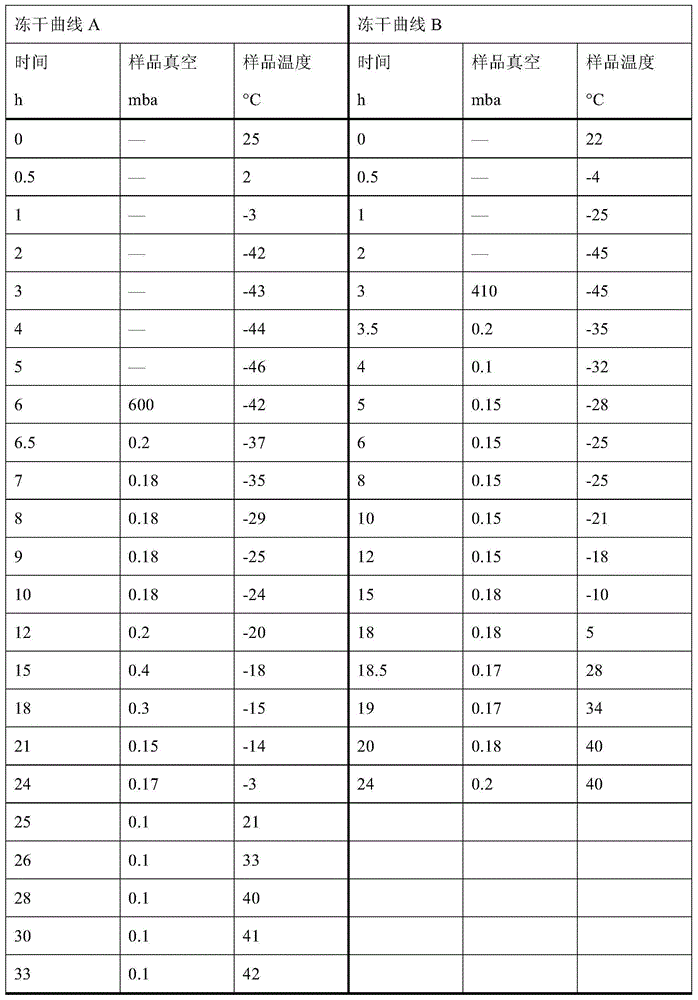
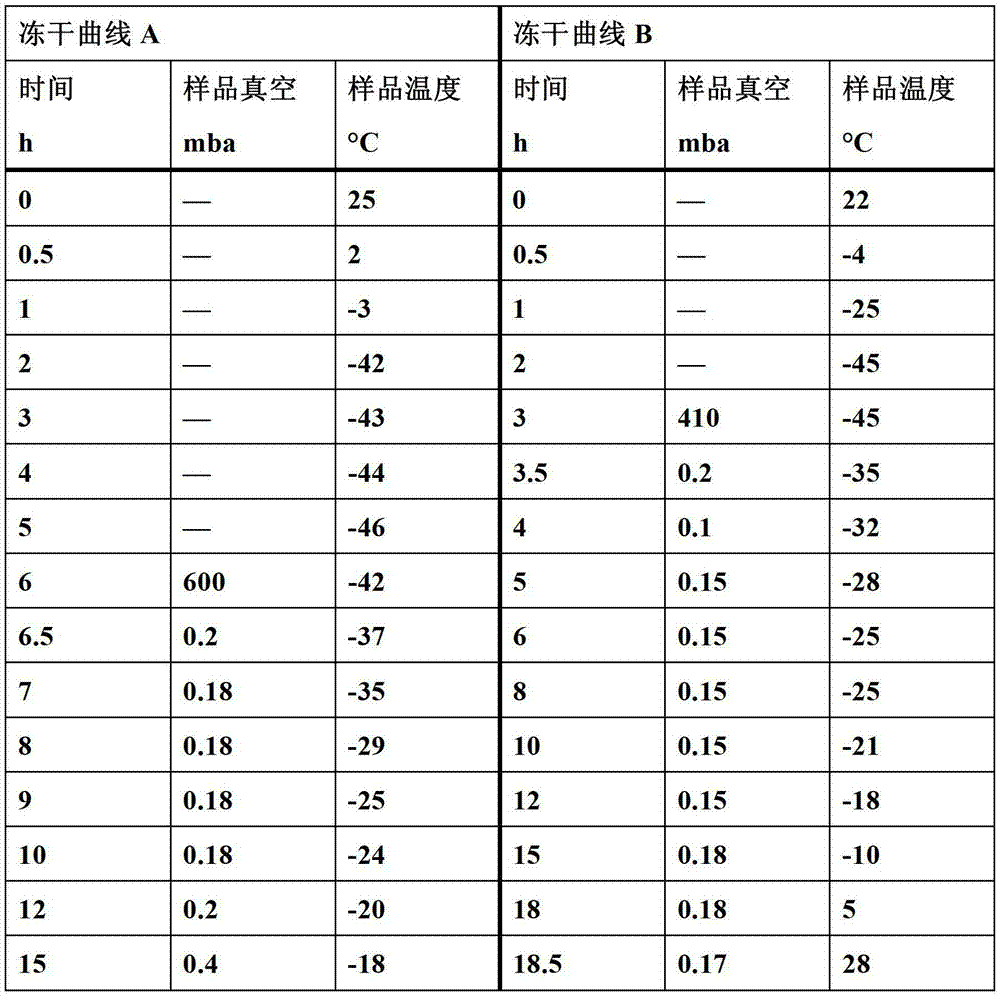
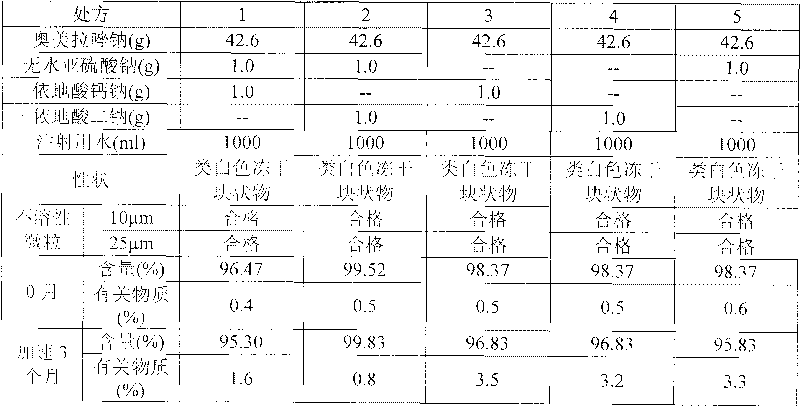Patents
Literature
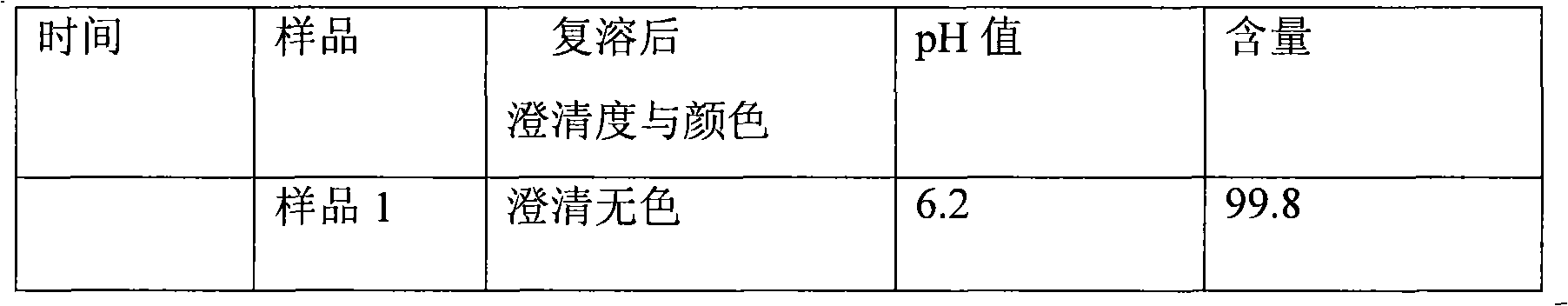
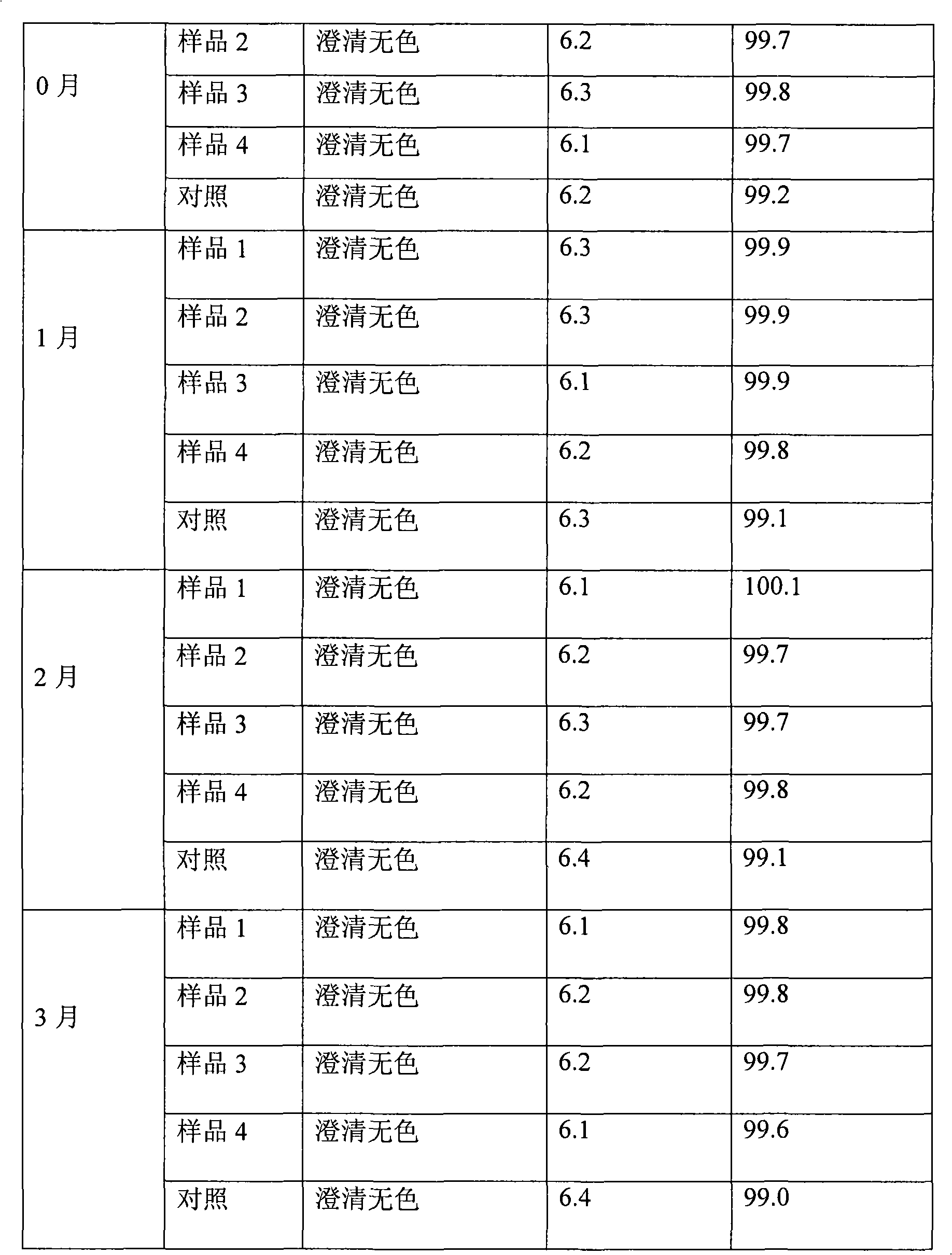
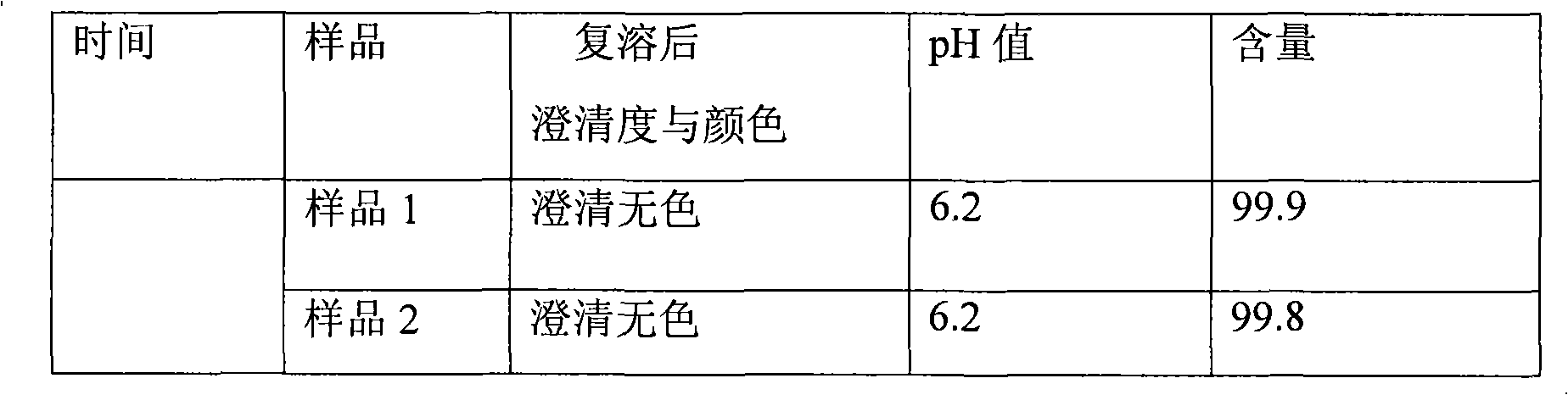
107 results about "Omeprazole Sodium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
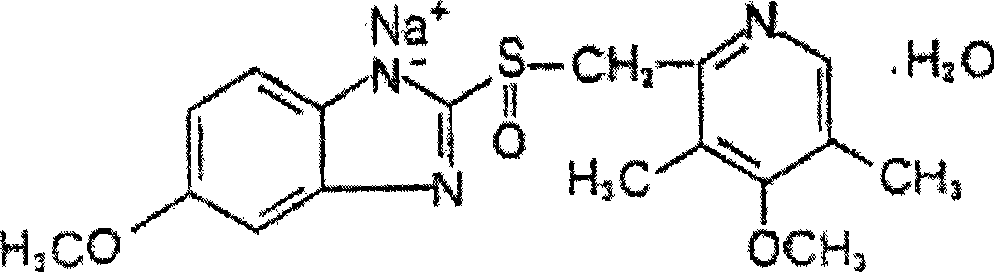
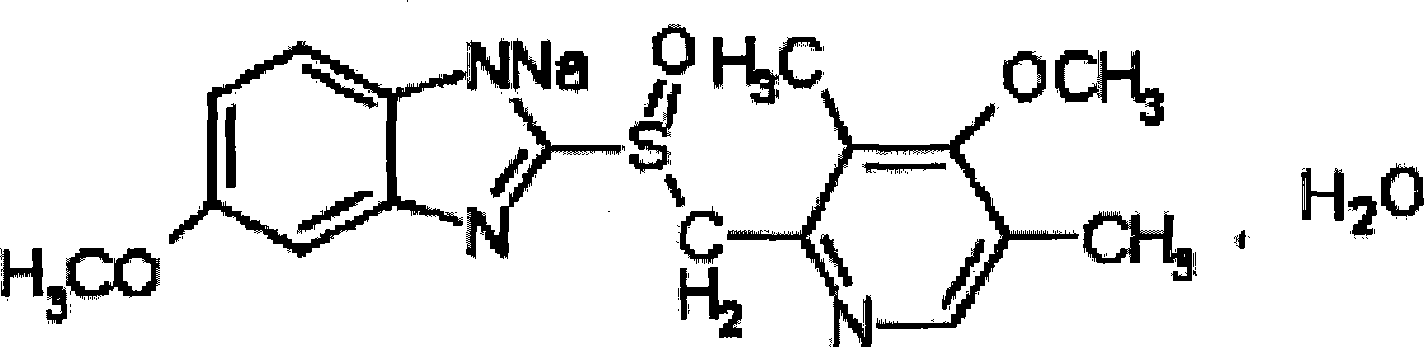
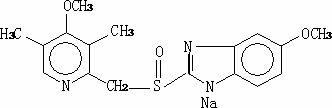
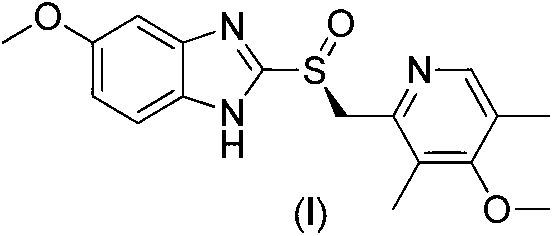
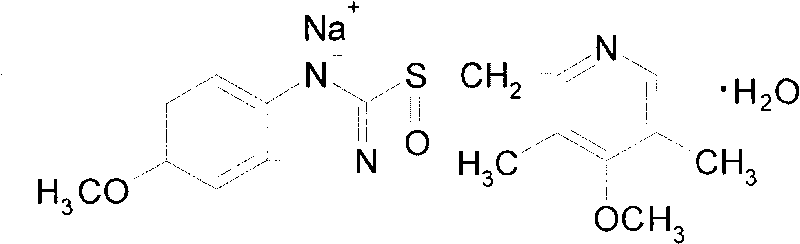
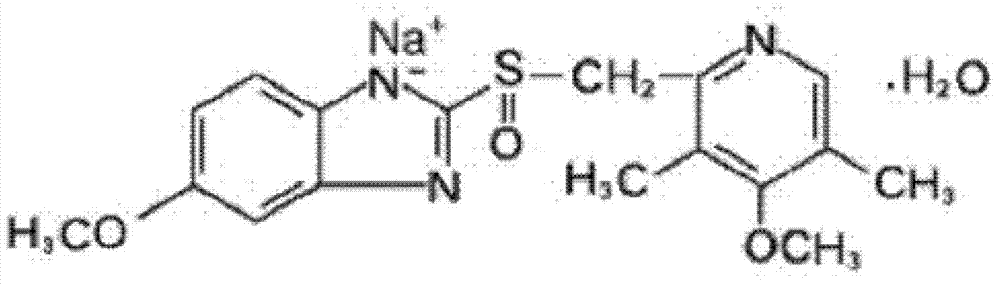
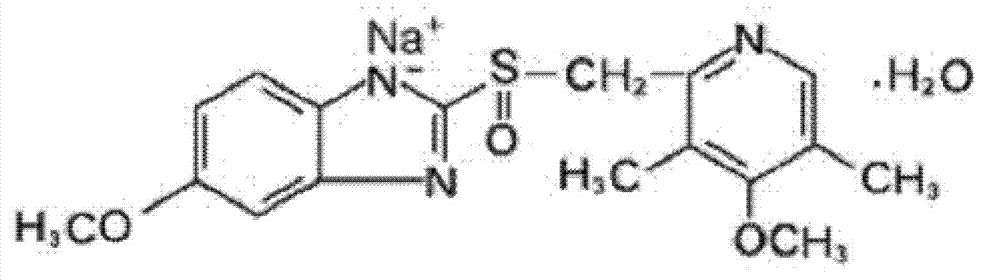
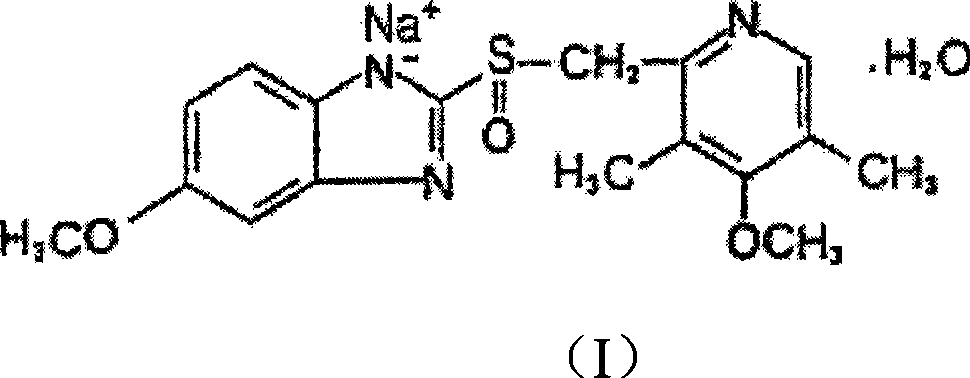
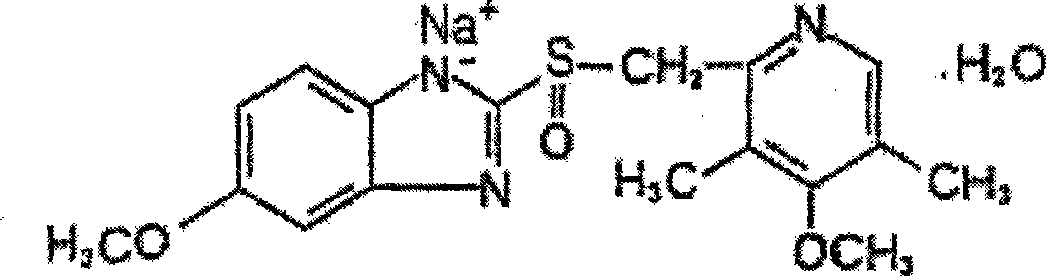
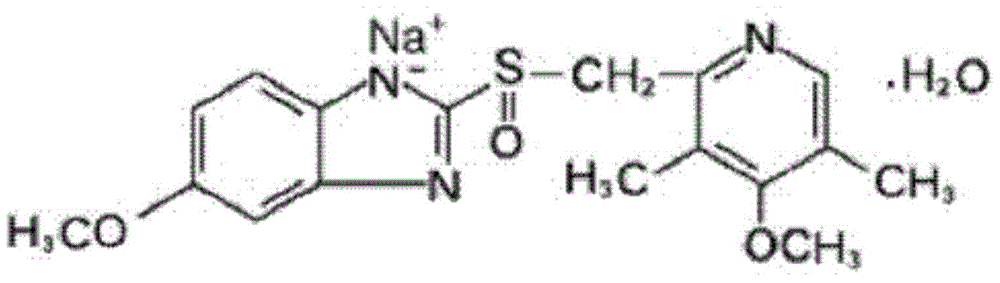
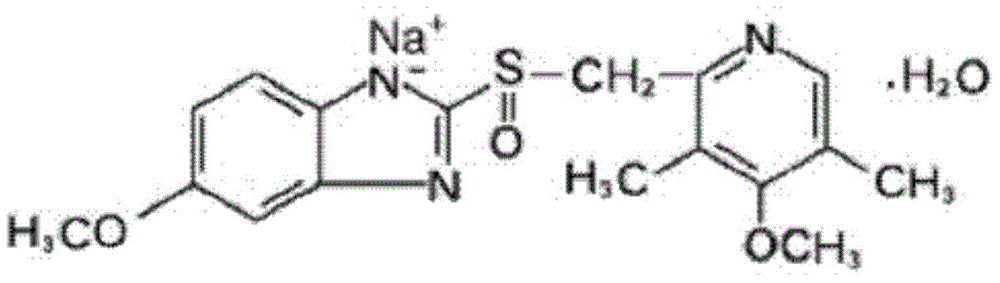
