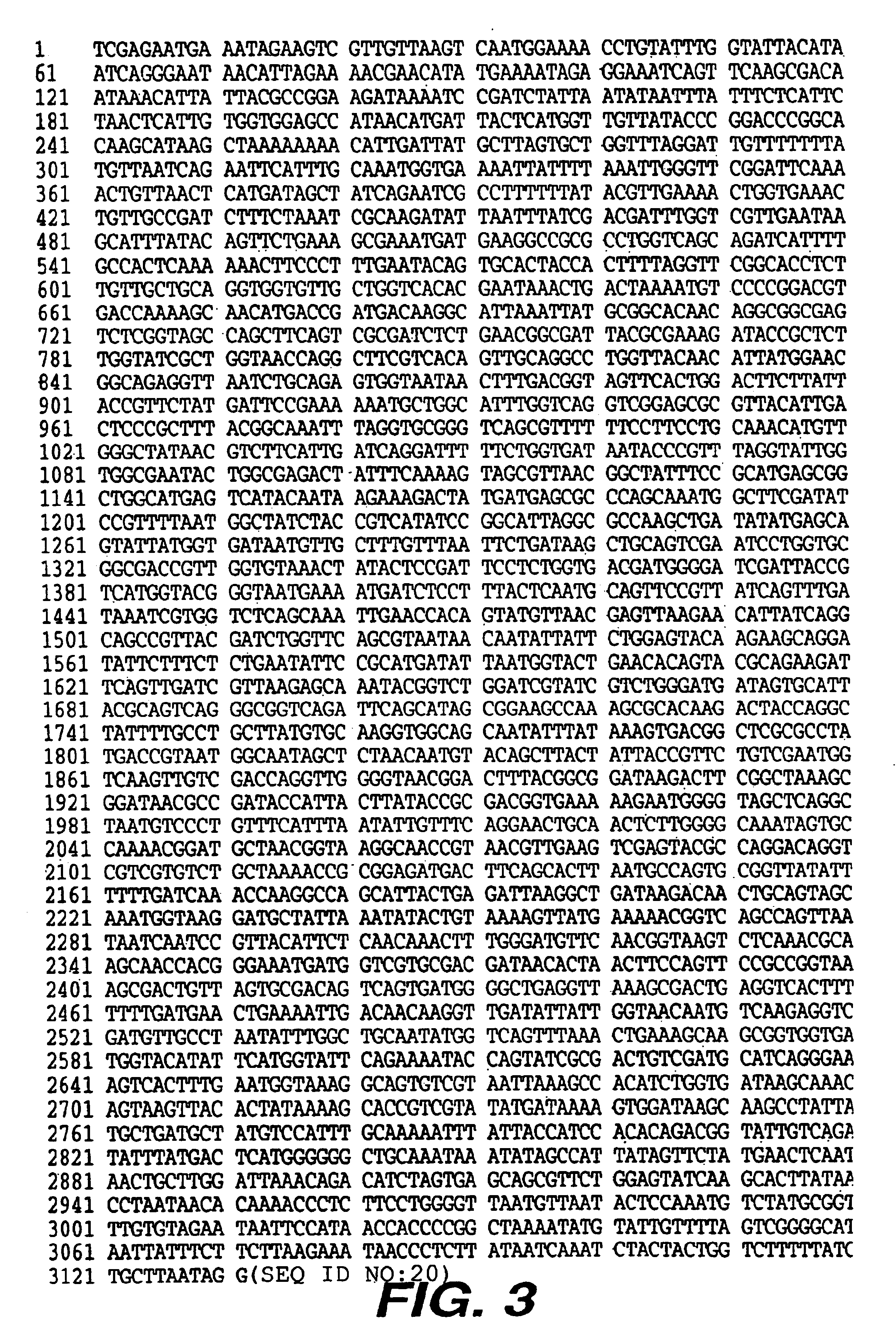
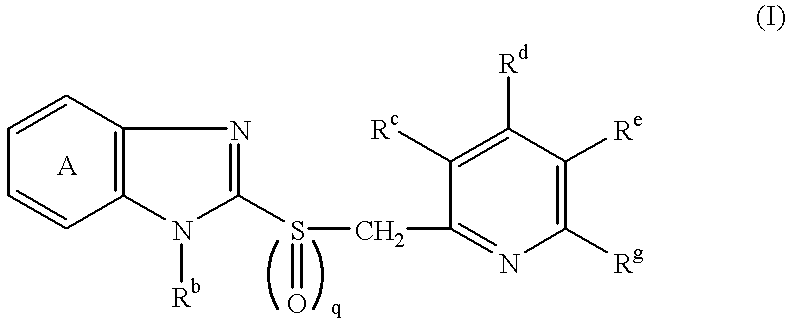
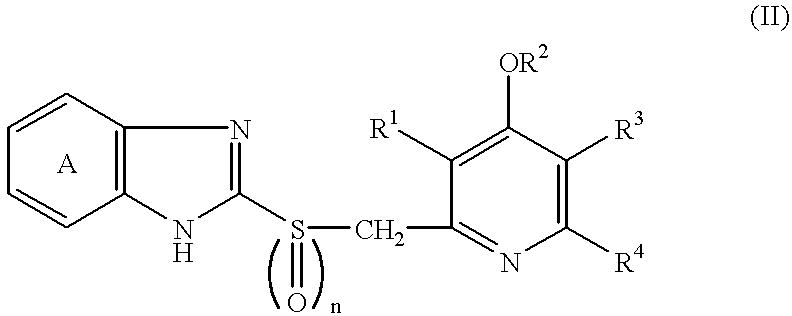
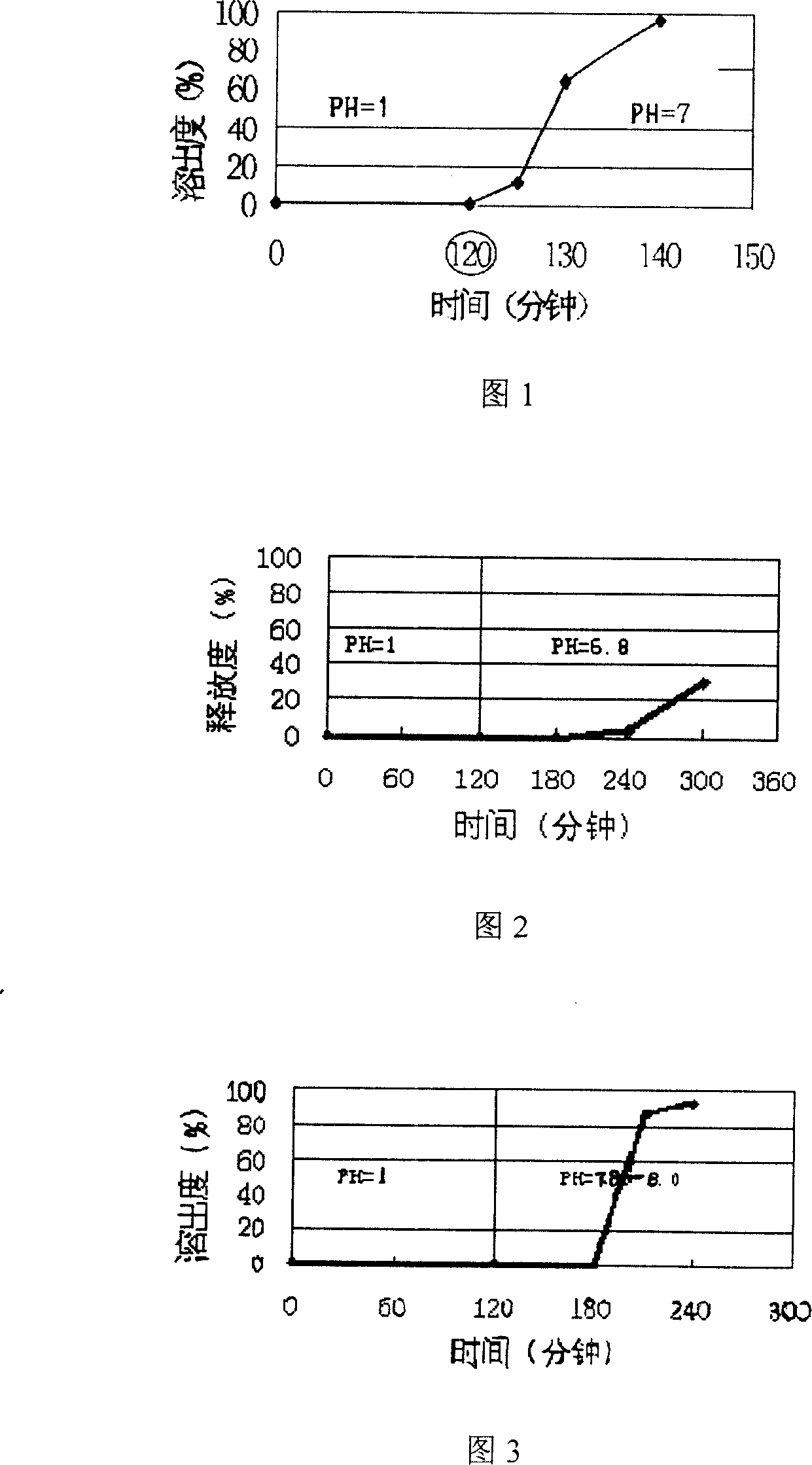
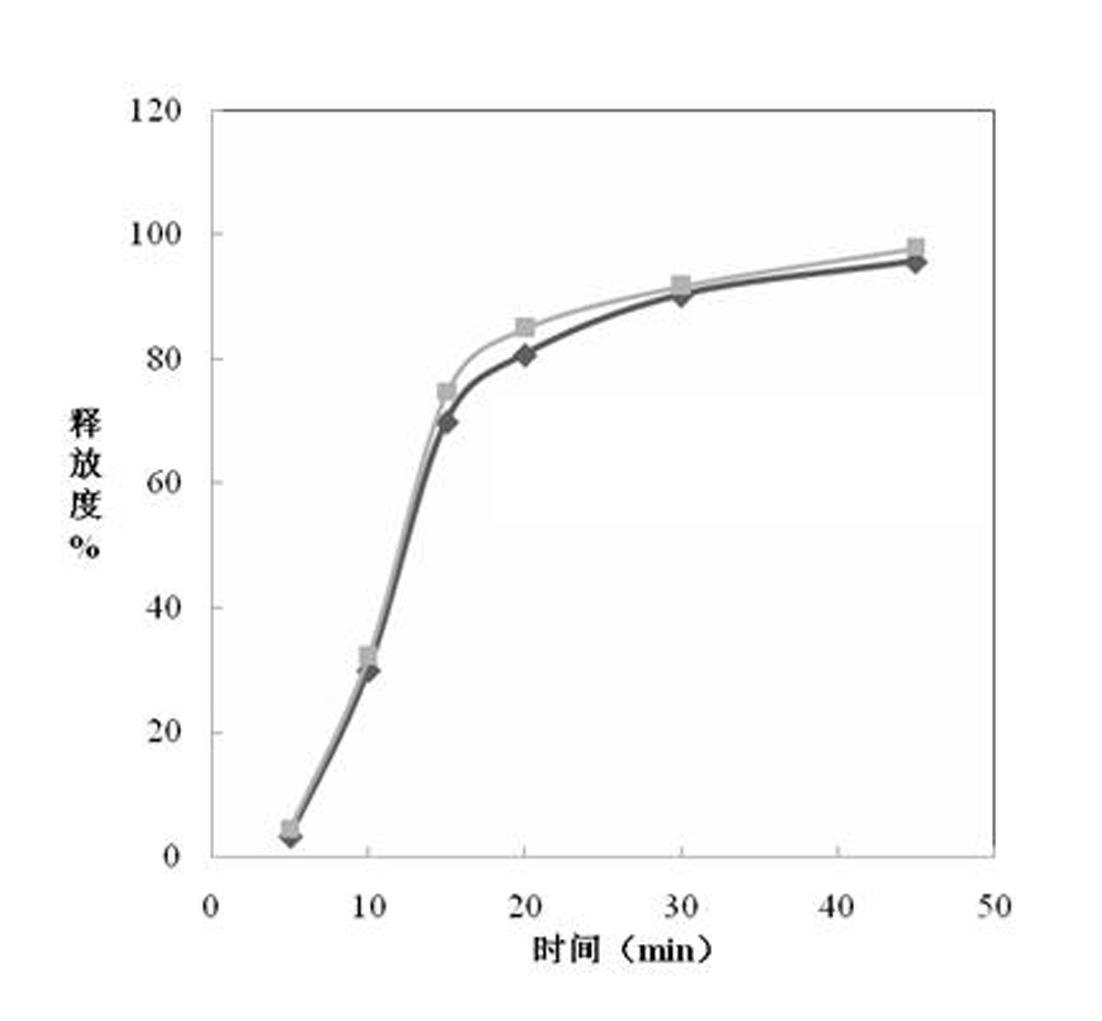
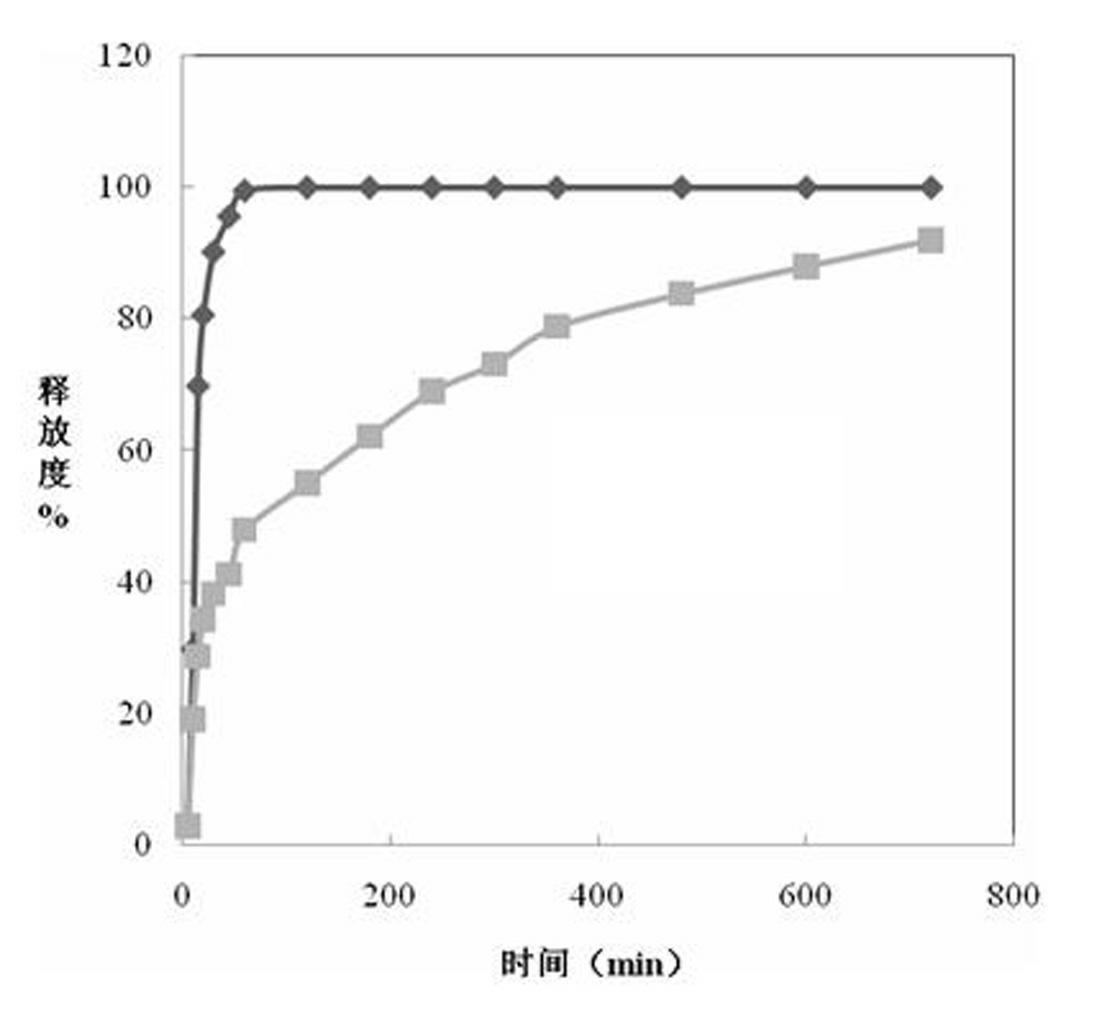
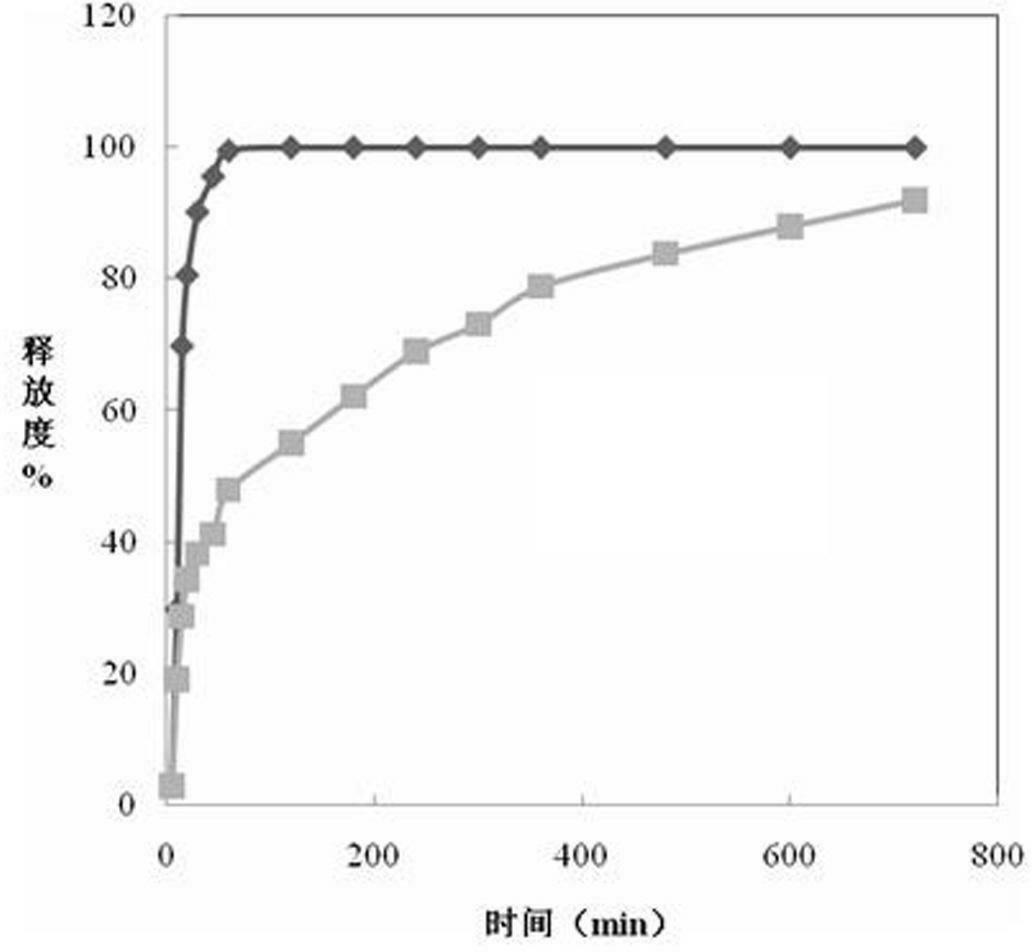
Patents
Literature
230 results about "Gastrointestinal mucosa" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Slowly-released compound acidifier for poultry and livestock feed, preparation method thereof and feed
ActiveCN102578387AMatching scienceDefinitelyClimate change adaptationAnimal feeding stuffDiseaseFeed conversion ratio
The invention discloses a slowly-released compound acidifier for poultry and livestock feed, a preparation method thereof and feed containing the same. The compound acidifier comprises the following compositions in part by weight: 30 to 80 parts of complex organic acid, 15 to 50 parts of supplementary materials and 5 to 20 parts of coating agents. The complex organic acid is citric acid, fumaric acid, malic acid, lactic acid, linoleic acid, crataegolic acid, ursolic acid, chlorogenic acid, glycyrrhizic acid and oleanolic acid, and the supplementary materials are silicon dioxide, hydroxypropyl methylcellulose and ethyl cellulose. The acidifier is scientifically proportioned, has strong efficiency and is long-acting and slow-releasing, nutrients in the feed are free from being damaged, the acidifier has good fluidity and is easy to be uniformly mixed with the feed, the digestive absorption of nutrients can be promoted, the conversion rate of the feed can be improved, the productivity of poultry and livestock can be improved, the time for domestic animals for sale can be effectively shortened, the health of gastrointestinal mucosa of the animals can be protected, the immunologic function of the animals can be strengthened, and diseases can be prevented.
Owner:BEIJING DABEINONG ANIMAL HEALTH TECH +1
Gastrointestinal mucosa-adherent pharmaceutical composition
InactiveUS6428813B1Improve security featuresEnhanced adhesion mucosaAntibacterial agentsPowder deliveryLow-substituted hydroxypropylcelluloseEfficacy
In order to provide a composition having a long gastroduodenal residence time and exhibiting an improved efficacy, is provided a gastrointestinal mucosa-adherent composition comprising an active ingredient and a material which swells a viscogenic agent capable of being viscous with water a (e.g. curdlan and / or a low-substituted hydroxypropylcellulose etc.).
Owner:TAKEDA PHARMA CO LTD
Oral liquid for alleviating hangover and protecting liver, and preparation method thereof
The invention provides oral liquid for alleviating a hangover and protecting a liver, and a preparation method thereof. The oral liquid is prepared from the following components in parts by weight: 5-15 parts of root of kudzu vine, 5-15 parts of semen hoveniae, 0.5-5 parts of ginseng, 2-10 parts of lalang grass rhizome, 0-5 parts of hawthorn, 0-3 parts of sealwort, 0-15 parts of pueraria flower, 3-20 parts of glucose, 1-10 parts of xylitol, 1-5 parts of L-arabinose, 0.2-1.0 part of taurine, 0-0.5 parts of glutamine, 0-5 parts of resistant dextrin, 0-1 part of decavitamin, and 0-2 parts of compound amino acid. The oral liquid has the beneficial effects of good hangover alleviating efficacy and good flavor and mouthfeel; the problems of dizzy giddy and scattered attention after excessive drinking are solved; damages to the liver and kidney caused by alcohol are reduced; and gastrointestinal mucosa is protected.
Owner:JINDA BIOENG BIOTECH DEV FUZHOU
Hydrophobic drug containing polyelectrolyte complex, its preparation method and application thereof
InactiveCN102813937APromote absorptionExtended stayPowder deliveryPharmaceutical non-active ingredientsRetention timeEntrapment
