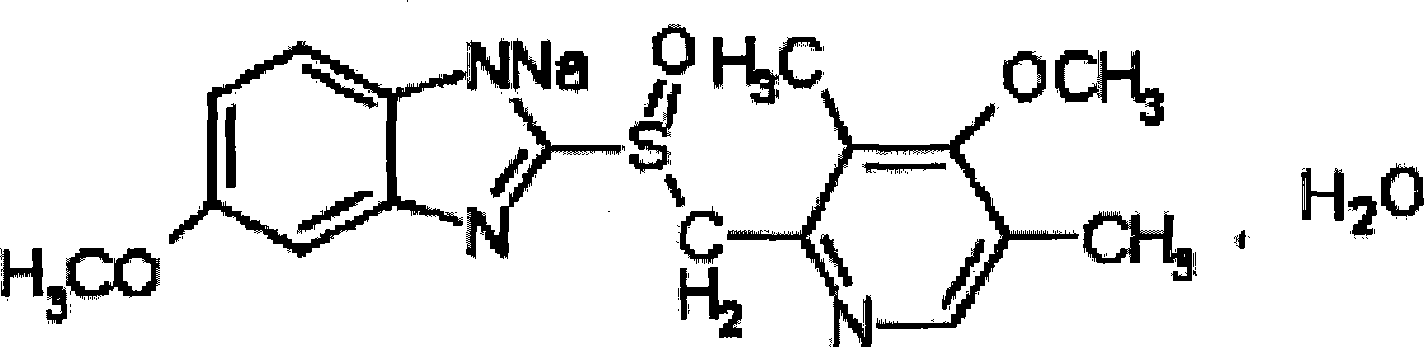
Omeprazole sodium freeze-dried powder injection and preparing method thereof
A technology of freeze-dried powder injection and omeprazole sodium, which is applied in the field of preparation of omeprazole sodium freeze-dried powder injection, can solve the problems of cumbersome preparation process, poor stability, and poor clarity, and achieve packaging Small size, good stability and excellent appearance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
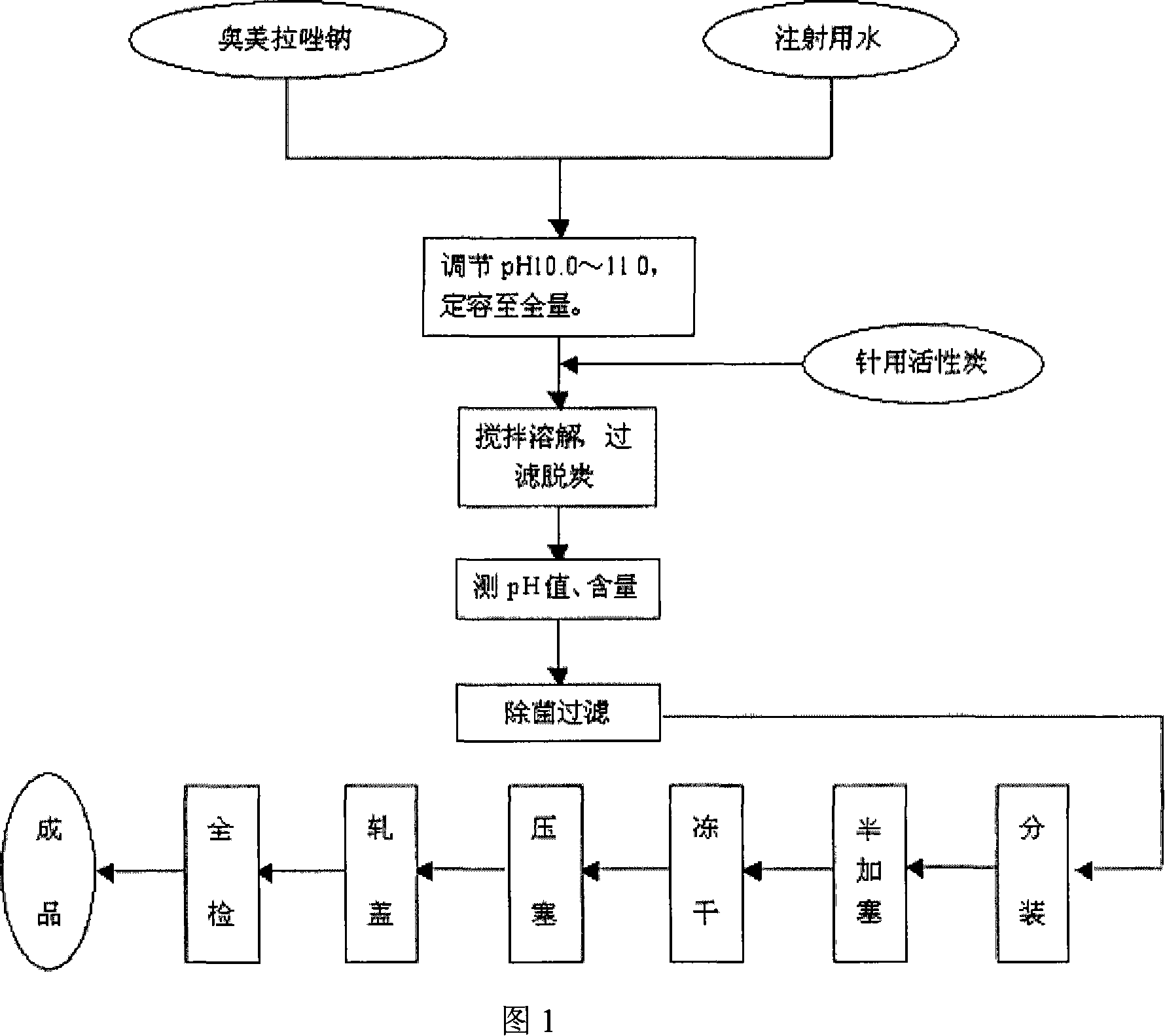
Image
Examples
Embodiment 1
[0075] 1. Prescription composition
[0076] 20mg
[0077] Omeprazole sodium 20g (calculated as omeprazole)
[0078] Add water for injection to 2000ml
[0079]
[0080] Freeze-dried to make 1000 vials
[0081] 2. Preparation method
[0082] Add the prescribed amount of omeprazole sodium into the liquid mixing tank, add 1500ml of water for injection and stir until completely dissolved, then adjust the pH to 11.0 with a sodium citrate solution with a concentration of 0.05mol / L, and add water for injection to 2000mL to obtain a mixed solution . Then add a total amount of 0.05% activated carbon for needles, stir for 15 minutes, and decarbonize by filtration. The obtained filtrate was fine-filtered with a 0.22 μm sterile microporous membrane, and the pH value, content and semi-plugging were measured. Then carry out freeze-drying: first lower the drug from normal temperature to -22.5°C, keep it warm for 60 minu...
Embodiment 2
[0084] 1. Prescription composition
[0085] 40mg
[0086] Omeprazole sodium 40g (calculated as omeprazole)
[0087] Add water for injection to 2000ml
[0088]
[0089] Freeze-dried to make 1000 vials