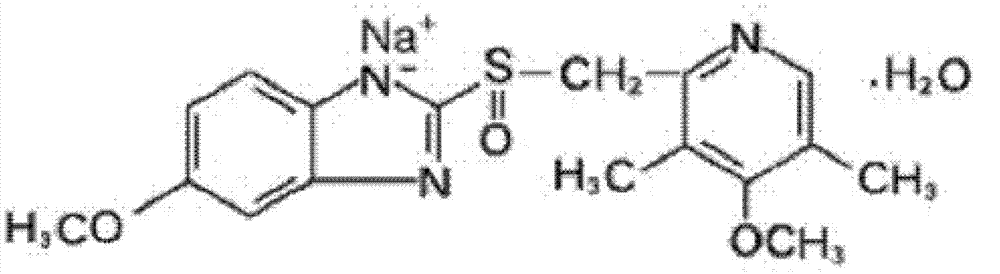
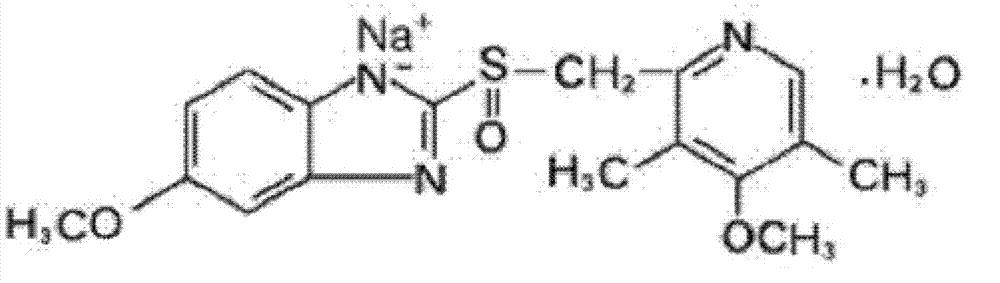
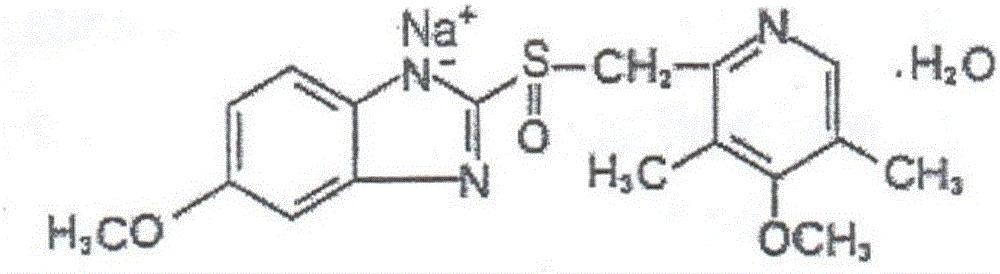
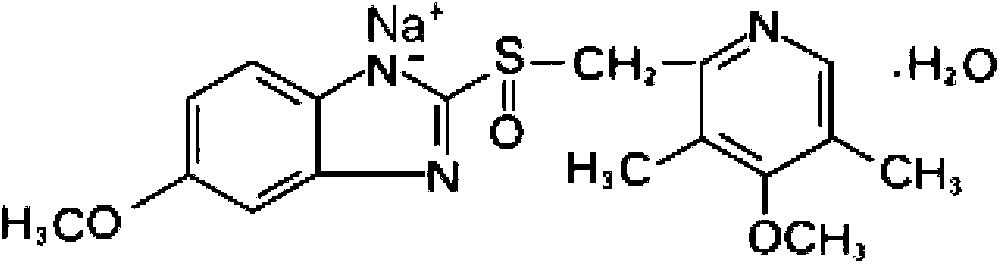
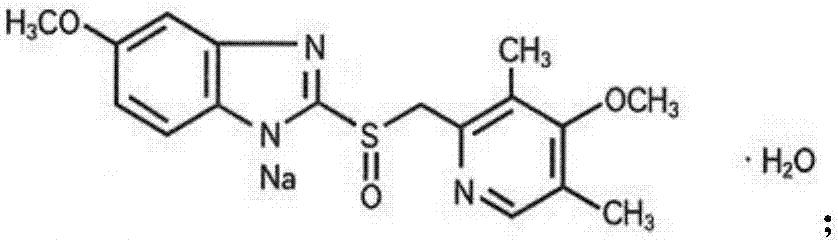
The sodium salt form of a benzimidazole with selective and irreversible proton pump inhibitor activity. In the acidic compartment of parietal cells, omeprazole is protonated and converted into the active achiral sulfenamide; the active sulfenamide forms one or more covalent disulfide bonds with the proton pump hydrogen-potassium adenosine triphosphatase (H+/K+ ATPase), thereby inhibiting its activity and the parietal cell secretion of H+ ions into the gastric lumen, the final step in gastric acid production. H+/K+ ATPase is an integral membrane protein of the gastric parietal cell.