The invention relates to a hydrophobic drug containing polyelectrolyte complex, its preparation method and an application thereof. The polyelectrolyte complex is formed by static compounding of polycation and electronegative nanoparticles formed by polyanion or its derivative entrapment or linkage of a hydrophobic drug. The particle size of the polyelectrolyte complex is 20-500 nm. The positive / negative charge ratio of the polycation to the polyanion is between 1.2 and 6.0. According to the drug-containing complex formed by static compounding, an external positive charge shell can protect stability of the complex in a gastric acidic environment, and in an intestinal alkaline environment, the complex disintegrates and releases the internal anion drug loaded nanoparticles so as to promote absorption of the drug through intestinal mucosa. In addition, the polycation shell has mucosa adhesiveness and retention time of the drug in gastrointestinal mucosa can be prolonged. Therefore, the complex is suitable for oral administration of hydrophobic drugs. Bioavailability of the hydrophobic drugs is increased, and pains caused by injection delivery can be minimized for patients.
Owner:TIANJIN UNIV
Endoscopic mucosal resectioning using purified inverse thermosensitive polymers
InactiveUS20110052490A1In-vivo radioactive preparationsSurgical adhesivesThermosensitive polymerEndoscopic Procedure
One aspect of the invention relates to use of a composition comprising a purified inverse thermosensitive polymer in an endoscopic procedure for gastrointestinal mucosal resectioning in a mammal. Another aspect of the invention relates to a method of gastrointestinal mucosal resectioning, comprising administering submucosally to a region of a gastrointestinal mucosa in a mammal an effective amount of a composition comprising a purified inverse thermosensitive polymer; and surgically resecting said region of gastrointestinal mucosa. Yet another aspect of the invention relates to a kit for use in gastrointestinal endoscopic mucosal resectioning in a mammal, comprising a composition comprising a purified inverse thermosensitive polymer; a syringe; and instructions for use thereof.
Owner:GENZYME CORP
Histidine-tagged intimin and methods of using intimin to stimulate an immune response and as an antigen carrier with targeting capability
InactiveUS6942861B2Lessens seriousnessPromotion of protective immune responseAntibacterial agentsOrganic active ingredientsAntigenInner membrane
The present invention describes the isolation and purification of histidine-tagged functional portions of intimin (his-tagged intimin or his-intimin), a protein associated with the ability of certain strains of pathogenic bacteria to adhere to epithelial cells. The invention further describes the use of intimin as an antigen to promote a protective immune response. In addition, the invention describes the combination of intimin with one or more other antigens and administration of the combination to promote a protective immune response against intimin and the one or more antigens.One aspect of the invention is the administration of intimin to target specific epithelial cells to promote a protective immune response to intimin proteins. Additional aspects of the invention include the use of intimin or intimin combined with one or more antigens and administration of the combination to target gastrointestinal mucosa and stimulate an immune response. Additionally, the invention describes administration of the combination of intimin combined with drugs, to provide a means for targeted delivery of drugs to specific epithelial cells. Other aspects of the invention include the production of antibodies directed against his-intimin and methods of using such antibodies to provide passive immune protection, and in an assay system.