[0090] 2. Preparation method (specification: 20mg; 40mg)
[0091] Add the prescribed amount of omeprazole sodium raw material into the liquid mixing tank, add 1500mL of water for injection and stir until completely dissolved, then adjust the pH to 10.5 with 0.05mol / L sodium citrate solution, and add water for injection to the prescribed amount. Add a total amount of 0.05% activated carbon for needles, stir for 15 minutes, and decarbonize by filtration. Finely filter the medicinal solution with a 0.22 μm sterile microporous membrane, and measure the pH value, content, and half stoppering. Finally, freeze-drying: first lower the drug from normal temperature to -22.5°C, keep it warm for 30 minutes, the...
Embodiment 3
[0093] 1. Prescription composition
[0094] 20mg:
[0095] Omeprazole Sodium 22.31g
[0096] Mannitol 100g
[0097] Add water for injection to 1500ml
[0098]
[0099] Makes 1000 bottles
[0100] 2. Preparation method
[0101] Add the prescribed amount of omeprazole sodium and mannitol raw materials into the liquid mixing tank, add 1000mL water for injection and stir until completely dissolved, then adjust the pH value to 10.0 with 0.15mol / L sodium citrate solution, and then add water for injection to 1500mL to obtain a diluted solution, then add 0.05% activated carbon for needles with a weight of 0.05% of the diluted solution weight, stir for 20 minutes, filter and decarbonize to obtain a filtrate. Fine filter the filtrate with a 0.22 μm sterile microporous membrane, and measure the pH value, content, and half stoppering. Finally, freeze-drying: first lower the drug from normal temperature to -22.5°C, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com