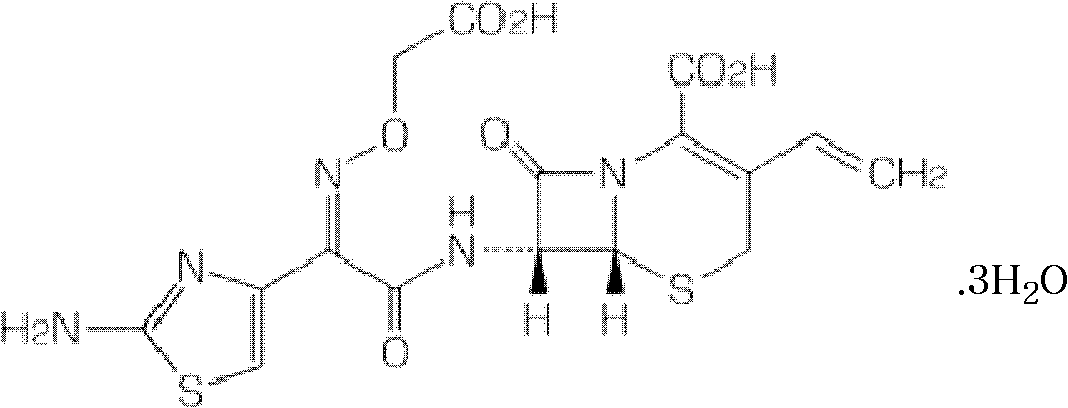
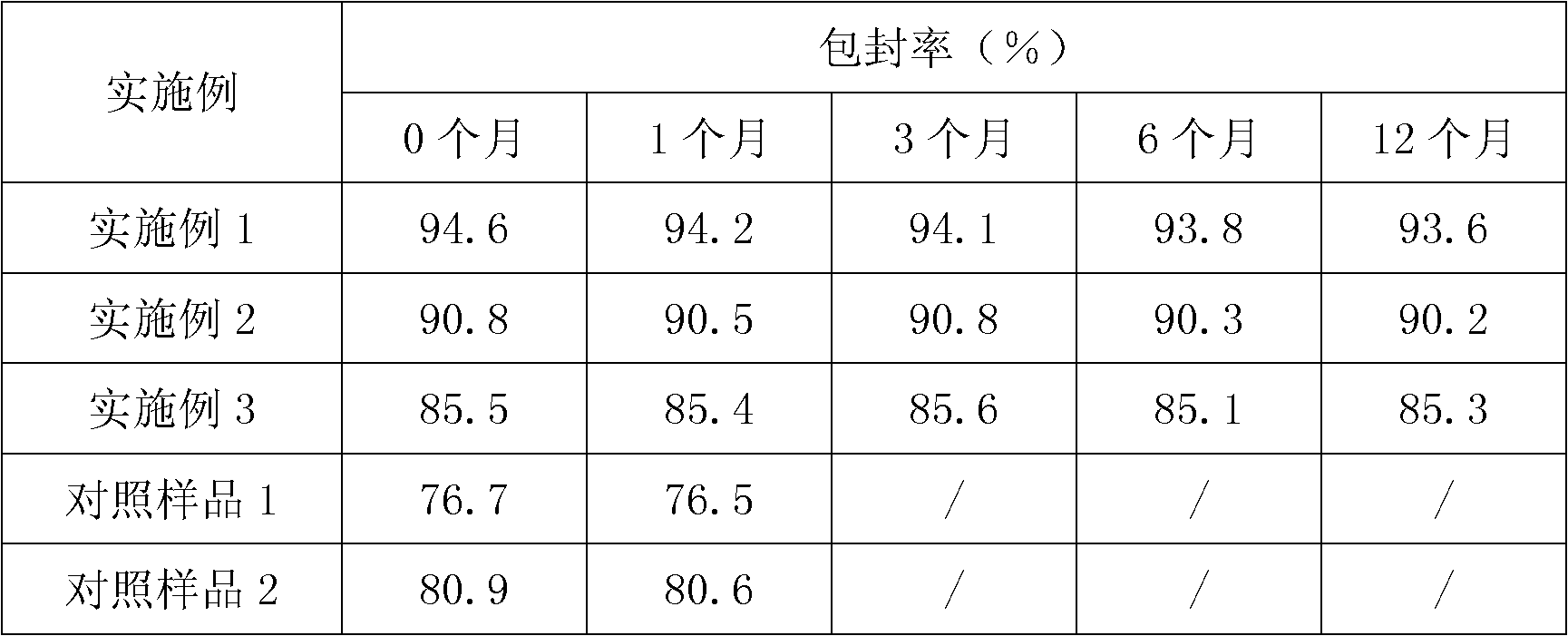