Owner:THE HENRY M JACKSON FOUND FOR THE ADVANCEMENT OF MILITARY MEDICINE INC
Cephalofruxin ester liposome, its preparation and medicinal composition containing it
ActiveCN1939305APromote dissolutionThe drug works quicklyAntibacterial agentsPharmaceutical non-active ingredientsMedicineCholesterol
A liposome of cefuroxime axetil used to prepare the tablet with high target performance to the gastrointestinal mucosa cells is prepared from cefuroxime axetil, soybean lecithin and chlosterol in weight ration of 1:2:2. Its preparing process is also disclosed.
Owner:CSPC OUYI PHARM CO LTD
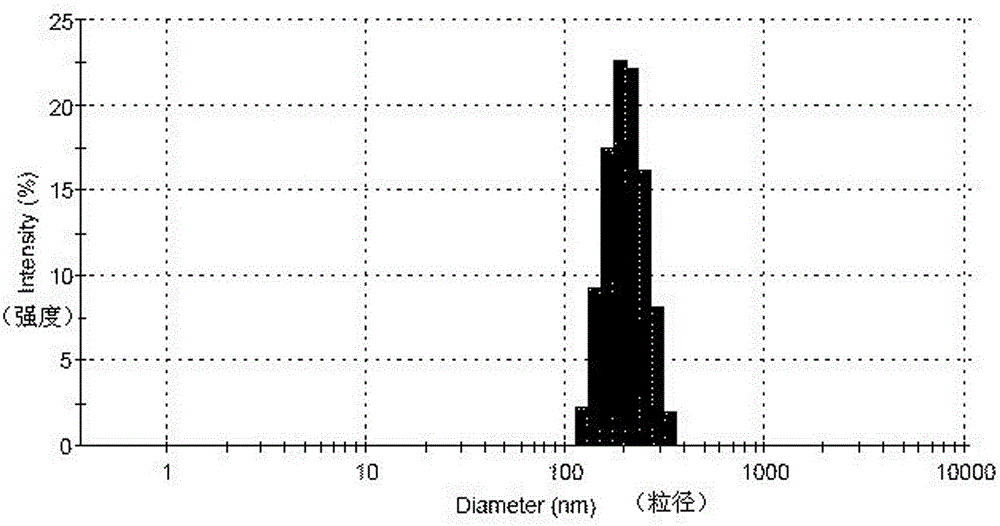
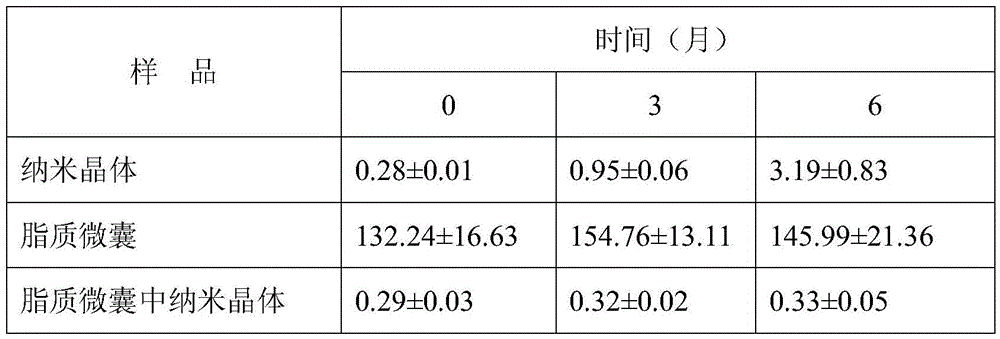
Loaded aprepitant nanocrystal lipid microcapsule and preparation method thereof
ActiveCN105456228AImprove stabilityMild preparation conditionsOrganic active ingredientsNervous disorderPhospholipidHigh pressure
The invention relates to a loaded aprepitant nanocrystal lipid microcapsule and a preparation method thereof, and belongs to the technical field of a medicine. The microcapsule is prepared by the method comprising the following steps: preparing an aprepitant nanocrystal from aprepitant by virtue of a grinding process, high-speed air jet pulverization or high-pressure homogenization, then mixing and emulsifying the aprepitant nanocrystal with phospholipid, removing a liquid phase and drying so as to obtain the aprepitant nanocrystal lipid microcapsule. The prepared loaded aprepitant nanocrystal lipid microcapsule can improve the stability of the aprepitant nanocrystal and the lipid loaded nanocrystal can increase the speed and the amount of being absorbed by gastrointestinal mucosa of a body, so that the effects of relatively high bioavailability and accelerated development of efficacy are achieved; and the preparation method of the loaded aprepitant nanocrystal lipid microcapsule is mild in condition, simple and controllable, low in preparation cost and is suitable for large-scale production.
Owner:FUREN PHARMA GROUP +2
Formulation comprising antibacterial substance and antiulcer substance
InactiveUS6319904B1Low prevalencePotent activityBiocideCarbohydrate active ingredientsSide effectRetention time
Owner:TAKEDA PHARMA CO LTD
Traditional Chinese medicinal composition for treating gastrointestinal tract and preparation thereof
InactiveCN101797347ARegulate secretionPromote gastrointestinal motilityPowder deliveryDigestive systemDiseaseMotility
The invention relates to a traditional Chinese medicinal composition for treating gastrointestinal tract, in particular to a medicinal composition for treating gastrointestinal tract diseases such as acute and chronic gastritis, enteritis, gastric ulcer, superficial gastritis, gastric acid, gastrectasia, indigestion and the like. The invention also relates to a preparation of the medicinal composition. The traditional Chinese medicinal composition for treating gastrointestinal tract comprises the medicaments in percentage by weight: 10-25 parts of red ginseng, 10-23 parts of poria, 8-20 parts of bighead atractylodes rhizome, 10-25 parts of rhizoma ligustici wallichii, 10-25 parts of rhizoma atractylodis, 10-25 parts of cyperus rotundus, 10-25 parts of medicated leaven, 10-25 parts of gardenia, 10-23 parts of betelnut, 8-20 parts of radix aucklandiae, 5-15 parts of liquorice, 5-8 parts of malt and 10-25 parts of hawthorn. The medicinal composition for treating the gastrointestinal tract is orally taken and has the efficacies of regulating the flow of qi, comforting the stomach, diminishing inflammation, killing pain, repairing gastrointestinal mucosa and ulceration, enhancing gastrointestinal motility, adjusting gastric acid secretion, promoting digestive absorption and the like.
