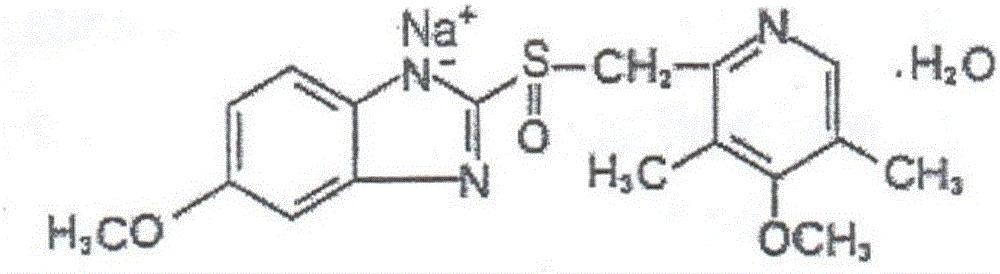
Omeprazole sodium microspheric freeze-dried preparation for injection and method for preparing omeprazole sodium microspheric freeze-dried preparation for injection
A technology of omeprazole sodium and freeze-dried preparations, applied in the field of medicine, can solve the problems of short duration of effective concentration, short drug action time, low drug utilization rate, etc., and achieves improved drug stability and high drug load. , sustained release effect and lasting effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The raw and auxiliary material proportioning of present embodiment 1 is as follows:
[0038]
[0039]
[0040] The preparation method of present embodiment 1 is as follows:
[0041] (1) Preparation of water phase: prepare 0.3% PVA aqueous solution, and add appropriate amount of surfactant Span 80.
[0042] (2) Preparation of the oil phase: Weigh 60g of PLGA and dissolve it in 1.5L of ethyl acetate, and weigh 15g of omeprazole sodium in 0.2L of propylene glycol, and mix the two into a homogeneous phase as the oil phase.
[0043] (3) Preparation of emulsion: Slowly add the oil phase into 50L of water, stirring constantly (speed is 1000r min -1 ) for 2 minutes, while keeping the emulsification temperature below 5°C in an ice bath.
[0044] (4) Preparation of microsphere freeze-dried preparation: Stir the emulsion prepared above at low speed for 4 hours, and gradually increase the system temperature to about 30°C, stir to remove the organic solvent until the microsp...
Embodiment 2
[0047] The raw and auxiliary material proportioning of present embodiment 2 is as follows:
[0048]
[0049]
[0050] The preparation method of present embodiment 2 is as follows:
[0051] (1) Preparation of water phase: prepare 0.3% PVA aqueous solution, and add appropriate amount of surfactant Span 80.
[0052] (2) Preparation of the oil phase: Weigh 70g of PLGA and dissolve it in 2L of ethyl acetate, and weigh 20g of omeprazole sodium in 0.2L of propylene glycol, and mix the two into a homogeneous phase as the oil phase.
[0053] (3) Preparation of emulsion: Slowly add the oil phase into 60L of water, stirring constantly (speed is 1000r min -1 ) for 2 minutes, while keeping the emulsification temperature below 5°C in an ice bath.
[0054] (4) Preparation of microsphere freeze-dried preparation: Stir the emulsion prepared above at low speed for 4 hours, and gradually increase the system temperature to about 30°C, stir to remove the organic solvent until the microsphere...
Embodiment 3
[0057] The raw and auxiliary material proportioning of present embodiment 3 is as follows:
[0058]
[0059]
[0060] The preparation method of present embodiment 3 is as follows:
[0061] (1) Preparation of water phase: prepare 0.3% PVA aqueous solution, and add appropriate amount of surfactant Span 80.
[0062] (2) Preparation of the oil phase: Dissolve 100g of PLGA in 3L of ethyl acetate, and weigh 30g of omeprazole sodium in 0.2L of propylene glycol, and mix the two into a homogeneous phase as the oil phase.
[0063] (3) Preparation of emulsion: slowly add the oil phase into 100L of water, and keep stirring (the speed is 1000r min -1 ) for 5 minutes, while keeping the emulsification temperature below 5°C in an ice bath.
[0064] (4) Preparation of microsphere freeze-dried preparation: Stir the emulsion prepared above for 6 hours at a low speed, and gradually increase the system temperature to about 30°C, stir to remove the organic solvent until the microspheres are...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com