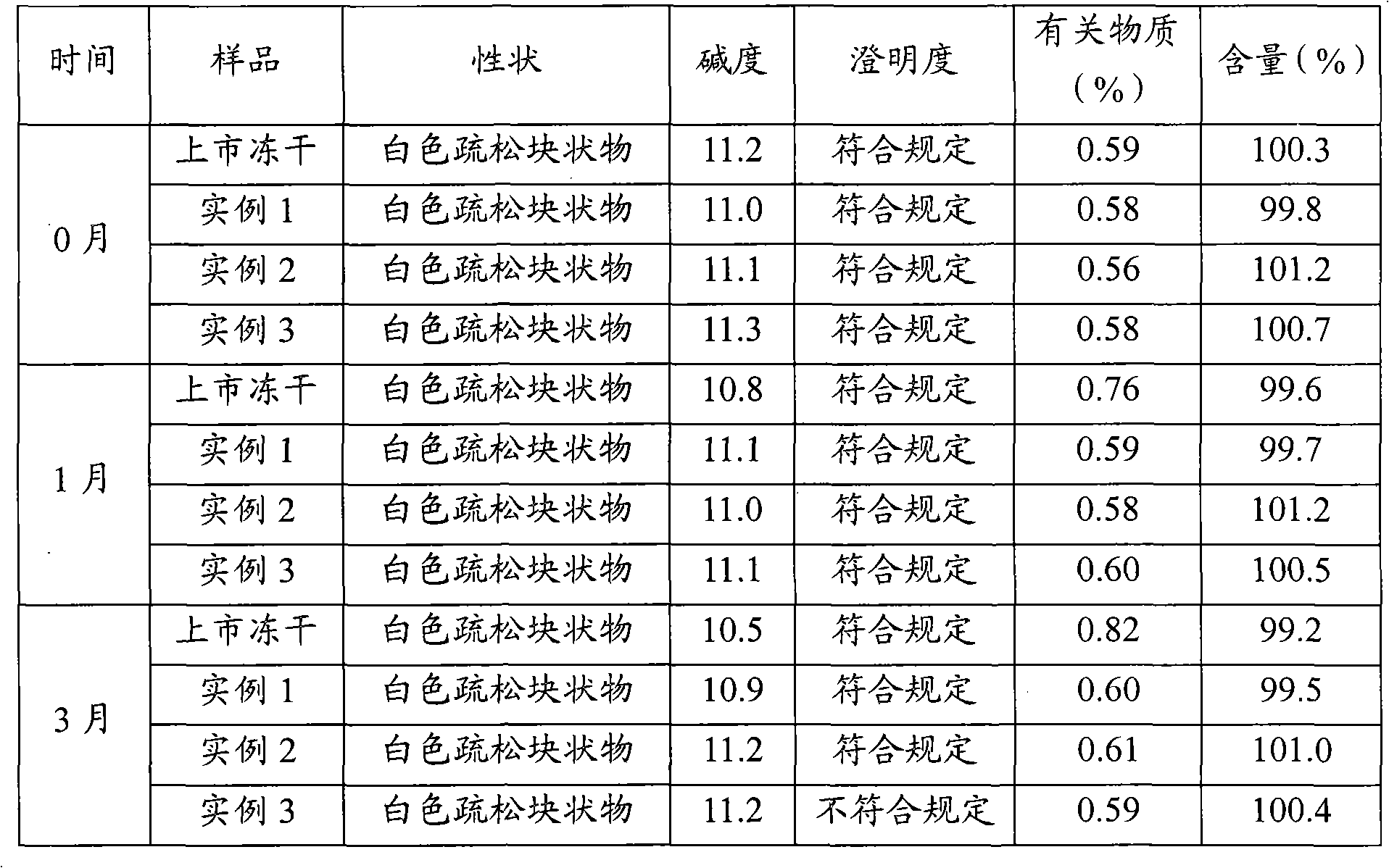
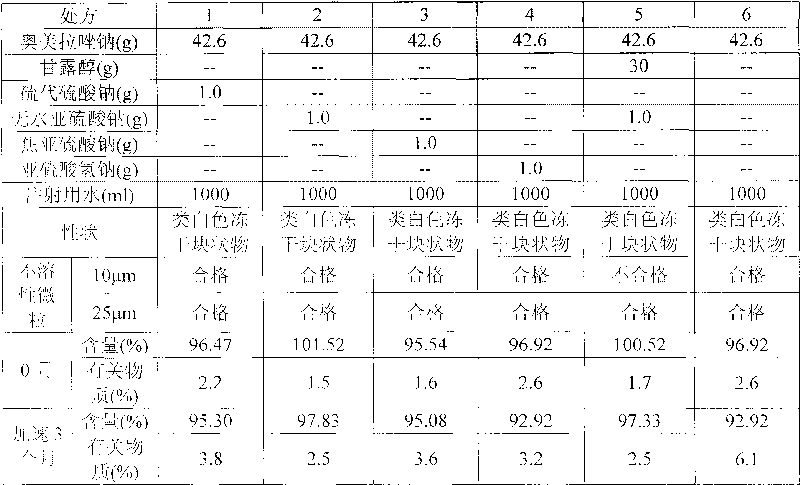
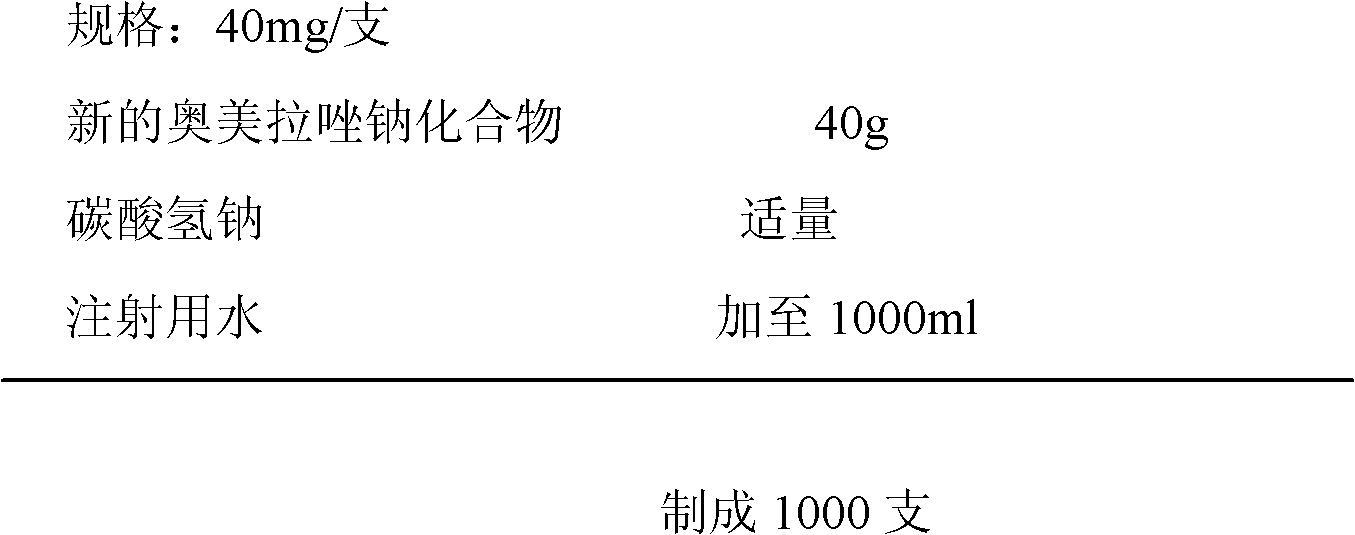
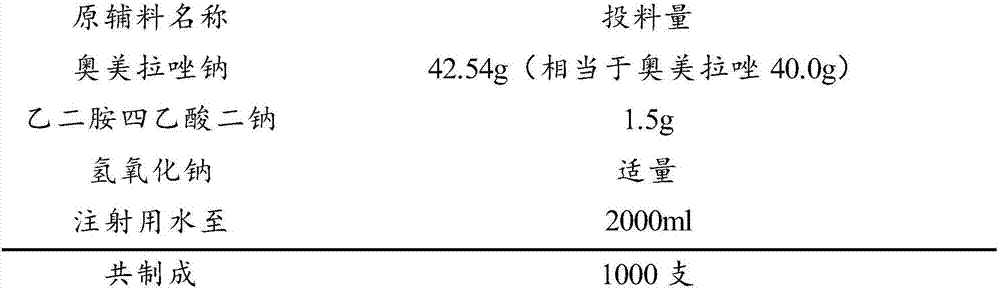
Stable omeprazol sodium preparation for injection
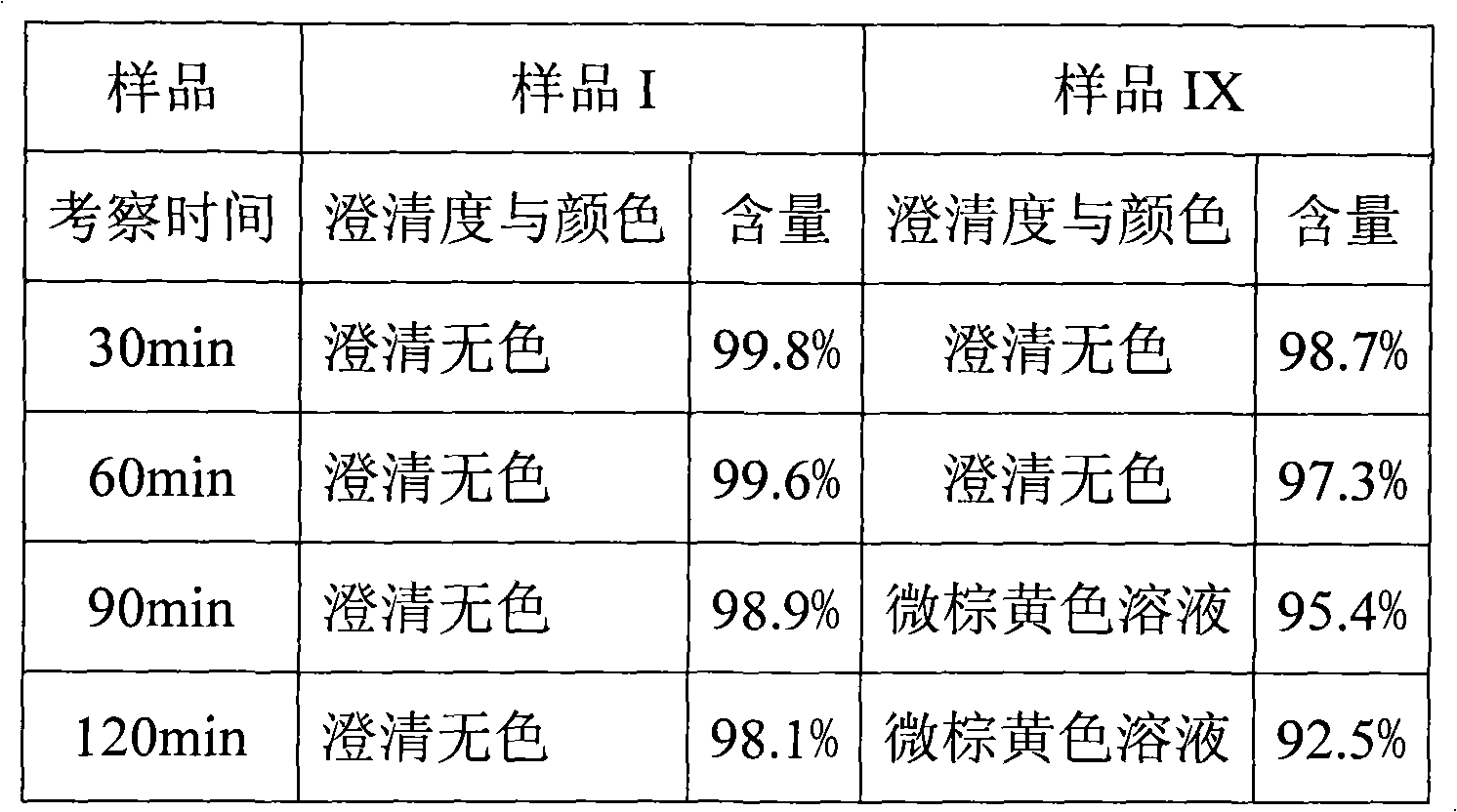
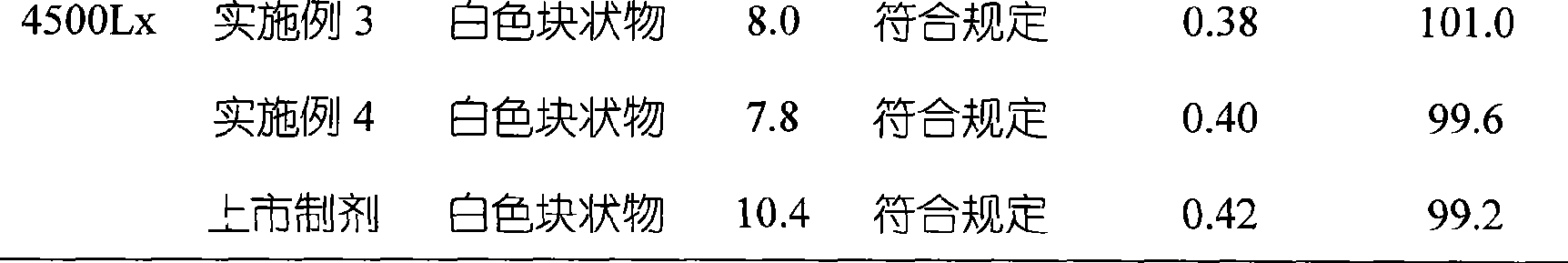
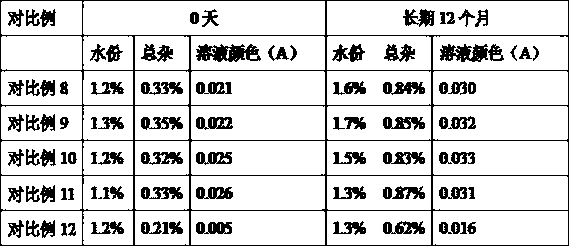
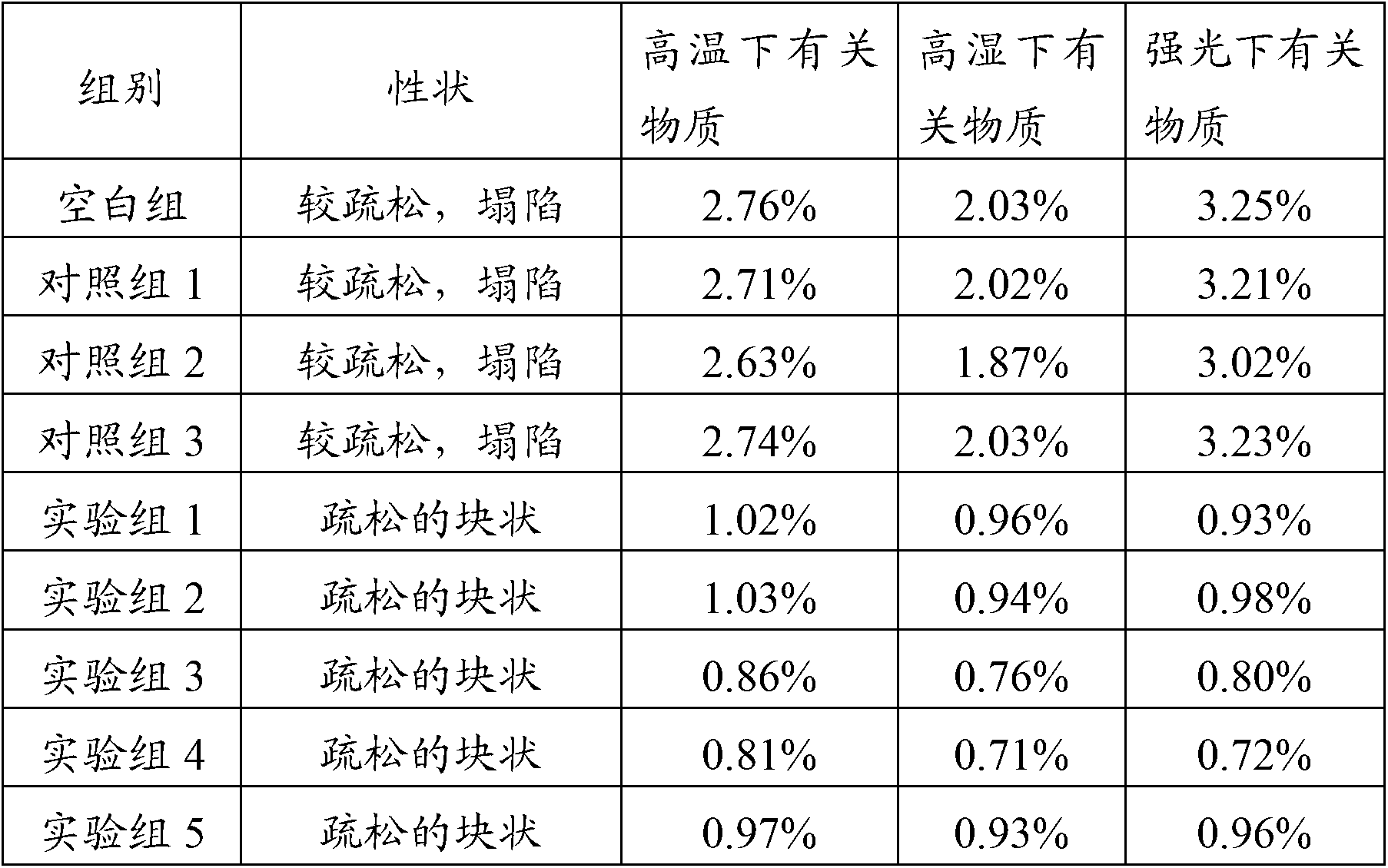
InactiveCN100998593AConvenient for clinical operationProduct quality unchangedOrganic active ingredientsDigestive systemOmeprazole SodiumAcetic acid
A high-stability freeze-dried powder injection of omeprazole sodium is proportionally prepared from omeprazole sodium, Na2EDTA and mannitol. Its preparing process is also disclosed.
Owner:黄玉明
Omeprazole freeze-dried powder injection and preparation method thereof
InactiveCN101283986AStable color and lusterLittle side effectsOrganic active ingredientsPowder deliveryOmeprazole SodiumSide effect
The invention provides an omeprazole sodium lyophilized powder for injection (pH of 10.8-11.2), comprising omeprazole sodium as the main active component, as well as excipient, metal ion complexing agent, stabilizer, antioxidant and pH regulator. The omeprazole sodium lyophilized powder for injection has higher active component content than that of the conventional omeprazole sodium injection, solves solution clarity problem, and effectively prevents loss of bone calcium due to complexation with calcium ion. The omeprazole sodium lyophilized powder for injection also has the advantages of stable color and properties, low side effects, good stability, good redissolution, and convenient application.
Owner:海南瑞基药物研究有限公司
Omeprazole sodium compound and method for synthesizing the same
InactiveCN101486706AHigh purityShort reaction timeOrganic chemistryDigestive systemOmeprazole SodiumSodium iodide
The invention relates to an omeprazole sodium compound and a synthetic method thereof; the synthetic method comprises the steps: in a reaction system, sodium iodide is added as a catalyst and ethanol and acetone are adopted as reactive solvents so that the reaction time is shortened and the product yield is significantly improved simultaneously and exceeds 90 percent; and in the generation step of omeprazole, peracetic acid is taken as a oxidizing agent. The synthetic method reduces costs, improves the oxidizing conditions, reduces side reaction, has relatively high product yield and product purity and obtains significant technical effect.
Owner:HAINAN LINGKANG PHARMA CO LTD
Omeprazole sodium freeze-dried powder injection and preparing method thereof
ActiveCN101229133ACatalysis against auto-oxidationHigh content of the main drugPowder deliveryOrganic active ingredientsOmeprazole SodiumDrug content
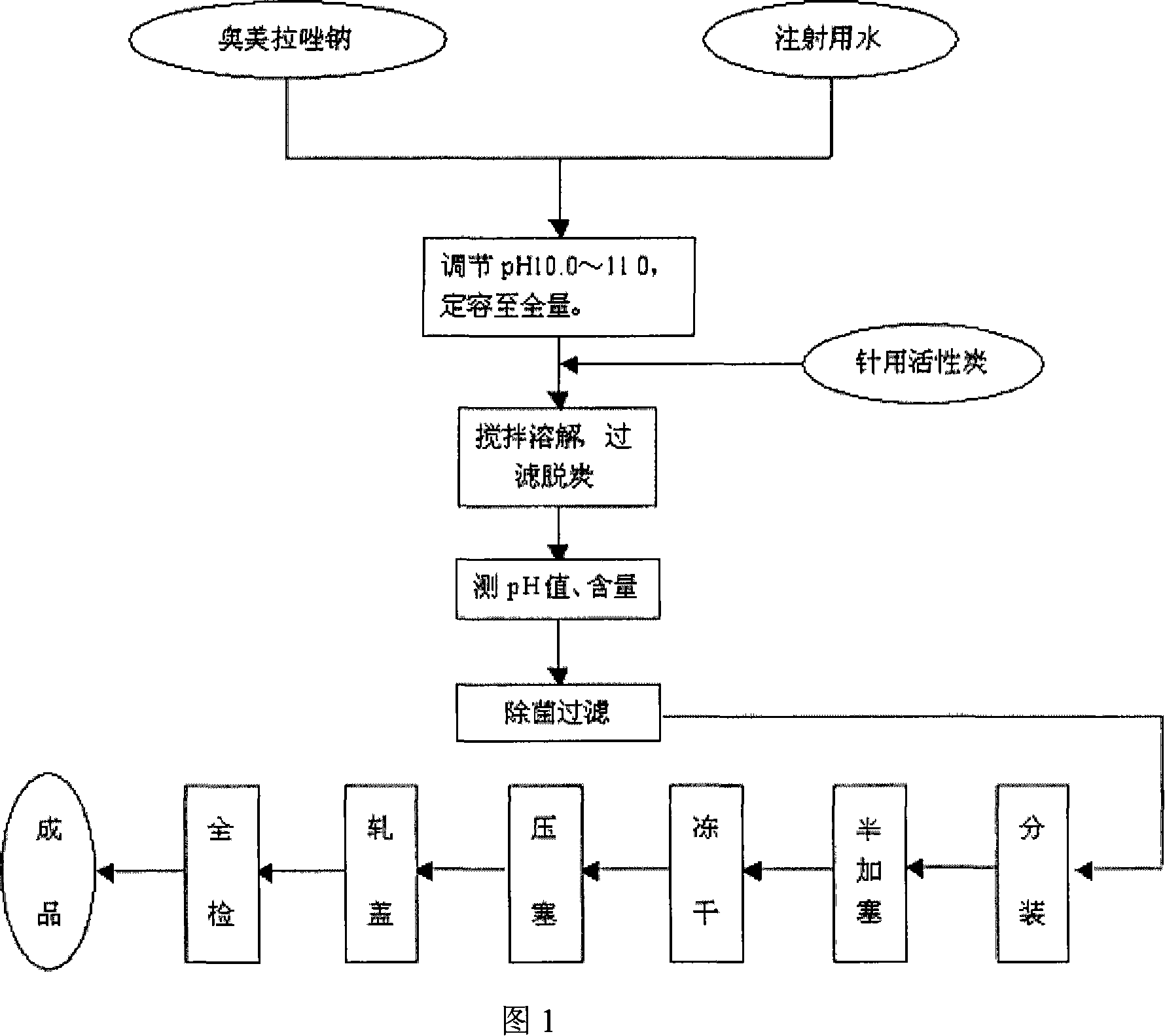
The invention provides an omeprazole sodium freeze-dried injection and a preparation method which is simple and feasible and the prepared freeze-dried injection is high in drug contents and low in related substances content. The method comprises the following steps: (1) the raw materials of the recipe quantity are stirred with injection water until the omeprazole sodium is completely dissolved to get omeprazole sodium solutions; sodium citrate solutions are put into the solutions got from step (1) and the pH value of the solutions is adjusted to 10.0 