Owner:王志海
Live microbial microbicides
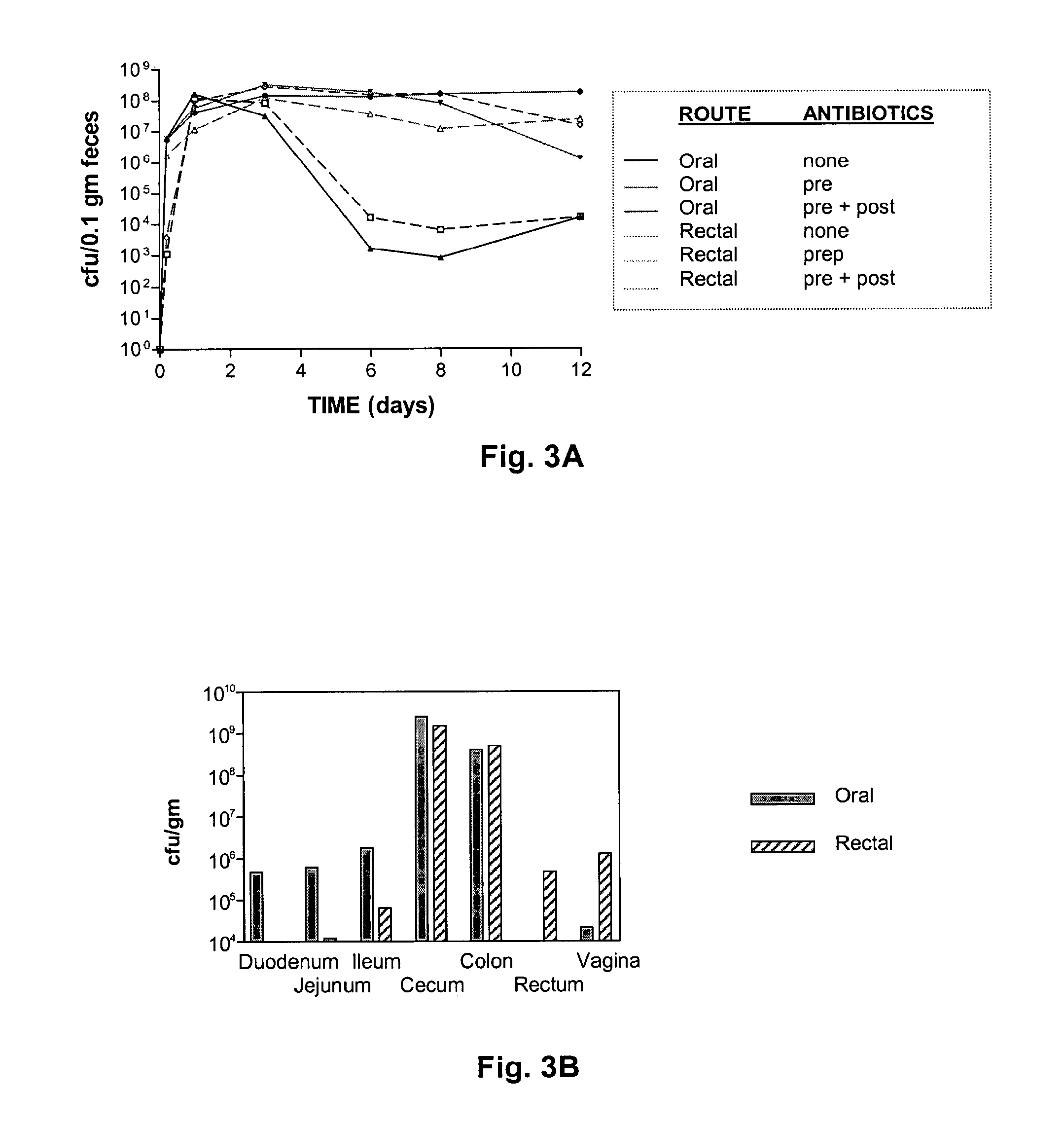
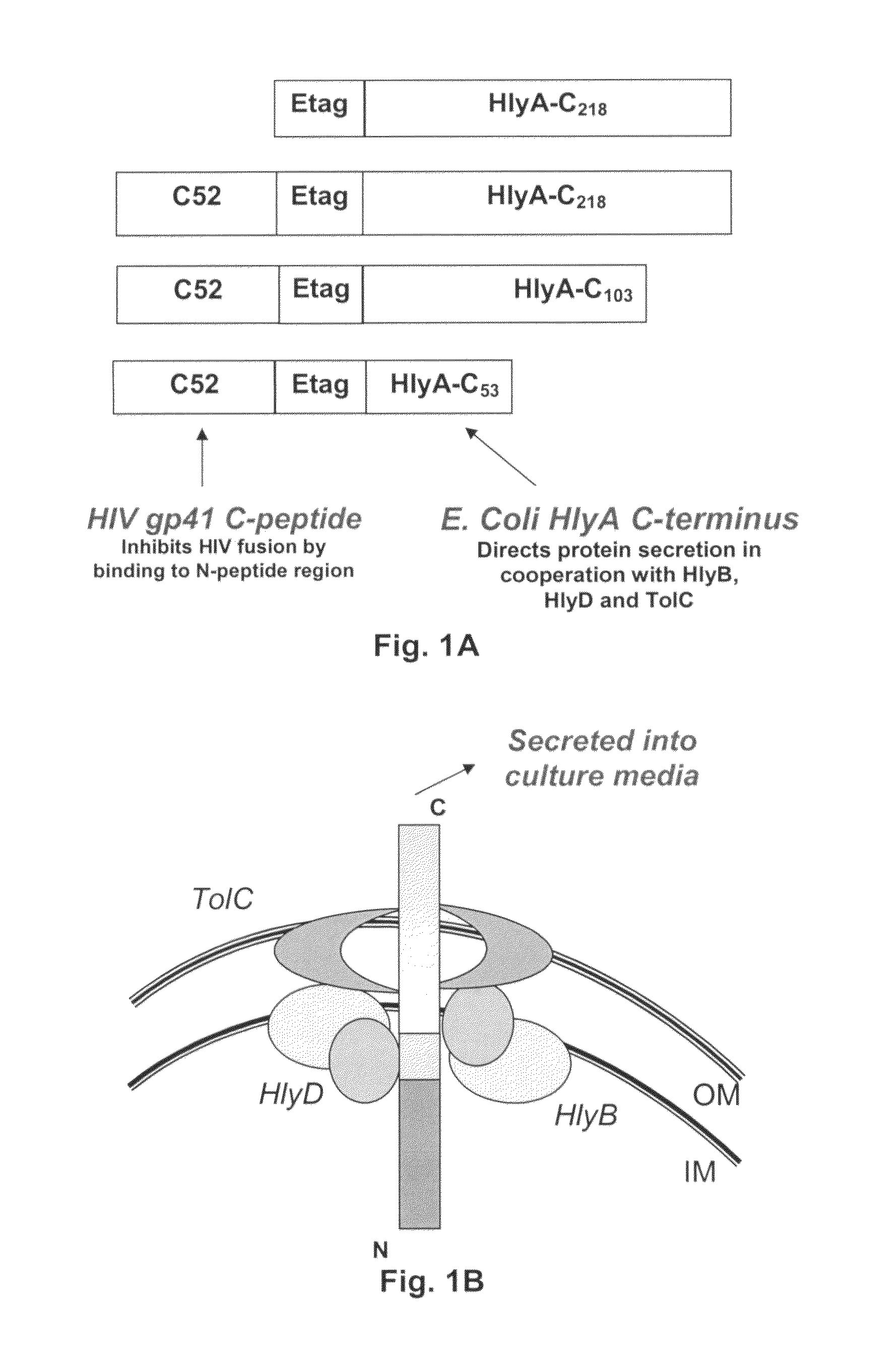
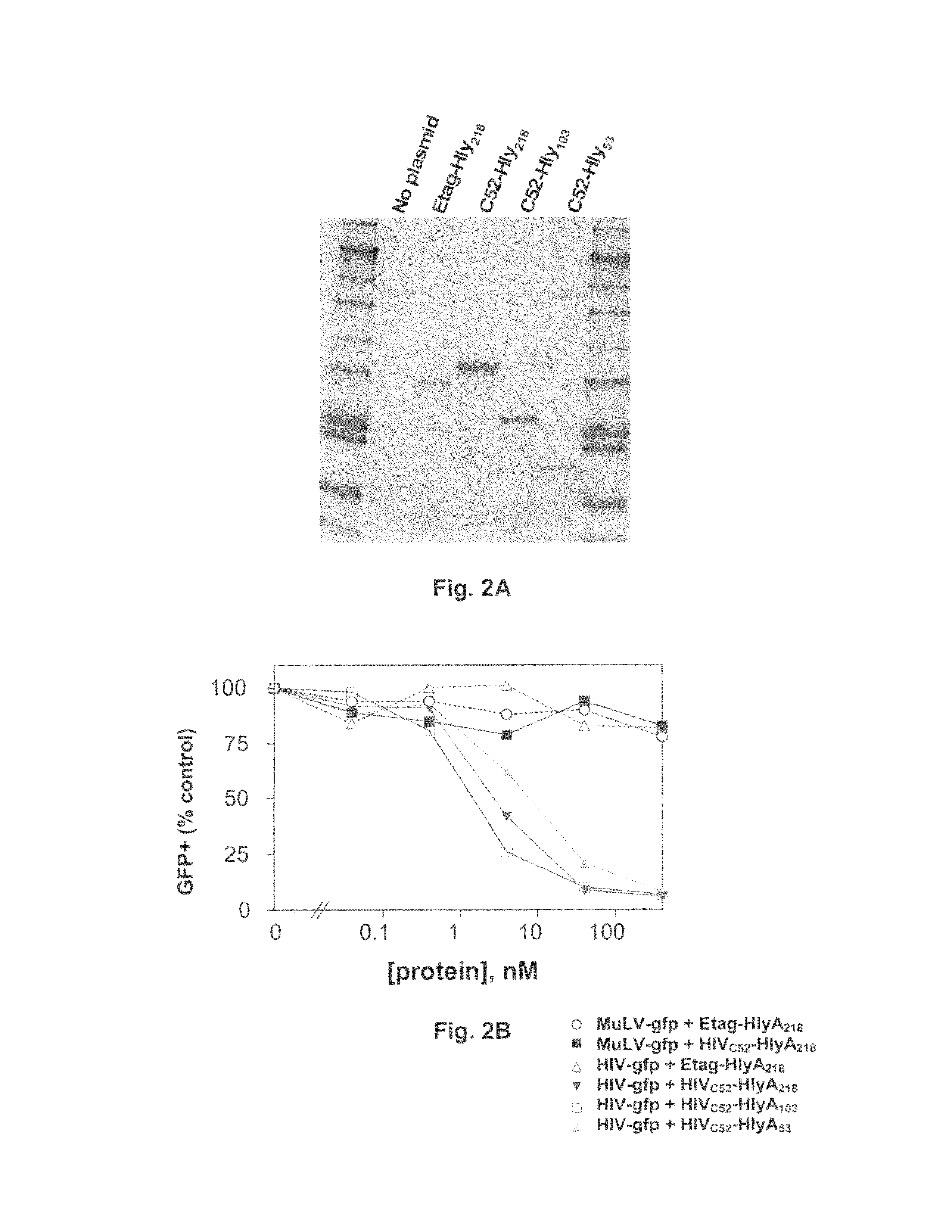
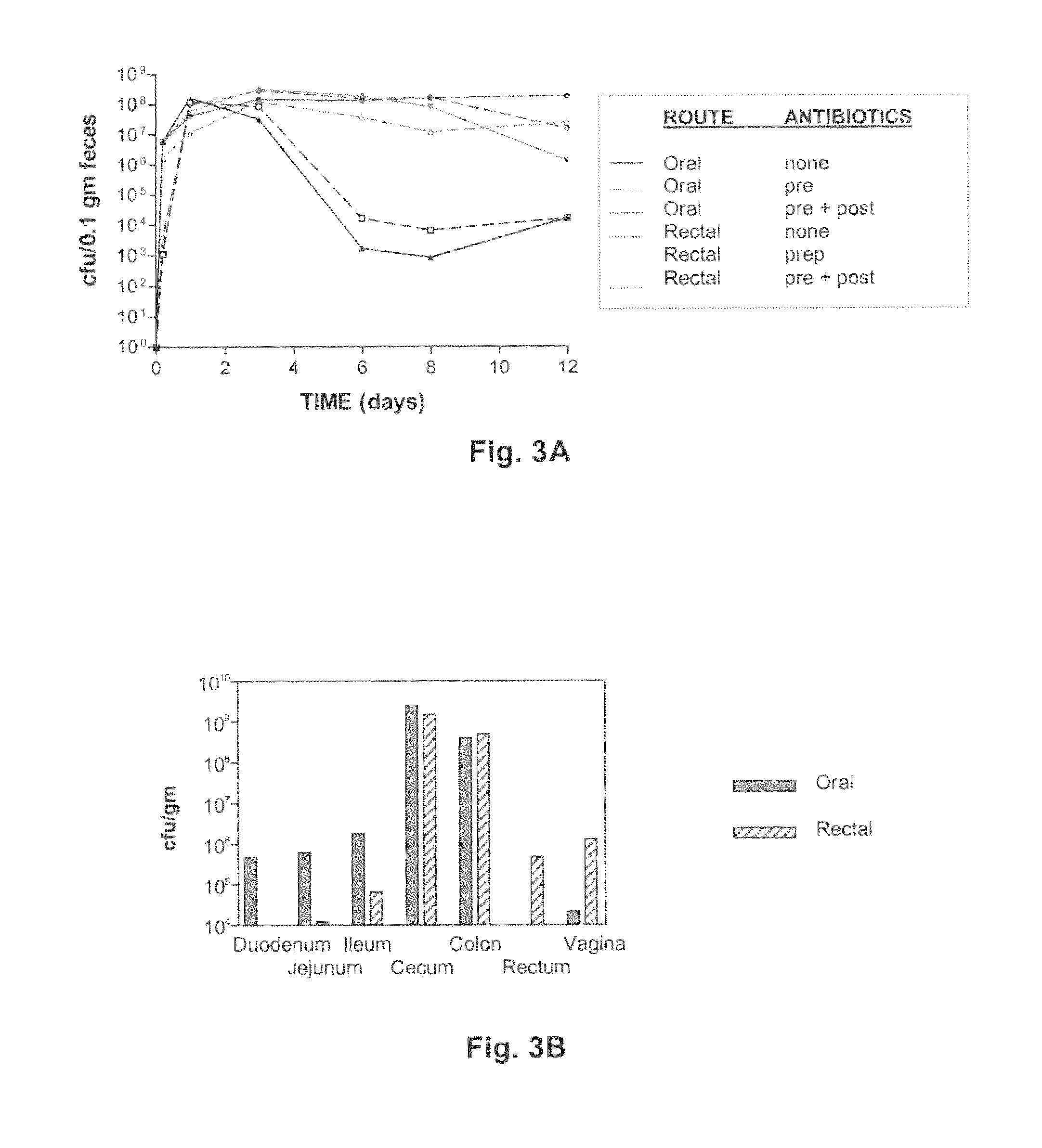
The present invention relates, e.g., to a commensal bacterium which can colonize the genitourinary and / or gastrointestinal mucosa, and which, under suitable conditions, secretes a heterologous antimicrobial polypeptide, wherein the secreted antimicrobial polypeptide is effective to inhibit infectivity by, or a pathogenic activity of, a pathogen. In a most preferred embodiment, the antimicrobial polypeptide inhibits HIV infection (e.g., fusion) and / or pathogenesis. Also described are preventive or therapeutic compositions comprising the commensal bacteria, and methods to inhibit infectivity and / or pathogenesis, using the bacteria.
Owner:UNITED STATES OF AMERICA
Production method of enteric-coated kitasamycin for feed
ActiveCN101611766AProtect weak alkaline antibioticsProlong the action timeAnimal feeding stuffAccessory food factorsAcrylic resinMicroparticle
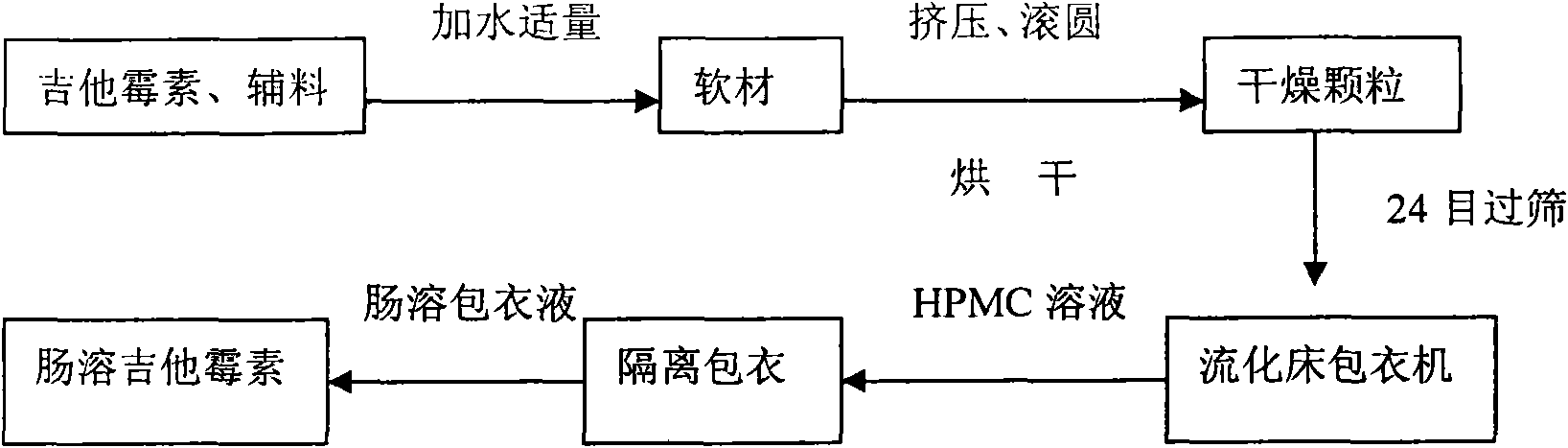
The invention discloses a production method of enteric-coated kitasamycin for feed, which comprises the following process steps: step one, the preparation of drug-loaded pellets; step two, inner layer sustained-release coating of the drug-loaded pellets, which prolongs the release and acting time of kitasamycin; and steps three, outer layer enteric coating of the drug-loaded pellets, which ensures the release in succus entericus and small or no release in gastric juice. The method has the advantages that the kitasamycin is subjected to pellets pelletizing and 99 percent of the prepared granulums can pass through 24 meshes, so the dust is greatly reduced and the fluidity is increased; the coating of the inner layer sustained-release agent (HPMC) prolongs the release and acting time of the kitasamycin, reduces medication times and reduces the medication cost; and a layer of enteric substance, namely acrylic resin-III is sprayed and coated on the outer layer of particles. The substance protects the kitasamycin which is a weakly alkaline antibiotic from being damaged by gastroc acid in stomach, quickly disintegrates after entering enteric canal and releases the kitasamycin; and then the kitasamycin is absorbed by gastrointestinal mucosa into blood drug to play a role of restraining the reproduction of pathogenic microorganism and preventing diarrhea. Insoluble in the stomach, kitasamycin coating formulations have no pessimal stimulation on the stomach, and cannot result in regurgitation and vomiting. In addition, the sustained-release formulation, namely the kitasamycin prolongs the acting time so the medication times is reduced, the medication cost of farmers is reduced, and the economic benefit is improved.
