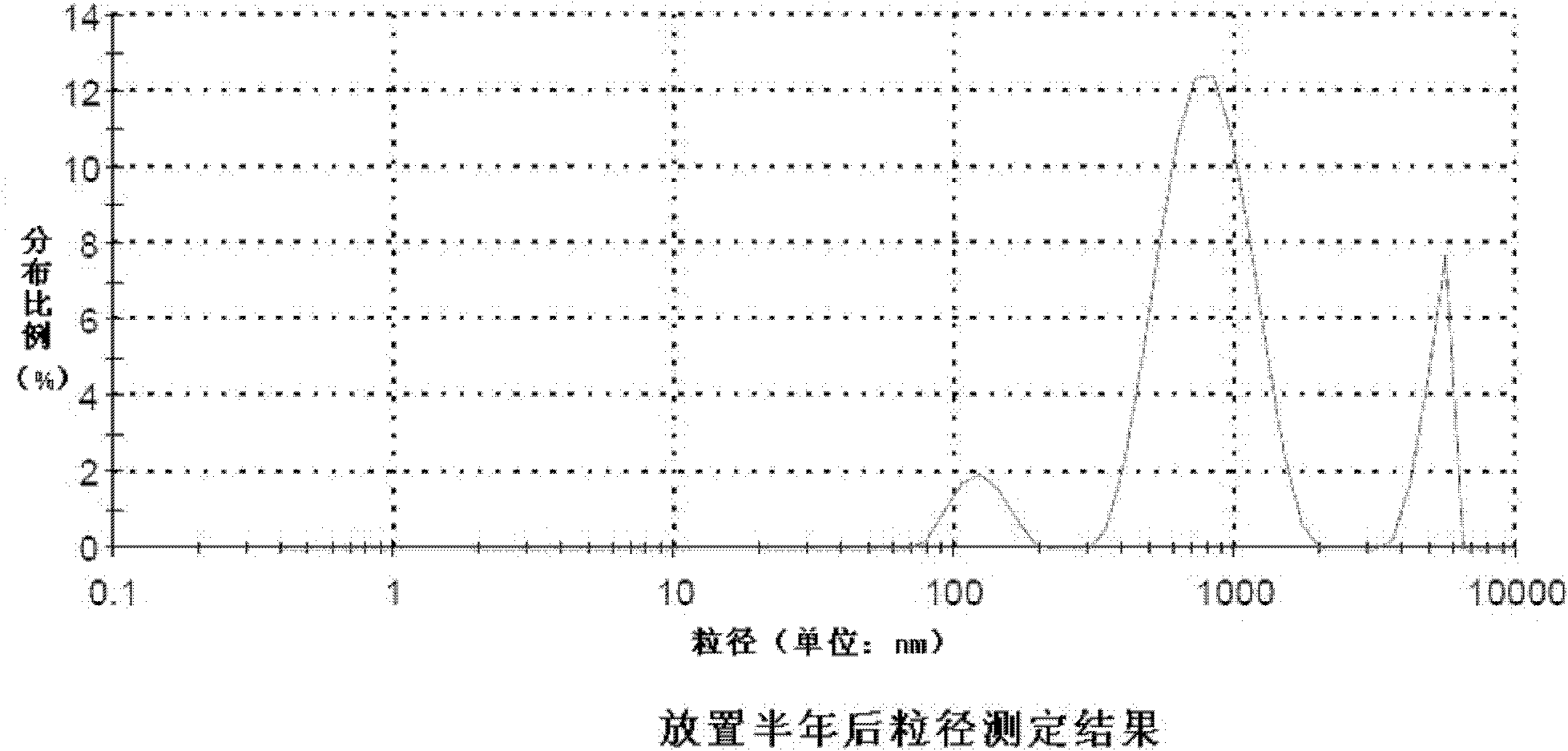
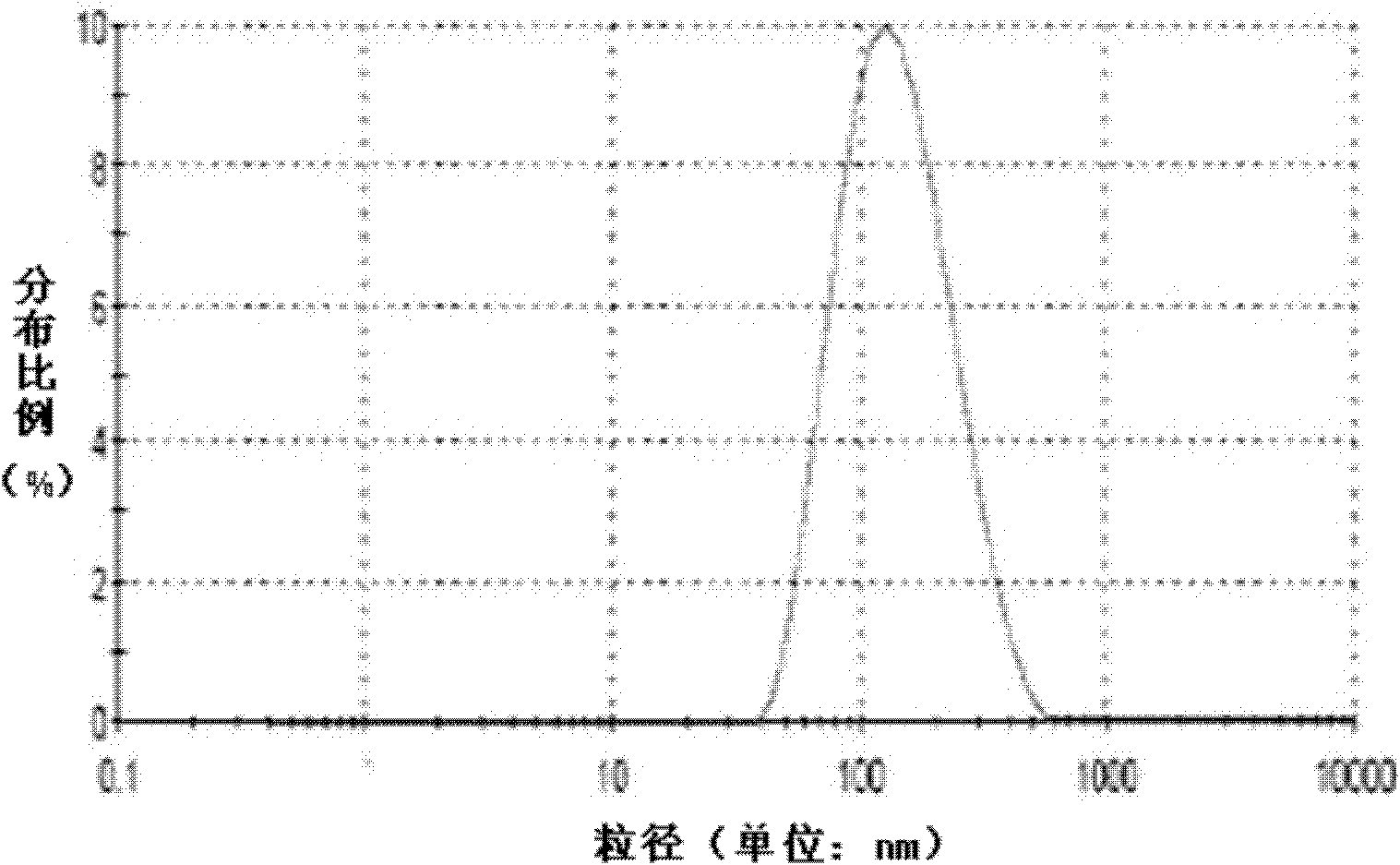
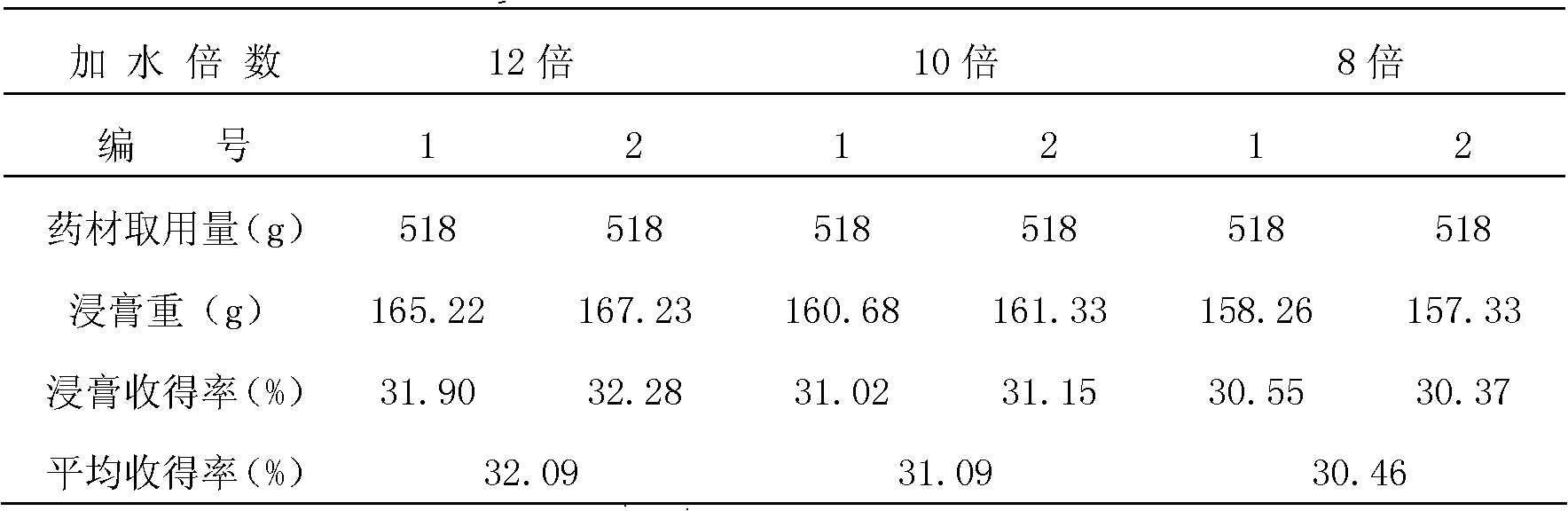
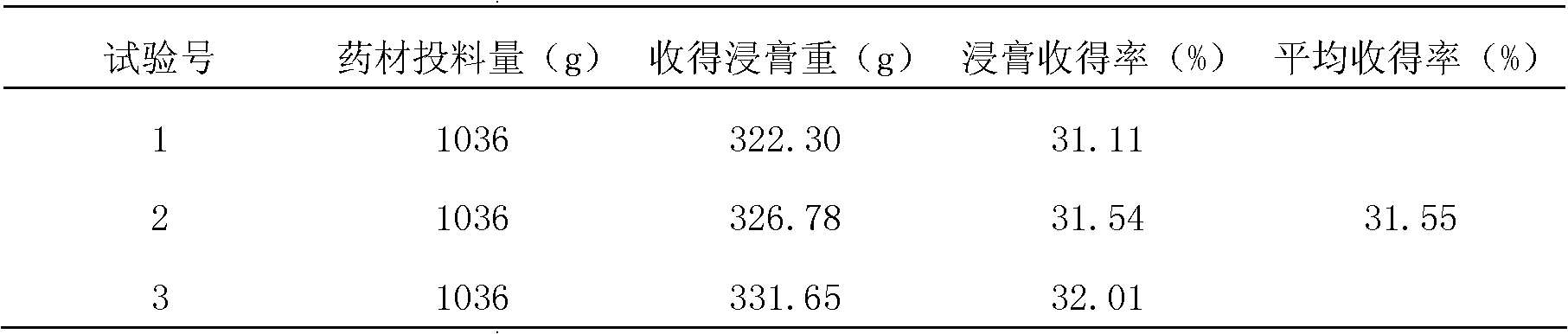
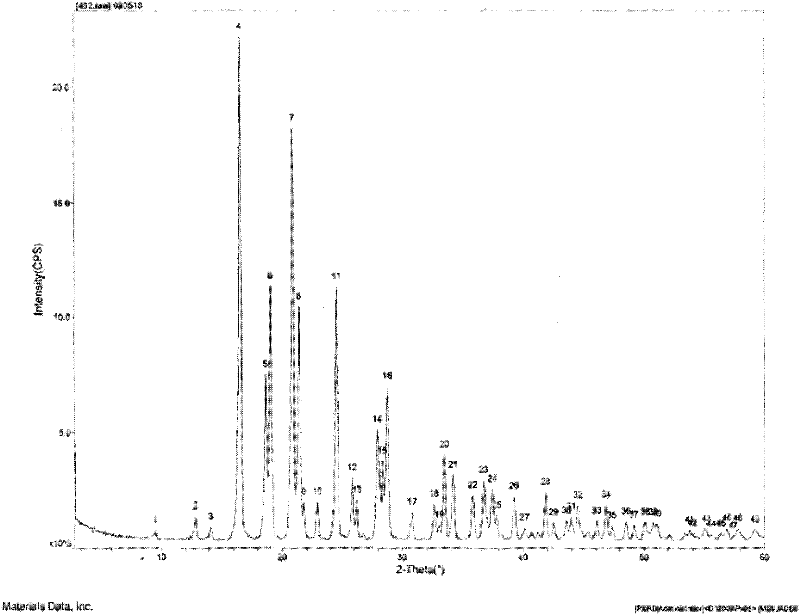
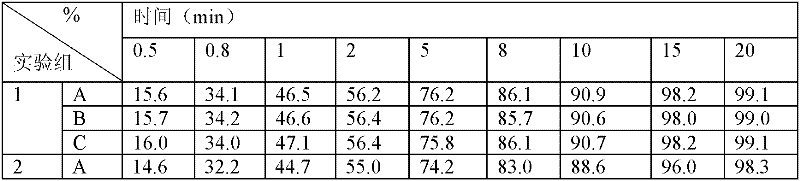
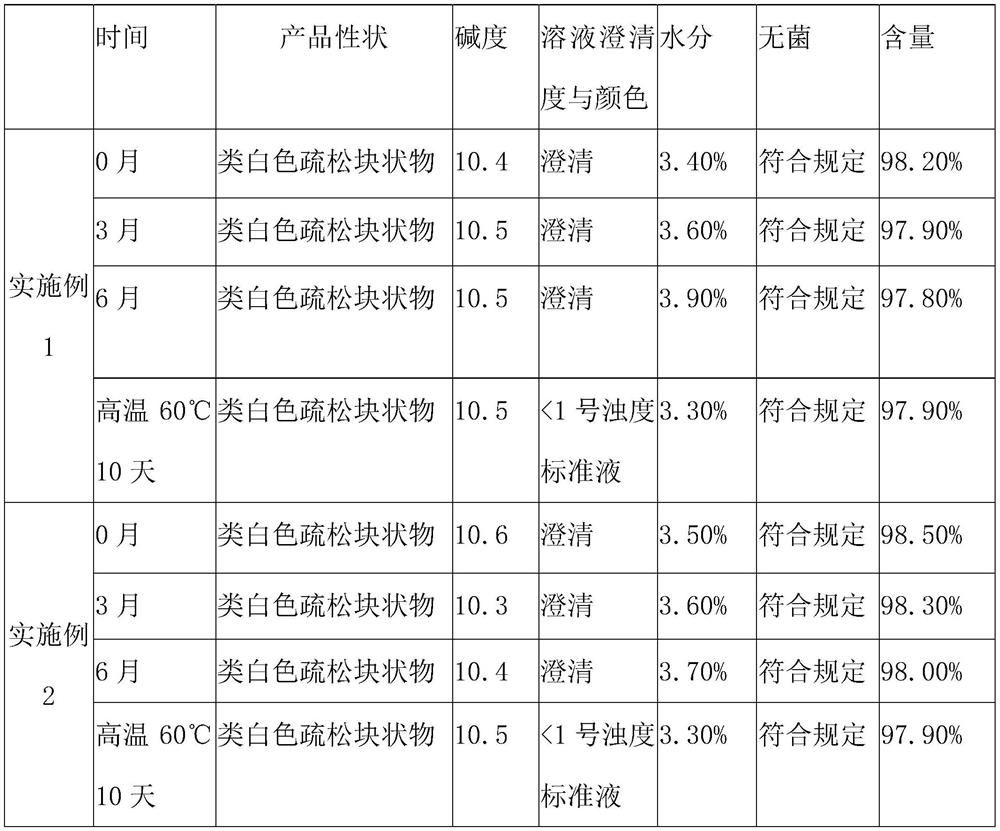
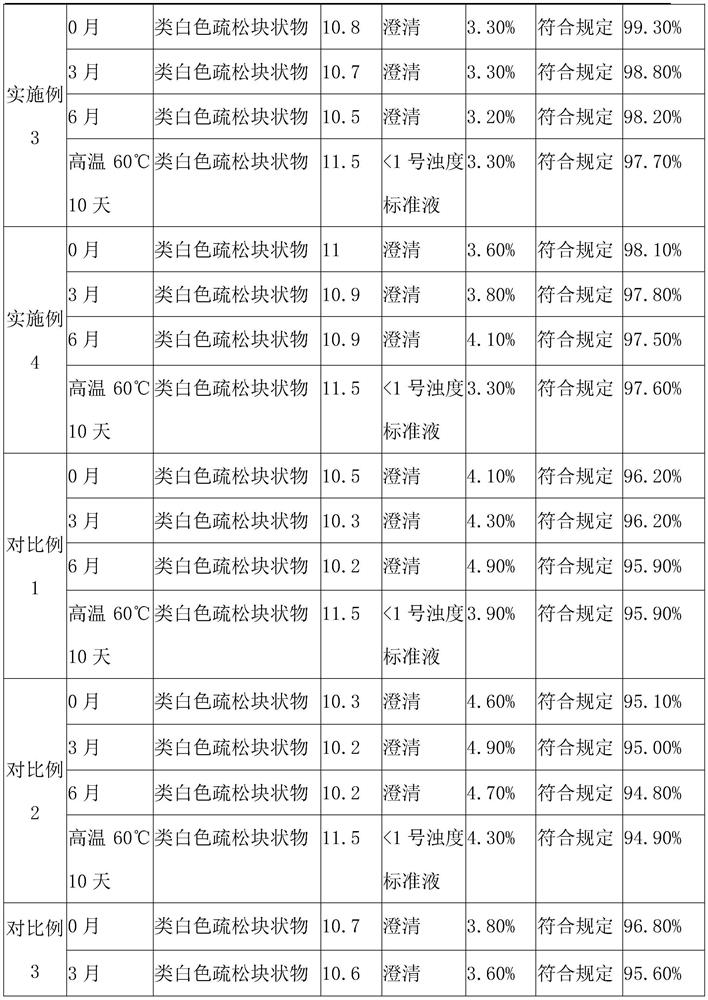
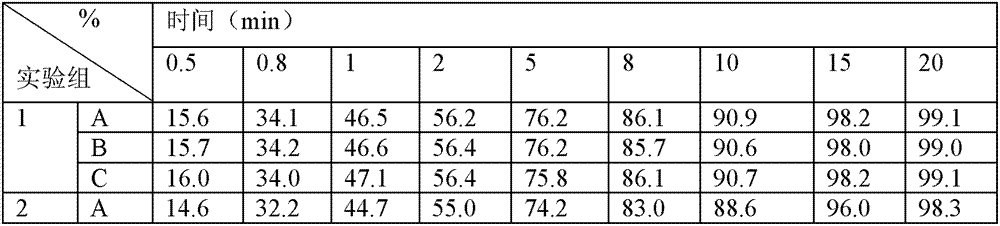
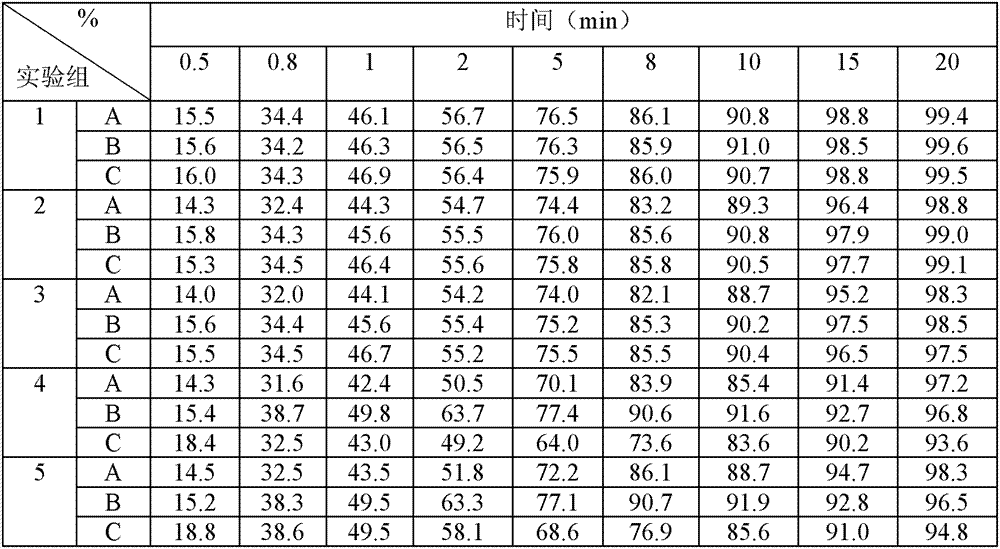
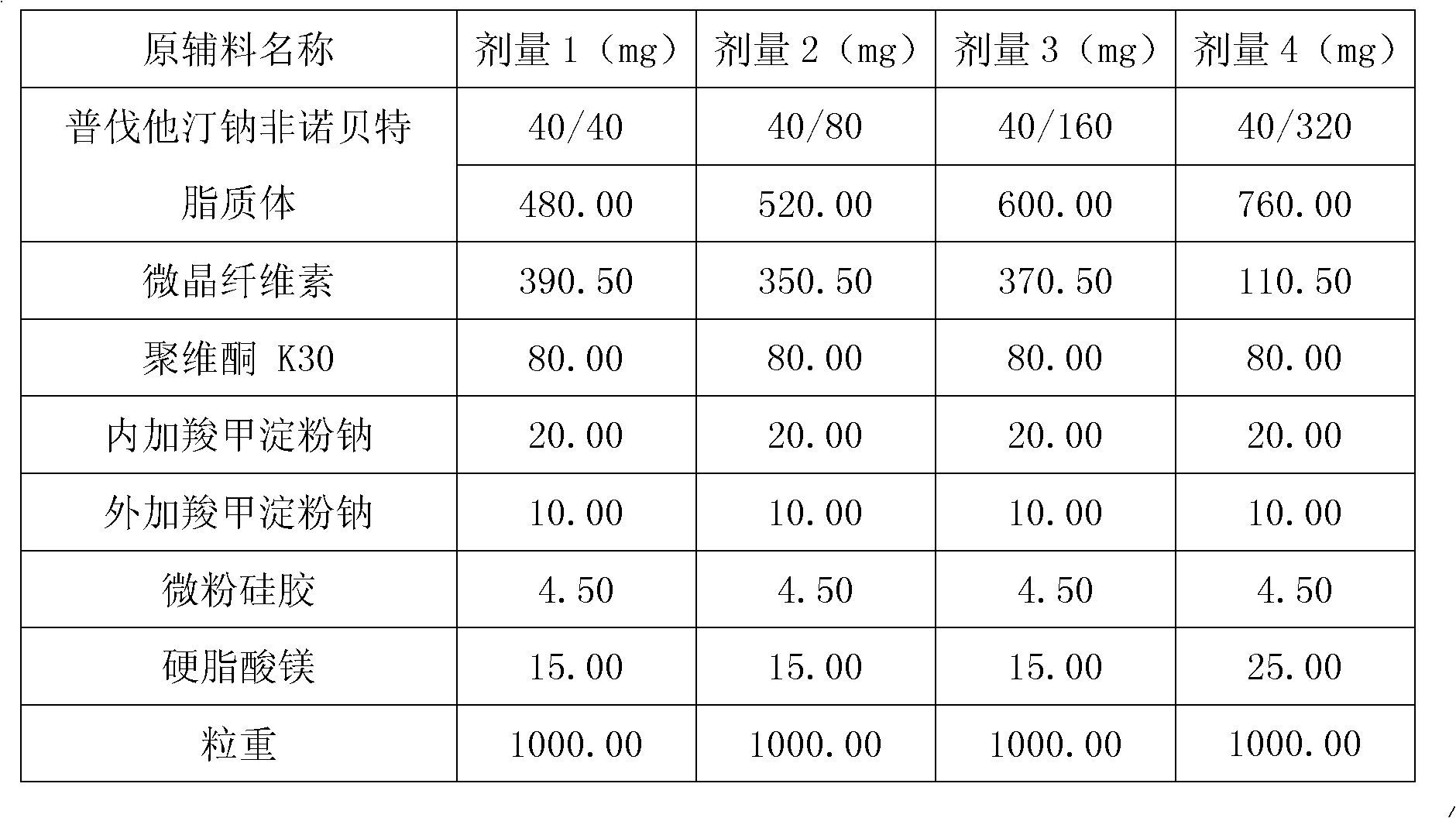Patents
Literature
36results about How to "High content of the main drug" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Brand-new oral solid medicinal composition and preparation method thereof
ActiveCN102335176AGuaranteed curative effectHigh content of the main drugOrganic chemistryCapsule deliveryCross-linkValsartan
The invention discloses a brand-new oral solid medicinal composition. The medicinal composition is an oral preparation prepared from hydrochlorothiazide, levamlodipine, valsartan and pharmaceutically acceptable auxiliaries, and the oral preparation comprises but is not limited to tablets or capsules. The composition comprises the following raw materials in parts by weight: 5-25 parts of the hydrochlorothiazide, 2.5-5 parts of the levamlodipine, 80-160 parts of the valsartan, 40-120 parts of microcrystalline cellulose, 30-90 parts of compressible starch, 5-25 parts of cross-linked sodium carboxymethylcellulose, 3-8 parts of silicon dioxide and 1-2 parts of stearic acid. The medicinal composition disclosed by the invention has the advantages of scientific and reasonable prescription, low auxiliary content and high bioavailability, and is a first choice of medicine for treating hypertension.