to 11.0; the injection water is put into the prepared products got from step (2) to the recipe quantity and then pin activated carbon is put into the prepared products, and filter decarburization is carried out after stirring to get filtrates; the filtrates got from step (3) are fine filling filtered by 0.22Mum removal bacteria microporous membrane, and the filtrates after fine filling filtering are put into a bottle which is partially stoppered, and then the filtrates are freeze-dried to get the freeze-dried injection.
Owner:SHANDONG YUXIN PHARMA CO LTD
Omeprazole sodium composition for injection
ActiveCN102151264AWon't releaseAvoid reactionPowder deliveryOrganic active ingredientsEthylene diamine tetra aceticEthylene diamine
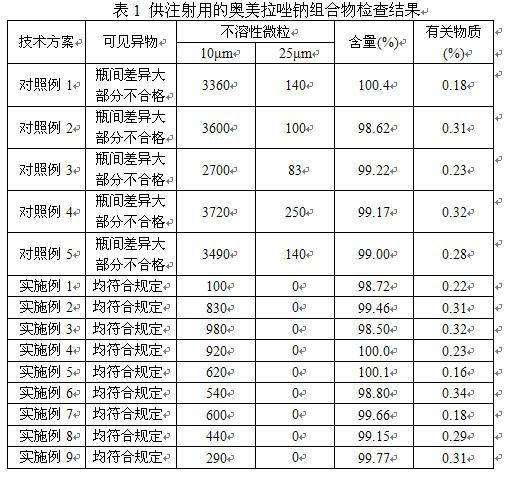
The invention provides an omeprazole sodium composition for injection. The omeprazole sodium composition for injection contains omeprazole sodium and ethylene diamine tetraacetic acid; and the weight ratio of the omeprazole sodium to the ethylene diamine tetraacetic acid is 1: (0.02-0.1). The composition is prepared by the following method: 1) preparing a liquid medicine: placing the omeprazole sodium and the ethylene diamine tetraacetic acid into a mixing pot, adding water for injection, stirring to dissolve the omeprazole sodium and the ethylene diamine tetraacetic acid and mixing uniformly, and adjusting the pH value to 11.0 to 12.0 by using sodium hydroxide; 2) processing by a rubber stopper; 3) performing aseptic filtering and packing; and 4) freezing and drying in vacuum so as to obtain the omeprazole sodium composition for injection. For medicaments, such as the omeprazole sodium which is easily reacted with an effusion from a capsule, the omeprazole sodium composition for injection can effectively guarantee that visible foreign matters and insoluble particles of the product meet the requirement of injection at the same time. By the omeprazole sodium composition for injection, the quality level is enhanced obviously, and potential hazards in clinical safe medication use of patients due to unqualified visible foreign matters and insoluble particles can be avoided; and the omeprazole sodium composition for injection has better curative effect and lower clinical side reaction.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Omeprazole sodium lyophilized preparation for injection and preparation method thereof
InactiveCN101352423AImprove stabilityGuarantee product qualityOrganic active ingredientsPowder deliveryOmeprazole SodiumFreeze-drying
The invention discloses an omeprazole sodium freeze-dried preparation used for injection, which is made by the raw materials with the following parts by weight: 20-40 parts of omeprazole sodium, 20-200 parts of glucan, 5-60 parts of stabilizing agent, 5-40 parts of sodium sulfite, 40-400 parts of polymethylmethacrylate and 10-500 parts of freeze-dried skeleton agent. The invention also discloses a preparation method of the omeprazole sodium freeze-dried preparation. When in the processing of forming emulsion and polymerized curing nanoparticles, the preparation causes the medicine to be wrapped into the nanoparticles or absorbed on the surface of the nanoparticles, thus greatly improving the stability of the omeprazole sodium and ensuring the quality of the products.