Owner:WUXI ZHENGDA POULTRY
Loop-type bile and pancreatic juice drainage tube
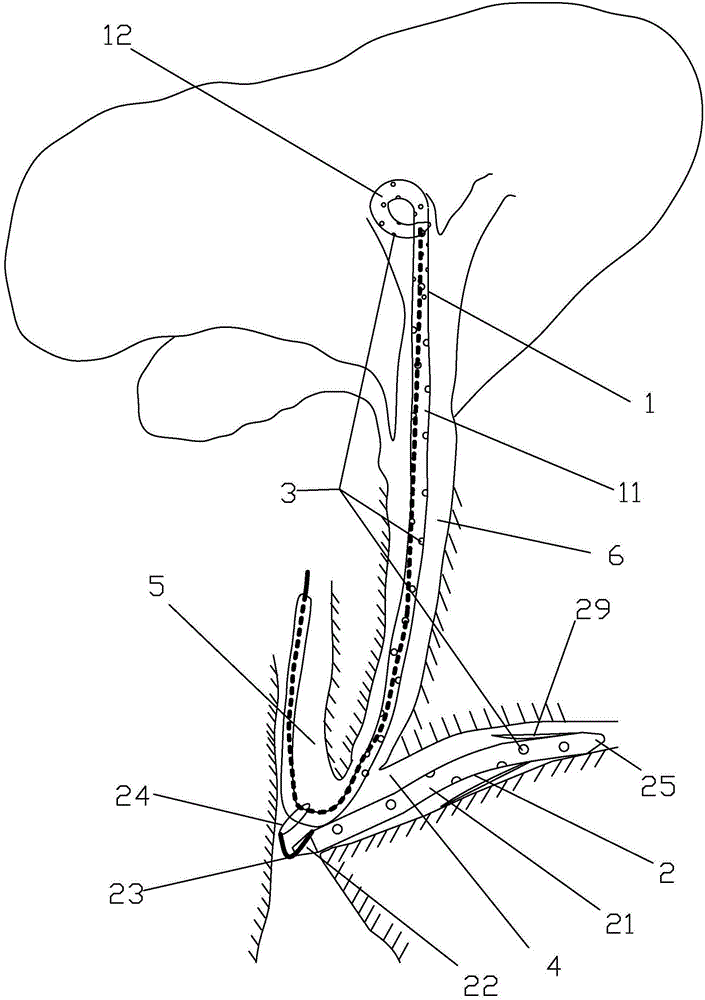
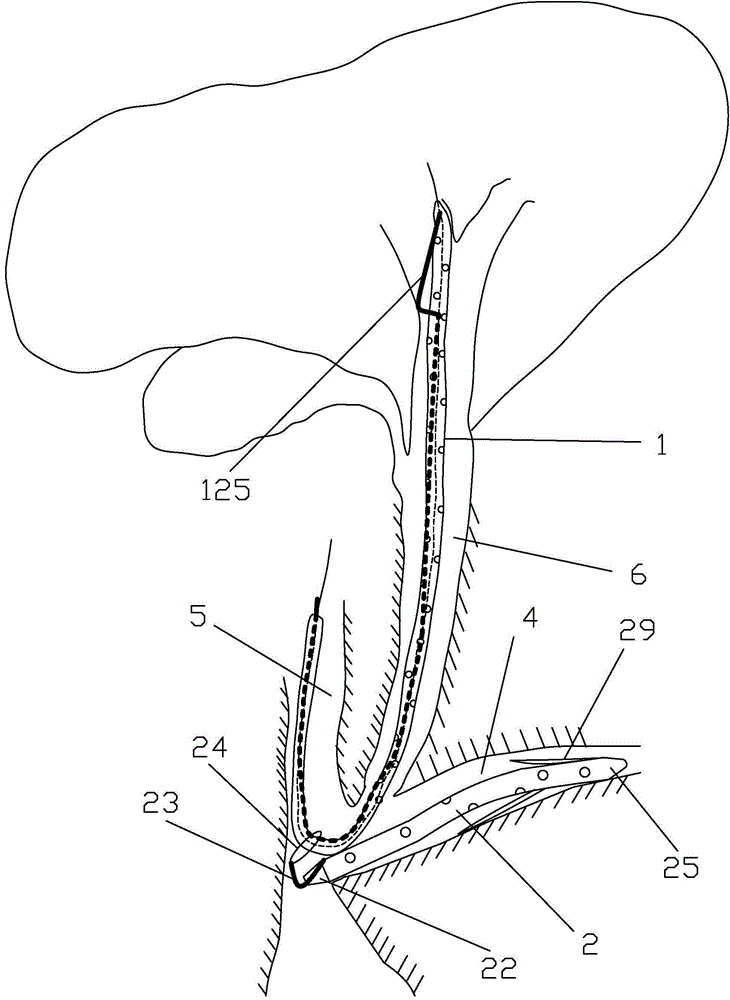
The invention discloses a loop-type bile and pancreatic juice drainage tube, which comprises a loop-type bile drainage tube and a pancreatic juice drainage tube, wherein the loop-type bile drainage tube comprises a straight tube and an elastic loop tube; the elastic loop tube is integrally of a cylindrical spiral line shape and is provided with an elastic loop tube head and an elastic loop tube tail; the pancreatic juice drainage tube comprises a drainage tube body and a drainage tube tail positioned on the lower end of the drainage tube body; the pancreatic juice drainage tube also comprises a second line, wherein one end of the second line is connected with the lower-end side wall of the drainage tube body, and the other end of the second line passes through the drainage tube tail to be connected with a coil or a metal ring. According to the loop-type bile and pancreatic juice drainage tube, the slippage probability of the loop-type bile drainage tube is lowered, and the loop-type bile and pancreatic juice drainage tube does not irradiate a bile duct mucous membrane, duodenal papilla, gastrointestinal mucosa and a throat mucous membrane; while the loop-type bile drainage tube is taken out, the pancreatic juice drainage tube can be simultaneously taken out so as to avoid a secondary ERCP (Endoscopic Retrograde Cholangiopancreatography) surgery and lower treatment cost and surgery risks; the loop-type bile and pancreatic juice drainage tube has a function that the pancreatic juice drainage tube can be prevented from inwards moving or separating.
Owner:DALIAN UNIV
Alimentary canal gel protective agent and use method and application thereof
InactiveCN108451973ATo achieve the effect of "isolation"Reach \"embedded\"Heavy metal active ingredientsOrganic active ingredientsSide effectCholesterol
The invention relates to the technical field of foods and pharmacy, and provides an alimentary canal gel protective agent. The alimentary canal gel protective agent comprises gels and an acid-solublecross-linking agent, wherein the gels are alginate, and the acid-soluble cross-linking agent is a multivalent metal salt or alkali which is insoluble under a neutral condition and soluble under an acidic condition; in addition, the invention further provides a use method and application of the alimentary canal gel protective agent. The alimentary canal gel protective agent and the use method and application thereof have the advantages that a stable gel protective layer is quickly formed on the gastrointestinal mucous membrane surface after the gel protective agent is taken, the absorption of food compositions by the alimentary canal mucous membrane is blocked or delayed through the modes of 'embedding' and 'isolation', particularly the absorption of fat, cholesterol, glucose, heavy metal ions and ethyl alcohol in the food can be reduced, the effects of losing weight, detoxifying emergently, avoiding alcohol, protecting peptic ulcer, reducing blood fat and protecting glucostasis are achieved, no toxic and side effects exist, the gel protective agent can be made into beverages, dissolved medicines and tablets for use, the production is simple and convenient, and the use is convenient.
Owner:南京健辉生物科技有限公司
Micro-ecology and Chinese herbal medicine compound preparation for treating gastrointestinal diseases and preparation method thereof
InactiveCN104984329AEliminate inflammationImprove defensePeptide/protein ingredientsDigestive systemDiseaseAdditive ingredient
The invention relates to a micro-ecology and Chinese herbal medicine compound preparation for treating gastrointestinal diseases and a preparation method thereof. 30-40 parts of micro-ecology probiotic viable bacteria and fermented compounds thereof, 5-10 parts of lactoferrin, 15-20 parts of hericium erinaceus extract, 10-15 parts of fructus mume extract and 10-15 parts of dandelion extract are mixed by weight at the normal temperature. Compared with the prior art, according to the micro-ecology and Chinese herbal medicine compound preparation for treating the gastrointestinal diseases and the preparation method thereof, the micro-ecology probiotic (lactobacilli and the like) viable bacteria and the fermented product compounds thereof aim at protecting gastrointestinal mucosae to restrain the activity of helicobacter pylori. Chinese herbal medicine ingredients enhance defense capability of gastrointestinal mucosa epithelial cells for H<+>, and meanwhile the repairing and nutritious functions for the mucosae are achieved.
Owner:上海善力健生物科技有限公司
Feed additive for preventing lactation-period-calf diarrhea and application method thereof
ActiveCN109770078APromote growthPromote repairAnimal feeding stuffAccessory food factorsAnti stressAnimal science
The invention discloses a feed additive for preventing lactation-period-calf diarrhea and an application method thereof. According to the feed additive, natural astaxanthin, sodium humate, beta-hydroxyl-beta-methylbutyric acid, glutamine, xylooligosaccharide and a Chinese herbal medicine additive (agrimonia pilosa ledeb, honeysuckle, Chinese pulsatilla roots, hawthorns, pomegranate bark, talc andlicorice roots) are compounded, the balance of gastrointestinal flora of lactation-period calves is conditioned, development of gastrointestinal mucosa is promoted, the damage and influence of pathogens and unfavorable factors on the calves are reduced, digestion and absorption of nutritional ingredients are increased, the body immunity is improved, the suffered oxidation stress and oxidative damage are relieved, the anti-stress ability is improved, and therefore the aims that occurrence of lactation-period-calf diarrhea is avoided, absorption of feed nutrients is promoted, and the health level and production performance of the calves are improved are achieved.
Owner:INST OF ANIMAL SCI & VETERINARY MEDICINE SHANDONG ACADEMY OF AGRI SCI
Preparation method of porcine epidemic diarrhea virus genetic engineering subunit oral vaccine
ActiveCN102988971AAvoid stressAvoid absorptionAntiviralsAntibody medical ingredientsStaphylococcus lactisLocal immunity
The invention discloses a preparation method of a porcine epidemic diarrhea virus genetic engineering subunit oral vaccine. The method comprises the following steps of: 1) synthesizing an M gene; 2) constructing an expression vector; 3) performing protein induction expression; 4) carrying out recombinant strain preservation experiment; and 5) preparing the vaccine. The oral immunization has the advantage of effectively stimulating local immune cells of the intestinal tract to generate secretion type IgA, is especially applicable to the infectious disease of the intestinal mucosa, and avoids stress and vaccine absorption problems resulting from conventional vaccine injection; and as a safe and non-toxic live vector system capable of living on the intestinal mucosa, the lactococcus lactis is the most suitable vector system. The oral vaccine performs antigen presentation through the gastrointestinal mucosa, and produces immune reaction similar to conventional injection through different immune pathways.
Owner:北京信得威特科技有限公司
Digestive tract contrast agent capable of relieving gastrointestinal convulsion and having hypotonic effect
InactiveCN103751810ALittle side effectsWith imaging functionDigestive systemX-ray constrast preparationsPhysiologyRaw material
The invention discloses a digestive tract contrast agent capable of relieving gastrointestinal convulsion and having hypotonic effect, which is prepared from 50-70 weight parts of barium sulfate dry suspension or compound cardiografin contrast agent and 20-30 weight parts of traditional Chinese medicinal extract. The traditional Chinese medicinal extract is prepared by mixing the following raw materials in parts by weight: 20-30 parts of Corydalis yanhusuo, 10-20 parts of Radix Bupleuri, 20-30 parts of processed Radix Glycyrrhizae, 20-30 parts of Salvia miltiorrhiza, 10-20 parts of Cyperus rotundus, 10-20 parts of Aucklandia lappa, 20-30 parts of Fructus Amomi, 20-30 parts of Evodia rutaecarpa, 10-20 parts of Rhizoma Coptidis, 10-20 parts of Radix Paeoniae Alba, 20-30 parts of Atractylodes macrocephala, 5-10 parts of Radix Codonopsis, 10-20 parts of Bletilla striata, and 5-15 parts of Panax pseudoginseng powder, soaking in water, decocting for 30-40 min, filtering to obtain a filtrate, concentrating the filtrate under reduced pressure, and freeze-drying. The digestive tract contrast agent disclosed by the invention has low adverse side effects and gastrointestinal imaging function, can relieve gastrointestinal convulsion to achieve clinically desired hypotonic effect, and has important value for imaging diagnosis of early gastrointestinal lesions. The digestive tract contrast agent has effects of relieving pain and swelling, dissipating blood stasis, stopping blooding, improving gastrointestinal mucosa microcirculation, promoting gastrointestinal fluid absorption, relieving cardiac ache and gastrointestinal convulsion, and nourishing and treating stomach, intestine, liver, spleen and other organs.
