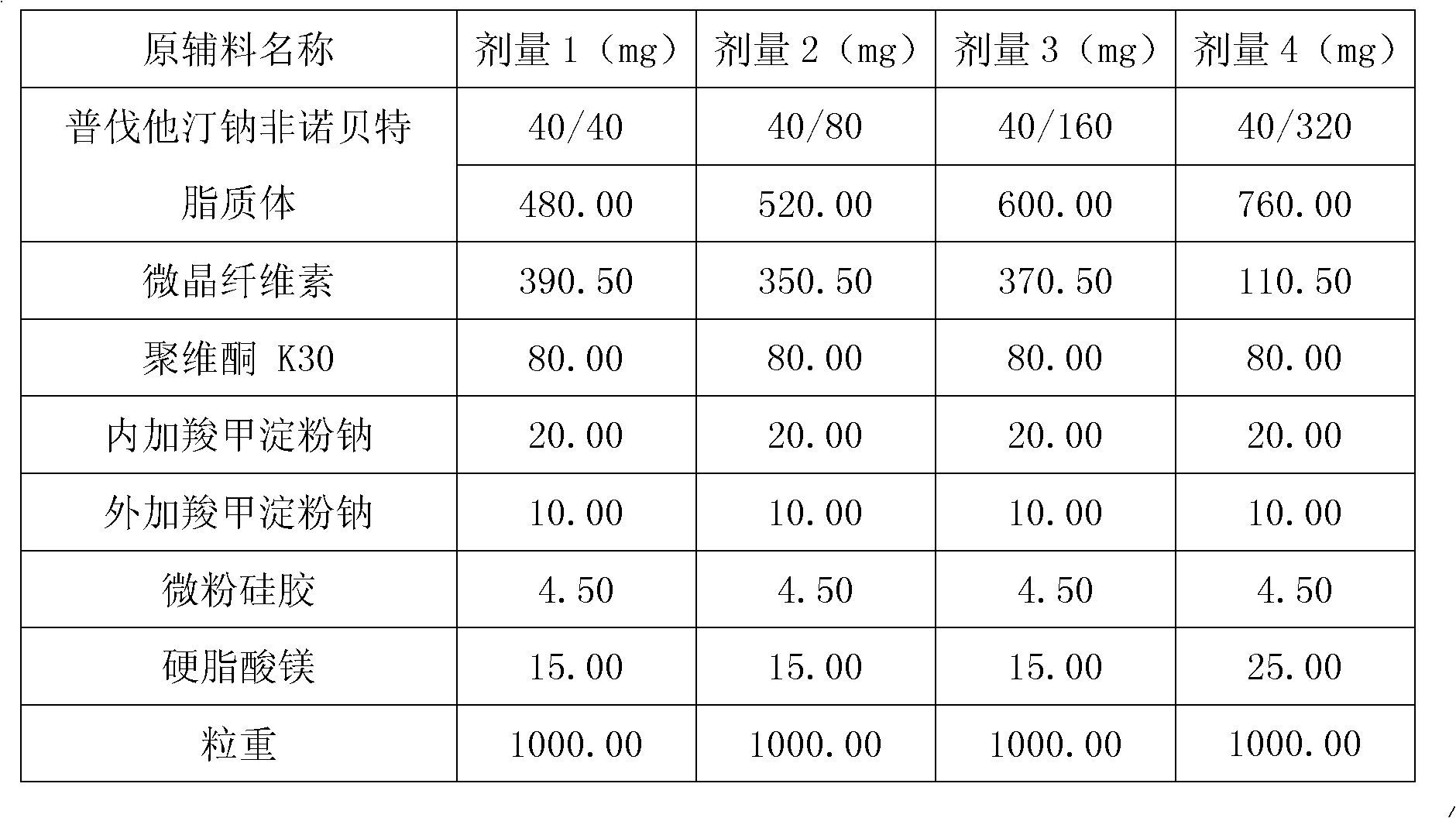Medicine composition containing pravastatin sodium fenofibrate liposome and preparation method of medicine composition
A technology of pravastatin sodium and fenofibrate, which is applied in the field of pharmaceutical compositions containing pravastatin sodium fenofibrate liposomes and its preparation, can solve the problem of easily forming lactone and the bioavailability of fenofibrate low bioavailability and low bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
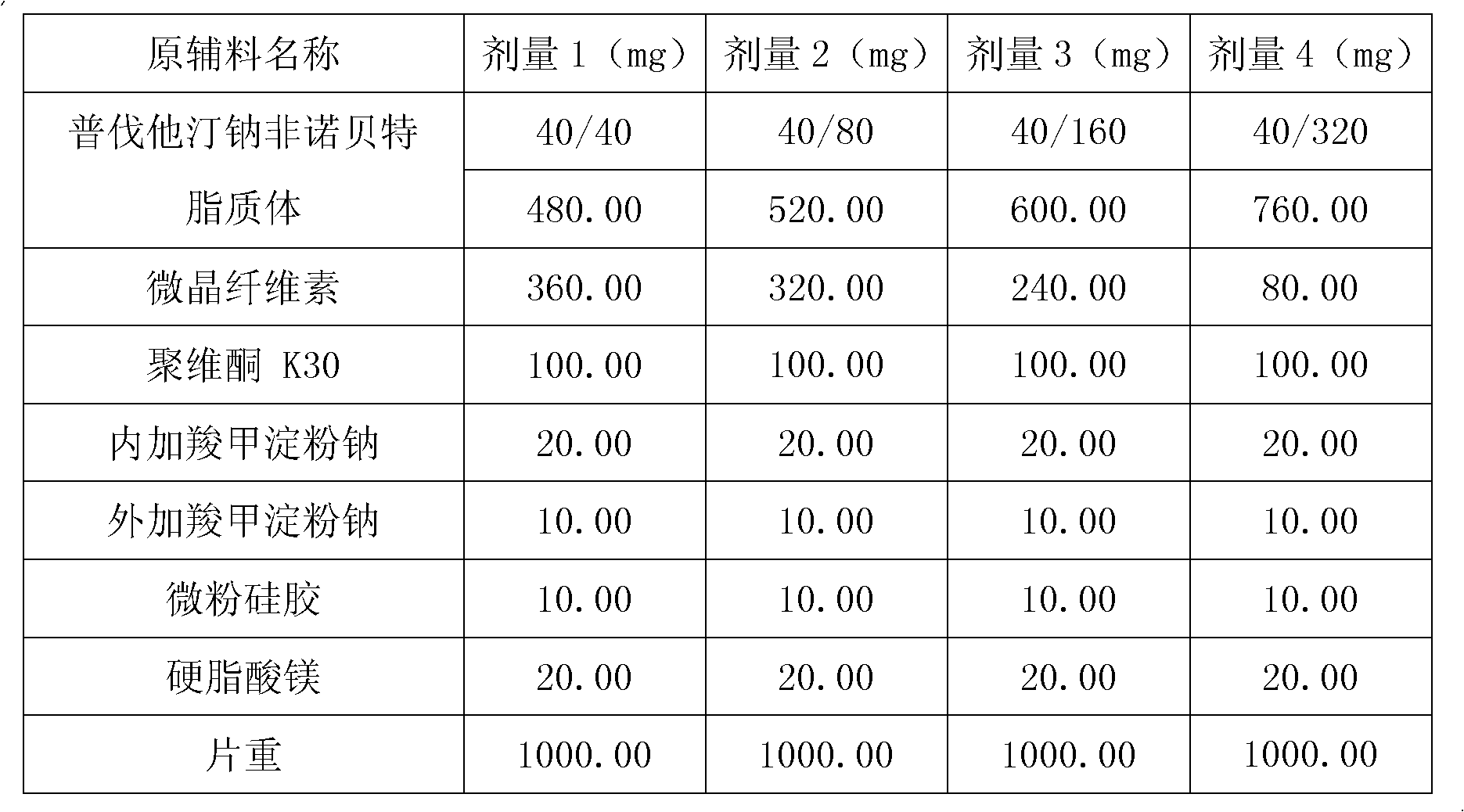
[0097] Embodiment 1: Preparation of pravastatin sodium fenofibrate liposome
[0098] Name of raw material
Dose 1 (mg)
Dose 2 (mg)
Dose 3 (mg)
Dose 4(mg)
40.00
40.00
40.00
40.00
40.00
80.00
160.00
320.00
Hydrogenated Soy Lecithin
100.00
100.00
100.00
100.00
Hydrogenated egg yolk lecithin
100.00
100.00
100.00
100.00
200.00
200.00
200.00
200.00
total
480.00
520.00
600.00
760.00
[0099] Preparation:
[0100] (1) Dissolve 1 part of pravastatin sodium, 1-8 parts of fenofibrate, 2.5 parts of hydrogenated soybean lecithin, 2.5 parts of hydrogenated egg yolk lecithin, and 5 parts of cholesterol in 25 parts of absolute ethanol, mix well, and record volume, filtered, evaporated to remove absolute ethanol to prepare phospholipid f...