Owner:HAINAN LINGKANG PHARMA CO LTD
Esomeprazole and preparation method of magnesium trihydrate of esomeprazole
The invention provides esomeprazole and a preparation method of magnesium trihydrate of the esomeprazole. The preparation method includes the following steps of subjecting racemization omeprazole and inorganic base to acid-base neutralization reaction in an alcoholic solution to obtain racemization omeprazole sodium salt; dissolving omeprazole sodium salt, organic metal coordination agents, chelating agents and organic base in an organic solvent for complex reaction to obtain esomeprazole complex; subjecting S-mandelic acid and the esomeprazole complex to condensation reaction to obtain an esomeprazole mandelate compound; dissolving the esomeprazole mandelate compound in an acetone solution, and performing filtering to obtain S-omeprazole-S-mandelate compound; and suspending the S-omeprazole-S-mandelate compound in a first solvent to obtain a suspension solution, and adjusting potential of hydrogen (pH) of the suspension solution to be 8-10 to obtain the esomeprazole, wherein the first solvent includes 30-32v / v% of an alkaline aqueous solution and 68-70v / v% of an organic solvent. By means of the preparation method, the technical problem that the yield and the purity of the esomeprazole in prior art are low is solved.
Owner:HUNAN FANGSHENG PHARMACEUTICAL CO LTD
Omeprazole sodium freeze-dried powder injection and preparation method thereof
ActiveCN101703483AReduce the risk of adverse reactionsComply with the requirements of human intravenous injectionPowder deliveryOrganic active ingredientsOmeprazole SodiumFiltration
The invention discloses an omeprazole sodium freeze-dried powder injection and a preparation method thereof. The omeprazole sodium freeze-dried powder injection contains an active ingredient, namely, omeprazole sodium monohydrate, and auxiliary materials, namely, calcium disodium edetate and sodium hydroxide. The preparation method of the omeprazole sodium freeze-dried powder injection is characterized by comprising the following steps: weighing the calcium disodium edetate of prescription amount and dissolving the calcium disodium edetate in water for injection, stirring, dissolving, and regulating pH value to 10.0-12.0 by using 10% of sodium hydroxide solution; weighing omeprazole sodium of the prescription amount and adding the omeprazole sodium in the mixture, stirring at room temperature for dissolution, supplementing and adding the water for injection to full amount; adding active carbon, stirring at room temperature for decoloration and endotoxin removal, conducting rough filtration to remove carbon firstly, and then conducting refining filtration by using a filter membrane of 0.22 Mum; taking refining filtrate to test intermediate, conducting encapsulation after meeting requirements; and freeze-drying and unboxing, thus obtaining the omeprazole sodium freeze-dried powder injection. The freeze-drying technology of the omeprazole sodium freeze-dried powder injection takes temperature below minus 40 DEG C as pre-freezing temperature; after pre-freezing for at least two hours, sublimation is started, wherein the sublimation temperature is 5-12 DEG C, the sublimation time is over 14 hours; and then drying is conducted for over 2 hours at the temperature of 20-35 DEG C. Unboxing is carried out after a stopper is added and a cover is put in place, thus obtaining the finished product of the omeprazole sodium freeze-dried powder injection.
Owner:HAINAN LEVTEC PHARMA
Omeprazole sodium freeze-dried powder injection, as well as preparation method and quality control method thereof
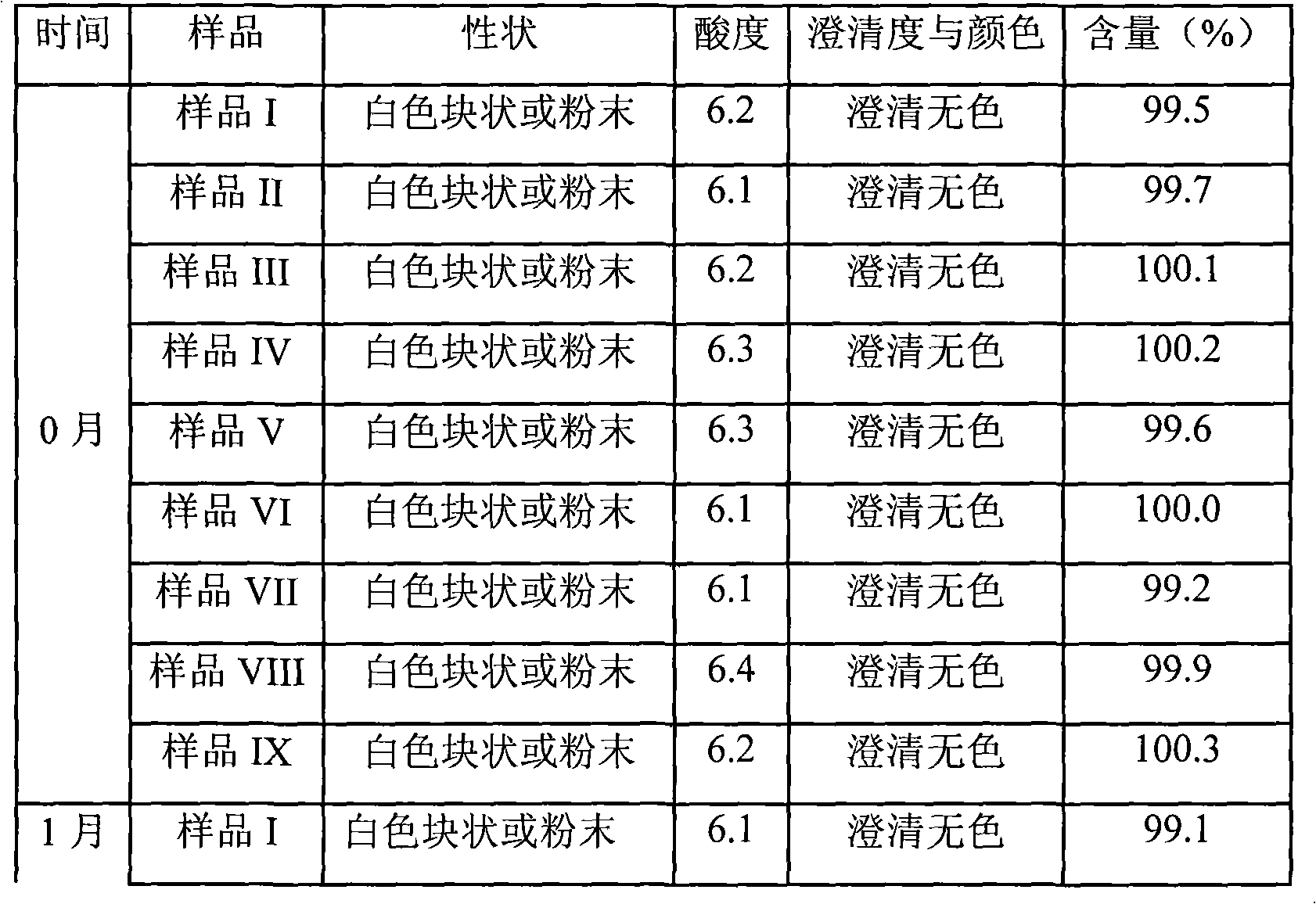
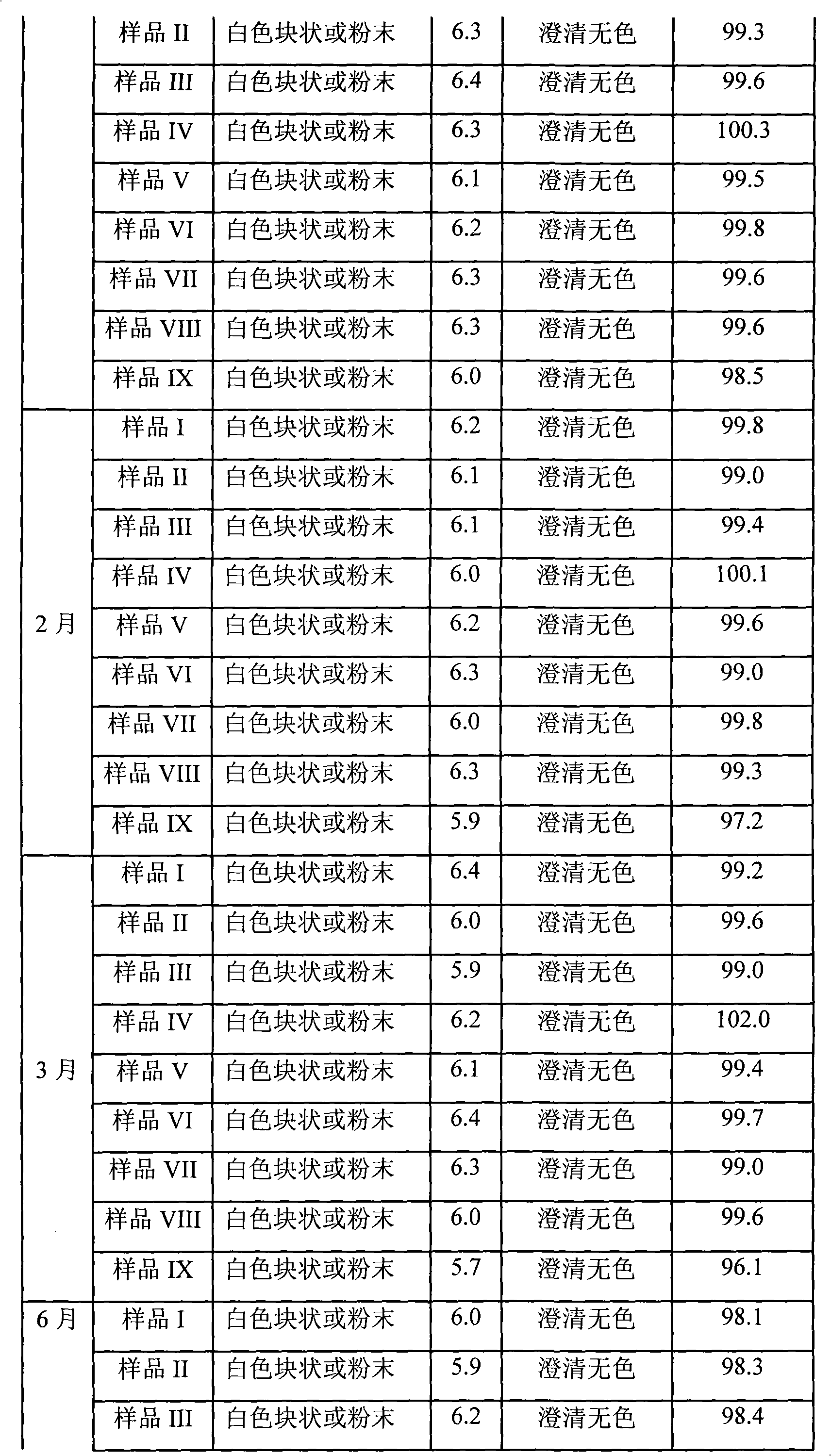
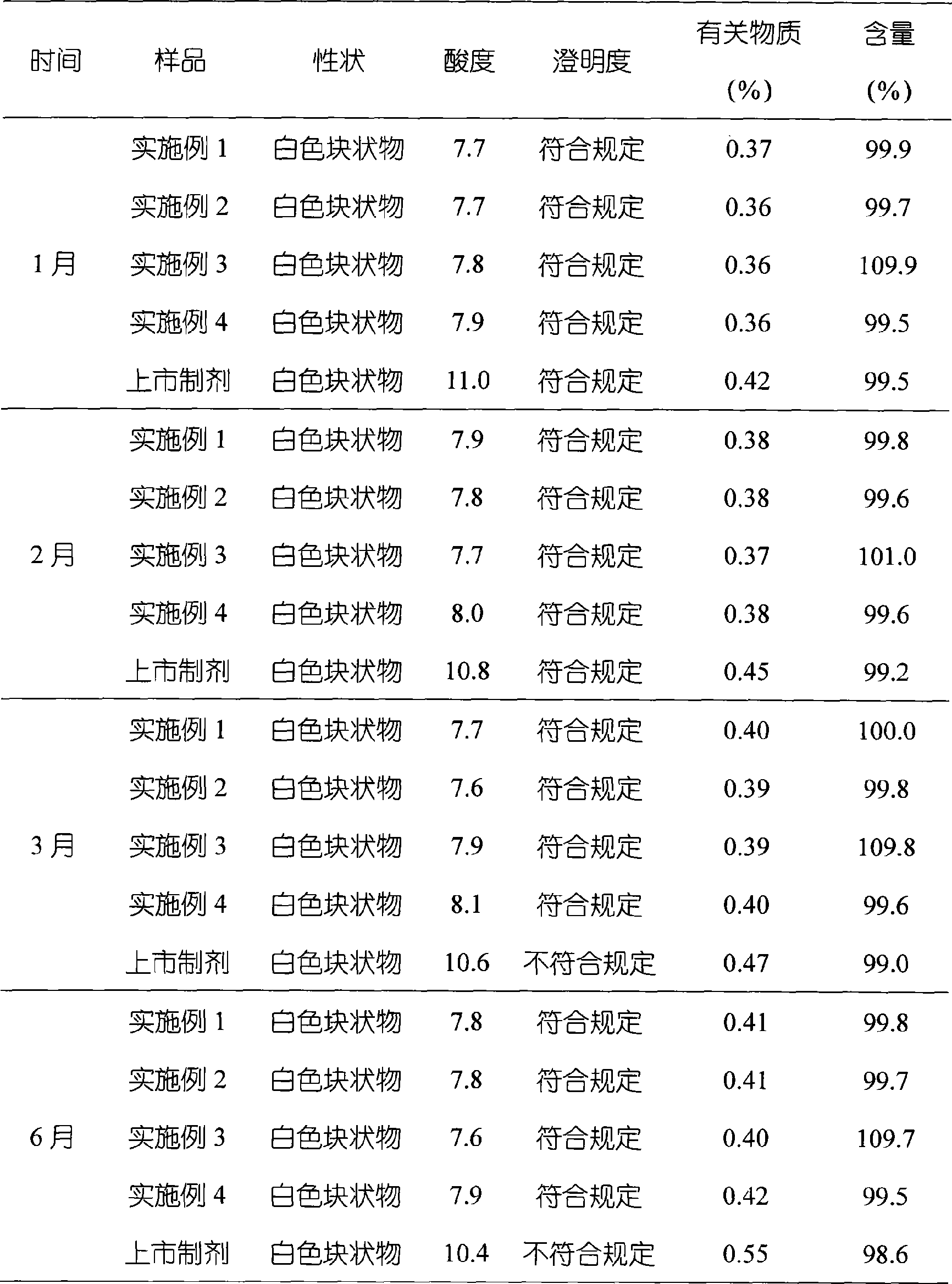
The invention relates to omeprazole sodium freeze-dried powder injection, as well as a preparation method and a quality control method thereof, belonging to the technical field of medicines. In the invention, the stability of the medicament is improved by utilizing the combined application of an antioxidant and a chelator, adjusting the pH value of an intermediate solution in the preparation process to be in a certain range and controlling the temperature in each phase of the freeze-drying process. In addition, the quality control method of the omeprazole sodium freeze-dried powder injection is researched, and a quality control method with high sensitivity and strong specificity is established so that the quality of the omeprazole sodium freeze-dried powder injection can be well controlled.