Owner:QIANFOSHAN HOSPITAL OF SHANDONG
Mucosa M-cell targeted viral myocarditis gene vaccine and preparation method thereof
ActiveCN103341179AValid responseEfficiently induces a responseGenetic material ingredientsAntiviralsWhole bodyViral Myocarditis
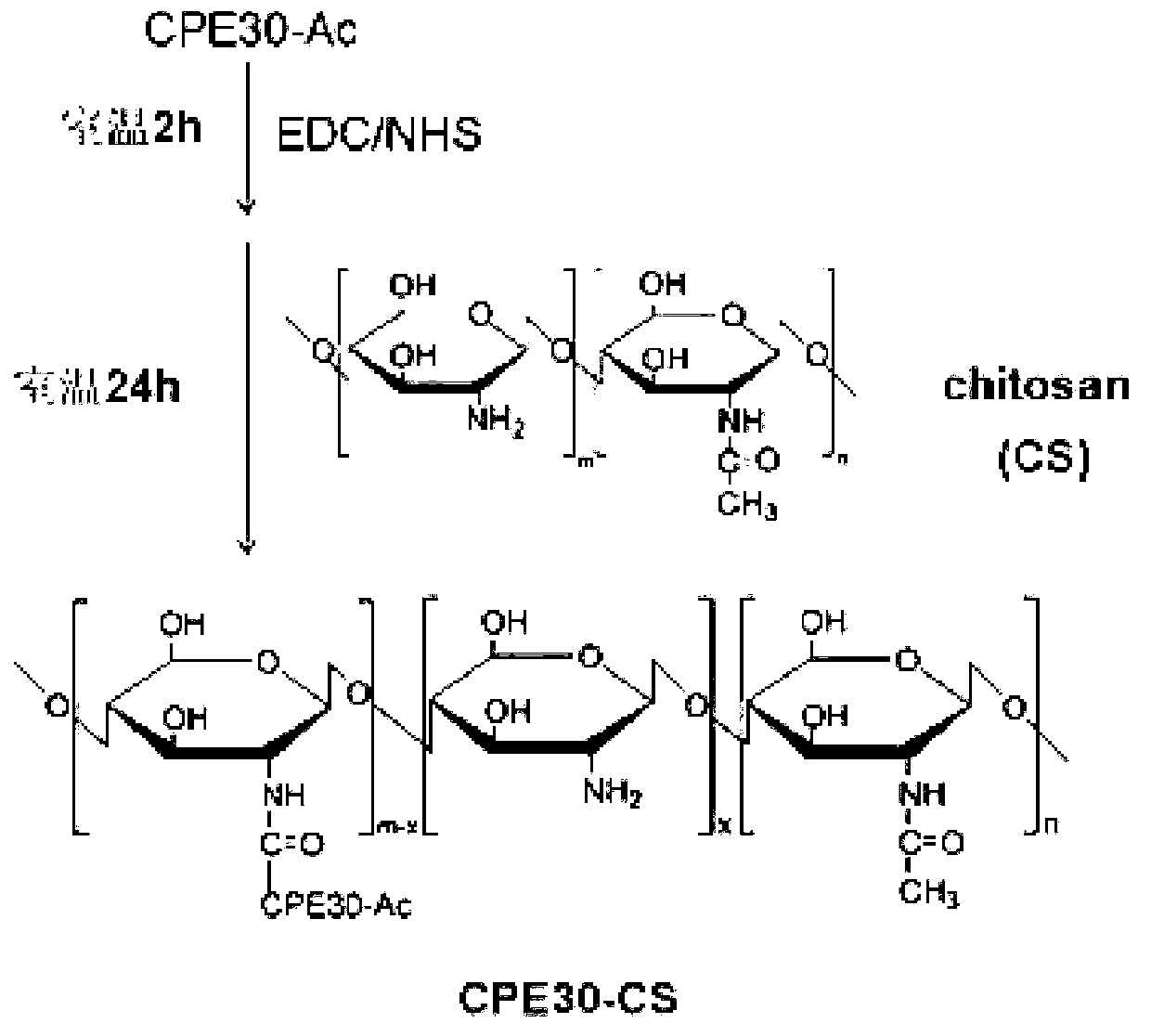
The invention discloses a mucosa M-cell targeted viral myocarditis gene vaccine. The vaccine is prepared by compounding a mucosa delivery system capable of targeting mucosa M-cells and B3-type coxsackie virus antigen encoding plasmids through cross-linking copolymerization. The invention further discloses a preparation method of the gene vaccine. The invention further discloses a preparation method of the mucosa delivery system capable of targeting the mucosa M-cells, and the mucosa delivery system is acquired after stable amide-ester bonds are formed by carboxyl groups and amino groups in deacetylated chitosan, wherein the carboxyl groups in peptide fragment CPE30 are targeted by M-cells which are activated by using EDC (Dichloroethane) and NHS (N-Hydroxysuccinimide). By nasally dropping the gene vaccine onto immunized mice, the gene vaccine is proved to be capable of effectively inducing the response between specific antigen serum and mucoantibody, obviously enhancing the local specific T-cell killing ability of the whole body and gastrointestinal mucosa and significantly improving the ability of mice for resisting B3-type coxsackie virus, thereby being an excellent prophylactic vaccine for viral myocarditis.
Owner:SUZHOU UNIV
Traditional Chinese medicine formulation for treating gastrosis and preparation method thereof
The invention relates to a Chinese patent medicine for treating gastric diseases. The Chinese patent medicine is characterized in that the medical prescription comprises prepared milkvetch root, cassia twig, medcinal evodia fruit, cassia bark, cuttlebone, peach seed, safflower, Chinese angelica, Szechuan lovage rhizome, red paeony root, salvia miltiorrhiza root, tree peony bark, rhizoma corydalis, wenyujin concise rhizome, common burreed rhizome, zedoary, leech, costustoot, bitter orange, combined spicebush root, golden thread, prepared liquoric root and rehmannia root. Compared with the prior Chinese patent medicines for treating the gastric diseases, the Chinese patent medicine of the invention has more reasonable formula, the synergy among various medicine components can promote the blood circulation of gastrointestinal mucosa, can rapidly recover the inflammation parts and the gastric ulcer surfaces thereof and can achieve the treatment effect on activating blood circulation, activating qi-flowing, relieving pain, nourishing qi, strengthening spleen and regulating spleen and stomach, the efficacy is remarkable and the Chinese patent medicine has no toxicity or side effects; the formula has the efficiency on swallowing acid, warming stomach, benefiting qi for warming stomach, dispelling cold, activating qi, activating blood circulation and relieving pain. The Chinese patent medicine is mainly used for gastric ulcer and duodenal ulcer caused by deficient cold of spleen and stomach, cold coagulation and blood stasis, as well as chronic atrophic gastritis.
Owner:陕西东泰制药有限公司
Lactic acid bacterium composition for protecting gastrointestinal mucosa and preparation method of lactic acid bacterium composition
InactiveCN104974963AImprove immunityImprove protectionBacteriaDigestive systemLactobacillus rhamnosusLactobacillus salivarius
The invention discloses a lactic acid bacterium composition for protecting the gastrointestinal mucosa. The lactic acid bacterium composition comprises the following components in parts by weight: 5-20 parts of bifidobacterium lactis, 0.5-2 parts of bifidobacterium infantis, 8-25 parts of lactobacillus rhamnosus, 3-20 parts of lactobacillus acidophilus, 3-20 parts of lactobacillus plantarum and 8-25 parts of lactobacillus salivarius. The invention further discloses a processing method for the lactic acid bacterium composition. The processing method comprises the following steps: (1), preparing sclerotia; (2), embedding non-reducing sugar; (3), embedding skimmed milk; and (4), freeze-drying. The lactic acid bacterium composition provided by the invention has the advantages of adjusting gastrointestinal microorganisms to be balanced and protecting the gastrointestinal mucosa.
Owner:HK GOOD ALLY BIOTECH CO LTD
Nutrient capable of strengthening human immunity and protecting cells of heat, brain, liver, kidney, stomach and intestine
InactiveCN101601812AImprove immunityGrowth inhibitionOrganic active ingredientsAnthropod material medical ingredientsIntestinal structureBlood sugar
The invention discloses a nutrient capable of strengthening the human immunity and protecting cells of the heat, the brain, the liver, the kidney, the stomach and the intestine, which is characterized by being prepared by the following raw materials based on parts by weight: 10-46 parts of reishi sporule powder, 6-16 parts of solomonseal polysaccharose powder, 8-22 parts of polyrhachis vicina roger ant and 5-16 parts of proanthocyanide. The invention has the advantages of even and comprehensive nutrition components, reasonable matching and lower price, is adapted to the demand of people and can adjust blood fat and blood sugar, protect the liver, resist fatigue, radiation, viruses and tumors, repair gastrointestinal mucosa, strengthen the human immunity and have the effect of protecting cells of the heat, the brain, the liver, the kidney, the stomach and the intestine.
Owner:黄刚
Thymus gland pentapeptide oral intestine-dissolved formulated product and method of preparing the same and use thereof
ActiveCN101108246ASolve the problem of oral drug deliveryImprove complianceOrganic active ingredientsPeptide/protein ingredientsIntestinal structureAdditive ingredient
The invention provides a Thymopentin Oral Enteric-coated Agent, which takes thymopentin of effective dosage as the active ingredient and enteric-coated agent as its accessories. Each dosage contains 5mg to 150 mg thymopentin. The invention also provides the preparation method and usage of the enteric-coated agent. The medicine effect tests prove that the medicine has the same indication and efficacy as the injections and can overcome that the gastrointestinal enzyme will easily degrade the thumopentin into amino acid and small peptide so as to lose the activity when orally taking the Thymopentin; the thumopentin can not easily penetrate the gastrointestinal mucosa, resulting in low bioavailability; and the liver has the First-pass effect on the thymopentin. The invention opens a new way to apply thumopentin and increases the patients' compliance.
Owner:CHENGDU DIAO JIUHONG PHARMA FAB
Nutritional agent for enhancing immunity, restoring gastrointestinal function and protecting heart, brain, liver and kidney cells
InactiveCN101810845ARepair immune functionRegulate immune functionOrganic active ingredientsAnthropod material medical ingredientsAnti virusAbnormal tissue growth
The invention discloses a nutritional agent for enhancing immunity, restoring a gastrointestinal function and protecting heart, brain, liver and kidney cells. The nutritional agent comprises the following raw materials in part by weight: 3 to 28 parts of ganoderan powder, 2 to 49 parts of protein powder, 1 to 24 parts of maitake extract and 4 to 22 parts of compound lactobacillus. The nutritional agent has the following advantages of remaining nutrients and active ingredients in each component of the raw materials to the maximum, supplementing various nutrients and proteins for a human body, and having the effects of anti-blood lipid, anti-blood glucose, anti-virus, anti-genetic mutation, anti-tumor, anti-radiation, liver-protection, anti-fatigue, gastrointestinal mucosa restoring, and the effect of helping to restore damaged heart, brain, liver and kidney cells of the human body.
Owner:黄刚
Processing method of nutritious spirulina rice
InactiveCN106174013AHigh vegetable proteinHigh nutritional valueFood ingredient functionsNutritive valuesRice grain
The invention discloses nutritious spirulina rice, and especially relates to a processing method of the nutritious spirulina rice. The nutritious spirulina rice is prepared from the following raw materials: long-shaped rice, polished round-grained non-glutinous rice, sticky rice, spirulina powder, carrot powder, rice-grain spouts, coix seeds, kelp powder and starch. The processing method of the nutritious spirulina rice comprises the following steps: carrying out crushing, carrying out mixing and homogenizing, carrying out puffing, carrying out water-milling, carrying out filter-pressing, carrying out granulating and drying, and carrying out packing. The prepared nutritious spirulina rice is relatively high in nutritive values and convenient to eat, and is soft, refreshing and smooth in taste; the nutrient substances in the nutritious spirulina rice are extremely good in effects of neutralizing gastric acid, repairing gastrointestinal mucosa, as well as promoting regeneration and normal secretion functions of the gastrointestinal mucosa; thus, the nutritious spirulina rice is especially suitable for patients with gastrointestinal diseases.