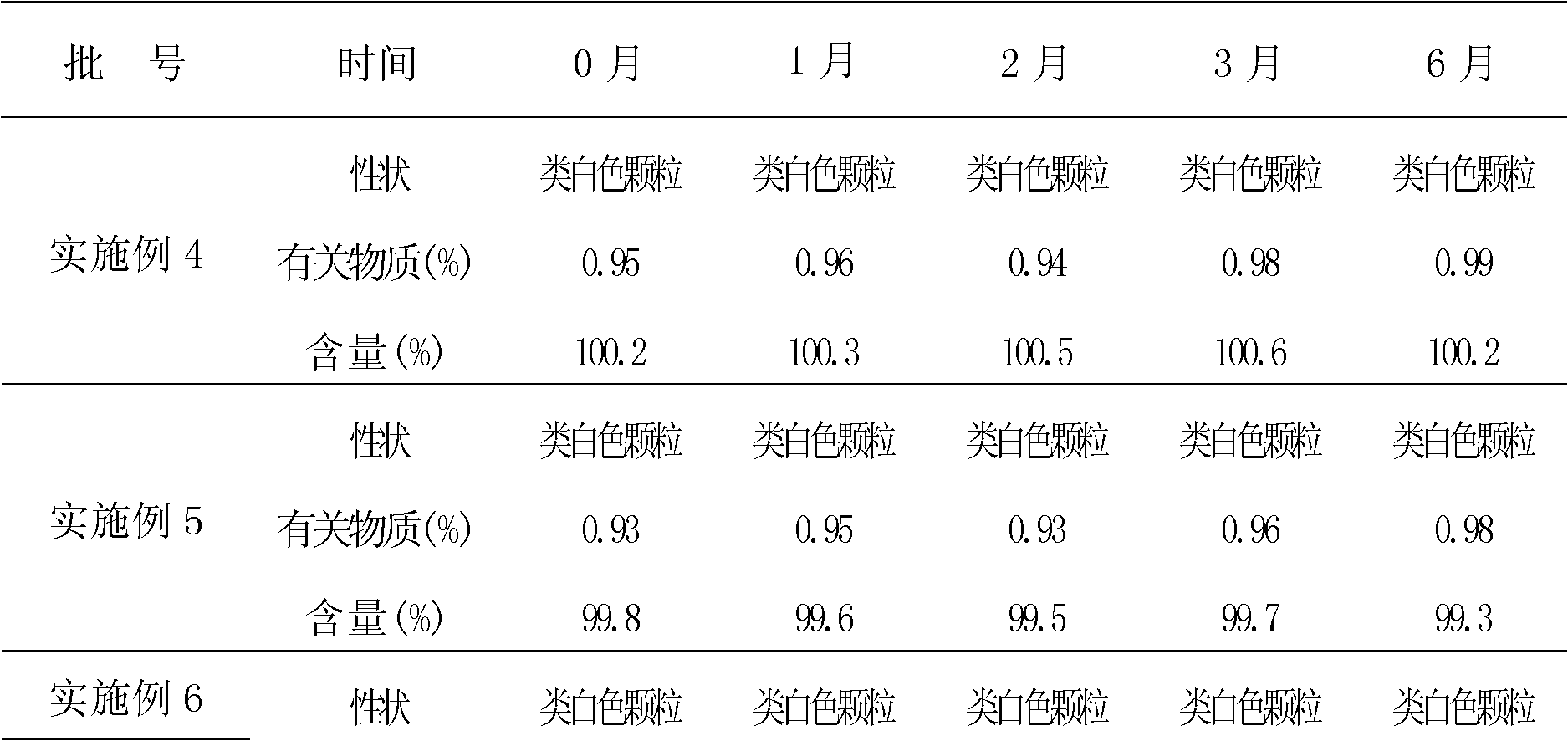