Owner:BEIJING SIHUAN KEBAO PHARM CO LTD
Preparation method for high-purity esomeprazole sodium
ActiveCN103288801ASolve the prone to titanium complex suspensionSolve the difficulty of splittingOrganic chemistrySodium bicarbonateOmeprazole Sodium
The invention discloses a preparation method for high-purity esomeprazole sodium. The preparation method comprises the steps of: including and splitting esomeprazole sodium and D-(-)-diethyl tartrate, titanium iso-propylate, triethylamine and L-(+)-mandelic acid in the presence of a proper amount of water, and separating to obtain an inclusion complex; dissolving the inclusion complex with ethyl acetate, washing inclusion complex with sodium carbonate water solution, carrying out ammonia hydroxide eluting on an ethyl acetate layer, slowly regulating the pH value to 6-7 with glacial acetic acid, then extracting with dichloromethane, and concentrating to obtain crude esomeprazole free alkali product; carrying out silica gel adsorption and elution on the crude product to obtain a pure esomeprazole free alkali product; and enabling the pure product and the methanol-ethanol-acetonitrile solution of sodium hydroxide to form salt, and then crystallizing with isopropyl ether to obtain the high-purity esomeprazole sodium. According to the preparation method, the difficulties that when inclusion and splitting are carried out, the titanium complex suspension body are difficult to split and the ammonia complex of titanium is difficult to remove can be solved, the industrialization production can be realized, the industrialized production cost is low, the product purity is high, the yield is high, and no harmful gas is generated.
Owner:SICHUAN BAILI PHARM CO LTD
Omeprazole sodium and preparation method
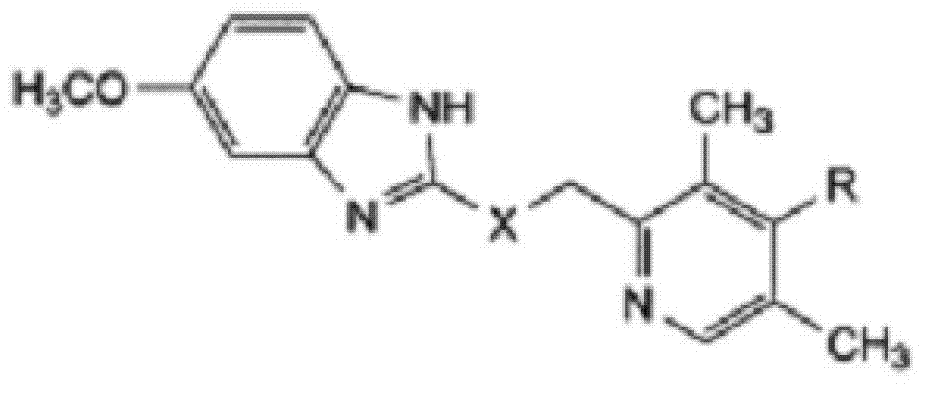
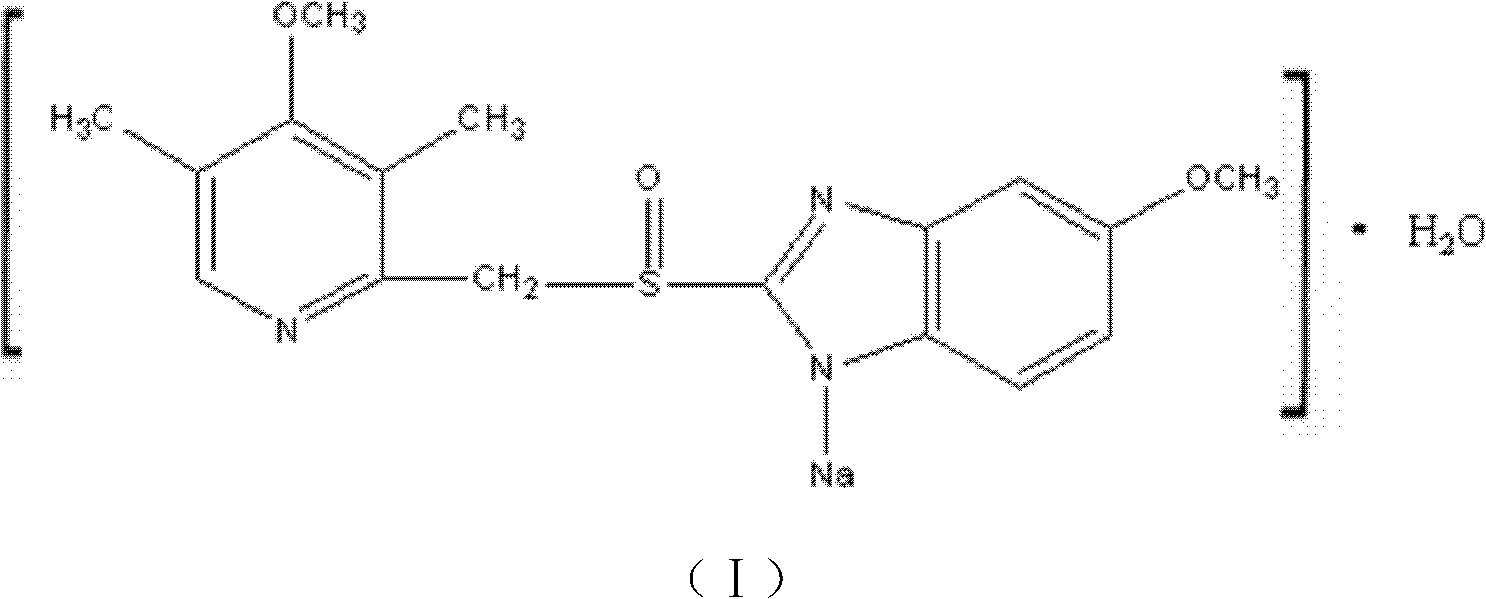
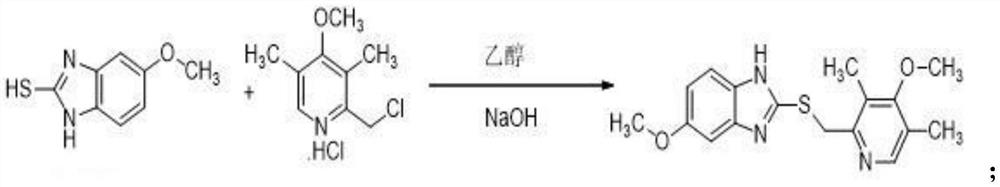
ActiveCN103204841APrevent reflux combined with aspirationPrevent rebleedingOrganic chemistryOmeprazole Sodium4-methoxypyridine
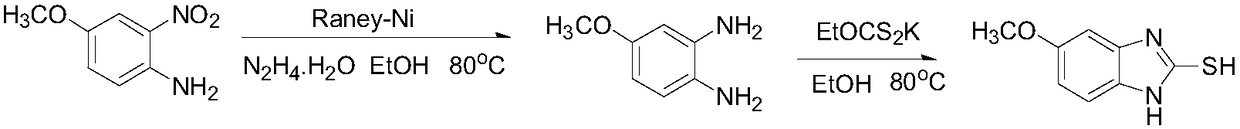
The invention relates to omeprazole sodium and a preparation method, and in particular relates to a method for preparing omeprazole or a salt thereof. The method comprises the following steps of: (1) reacting 2-chlorinated methyl-3,5-dimethyl-4-methoxypyridine or a salt thereof and 2-mercapto-5-methoxy-1H-benzimidazole to generate 5-methoxy-2-[(4-methoxy-3,5-dimethylpyridine-2-yl)methylmercapto]-1H-benzimidazole; and (2) oxidizing 5-methoxy-2-[(4-methoxy-3,5-dimethylpyridine-2-yl)methylmercapto]-1H-benzimidazole to generate omeprazole. The invention further relates to omeprazole prepared by the invention or a sodium salt thereof. A product prepared by the method has high purity.