Embodiment 2
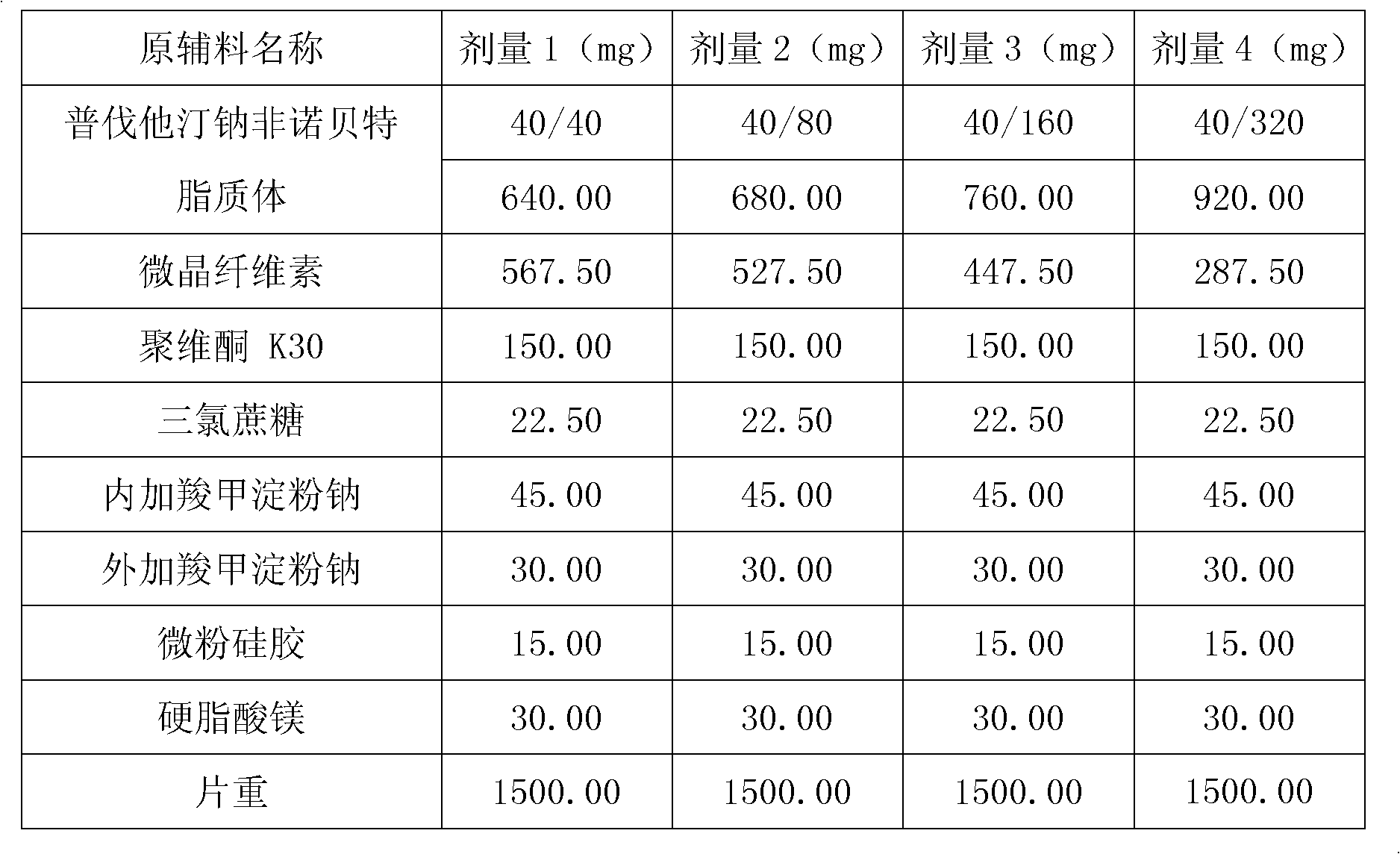
[0103] Embodiment 2: Preparation of pravastatin sodium fenofibrate liposome
[0104] Name of raw material
Dose 1 (mg)
Dose 2 (mg)
Dose 3 (mg)
Dose 4(mg)
40.00
40.00
40.00
40.00
40.00
80.00
160.00
320.00
Hydrogenated Soy Lecithin
140.00
140.00
140.00
140.00
Hydrogenated egg yolk lecithin
140.00
140.00
140.00
140.00
cholesterol
280.00
280.00
280.00
280.00
total
640.00
680.00
760.00
920.00
[0105] Preparation:
[0106] (1) Dissolve 1 part of pravastatin sodium, 1-8 parts of fenofibrate, 3.5 parts of hydrogenated soybean lecithin, 3.5 parts of hydrogenated egg yolk lecithin, and 7 parts of cholesterol in 30 parts of absolute ethanol, mix well, Record the volume, filter, evaporate and remove absolute ethanol to prepare a phospholipid f...
Embodiment 3
[0109] Embodiment 3: the preparation of pravastatin sodium fenofibrate liposome
[0110] Name of raw material
Dose 1 (mg)
Dose 2 (mg)
Dose 3 (mg)
Dose 4(mg)
10.00
20.00
40.00
80.00
[0111] Fenofibrate
160.00
160.00
160.00
160.00
Hydrogenated Soy Lecithin
80.00
80.00
80.00
80.00
Hydrogenated egg yolk lecithin
80.00
80.00
80.00
80.00
cholesterol
160.00
160.00
160.00
160.00
total
480.00
520.00
600.00
760.00
[0112] Preparation:
[0113] (1) Dissolve 1 part of pravastatin sodium, 2-16 parts of fenofibrate, 1-8 parts of hydrogenated soybean lecithin, 1-8 parts of hydrogenated egg yolk lecithin, and 2-16 parts of cholesterol in 20 parts of absolute ethanol In, mix evenly, record the volume, filter, evaporate and remove absolute ethanol t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com