Owner:HAINAN JINRUI PHARMA
Coated thioctic acid tablet for treating and controlling diabetic neural lesion
InactiveCN1739501ALess easy to takeEasy to takeOrganic active ingredientsNervous disorderPlasticizerThioctic Acid
The present invention is coated thioctic acid tablet for treating and controlling diabetic neural lesion and belongs to the field of medicine technology. The tablet core consists of thioctic acid 600 mg, stuffing / diluent 150-180 mg, adhesive 20-25 mg, disintegrant 20-25 mg and lubricant 80-90 mg, and has total weight of 889.6-938.2 mg. The coating consists of skeleton / filming agent 10-12 mg, opacifier 5-6 mg, coloring agent 0.1-0.2 mg, surfactant 0.5-1 mg and plasticizer 5-6 mg, and has total weight of 20.10-24.20 mg. The present invention has less excipient for easy orally taking, film to screen light for long preservation period and high medicine content.
Owner:SHANGHAI JIAO TONG UNIV
Pectin-adriamycin coniuncate lyophilized preparation, and preparation method thereof
ActiveCN102232932ASmall particle sizeImprove solubilityOrganic active ingredientsPowder deliveryEnhanced bioavailabilityBiomass
The invention belongs to the field of pharmacy, and specifically relates to a pectin-adriamycin coniuncate lyophilized preparation, and a preparation method thereof. In order to solve the dissolving difficulty of pectin-adriamycin coniuncate (PAC for short), to improve the biomass utilance, and to produce preparations conveniently, the inventor produces PAC into a nano-sized suspension. However, because the long-term stability of the nano-sized suspension is poor, the nano-sized suspension is then produced into a lyophilized preparation. The preparation method comprises the steps that: PAC which is hard to dissolve is prepared into a suspension or a nano-sized suspension; a lyophilizing proppant is added to the suspension or the nano-sized suspension; lyophilization is carried out upon the suspension, and a lyophilized preparation is obtained. When the nano-sized suspension is prepared into the lyophilized preparation, stability of nano-particle size is improved, and stability of drugloading capacity is improved. The invention assists in providing a novel scheme for the clinic application of PAC.
Owner:SICHUAN YINGRUI PHARMA TECH CO
Lipid-lowering dispersible tablets and preparation technology thereof
ActiveCN102319357AKeep active ingredientsImprove extraction efficiencyMetabolism disorderPharmaceutical non-active ingredientsSide effectAdditive ingredient
The invention discloses lipid-lowering dispersible tablets and a preparation technology thereof. The lipid-lowering dispersible tablets comprise the following ingredients: 177-266 weight portions of prepared polygonum multiflorum, 177-266 weight portions of wolfberry, 236-355 weight portions of polygonatum rhizome, 118-178 weight portions of haw, 35-53 weight portions of cassia seed, 88-132 weight portions of sodium carboxy methyl starch, 144-216 weight portions of silica, and 1.6-2.4 weight portions of magnesium stearate. The present invention has the advantage of convenient administration, safety, reliable and fast effect, high bioavailability, capability of being used for a long period, and no toxic and side effect, and is the first choice in hyperlipidemia patients. The invention also has the advantages of simple preparation, low cost, and the like, and is suitable to people of all ages.
Owner:ZHEJIANG WECOME MEDICINE IND
Granule for dredging cardiovascular and cerebrovascular blood vessels and method for preparing same
InactiveCN1883583AHigh content of the main drugSmall dosage formAnthropod material medical ingredientsGranular deliveryDiseaseMedicine
The invention relates to medicinal granules for treating cardio-cerebrovascular diseases, wherein the raw materials include dried medicinal powder, sodium carboxymethylstarch, carmethose, xanthan gum and lactose by a predetermined proportion.
Owner:南京海辰药业股份有限公司
Novel oral solid medicinal composition and preparation method thereof
ActiveCN102342942AReduce dosageSolving Quality Control IssuesOrganic active ingredientsOrganic chemistryCandesartanLevamlodipine
The invention discloses a novel oral solid medicinal composition. The novel oral solid medicinal composition is an oral preparation prepared from hydrochlorothiazide, levamlodipine besylate, candesartan cilexetil and pharmaceutically acceptable auxiliary materials. The novel oral solid medicinal composition can be processed into tablets, capsules and the like. Specifically, the novel oral solid medicinal composition comprises: be weight, 5 to 25 parts of hydrochlorothiazide, 2.5 to 5 parts of levamlodipine besylate, 4 to 20 parts of candesartan cilexetil, 30 to 60 parts of microcrystalline cellulose, 30 to 60 parts of compressible starch, 30 to 50 parts of crosslinked polyvinylpyrrolidone, 1 to 2 parts of silica and 0.5 to 2 parts of magnesium stearate. The novel oral solid medicinal composition has a scientific and reasonable formula, low auxiliary material content and high bioavailability. Therefore, the novel oral solid medicinal composition is a drug of first choice for the treatment of hypertension.
Owner:HAINAN JINRUI PHARMA
Dexibuprofen sustained-release tablet and preparation process thereof
InactiveCN104546732AHigh content of the main drugHigh particle yieldOrganic active ingredientsAntipyreticDexibuprofenTableting
The invention discloses a dexibuprofen sustained-release tablet and a preparation process thereof. According to the preparation process, colloidal silicon dioxide is used as an antiplastering aid, and dry granulation is carried out by using a high-speed rotary tablet press; the prepared dry granules are respectively mixed with auxiliaries in specific quick-release and sustained-release layers to press a dual-layer tablet which is the dexibuprofen sustained-release tablet. The preparation process can be used for effectively reducing the sticking phenomenon caused by temperature increase when tabletting is carried out for a long time, and has simple process and relatively high efficiency; and the prepared sustained-release tablet has high bioavailability and good stability.
Owner:BEIJING HANMI PHARMA CO LTD
Amlodipine besylate medicinal preparation and preparation method thereof
InactiveCN102988317AThere are few varieties to chooseImprove securityOrganic active ingredientsPill deliveryDrug contentDrugs preparations
The invention discloses an amlodipine besylate medicinal preparation and a preparation method thereof. An amlodipine besylate tablet with stable main drug content and high bioavailability is prepared by reasonable matching of main and auxiliary materials. The method disclosed by the invention is simple and convenient in technological operation, low in device requirement, low in production cost, and suitable for industrial production.