Owner:CHENGDU TIANTAISHAN PHARMA
Novel omeprazole sodium compound and medicinal composition thereof
ActiveCN102351846AHigh thermodynamic stabilityHigh yieldOrganic active ingredientsPowder deliveryOmeprazole SodiumFreeze-drying
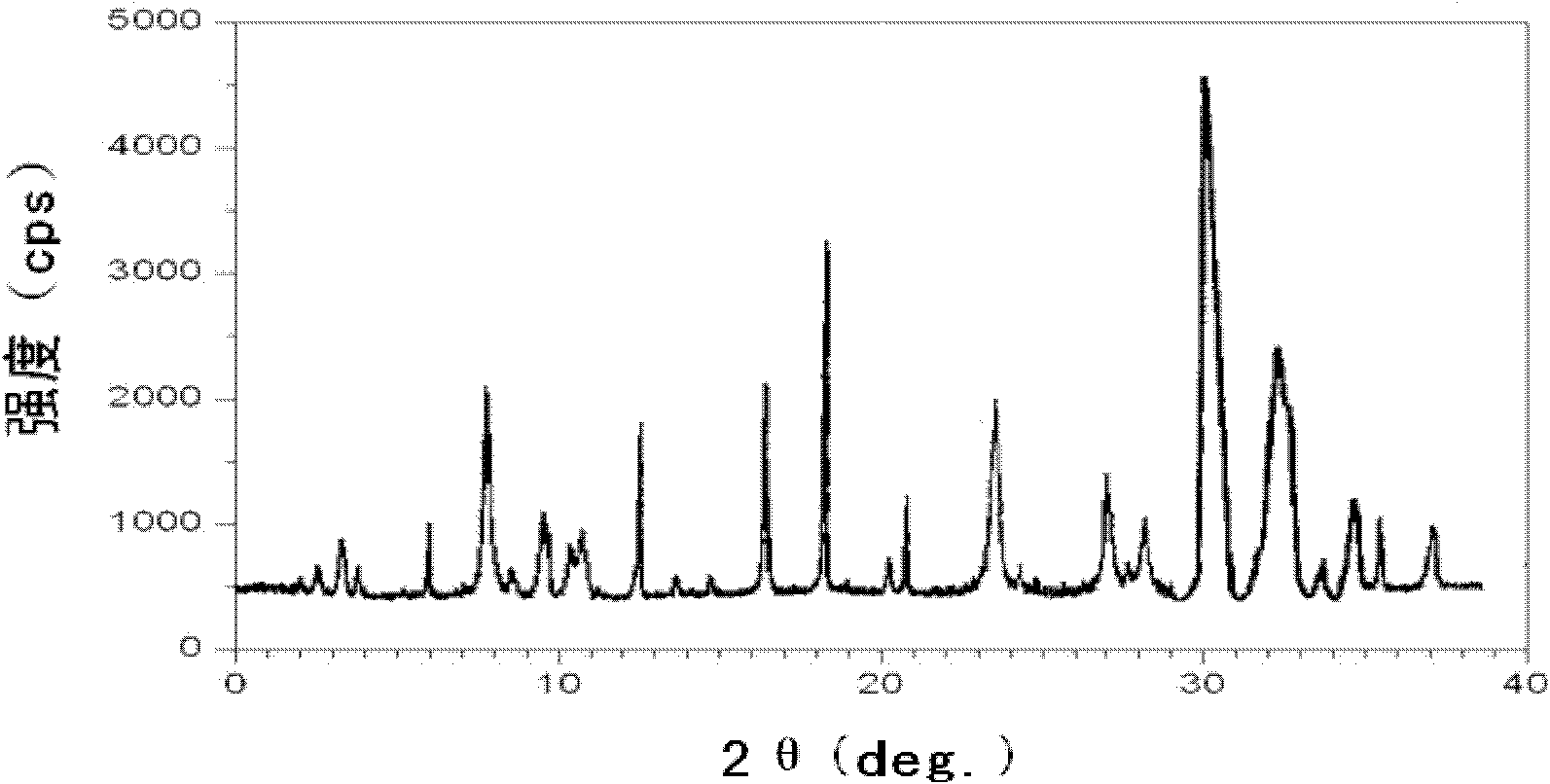
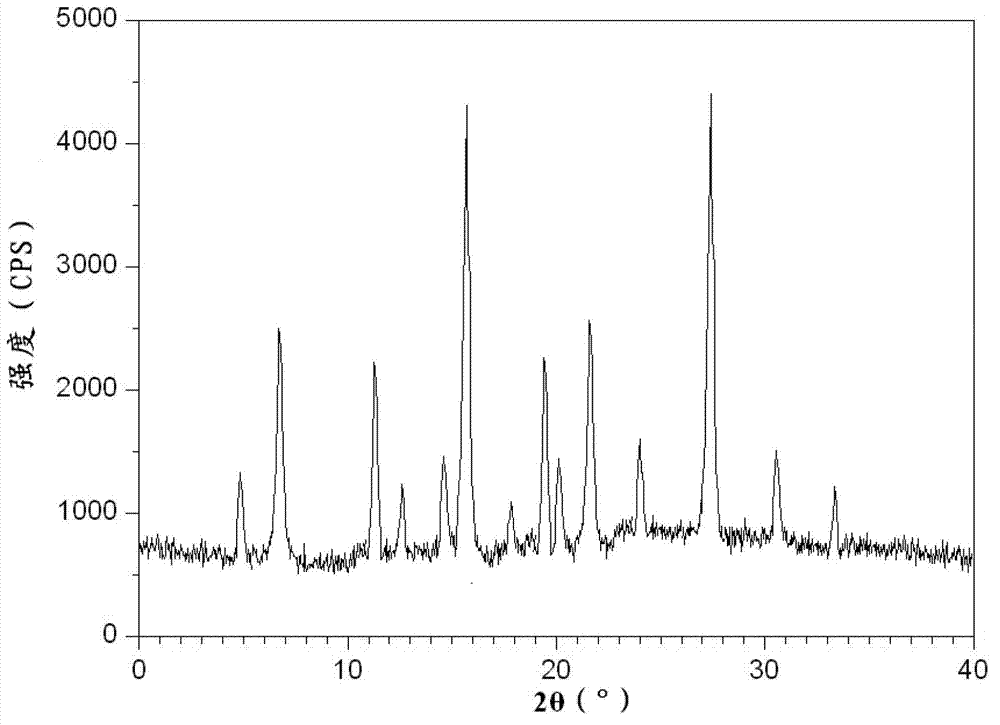
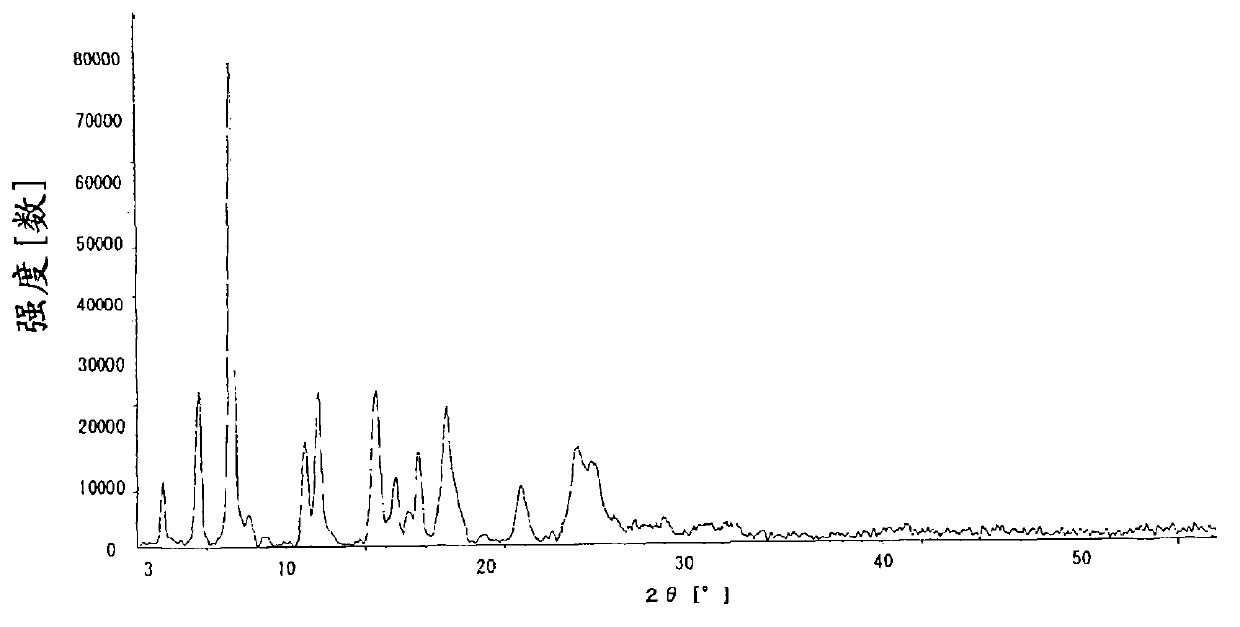
The invention discloses a novel omeprazole sodium compound. Characteristic peaks of the omeprazole sodium compound in an X-ray powder diffraction pattern obtained through Cu-K alpha-ray measurement are displayed when 2 theta is 3.2, 5.9, 7.6, 9.2, 10.5, 10.6, 12.3, 16.2, 18.2, 20.8, 23.4, 27.1, 30.4, 32.3 and 34.6. The omeprazole sodium compound has a new crystal form different from the prior art, has the thermodynamic stability higher than that with known crystal form, and has the advantage of not absorbing moisture basically. The invention also discloses a medicinal composition, which comprises the novel omeprazole sodium compound. Omeprazole sodium freeze-dried powder injection can be directly prepared under the condition of not adding lyoprotectants, and the omeprazole sodium freeze-dried powder injection has the advantages of high stability and high re-dissolubility.
Owner:周晓东
Esomeprazole sodium and lyophilized preparation comprising same
InactiveCN109456306AImprove manufacturing precisionPreparation precision safetyOrganic active ingredientsPowder deliveryOmeprazole SodiumEsomeprazole Sodium
The invention provides esomeprazole sodium and a lyophilized preparation comprising the same. A preparation method of esomeprazole sodium includes following steps: 1), synthesizing an intermediate; 2), synthesizing esomeprazole sodium; 3), roughly preparing esomeprazole sodium; 4), finely preparing esomeprazole sodium. Esomeprazole sodium prepared by the method is higher in preparation accuracy and safer to use; the preparation process is easier to control, and step decomposition brings convenience to quality control of middle steps, so that ensuring of preparation accuracy of esomeprazole sodium is facilitated, and drug prepared from esomeprazole sodium is safer.
Owner:SHANXI PUDE PHARMA CO LTD
Omeprazole sodium combined medicament and preparation method thereof
InactiveCN101766614AGood treatment effectAvoid allergic reactionsOrganic active ingredientsDigestive systemOmeprazole SodiumMedicine
The invention discloses an omeprazole sodium combined medicament and a preparation method thereof. The omeprazole sodium combined medicament comprises the following medicinal effect compositions in part by weight: 17.3 to 34.8 parts of the omeprazole sodium, 8.7 to 13.0 parts of reduced glutathione and 43.5 to 52.2 parts of sodium glutamate. The omeprazole sodium combined medicament provided by the invention has the effect of resisting drug allergy and can lighten the damage effect and the adverse reaction of the omeprazole sodium on liver; the medicament has high quality; and the preparation method is energy-saving and environment-friendly.
Owner:蔡海德
Omeprazole sodium freeze-dried lipidosome preparation and preparation method thereof
InactiveCN101530392AGuaranteed bioavailabilityFull appearanceOrganic active ingredientsDigestive systemOmeprazole SodiumCholesterol
The invention relates an omeprazole sodium freeze-dried lipidosome preparation and a preparation method thereof. The omeprazole sodium freeze-dried lipidosome preparation is characterized by comprising the following components in portion by weight: 10 to 20 portions of omeprazole sodium, 10 to 40 portions of phospholipids, 0 to 10 portions of cholesterol, 0 to 10 portions of antioxidant, and 5 to 20 portions of cryoprotectant.
Owner:HAINAN LINGKANG PHARMA CO LTD
Omeprazole sodium compound and preparation thereof
InactiveCN101412710AImprove stabilityImprove clinical efficacyOrganic chemistryDigestive systemOmeprazole SodiumOrganic solvent
The invention relates to an omeprazole sodium compound and a method for preparing the same. The method comprises the following steps: dissolving an omeprazole sodium crude product in water, adding solution of a solid acid salt into the mixture, collecting the precipitated solid, dissolving the solid by using an organic solvent, eluting and purifying the mixture by an eluant through macroporous resin, collecting the eluate, adding an alkaline solution into the eluate to regulate the pH value to be alkaline, collecting the precipitated solid, and performing vacuum drying on the solid after washing to obtain the refined product of the omeprazole sodium. The purity of the omeprazole sodium prepared by the method is more than 99.8 percent, thus the purity and the preparation stability of the omeprazole sodium product are greatly improved.
Owner:HAINAN LINGKANG PHARMA CO LTD
Omeprazole sodium crystal compound and medicine composition containing omeprazole sodium crystal compound
The invention relates to an omeprazole sodium crystal compound, which shows characteristic diffraction peaks at 4.8-degree, 6.8-degree, 11.5-degree, 12.6-degree, 14.6-degree, 15.8-degree, 17.9-degree, 19.4-degree, 20.2-degree, 21.8-degree, 24.0-degree, 27.4-degree, 30.5-degree and 33.3-degree angles in an X-ray powder diffraction pattern which is expressed by a 2theta plus or minus 0.2-degree diffraction angle. The invention additionally relates to a preparation method of the crystal compound and a medicine composition containing the crystal compound. The crystal compound has better stability; and after a freeze-dried powder injection which is prepared by using the crystal compound is combined with four kinds of injections, the quantity of insoluble particles is fewer and the change of the quantity of the insoluble particles within 4h after combination is smaller.
Owner:SHANDONG YUXIN PHARMA CO LTD +1
Injection with omeprazole and domperidone
InactiveCN1736378AAcid reflux reductionReduce tensionPowder deliveryOrganic active ingredientsOmeprazole SodiumDuodenal ulcer
The invention discloses an Omeprazole sodium and domperidone injection, wherein the active constituents include Omeprazole sodium and domperidone by the weight ratio of 4:1-2:3, the injection also comprises excipient and stabilizing agent. The injection can be used for treating gastric ulcer, duodenal ulcer, stomach and gullet reverse-current, and functional dyspepsia.
Owner:张志生
Pharmaceutical composition containing esomeprazole sodium and preparation method thereof
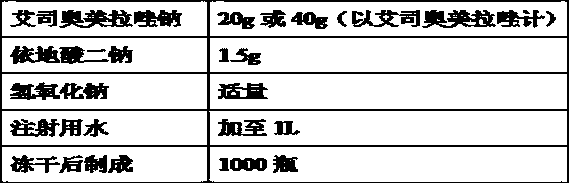
InactiveCN108785259AInhibit growthReduced stabilityPowder deliveryOrganic active ingredientsOmeprazole SodiumDesorption
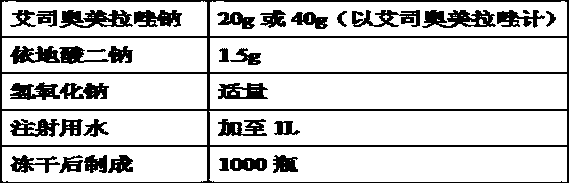
The invention relates to a pharmaceutical composition containing esomeprazole sodium and a preparation method thereof. The pharmaceutical composition is freeze-dried powder for injection, and for every 1000 bottles, the pharmaceutical composition is composed of the following pharmaceutical ingredients of 20-40 grams of the esomeprazole sodium, 1.5 grams of disodium edetate, and 1 liter of water for injection. The preparation method of the pharmaceutical composition containing the esomeprazole sodium includes the steps of liquid preparation and freeze drying, wherein in the process of liquid preparation, the esmeprazole sodium, the disodium edetate, and the water for injection are mixed to prepare a liquid of a pH of 11.0 to 11.5; the step of freeze drying includes freeze control 1, freezecontrol 2, sublimation drying 1, sublimation drying 2 and desorption drying. The pharmaceutical composition containing the esomeprazole sodium has various advantages, such as having low moisture content, few metal ions and colored impurities and good stability, and having no significant increase in impurities accompanied by changes in solution color after long-term placement.
Owner:CHENGDU GUOHONG PHARMA
Omeprazole sodium freeze drying powder injection pharmaceutical composition for injection
InactiveCN104666255APrevent reflux combined with aspirationPrevent rebleedingPowder deliveryOrganic active ingredientsOmeprazole SodiumBiological property
The invention relates to an omeprazole sodium freeze drying powder injection pharmaceutical composition for injection. In particular, on the one hand, the invention relates to a freeze drying powder injection pharmaceutical composition which comprises omeprazole sodium, ethylenediamine tetraacetic acid disodium salt and an optional acidifying or alkalizing agent; on the other hand, the invention further relates to a method for preparing the omeprazole sodium freeze drying powder injection pharmaceutical composition for injection. The method is a conventional preparation method of the omeprazole sodium freeze drying powder injection pharmaceutical composition for injection. The omeprazole sodium freeze drying powder injection pharmaceutical composition for injection provided by the invention can be used for treating gastropathy and has an anticipated good property. For example, the omeprazole sodium freeze drying powder injection pharmaceutical composition for injection not only has excellent physical and chemical stabilities, but also has an excellent biological property.