Owner:GUANGDONG JINYOU RICE
Compound diclofenac sodium slow-release preparation and preparation method thereof
InactiveCN102526049AImprove complianceReduce dosing frequencyOrganic active ingredientsAntipyreticAdditive ingredientDiclofenac Sodium
The invention discloses a compound diclofenac sodium slow-release preparation and a preparation method of the compound diclofenac sodium slow-release preparation. The compound preparation consists of the following ingredients in percentage by mass: 20 to 30 percent of diclofenac sodium, 4 to 8 percent of omeprazole or sodium salt of the omeprazole and the balance of auxiliary materials allowed on the pharmacy. The preparation method of the compound preparation comprises the steps that: the diclofenac sodium and the omeprazole are respectively prepared into enteric granules or pellets, the prepared omeprazole enteric granules or pellets are mixed with the diclofenac sodium enteric granules or pellets, and the tabletting or the capsule filling is carried out. In compound diclofenac sodium enteric slow-release tablets or enteric slow-release capsules prepared by the preparation method, the medicine release of the main omeprazole medicine is carried out at the normal release speed, but the diclofenac sodium is slowly released from the preparation. Therefore, the omeprazole or the sodium salt of the omeprazole is firstly released for realizing the effect of protecting the gastrointestinal mucosa, the diclofenac sodium is prevented from irritating the gastrointestinal mucosa of a patient in the medicine release process, and the gastrointestinal tract adverse reaction occurrence rate of the diclofenac sodium is reduced.
Owner:SHINEWAY PHARMA GRP LTD
Hypotonic microbicidal formulations and methods of use
ActiveUS10092509B2Preventing and decreasing HIV infectionEnhance cleansing, buffers, or preservativesCosmetic preparationsToilet preparationsPreservativeSURFACTANT BLEND
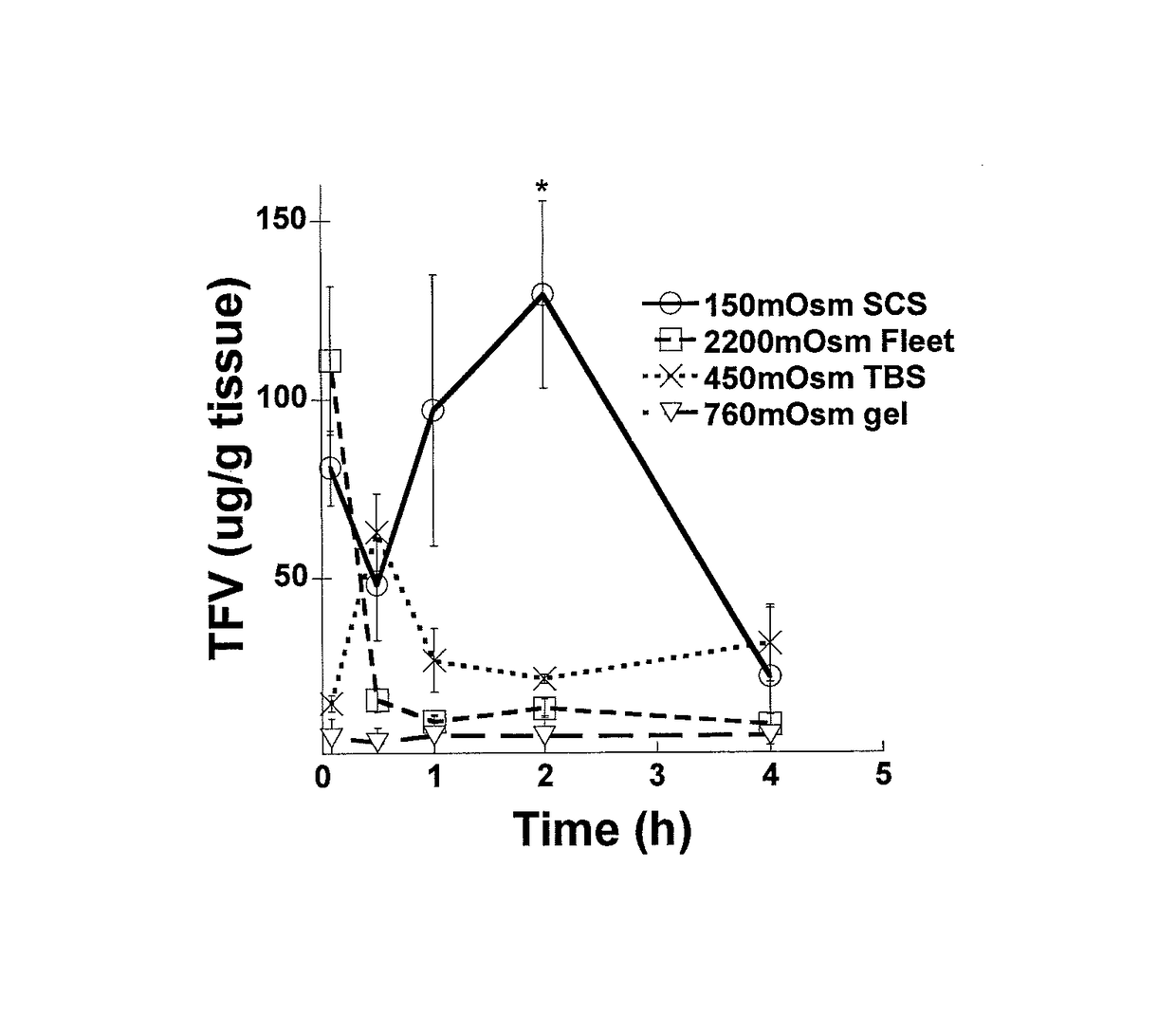
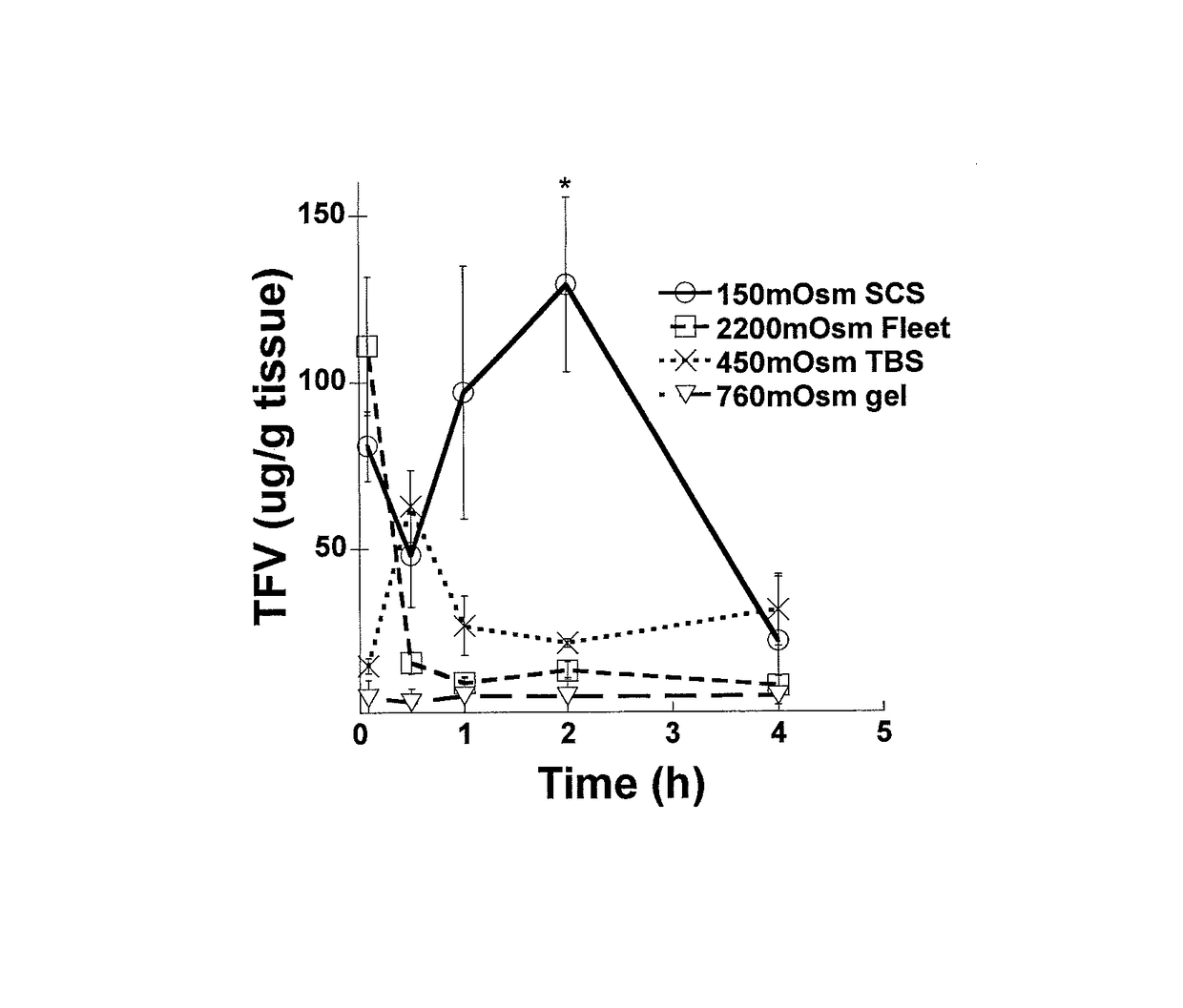
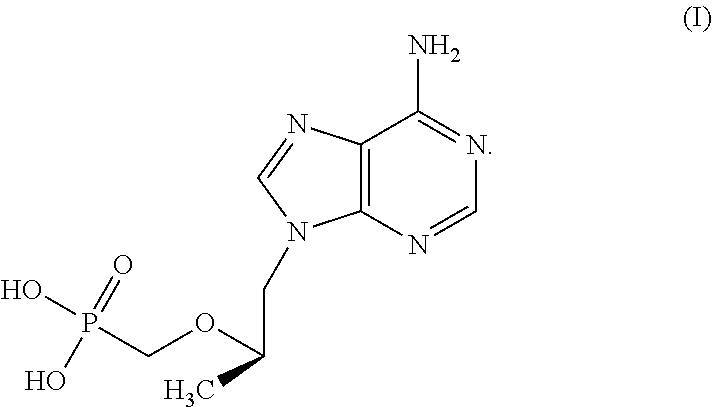
Hypotonic microbicidal compositions including an antimicrobial, such as an antiviral compound, and a pharmaceutically acceptable carrier in a solution formulation having hypotonic osmolarity have been developed for administration rectally to the gastrointestinal mucosa. In a preferred embodiment for use in preventing or decreasing HIV infection, the microbiocidal is tenofovir, or a prodrug or derivative thereof. The formulations may include additional agents such as surfactants to enhance cleansing, buffers, or preservatives. Polymers may be included for osmolarity as well as comfort.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Nasointestinal tube
The invention discloses a nasointestinal tube and belongs to the field of medical equipment. A bag is sealed at the top of the front end of a nasointestinal tube in the prior art. In the intubation process, the bag turns back to increase push resistance, the gastrointestinal mucosa is damaged easily, and the time for a catheter to enter intestines cannot be controlled actively, so that a curative effect is delayed. The nasointestinal tube comprises a catheter for being inserted into the stomach and intestine, the top of the front end of the catheter is provided with a metal body having a certain rigidity, the metal body is in a streamline shape, the outside of the metal body is covered with a layer of flexible film having a certain elasticity, and the metal body is made of a material capable of sensing a magnetic field. The metal body is in the streamline shape, metal has rigidity and is not bent easily, and the streamline shape reduces push resistance; the outside of the metal body is covered with the layer of flexible film, the surface is soft, so that when the catheter is pushed to move forwards, the front end of the catheter has a certain elasticity while having a certain rigidity, and the gastrointestinal mucosa is not damaged easily. The magnetic field and a guiding wire are utilized to drive the catheter to move, so that the moving process is smoother and quicker.