Owner:KAMP PHARMA
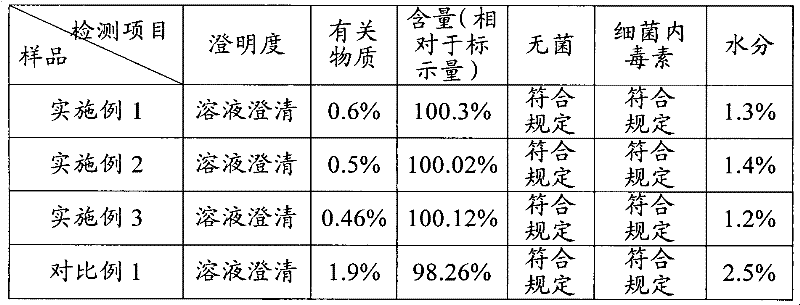
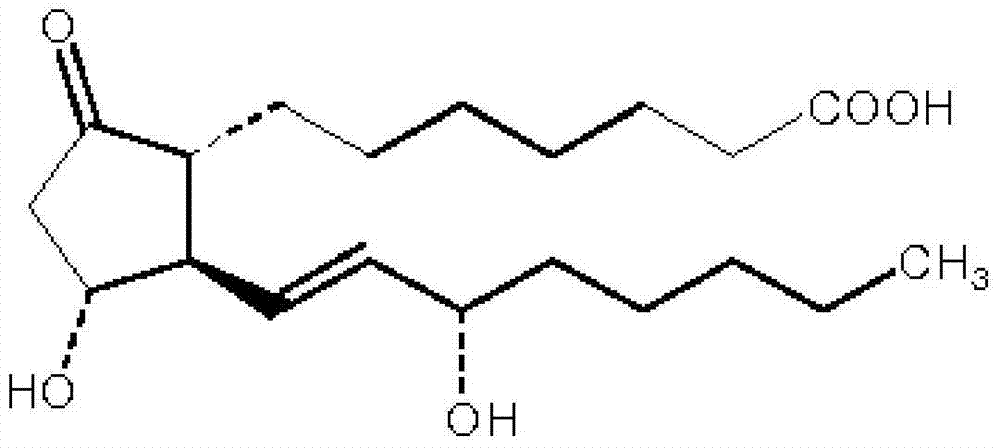
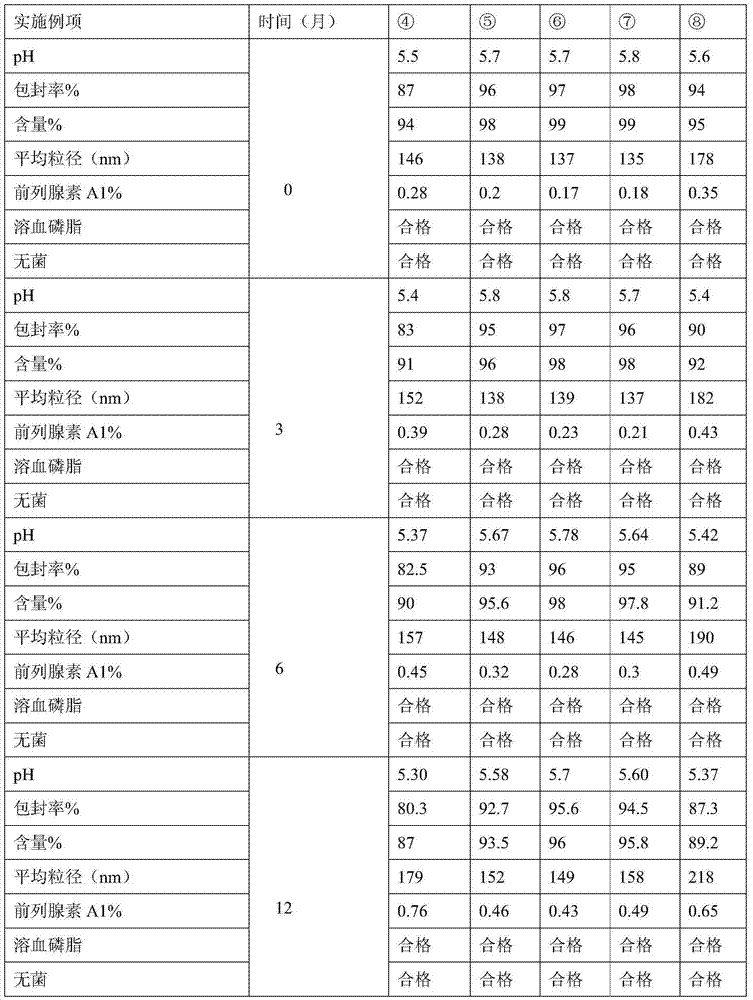
Alprostadil injection and preparation method thereof
ActiveCN103599066AHigh content of the main drugLow related substancesOrganic active ingredientsNervous disorderDrugSOYBEAN SEED OIL
The invention provides an alprostadil injection. Every 1000ml of alprostadil injection comprises the following raw materials: 5mg of alprostadil, 90-110g of soybean oil, 15-20g of phosphatide, 2-3g of oleic acid, 22.1-25g of isoosmotic agent, and appropriate pH modifier, wherein the content of the pH modifier is enough to regulate the pH of the injection to be 5.0-6.0. The invention also provides a preparation method for the alprostadil injection. Compared with the prior art, the alprostadil injection prepared by the method has higher encapsulation efficiency and content of main drug, contains fewer related substances, has good stability, and provides safety guarantee for clinical medication.
Owner:SICHUAN KELUN PHARMA CO LTD
Fully-novel oral solid pharmaceutical composition and preparation method thereof
ActiveCN102327272AHigh content of the main drugEasy to takeOrganic active ingredientsCapsule deliverySodium bicarbonateLevamlodipine
The invention discloses a fully-novel oral solid pharmaceutical composition. The pharmaceutical composition is an oral preparation prepared from hydrochlorothiazide, L-amlodipine, telmisartan and pharmaceutically acceptable auxiliary materials; and the oral preparation includes but is not limited to tablets or capsules. The composition is prepared from the following raw materials in parts by weight: 5-25 parts of hydrochlorothiazide, 2.5-5 parts of L-amlodipine, 20-80 parts of telmisartan, 0.1-0.5 part of sodium bicarbonate, 40-120 parts of microcrystalline cellulose, 20-60 parts of hydroxypropyl cellulose, 30-100 parts of lactose, 30-90 parts of compressible starch, 2-5 parts of low-substituted hydroxypropyl cellulose, 10-45 parts of crosslinked polyvinylpyrrolidone and 1-2 parts of magnesium stearate. The pharmaceutical composition disclosed by the invention has the advantages of reasonable and scientific prescription, low auxiliary material content and high bioavailability, and is a drug of first choice for treating hypertensive.
Owner:HAINAN JINRUI PHARMA
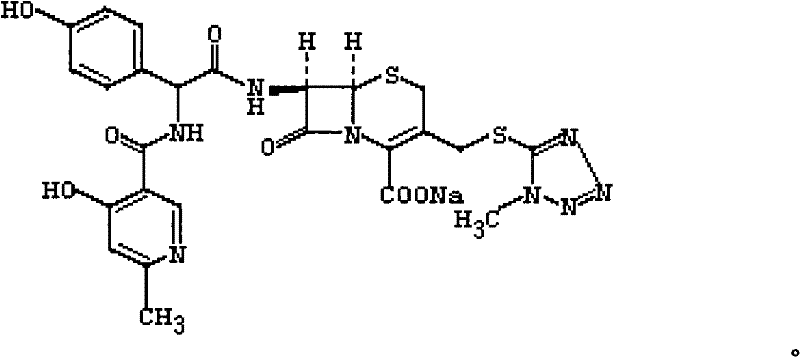
Levofloxacin hydrochloride injection and preparation method thereof
ActiveCN108685846AReduce adsorptionIncrease contentAntibacterial agentsOrganic active ingredientsActivated carbonForeign matter
The invention relates to a preparation method of a levofloxacin hydrochloride injection. The method comprises the following steps: adding glycerin, lipoic acid and / or sodium calcium edetate, and sodium sulfite into water for injection, adding pre-treated activated carbon used for needles in two batches, and decarbonizing to obtain a solution A; adding levofloxacin hydrochloride into the water forinjection, dissolving, then adding the pre-treated activated carbon used for needles, and decarbonizing to obtain a solution B; adding the solution B into the solution A, supplementing the water for injection to a full dose, adjusting the pH to 4.0-4.6, filtering, carrying out ultrafiltration, sampling and inspecting ultrafiltrate, filling after the product is qualified, filling nitrogen, carryingout fused sealing, sterilizing, carrying out lamp inspection after cooling, packaging, and testing to obtain the finished product. The product prepared by the method is high in content of principal agents and good in stability, no visible foreign matter appears in a storage process, and the color of the product does not change.
Owner:JIANGXI GUOYAO PHARMA LLC
Brand-new oral solid pharmaceutical composition and preparation method thereof
ActiveCN102335178AReduce dosageSolving Quality Control IssuesOrganic active ingredientsOrganic chemistryOlmesartanLevamlodipine
The invention discloses a brand-new oral solid pharmaceutical composition. The pharmaceutical composition is an oral preparation prepared from hydrochlorothiazide, l-amlodipine, olmesartan medoxomil and pharmaceutically acceptable auxiliary materials, and the oral preparation comprises but is not limited to tablets or capsules. The composition comprises the following raw materials in parts by weight: 5-25 parts of hydrochlorothiazide, 2.5-5 parts of l-amlodipine, 20-40 parts of olmesartan medoxomil, 40-120 parts of microcrystalline cellulose, 30-90 parts of pregelatinized starch, 15-40 parts of low-substituted hydroxypropyl cellulose, 10-45 parts of crosslinked polyvinylpyrrolidone, 3-8 parts of silica and 1-2 parts of magnesium stearate. The pharmaceutical composition disclosed by the invention has the advantages of scientific and reasonable prescription, low auxiliary material content and high bioavailability, and is a drug of first choice for treating hypertension.
Owner:HAINAN JINRUI PHARMA CO LTD
Glipizide sustained release tablet and preparation method thereof
InactiveCN104739795AReduce the number of dosesImprove complianceMetabolism disorderSulfonylurea active ingredientsSustained Release TabletPharmacology
The invention discloses a glipizide sustained release tablet and a preparation method thereof. The glipizide sustained release tablet comprises 1-10wt% of glipizide, 35-60wt% of a sustained release material, 1-60wt% of a disintegrating agent and 5-10wt% of a lubricant. The glipizide sustained release tablet has the advantages of uniform drug concentration, good stability and high drug concentration through repeatedly proportioning, carrying out a strict preparation process, using a sieve with a specific mesh and strictly controlling the temperature and the tablet weight, so the administration frequency is reduced, and the compliance of patients is enhanced.
Owner:KAMP PHARMA
Omeprazole sodium freeze-dried powder injection and preparation method thereof
PendingCN112807282AHigh content of the main drugRecovery propertiesOrganic active ingredientsPowder deliveryOmeprazole SodiumXylylene
The invention provides an omeprazole sodium freeze-dried powder injection and a preparation method thereof. The preparation method comprises the following steps of: (1) adding sodium xylene sulfonate and sodium hydrogen sulfite into sterile injection water, stirring and dissolving to obtain first mixed solution; (2) adding omeprazole sodium into the sterile injection water, stirring and dissolving at the normal temperature to obtain second mixed solution; (3) adding the first mixed solution obtained in the step (1) into the second mixed solution obtained in the step (2) to obtain total mixed solution, adding the sterile injection water, then adding sodium citrate to adjust the pH value, and carrying out stirring and dissolving, wherein the mass ratio of the total mixed solution to the sterile injection water is 1: (8-12); and (4) filtering the mixed solution prepared in the step (3), pouring the filtered mixed solution into a glass bottle, and freeze-drying to obtain the omeprazole sodium freeze-dried powder injection. The omeprazole sodium freeze-dried powder injection prepared by the invention is clear in solution, good in stability and high in main drug content.
Owner:HAINAN HULUWA PHARMA GRP CO LTD
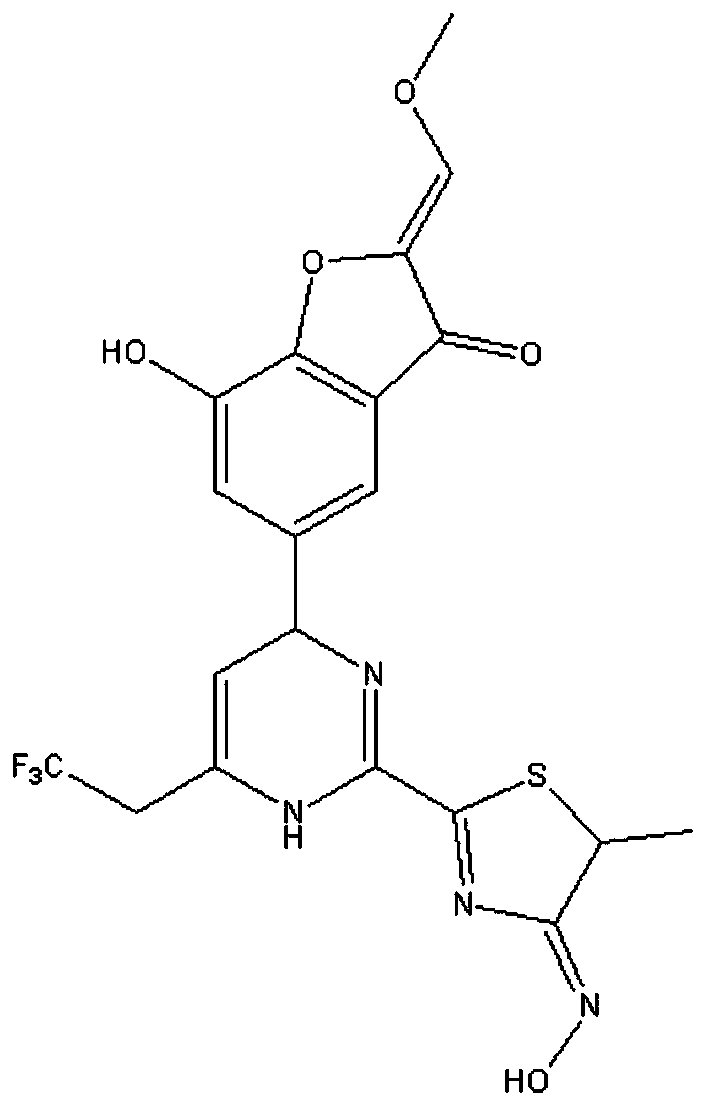
Novel medicine composition for treating hepatitis B virus and preparation method
InactiveCN110354129AThe preparation method is simpleInhibition of replicationOrganic active ingredientsDigestive systemAntigenDual effect
The invention discloses a novel medicine composition for treating the hepatitis B virus and a preparation method. The novel medicine composition comprises a compound shown in a formula I or medicinalsalt of the compound and pharmaceutically acceptable auxiliary materials. The novel medicine composition is prepared into oral tablets, is convenient to take, has the advantages of a high dissolutionrate and a quick effect, has the dual effects of inhibiting the hepatitis B virus, stabilizing illness states, reducing toxicity and enhancing efficacy, has a significant inhibition effect on secretion of S antigens and e antigens of hepatitis B by HepG2.2.15 cells and significant effects of reducing toxicity and enhancing efficacy, and is safe, reliable and beneficial for application and popularization.