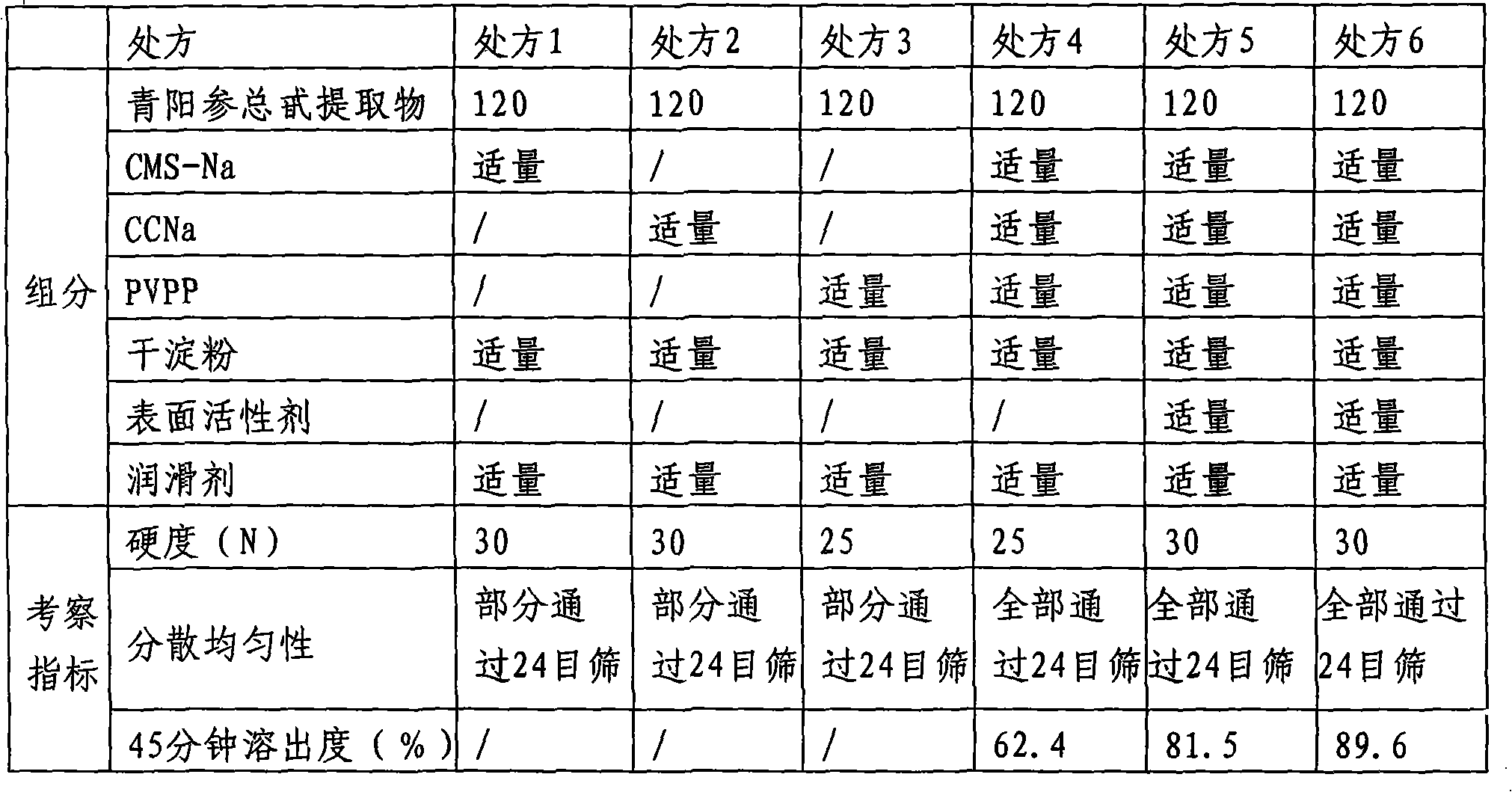