Owner:山东北大高科华泰制药有限公司
Omeprazole sodium freeze-dried powder injection for injection
ActiveCN103169674APrevent reflux combined with aspirationPrevent rebleedingPowder deliveryOrganic active ingredientsOmeprazole SodiumFreeze-drying
The invention relates to an omeprazole sodium freeze-dried powder injection for injection. Specifically, the invention relates to the freeze-dried powder injection containing omeprazole sodium, disodium edetate and arbitrarily selected pH regulator. The omeprazole sodium freeze-dried powder injection for injection, disclosed by the invention, can be used for treating stomach diseases and further has exciting good properties.
Owner:成都天台山制药股份有限公司
New application of injection omeprazole sodium for treating gastric mucosa-associated lymphoid tissue lymphoma
InactiveCN101632667AImprove stabilityGood resolubilityOrganic active ingredientsPowder deliveryOmeprazole SodiumSide effect
The invention relates to new application of injection omeprazole sodium for treating gastric mucosa-associated lymphoid tissue lymphoma. The injection omeprazole sodium for treating gastric mucosa-associated lymphoid tissue lymphoma comprises omeprazole sodium, filling agent, metallic ion complexing agent, antioxidant and pH regulator. The invention has the advantages of stable characters and color, little side effects, high security, good stability and favorable resolubility, and has higher content of omeprazole sodium than that of the common omeprazole sodium injection.
Owner:邓菊娟
Ao meilazole sodium injection liquid
InactiveCN1686124AImprove solubilityImprove work efficiencyOrganic active ingredientsDigestive systemOmeprazole SodiumPolyethylene glycol
A liquid injection of omeprazole sodium is prepared from omeprazole sodium, Na2EDTA, and polyethanediol-400. Its preparing process is also disclosed.
Owner:马志民
Omeprazole sodium microspheric freeze-dried preparation for injection and method for preparing omeprazole sodium microspheric freeze-dried preparation for injection
InactiveCN105997900AGood sustained release effectHigh drug loadingOrganic active ingredientsPowder deliveryOmeprazole SodiumSide effect
The invention relates to a freeze-dried preparation of omeprazole sodium long-acting slow-release microspheres for injection and a preparation method thereof, comprising 15-30 parts of omeprazole sodium, polylactic-glycolic acid (PLGA) in parts by weight ) 60-100 parts, polyvinyl alcohol (PVA) 50-80 parts and surfactant 1-2 parts. The omeprazole sodium long-acting sustained-release microsphere freeze-dried preparation for injection of the present invention has a long-lasting slow-release time, reduces drug side effects and administration times, and has good drug stability. The carrier material used is biodegradable, has high safety and is freeze-dried. Rapid freeze-drying is adopted in the process, and the product has fine texture and good resolubility.
Owner:ZHEJIANG YATAI PHARMA
Novel omeprazole compound and pharmaceutical composition thereof
InactiveCN103059000AImprove stabilityHigh purityOrganic active ingredientsOrganic chemistryOmeprazole SodiumVitamin C
The invention discloses a novel omeprazole compound. The purity of the novel omeprazole compound reaches more than 99.8%. Meanwhile, the invention also discloses a pharmaceutical composition of the novel omeprazole compound. The pharmaceutical composition comprises the following components in parts by weight: 62-90 parts of omeprazole sodium, 10-25 parts of sodium glutamate, 1-4 parts of sodium tartrate and 7-12 parts of vitamin C. According to the pharmaceutical composition of the novel omeprazole compound, a synergistic effect is generated between sodium glutamate and sodium tartrate, so that the stability of omeprazole sodium is remarkably improved, and the pharmaceutical composition has important significance for omeprazole sodium in clinical popularization and application.
Owner:黄明芳
Omeprazole soluble powder for livestock and preparation process of omeprazole soluble powder
InactiveCN107397719AImprove securityImprove solubilityPowder deliveryOrganic active ingredientsOmeprazole SodiumSolubility
The invention discloses omeprazole soluble powder for livestock and a preparation process of the omeprazole soluble powder. The omeprazole soluble powder for livestock contains the following components: 0.5-6g of omeprazole sodium or omeprazole, 1.25-15g of an alkali cosolvent, 1-6g of a macromolecule solubilizer, 1-7.5g of a stabilizer, 0.1-2g of a sweetening agent and 63.5-96.15g of auxiliary materials, wherein the total weight of the mixed components is 100g. Defects that omeprazole is poor in water solubility and cannot be taken in a mixed drinking manner are overcome, due to adding of the cosolvent, the water solubility of the omeprazole is improved; due to adoption of the stabilizer, the stability of the omeprazole is improved; water drinking administration is achieved, and thus the omeprazole soluble powder is convenient to use, good in symptomatic treatment on pig gastritis and proventriculitis, good and stable in water solubility, low in production and application cost and convenient in packaging and transportation.
Owner:洛阳市兽药厂
Omeprazole sodium crystalline compound, preparation method and medicine composition thereof
ActiveCN103012371AImprove performanceImprove solubilityOrganic active ingredientsOrganic chemistryOmeprazole SodiumMedicine
The invention provides a V-type crystal of omeprazole sodium with diffraction angles of 3.8 DEG, 5.4 DEG, 7.2 DEG, 11.7 DEG, 14.6 DEG, 18.1 DEG, 23.5 DEG and 26.8 DEG expressed by 2 theta in powder X-ray diffraction analysis. The V-type crystal has excellent stability and solubility and low hygroscopicity. The invention also provides a preparation method of the crystal, a medicine composition and a medicine preparation comprising the V-type crystal, especially a powder injection.
Owner:双鹤药业(海南)有限责任公司
Omeprazole sodium freeze-dried powder injection preparation method
InactiveCN107260691AReduce the impactLess prone to structurePowder deliveryOrganic active ingredientsOmeprazole SodiumFreeze-drying
The invention provides an omeprazole sodium freeze-dried powder injection preparation method. The method includes: A) mixing a metal ion complexing agent with part of water for injection, and adjusting a pH value to 9.5-11 to obtain first mixed solution; B) mixing omeprazole sodium with the first mixed solution, and adjusting a pH value to 9.9-11.1; C) adding the remaining water for injection to obtain omeprazole sodium solution; D) prefreezing the omeprazole sodium solution for 2-3h at a prefreezing temperature ranging from -35 DEG C to -40 DEG C; E) after prefreezing is finished, heating to 0 DEG C at a speed of 7-9DEG C / h, then heating from 0 DEG C to 30 DEG C at a speed of 3-5DEG C / h, and heating from 30 DEG C to 35-40 DEG C at a speed of 1-3DEG C / h to obtain omeprazole sodium powder; F) subjecting the omeprazole sodium powder to heat preservation at 35-40 DEG C for 3-5h. Quality and stability of a finished product are improved finally through regulation of parameters at steps of prefreezing, sublimation, heat-preservation drying and the like.
Owner:HUNAN KELUN PHARMA
Preparing method for esomeprazole magnesium
The invention relates to a preparing method for esomeprazole magnesium. The method comprises esomeprazole magnesium crude product manufacturing steps that 1, omeprazole thioether is synthesized; 2, obtained omeprazole thioether is oxidized under the effects of phthalic anhydride and oxidizing agents to obtain omeprazole, and omeprazole is salinized in a sodium mode to obtain omeprazole sodium; 3, omeprazole sodium is complexed to obtain an omeprazole sodium complex; 4, solvent A,N,N-diethyl ethylamine and 1-phenol acetic acid are added into the omeprazole sodium complex, and the mixture reacts to obtain an esomeprazole sodium complex; 5, alkali, solvent B and purified water are added into the esomeprazole sodium complex, the mixture is stirred for two hours and layered, after an alkaline water layer is acidized, the solvent B is used for extraction, and the mixture is dried and evaporated to dryness in a pressure reducing mode to obtain an esomeprazole magnesium crude product. According to the preparing method, the reaction is mild, the yield is high, and the requirement of esomeprazole magnesium serving as a crude drug can be met; no toxic reagent is needed, and environmental friendliness is achieved; operation is easy, reagents participating in the reaction are common reagents, and cost is controllable.
Owner:ZHEJIANG DAVI PHARMA
Synthesis method of esomeprazole sodium
PendingCN113512026AThe synthesis process is simpleLow costOrganic chemistryOmeprazole SodiumEsomeprazole Sodium
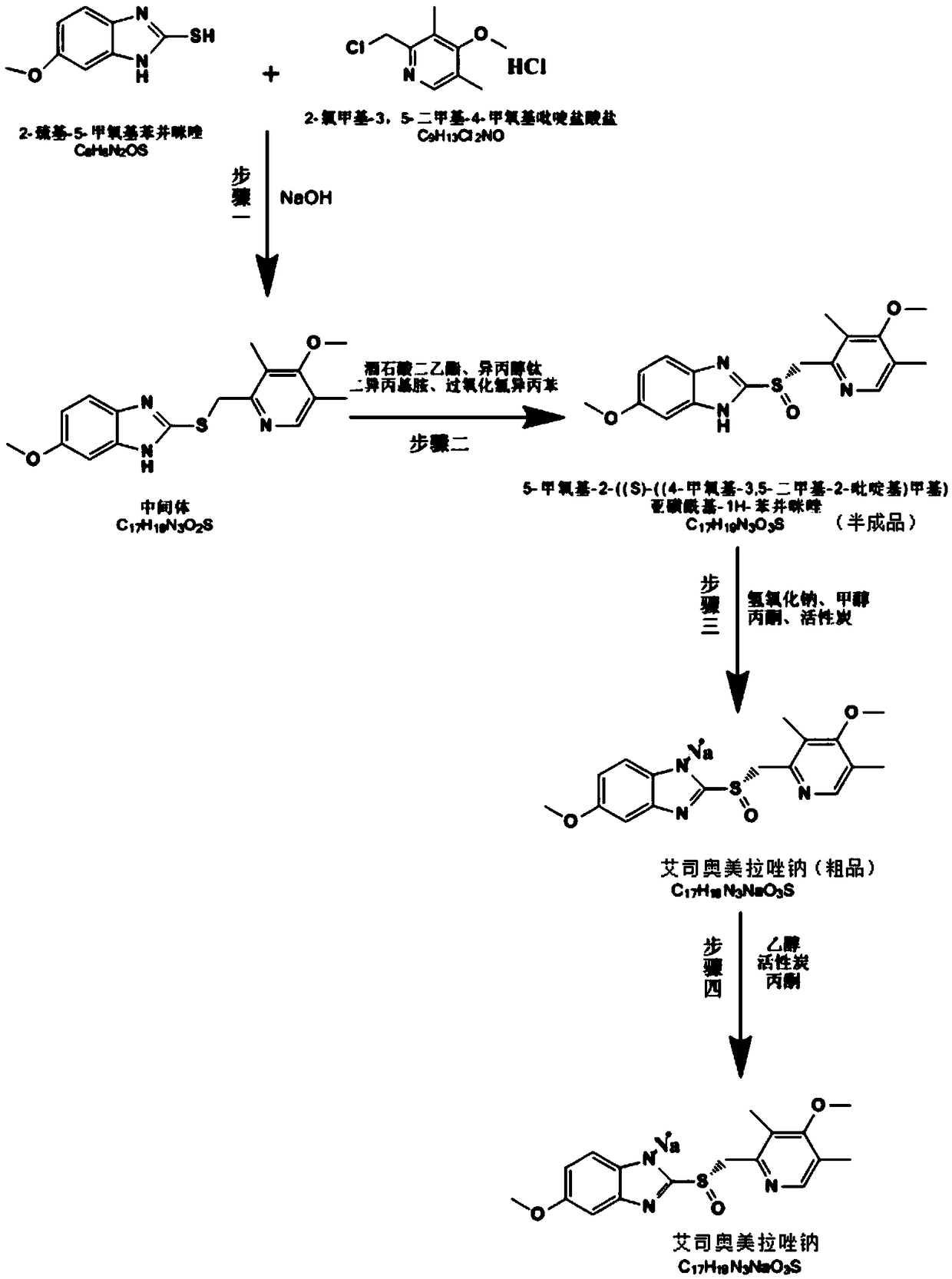
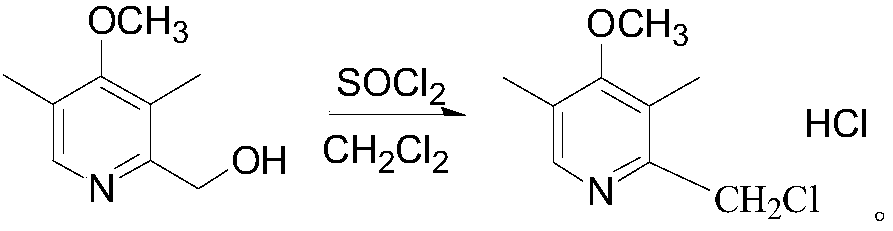
The invention discloses a synthesis method of esomeprazole sodium, and belongs to the field of organic synthesis. The method comprises the following steps: carrying out a heating reaction on 5-methoxy-2-mercaptobenzimidazole and 2-chloromethyl-3, 5-dimethyl-4-methoxypyridine hydrochloride in the presence of an ethanol-sodium hydroxide solution to obtain omeprazole thioether, adding alkali and carrying out a symmetric oxidation reaction to obtain a methyl isobutyl ketone solution of esomeprazole, and reacting with sodium hydroxide to obtain the esomeprazole sodium. According to the synthesis method, the raw material utilization rate is high, the content and variety of impurities are reduced, the extraction rate of the esomeprazole sodium is greatly increased, the yield can reach 75%, the purity is larger than 99%, the synthesis route is short, and the synthesis cost is greatly reduced.
Owner:SICHUAN ZIREN PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com