Owner:苏州扬厉医药科技有限公司
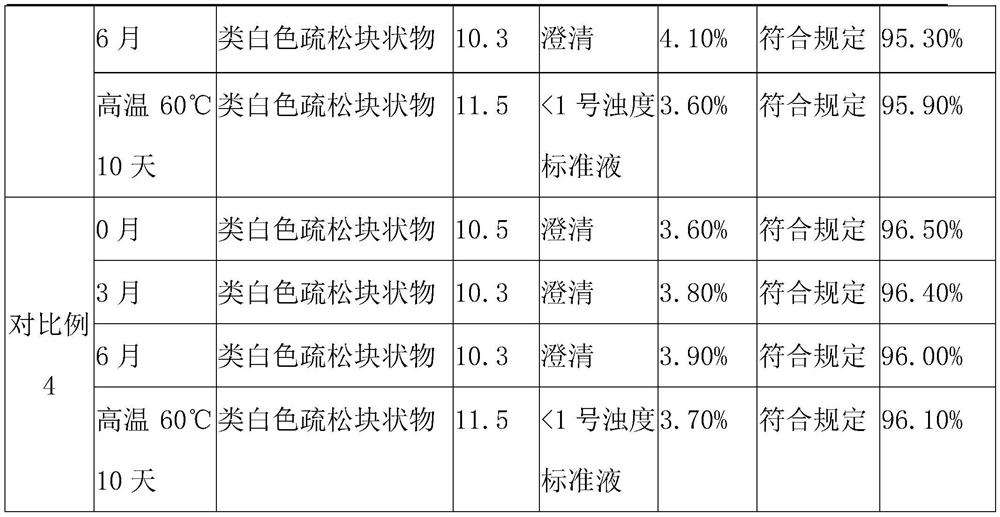
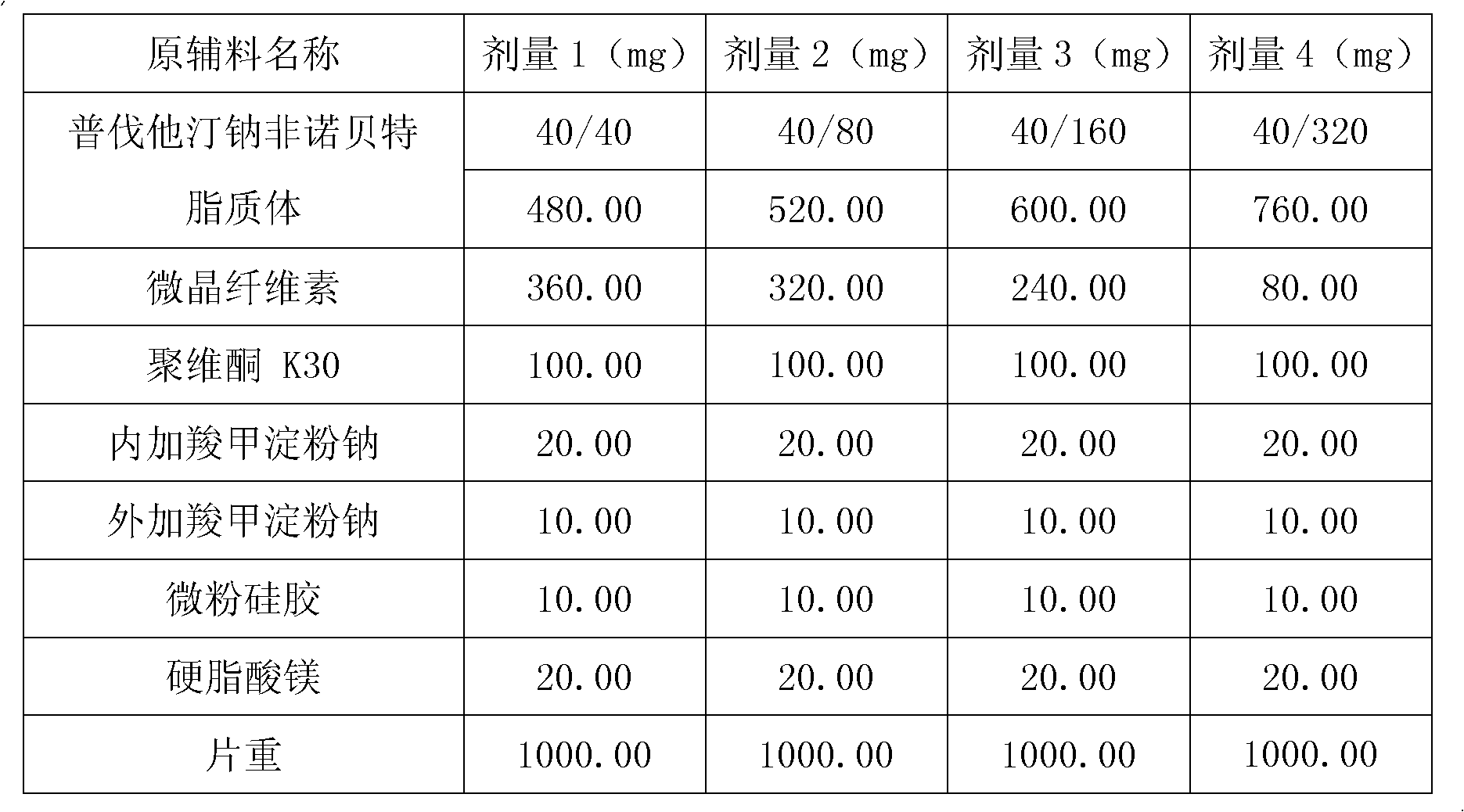
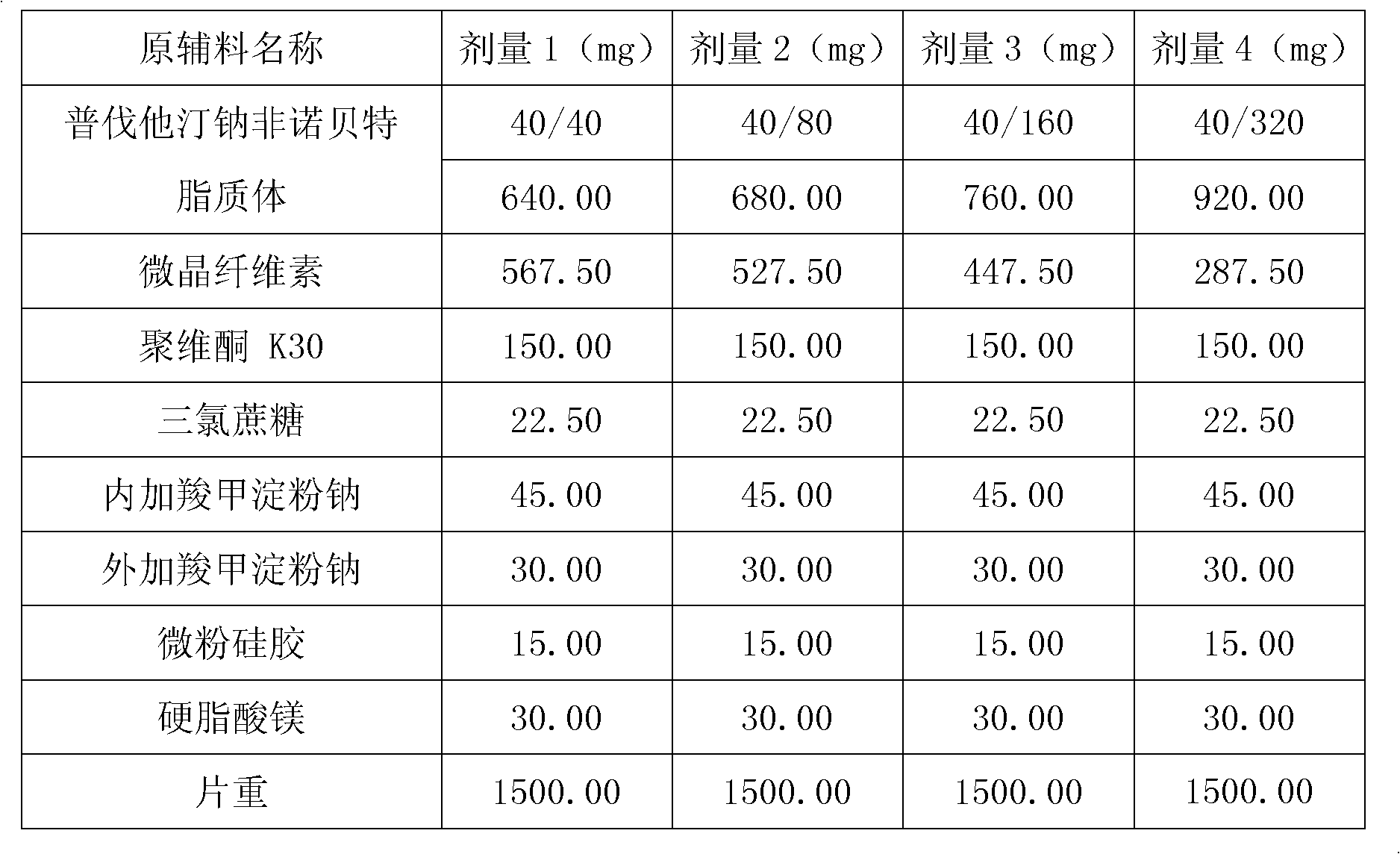
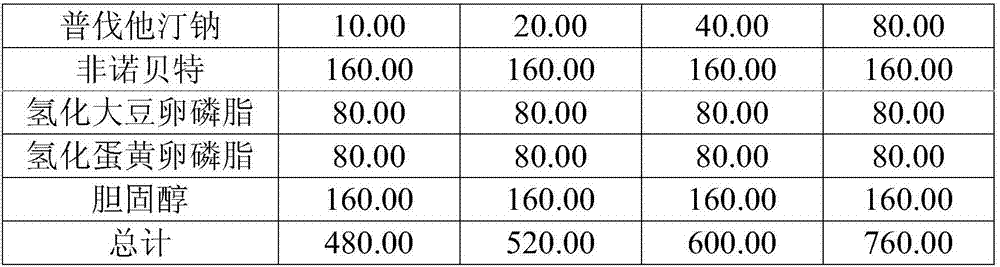
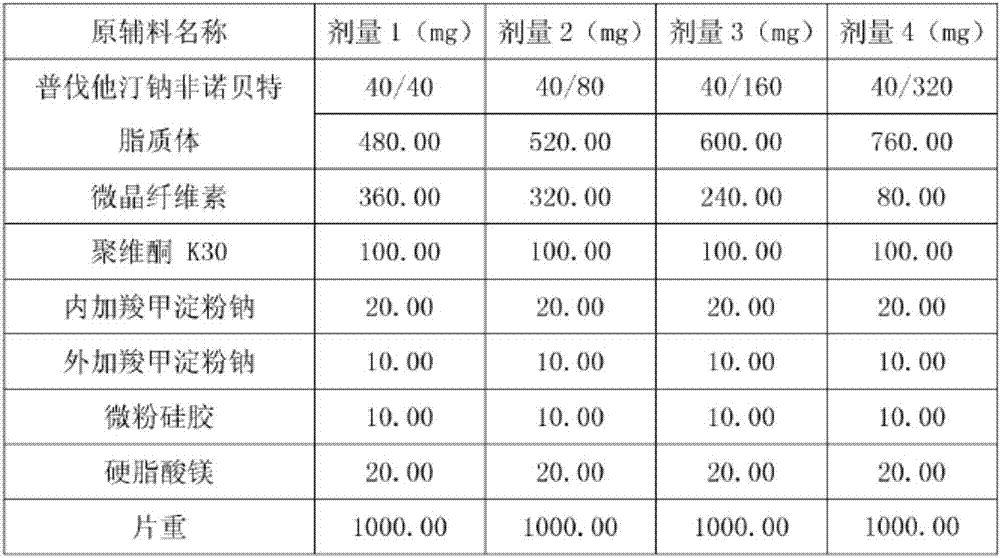
Medicine composition containing pravastatin sodium fenofibrate liposome and preparation method of medicine composition
InactiveCN102552143AHigh content of the main drugHigh dissolution rateMetabolism disorderPharmaceutical non-active ingredientsCholesterolDissolution
The invention relates to a medicine composition containing a pravastatin sodium fenofibrate liposome and a preparation method of the medicine composition. The medicine composition comprises a pravastatin sodium fenofibrate liposome and at least one pharmaceutically acceptable vector, wherein the pravastatin sodium fenofibrate liposome comprises pravastatin sodium, fenofibrate, phospholipid and cholesterol. The medicine composition prepared by the pravastatin sodium fenofibrate liposome not only satisfies the requirements of Chinese Pharmacopoeia and has the advantages of faster dissolution, faster medicine efficacy, high bioavailability, good stability as well as worth of being widely promoted and applied when being compared with the common pravastatin sodium fenofibrate medicine composition.
