Compound diclofenac sodium slow-release preparation and preparation method thereof
A technology of diclofenac sodium and sustained-release preparations, applied in anti-inflammatory agents, pill delivery, pharmaceutical formulations, etc., can solve problems such as adverse reactions, gastrointestinal bleeding, and increased bleeding risk of gastrointestinal ulcers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
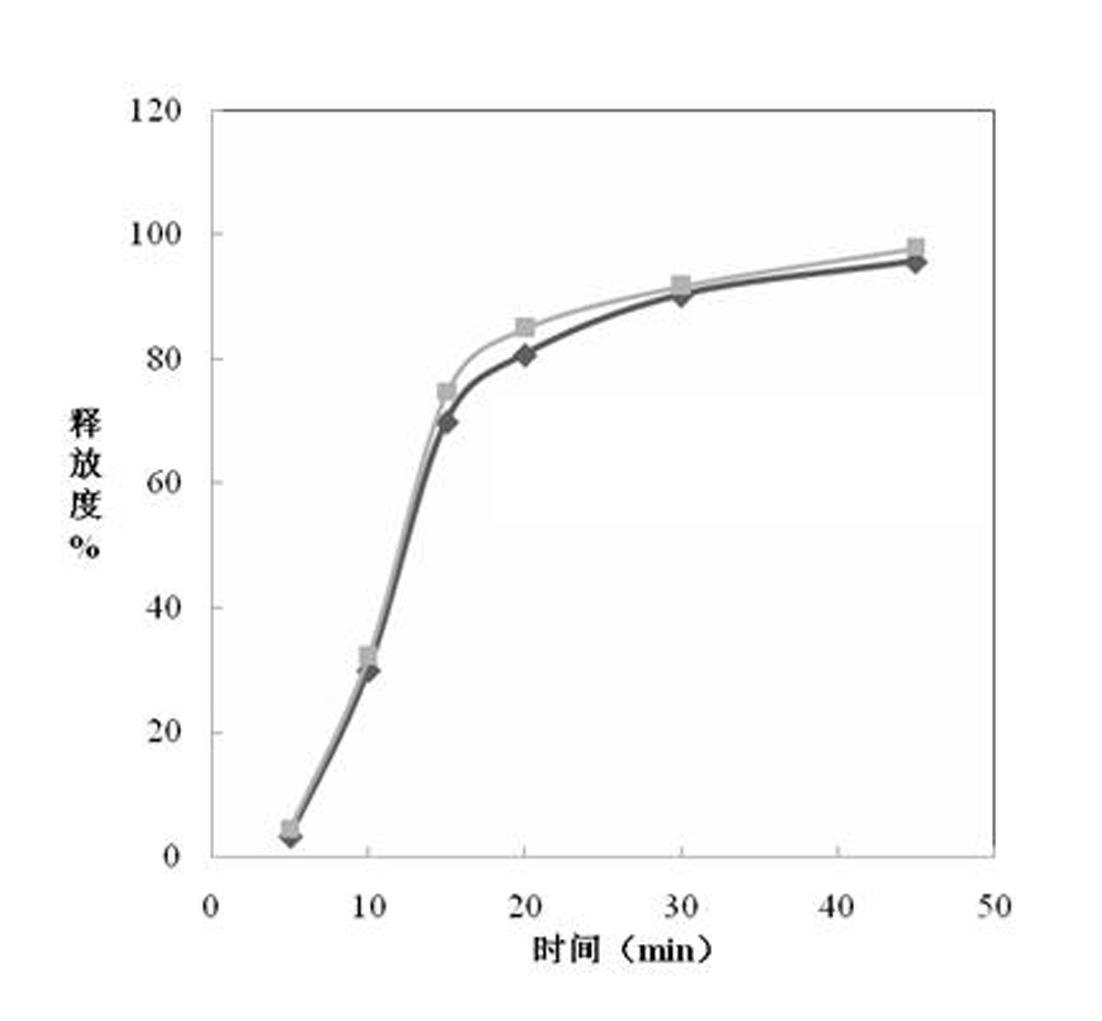
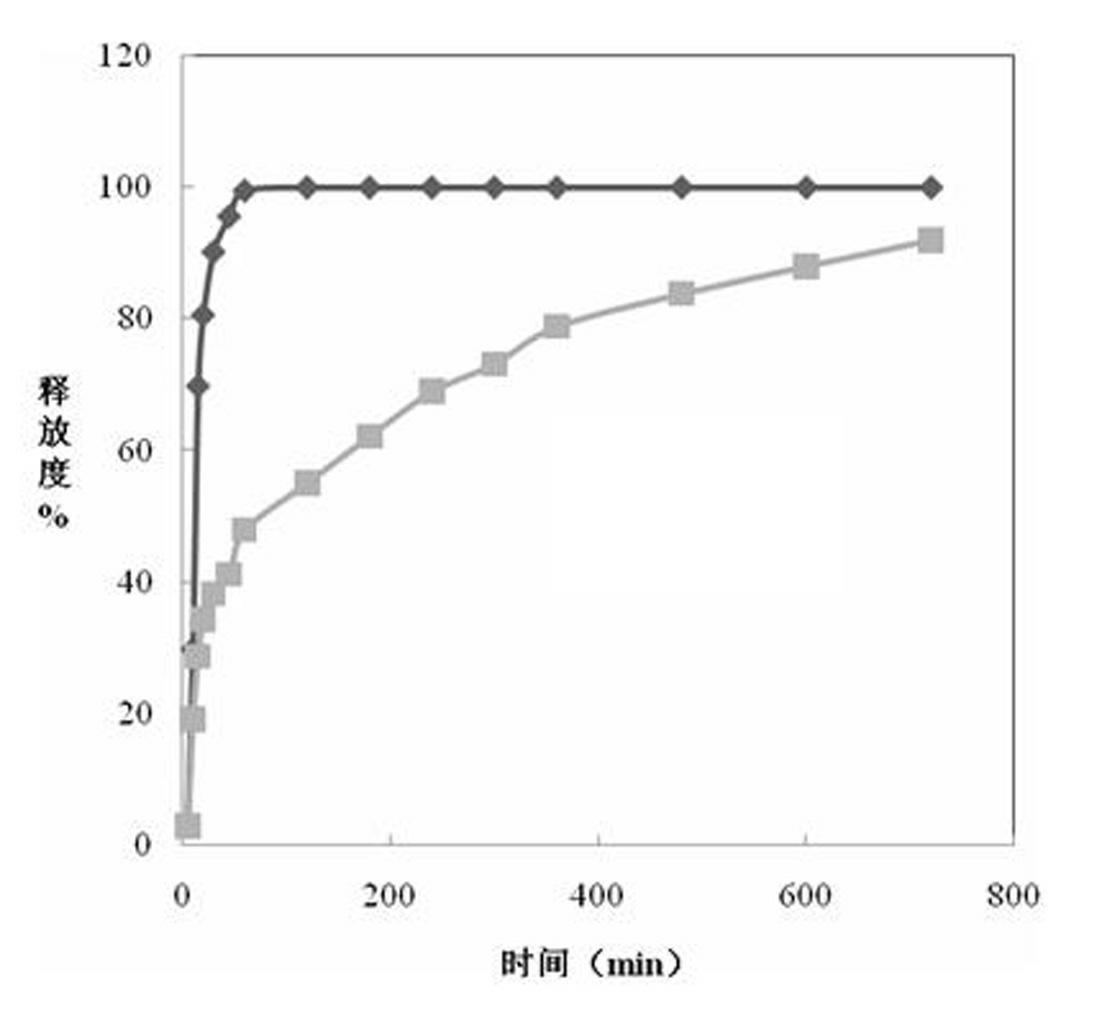
Image
Examples
Embodiment 1
[0038] a. Weigh the following components:
[0039] Diclofenac sodium 28.5g, omeprazole 7.6g, mannitol 8.0g, talcum powder 8.0g, polyvinylpyrrolidone K30 1.5g, sodium dihydrogen phosphate 1.2g, lactose 10.4g, hydroxypropyl methylcellulose 5.0g, 25g of microcrystalline cellulose, 2.4g of casing material, and 2.4g of insulating material. The casing material is a mixture of acrylic resin No. II and diethyl phthalate in a ratio of 100:4 by weight. The insulating material is hydroxypropyl methylcellulose.
[0040] b. The polyvinylpyrrolidone K30 described in step a is prepared into an aqueous solution with a mass ratio concentration of 10% for subsequent use;
[0041] c. Mix diclofenac sodium with lactose, hydroxypropyl methylcellulose, and microcrystalline cellulose evenly, add 1 / 2 amount of polyvinylpyrrolidone K30 aqueous solution prepared in step b, make it into pellets, and dry them for backup use;
[0042] d. Omeprazole is mixed with mannitol, talcum powder, and sodium dih...
Embodiment 2
[0048] a. Weigh the following components:
[0049] Diclofenac sodium 28.5g, omeprazole 7.6g, mannitol 6.0g, talc 8.0g, polyvinylpyrrolidone K302g, sodium dihydrogen phosphate 1.2g, starch 12g, hydroxypropyl methylcellulose 12g, microcrystalline cellulose 19.1g, casing material 1.8g, insulation material 1.8g. The enteric coating material is a mixture of acrylic resin No. II and diethyl phthalate in a proportion of 100:8 by weight. The insulating material is hydroxypropyl methylcellulose.
[0050] B, the polyvinylpyrrolidone K30 described in a step operation is mixed with the aqueous solution that mass ratio concentration is 10% for subsequent use;
[0051] C, mix diclofenac sodium with starch, hydroxypropyl methylcellulose, microcrystalline cellulose, add the polyvinylpyrrolidone K30 aqueous solution of 1 / 2 amount prepared by b step operation, make it into granules, after drying for subsequent use ;
[0052] D, omeprazole is mixed with mannitol, talcum powder, sodium dihydr...
Embodiment 3
[0058] a. Weigh the following components:
[0059] Diclofenac sodium 35g, omeprazole sodium salt 4.2g, mannitol 7g, talc 8.0g, polyvinylpyrrolidone K302g, sodium bicarbonate 1.2g, pre-interleaved starch 10g, magnesium stearate 2g, sodium carboxymethyl starch 12. Microcrystalline cellulose 15g, casing material 1.8g, isolation material 1.8g. The enteric coating material is a mixture of acrylic resin No. II, diethyl phthalate and polysorbate in a ratio of 100:8:2 by weight. The insulating material is hydroxypropyl methylcellulose.
[0060] B, the polyvinylpyrrolidone K30 described in a step operation is mixed with the aqueous solution that mass ratio concentration is 10% for subsequent use;
[0061] c, mix diclofenac sodium with pre-interleaved starch methyl starch sodium, microcrystalline cellulose, add the polyvinylpyrrolidone K30 aqueous solution of 1 / 2 amount prepared by step b, make it into granules, add hard Magnesium fatty acid for use;
[0062] D, omeprazole sodium sa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com