Owner:陈一奇
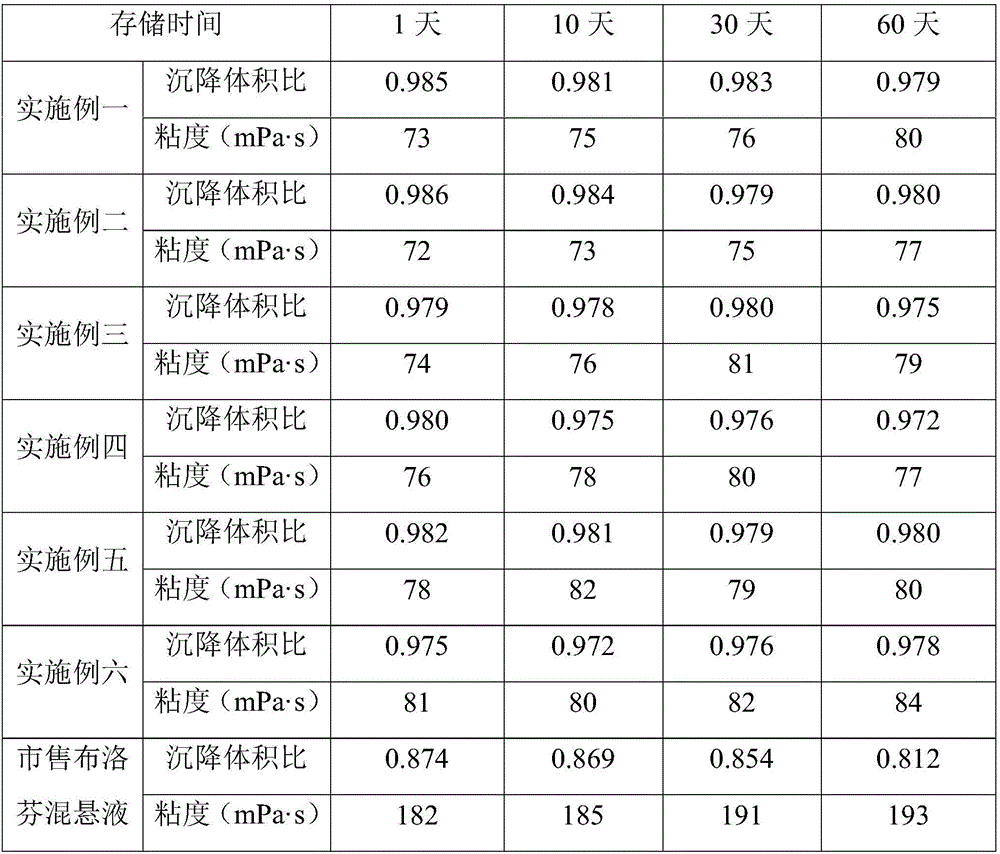
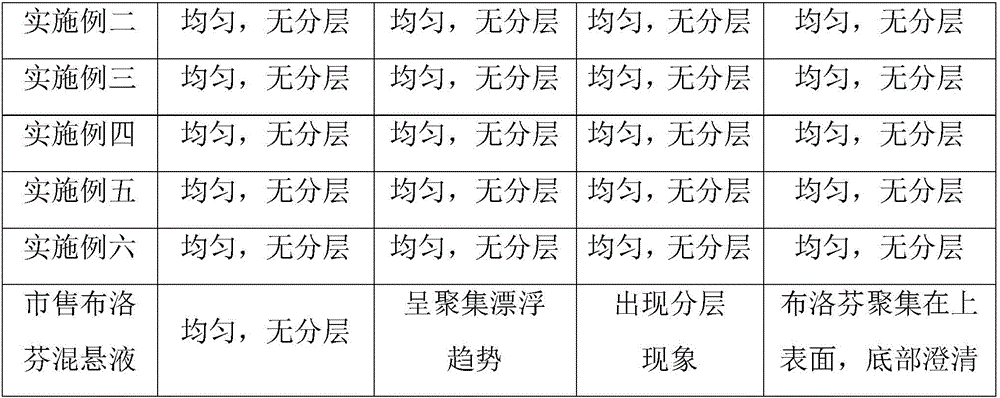
Ibuprofen oral suspension and preparation method thereof
InactiveCN106727303AImprove stabilityImprove uniformityOrganic active ingredientsAnthropod material medical ingredientsIbuprofen Oral SuspensionDentistry
The invention relates to an ibuprofen oral suspension and a preparation method thereof. The ibuprofen oral suspension is characterized in that a raw material comprises a first component and a second component; based on every 100 mL ibuprofen oral suspension, the first compound comprises 1.0-5.0 g of ibuprofen; and the second compound comprises 8-12 g of glycerinum, 25-35 g of saccharose, 1.1-1.5 g of pregelatinized starch, 0.15-0.25 g of xanthan gum, 0.03-0.07 g of twain 80, 0.15-0.25 g of citric acid, 0.15-0.25 g of sodium benzoate, 0.1-0.3 g of essence, and 0.003-0.006 g of haematochrome. According to the ibuprofen oral suspension, the stability of the ibuprofen oral suspension after the ibuprofen oral suspension is stored for a long time can be improved, and floating or settling can be avoided; the effects of relieving pain and relieving fever can be improved; and the damage and stimulus to gastrointestinal mucosa is reduced after the ibuprofen oral suspension is orally taken.
Owner:TIANDA PHARMA ZHUHAI
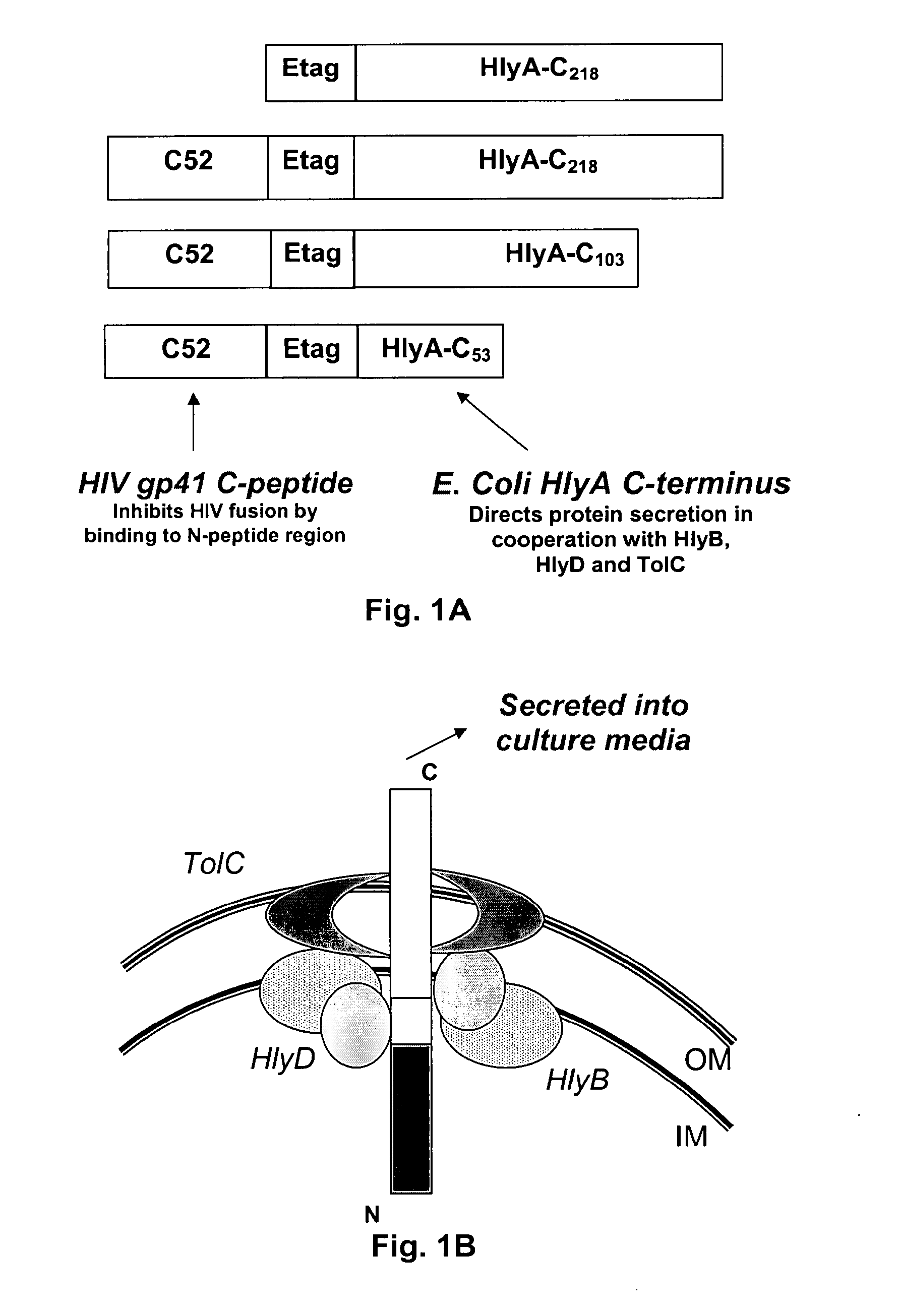
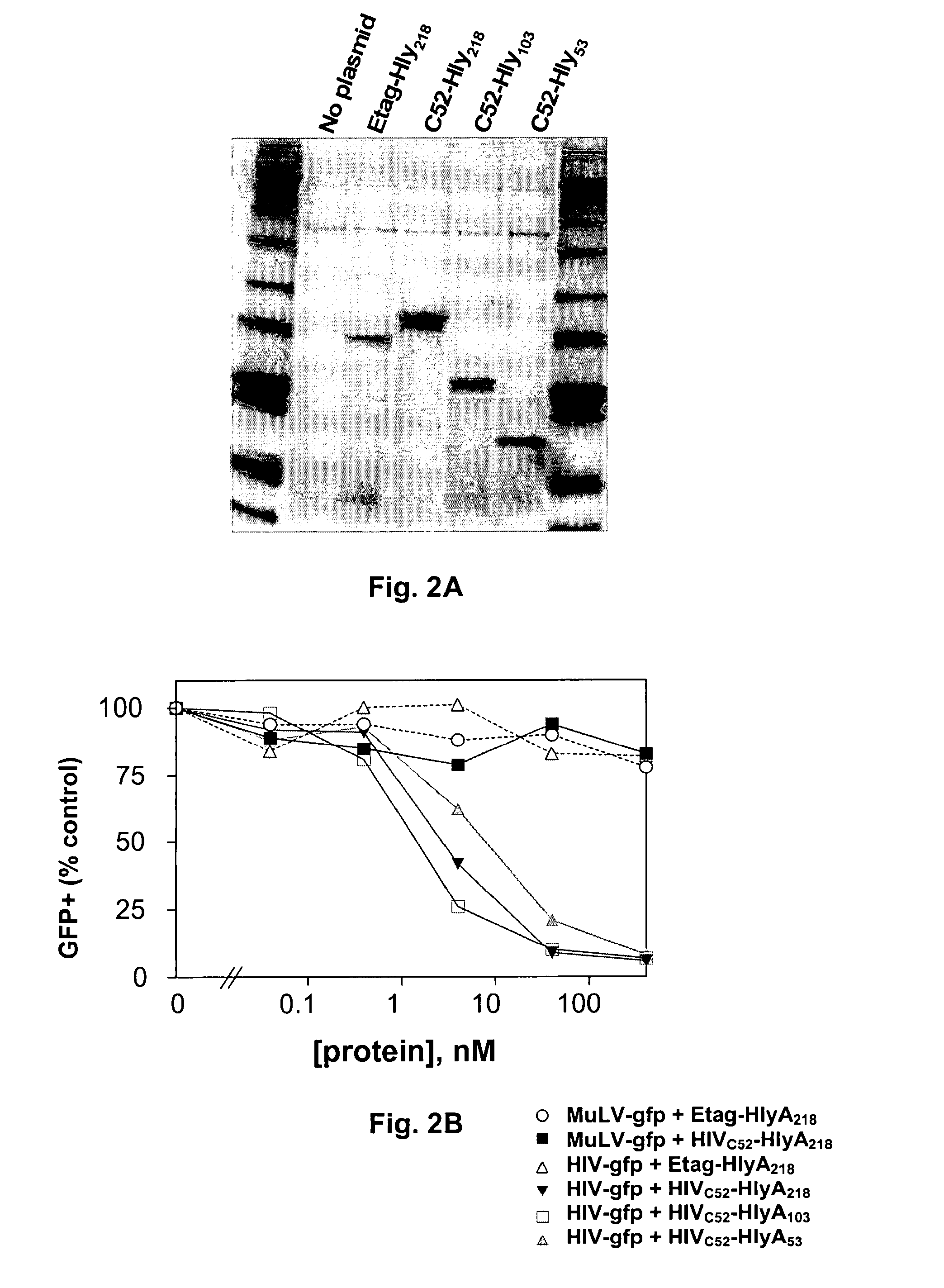
Commensal strain of E. coli encoding an HIV GP41 protein
The present invention relates, e.g., to a commensal bacterium which can colonize the genitourinary and / or gastrointestinal mucosa, and which, under suitable conditions, secretes a heterologous antimicrobial polypeptide, wherein the secreted antimicrobial polypeptide is effective to inhibit infectivity by, or a pathogenic activity of, a pathogen. In a most preferred embodiment, the antimicrobial polypeptide inhibits HIV infection (e.g., fusion) and / or pathogenesis. Also described are preventive or therapeutic compositions comprising the commensal bacteria, and methods to inhibit infectivity and / or pathogenesis, using the bacteria.
Owner:UNITED STATES OF AMERICA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com