Owner:海南欣莱医药科技股份有限公司
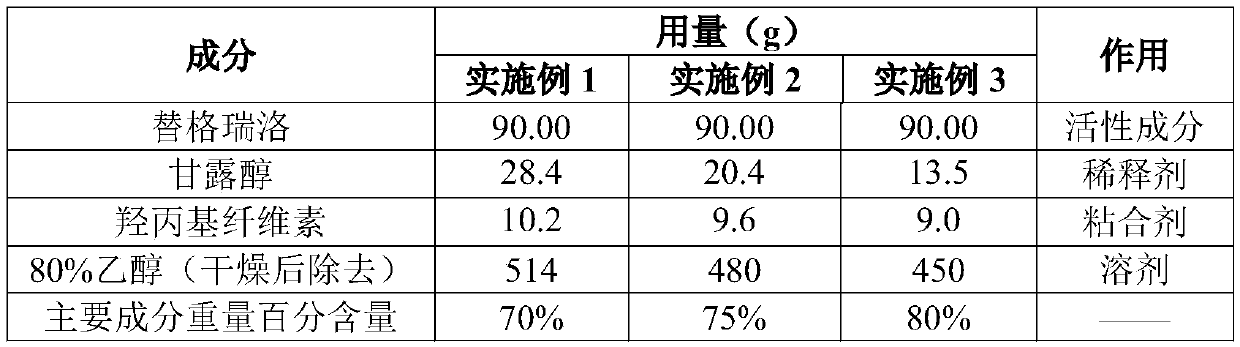
Ticagrelor preparation composition and preparation method thereof
ActiveCN109700773AImprove liquidityImprove solubilityOrganic active ingredientsPill deliveryMedicineTicagrelor
The invention provides a ticagrelor preparation composition. The composition is prepared from the following components in percentage by weight: 25-35 percent of ticagrelor granules, 60-70 percent of filler, 3 percent of a disintegrating agent and 2 percent of a lubricating agent. The ticagrelor granules are prepared by a spray-drying method, the content of main drugs in the granules can be increased, and the tablet weight can be reduced to improve the compliance of patients on the one hand; and on the other hand, the ticagrelor granules are prepared by the spray-drying method, the process is smooth, and the material mobility is good, and the process feasibility during high-dose drug loading is improved.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD
Brand-new oral solid pharmaceutical composition and preparation method thereof
ActiveCN102335177AHigh content of the main drugEasy to takeOrganic active ingredientsOrganic chemistryLevamlodipineIrbesartan
The invention discloses a brand-new oral solid pharmaceutical composition. The pharmaceutical composition is an oral preparation prepared from hydrochlorothiazide, l-amlodipine, irbesartan and pharmaceutically acceptable auxiliary materials, and the oral preparation comprises but is not limited to tablets or capsules. The composition comprises the following raw materials in parts by weight: 5-25 parts of hydrochlorothiazide, 2.5-5 parts of l-amlodipine, 75-350 parts of irbesartan, 30-100 parts of lactose, 10-80 parts of microcrystalline cellulose, 10-80 parts of pregelatinized starch, 5-25 parts of croscarmellose sodium, 1.5-6 parts of hydroxypropyl methylcellulose, 0.5-5 parts of silica and 1-5 parts of magnesium stearate. The pharmaceutical composition disclosed by the invention has theadvantages of scientific and reasonable prescription, low auxiliary material content and high bioavailability, and is a drug of first choice for treating hypertension.
Owner:HAINAN JINRUI PHARMA
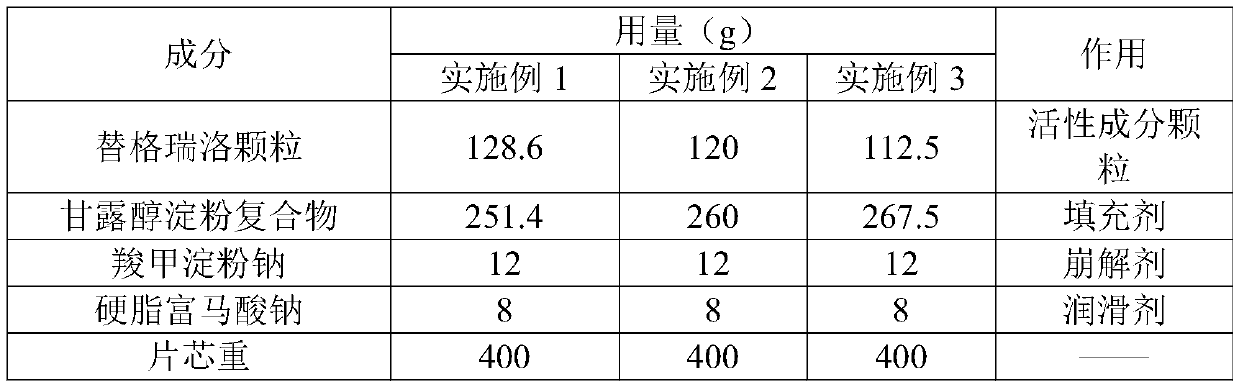
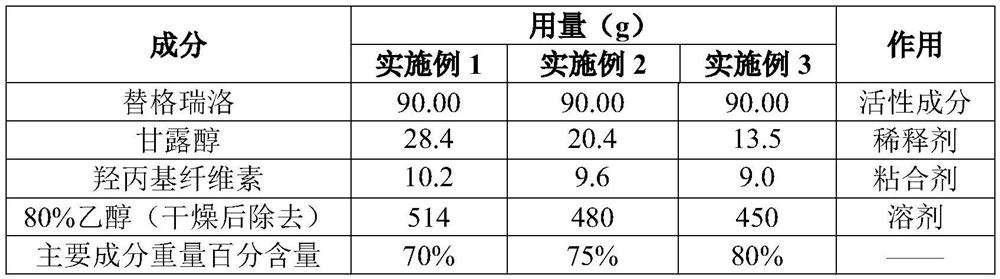
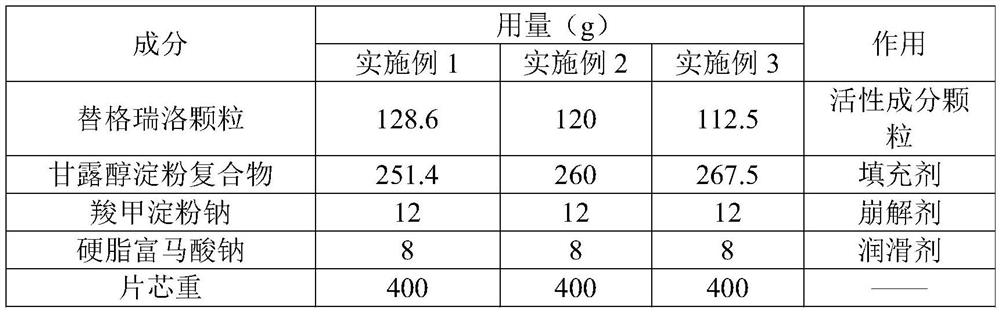
A kind of ticagrelor preparation composition and preparation method thereof
ActiveCN109700773BReduce liquidityFluffy appearanceOrganic active ingredientsPharmaceutical non-active ingredientsPatient complianceTicagrelor
A ticagrelor preparation composition, which comprises ticagrelor granules, fillers, disintegrants and lubricants, wherein the ticagrelor granules account for 25 to 35% by weight of the ticagrelor preparation composition %, the filler accounts for 60 to 70% by weight of the ticagrelor preparation composition, the disintegrant accounts for 3% by weight of the ticagrelor preparation composition, and the lubricant accounts for 60% by weight of the ticagrelor preparation composition. The percentage by weight of the composition of the rello preparation is 2%. The present invention prepares ticagrelor granules by spray drying, on the one hand, it can increase the main drug content in the granules, reduce the tablet weight to increase patient compliance; on the other hand, prepare ticagrelor granules by spray drying, The process is smoother and the fluidity of the material is better, which improves the feasibility of the process when the drug is loaded at a high dose.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD
A new oral solid pharmaceutical composition and its preparation method
ActiveCN102342942BHigh content of the main drugEasy to takeOrganic active ingredientsOrganic chemistryCaplet Dosage FormLevamlodipine
Owner:HAINAN JINRUI PHARMA CO LTD
Brand new oral solid medicinal composition and its preparation method
InactiveCN102342943AHigh content of the main drugEasy to takeOrganic active ingredientsOrganic chemistryLevamlodipineSilicon dioxide
The invention discloses a brand new oral solid medicinal composition and its preparation method, the medicinal composition is an oral preparation prepared by hydrochlorothiazide, levamlodipine, losartan potassium and pharmaceutically-accepted auxiliary materials, the oral preparation is including but not limited to tablets or capsules. The composition comprises the following raw materials by weight part: 5-25 parts of hydrochlorothiazide, 2.5-5 parts of levamlodipine, 20-120 parts of losartan potassium, 30-90 parts of lactose, 20-60 parts of microcrystalline cellulose, 20-60 parts of pregelatinized starch, 2-5 parts of hydroxypropylcellulose, 1-3 parts of silica and 1-2 parts of magnesium stearate. The medicinal composition has the advantages of scientific and reasonable prescription, lowauxiliary materials content and high biological availability, and is a preferred medicine for treating hypertension.
Owner:HAINAN JINRUI PHARMA CO LTD
Pharmaceutical composition containing pravastatin sodium fenofibrate liposome and preparation method thereof
InactiveCN102552143BHigh content of the main drugHigh dissolution rateMetabolism disorderPharmaceutical non-active ingredientsPharmacologyPravastatin Sodium
Owner:海南欣莱医药科技股份有限公司
Oral solid pharmaceutical composition and preparation method thereof
ActiveCN102335178BHigh content of the main drugEasy to takeOrganic active ingredientsOrganic chemistryAMLODIPINE/OLMESARTANLow-substituted hydroxypropylcellulose
The invention discloses a brand-new oral solid pharmaceutical composition. The pharmaceutical composition is an oral preparation prepared from hydrochlorothiazide, l-amlodipine, olmesartan medoxomil and pharmaceutically acceptable auxiliary materials, and the oral preparation comprises but is not limited to tablets or capsules. The composition comprises the following raw materials in parts by weight: 5-25 parts of hydrochlorothiazide, 2.5-5 parts of l-amlodipine, 20-40 parts of olmesartan medoxomil, 40-120 parts of microcrystalline cellulose, 30-90 parts of pregelatinized starch, 15-40 parts of low-substituted hydroxypropyl cellulose, 10-45 parts of crosslinked polyvinylpyrrolidone, 3-8 parts of silica and 1-2 parts of magnesium stearate. The pharmaceutical composition disclosed by the invention has the advantages of scientific and reasonable prescription, low auxiliary material content and high bioavailability, and is a drug of first choice for treating hypertension.
Owner:HAINAN JINRUI PHARMA CO LTD
Brand-new oral solid pharmaceutical composition and preparation method thereof
ActiveCN102335177BHigh content of the main drugEasy to takeOrganic active ingredientsOrganic chemistryCaplet Dosage FormIrbesartan
The invention discloses a brand-new oral solid pharmaceutical composition. The pharmaceutical composition is an oral preparation prepared from hydrochlorothiazide, l-amlodipine, irbesartan and pharmaceutically acceptable auxiliary materials, and the oral preparation comprises but is not limited to tablets or capsules. The composition comprises the following raw materials in parts by weight: 5-25 parts of hydrochlorothiazide, 2.5-5 parts of l-amlodipine, 75-350 parts of irbesartan, 30-100 parts of lactose, 10-80 parts of microcrystalline cellulose, 10-80 parts of pregelatinized starch, 5-25 parts of croscarmellose sodium, 1.5-6 parts of hydroxypropyl methylcellulose, 0.5-5 parts of silica and 1-5 parts of magnesium stearate. The pharmaceutical composition disclosed by the invention has theadvantages of scientific and reasonable prescription, low auxiliary material content and high bioavailability, and is a drug of first choice for treating hypertension.
Owner:HAINAN JINRUI PHARMA CO LTD
Cefpiramide sodium powder injection and preparation method thereof
ActiveCN101849916BFull appearanceUniform appearanceAntibacterial agentsPowder deliverySodium bicarbonateFreeze-drying
Owner:HAINAN HULUWA PHARMA GRP CO LTD
Alprostadil injection and preparation method thereof
ActiveCN103599066BHigh encapsulation efficiencyHigh content of the main drugOrganic active ingredientsNervous disorderMedicineOleic Acid Triglyceride
The invention provides an alprostadil injection. Every 1000ml of alprostadil injection comprises the following raw materials: 5mg of alprostadil, 90-110g of soybean oil, 15-20g of phosphatide, 2-3g of oleic acid, 22.1-25g of isoosmotic agent, and appropriate pH modifier, wherein the content of the pH modifier is enough to regulate the pH of the injection to be 5.0-6.0. The invention also provides a preparation method for the alprostadil injection. Compared with the prior art, the alprostadil injection prepared by the method has higher encapsulation efficiency and content of main drug, contains fewer related substances, has good stability, and provides safety guarantee for clinical medication.
Owner:SICHUAN KELUN PHARMA CO LTD
Lipid-lowering dispersible tablets and preparation technology thereof
ActiveCN102319357BKeep active ingredientsImprove extraction efficiencyMetabolism disorderPharmaceutical non-active ingredientsBiotechnologyCassia
The invention discloses lipid-lowering dispersible tablets and a preparation technology thereof. The lipid-lowering dispersible tablets comprise the following ingredients: 177-266 weight portions of prepared polygonum multiflorum, 177-266 weight portions of wolfberry, 236-355 weight portions of polygonatum rhizome, 118-178 weight portions of haw, 35-53 weight portions of cassia seed, 88-132 weight portions of sodium carboxy methyl starch, 144-216 weight portions of silica, and 1.6-2.4 weight portions of magnesium stearate. The present invention has the advantage of convenient administration, safety, reliable and fast effect, high bioavailability, capability of being used for a long period, and no toxic and side effect, and is the first choice in hyperlipidemia patients. The invention alsohas the advantages of simple preparation, low cost, and the like, and is suitable to people of all ages.
Owner:ZHEJIANG WECOME MEDICINE IND
Conjugate and application thereof in treating inner ear diseases
ActiveCN114848836AImprove delivery efficiencyAchieve targetedOrganic active ingredientsSenses disorderDiseaseInner ear structure
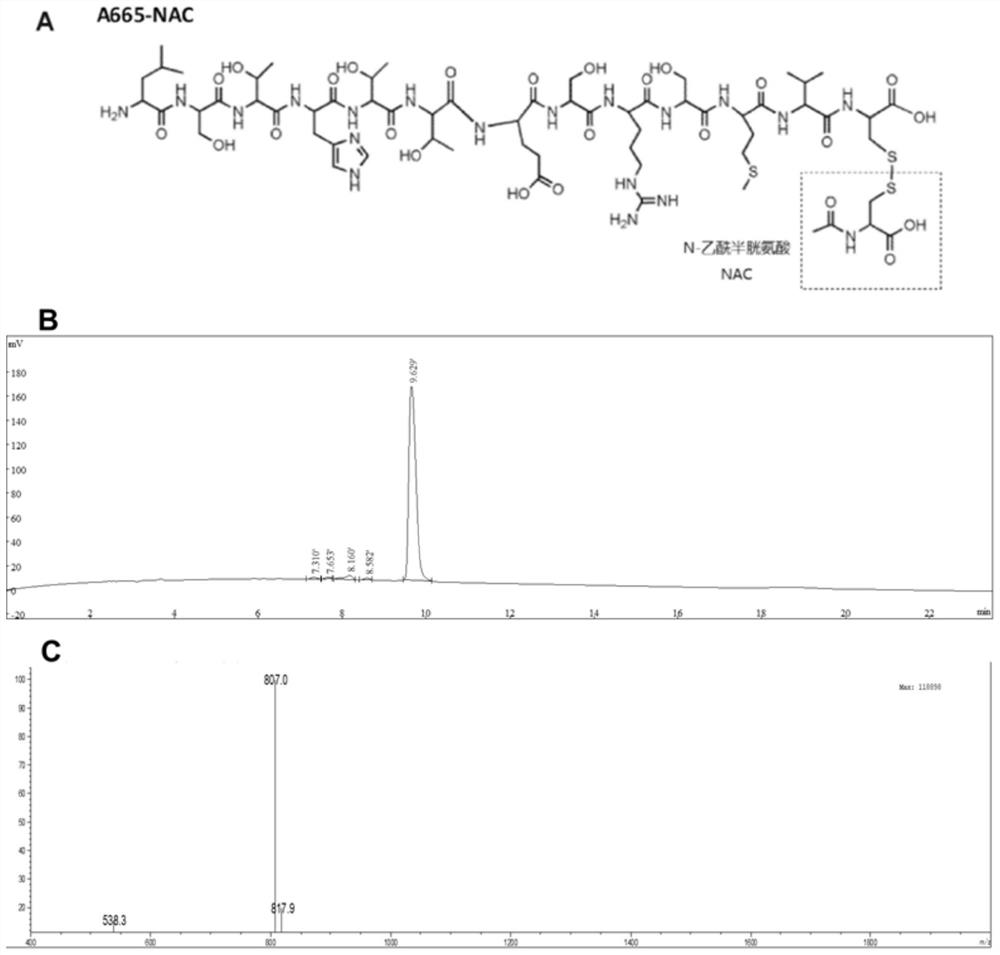
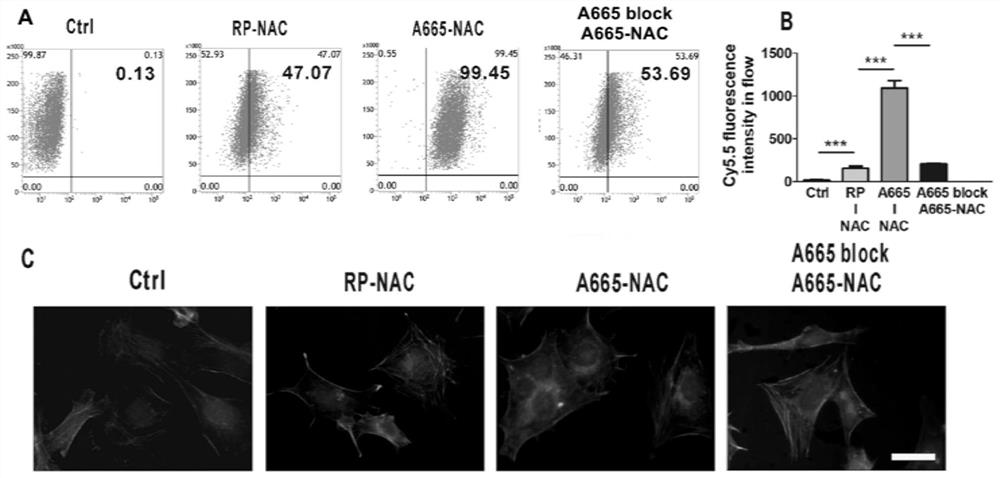
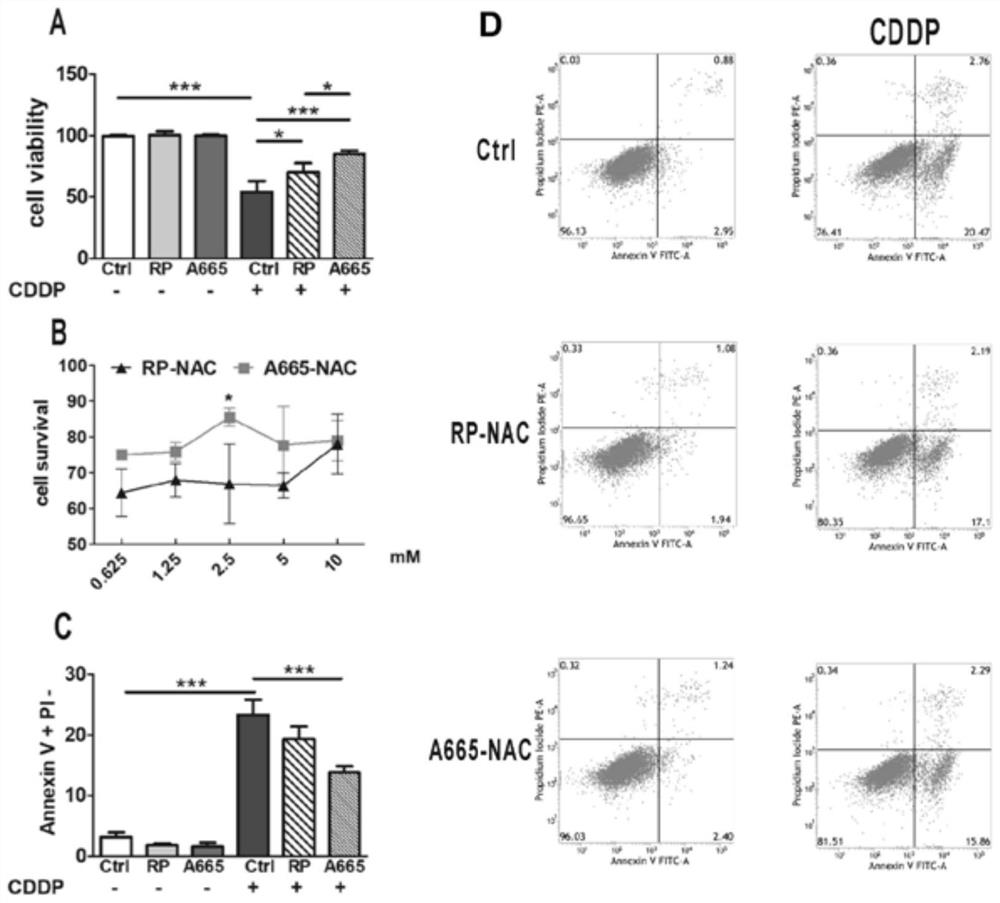
The invention discloses a conjugate and application thereof in inner ear diseases, the conjugate is formed by combining an inner ear targeting polypeptide and a main drug for diagnosing, preventing or treating the inner ear diseases, so that the main drug is assisted to target an inner ear or a specific structure and cells in the inner ear by virtue of the inner ear targeting polypeptide, the enrichment of the main drug is realized, and the inner ear diseases are prevented and treated. Therefore, targeted inner ear structures or cells can be accurately acted on; the efficiency of transporting the medicine to the inner ear can be improved through the inner ear targeting polypeptide, so that the content of the medicine reaching the inner ear is remarkably improved, and the inner ear bioavailability of the medicine is improved. The invention further provides application of the conjugate to the inner ear diseases, and diagnosis, prevention or treatment of the inner ear diseases can be conveniently achieved through the main medicine in the conjugate. More specifically, the delivery and medicinal process of the conjugate targeting inner ear outer hair cells is realized by locally delivering the conjugate A665-NAC. When the conjugate is applied to an inner ear acted by cis-platinum, hearing loss caused by cis-platinum can be reduced, and the cis-platinum ear toxicity prevention and treatment effects are achieved.
Owner:SUN YAT SEN MEMORIAL HOSPITAL SUN YAT SEN UNIV
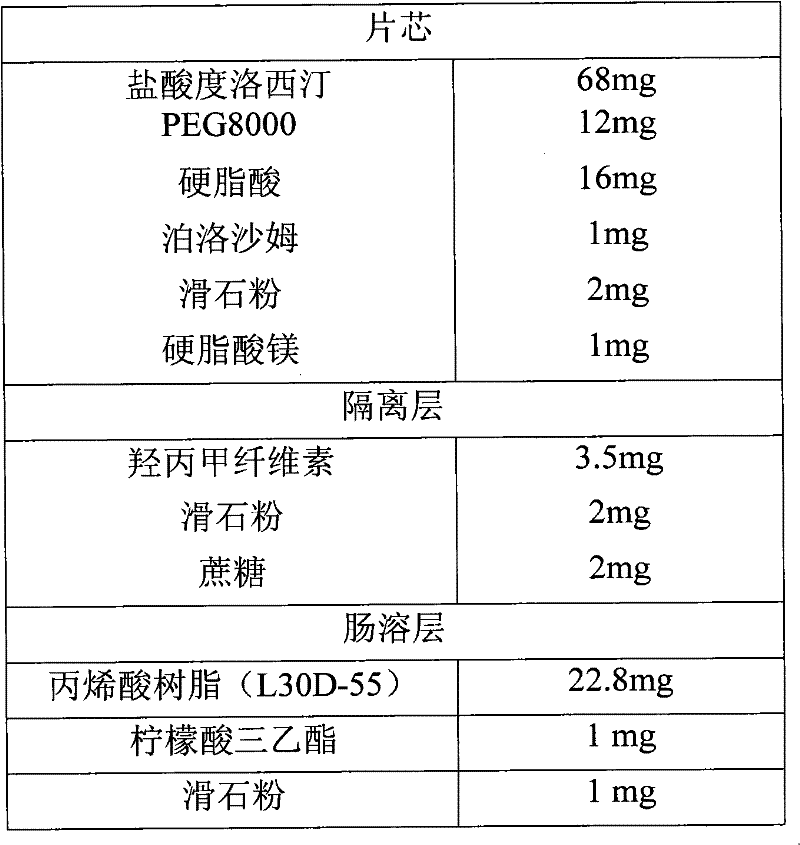
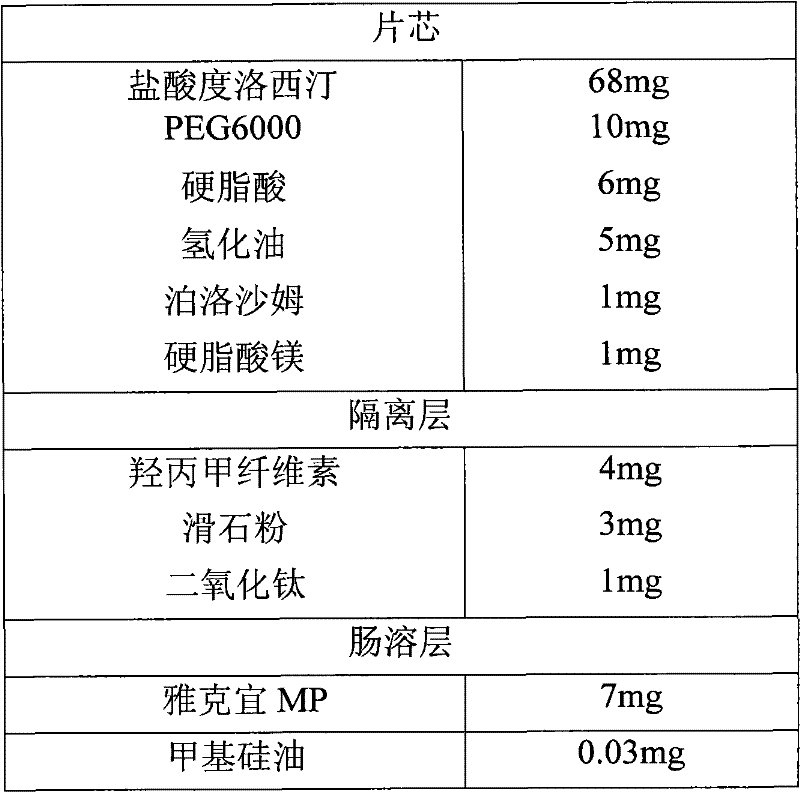
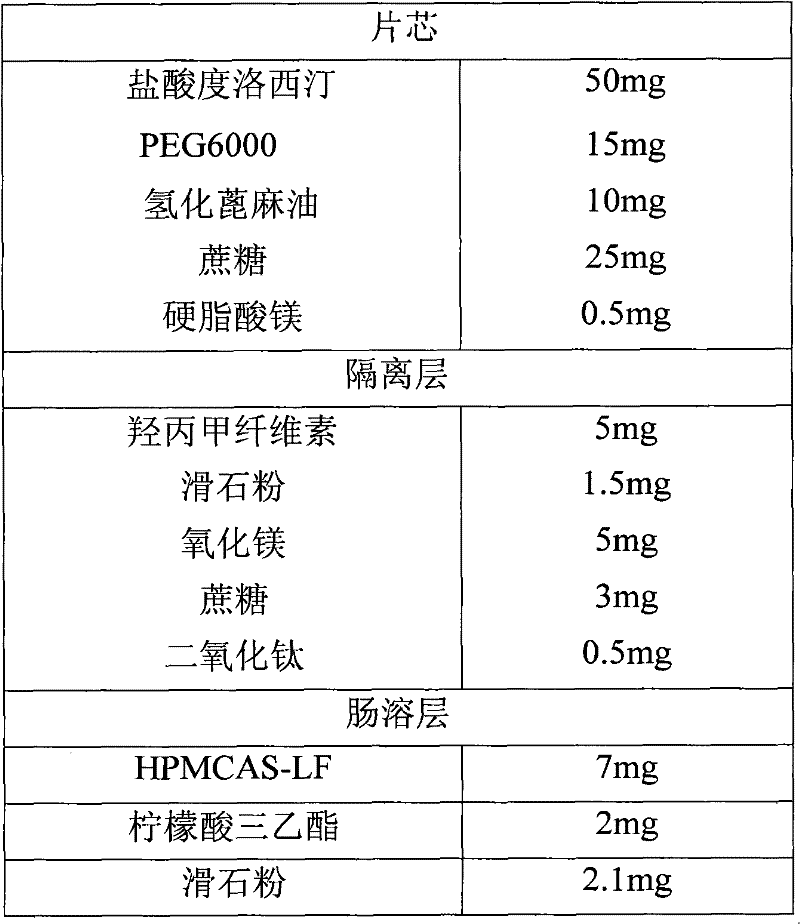
A sustained-release enteric-coated preparation of duloxetine, core material and preparation method thereof
ActiveCN101766579BReduce degradationAdjust the dissolution rateOrganic active ingredientsNervous disorderDuloxetineOrganic solvent
The invention discloses a Duloxetine nteric-coated sustained release preparation as well as a core material and a preparation method thereof. The core material consists of Duloxetine as active medicinal ingredient or salts thereof and pharmaceutically acceptable auxiliary, wherein the pharmaceutically acceptable auxiliary contains a hot-melt material which at least contains a water-soluble hot-melt material and a hydrophobic hot-melt material, and the content of the hot-melt material is 20%-35%, the content of Duloxetine or salts thereof is 50%-75%, the rest is other pharmaceutically acceptable auxiliary the percentage of which is the mass percentage thereof based on the total amount of the core material. According to the invention, the core material of the Duloxetine nteric-coated sustained release preparation is prepared by adopting hot-melt technology, which avoids the introduction of water or organic solvents during preparation, lessens the degradation of principal and residual solvent, achieves a proper delay on the release of Duloxetine in intestines, namely 4-hour release amount in phosphate buffer with 6.8 of pH accounting for over 70% of the total amount, and is favorable for reducing adverse reaction of patients.
Owner:SHANGHAI ZHONGXI PHARMA
Levofloxacin hydrochloride injection and preparation method thereof
ActiveCN108685846BReduce adsorptionIncrease contentAntibacterial agentsOrganic active ingredientsSulfite saltUltrafiltration
The invention relates to a preparation method of a levofloxacin hydrochloride injection. The method comprises the following steps: adding glycerin, lipoic acid and / or sodium calcium edetate, and sodium sulfite into water for injection, adding pre-treated activated carbon used for needles in two batches, and decarbonizing to obtain a solution A; adding levofloxacin hydrochloride into the water forinjection, dissolving, then adding the pre-treated activated carbon used for needles, and decarbonizing to obtain a solution B; adding the solution B into the solution A, supplementing the water for injection to a full dose, adjusting the pH to 4.0-4.6, filtering, carrying out ultrafiltration, sampling and inspecting ultrafiltrate, filling after the product is qualified, filling nitrogen, carryingout fused sealing, sterilizing, carrying out lamp inspection after cooling, packaging, and testing to obtain the finished product. The product prepared by the method is high in content of principal agents and good in stability, no visible foreign matter appears in a storage process, and the color of the product does not change.
Owner:JIANGXI GUOYAO PHARMA LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com