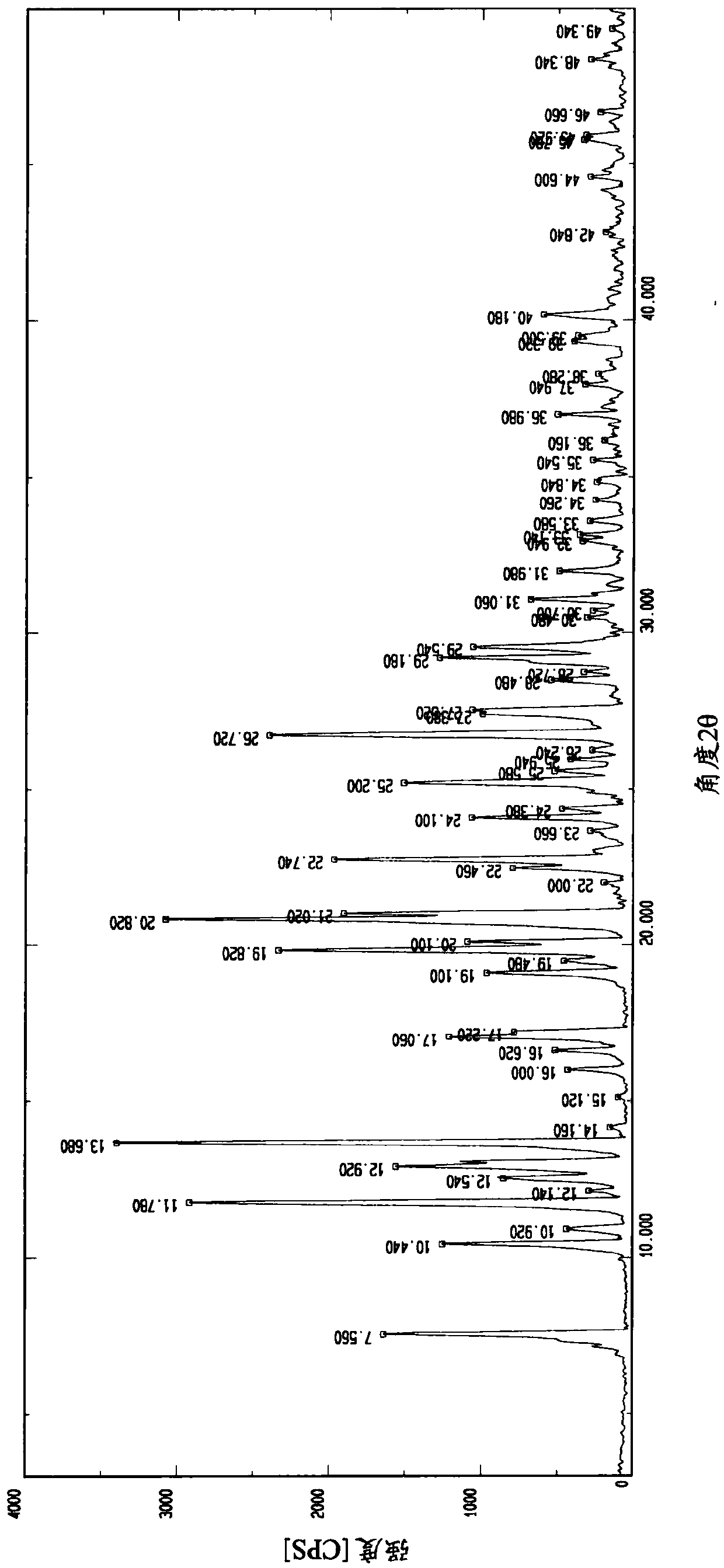
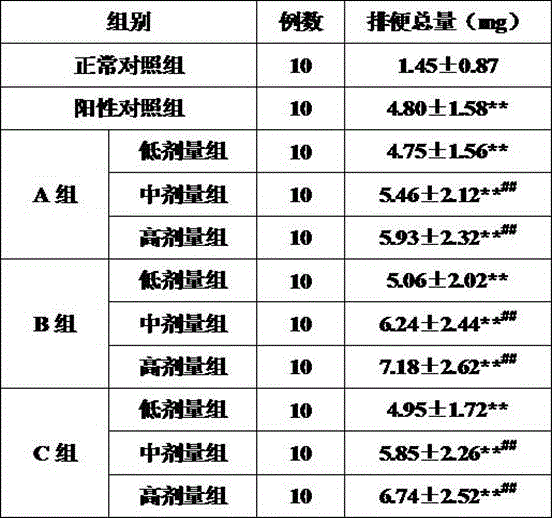
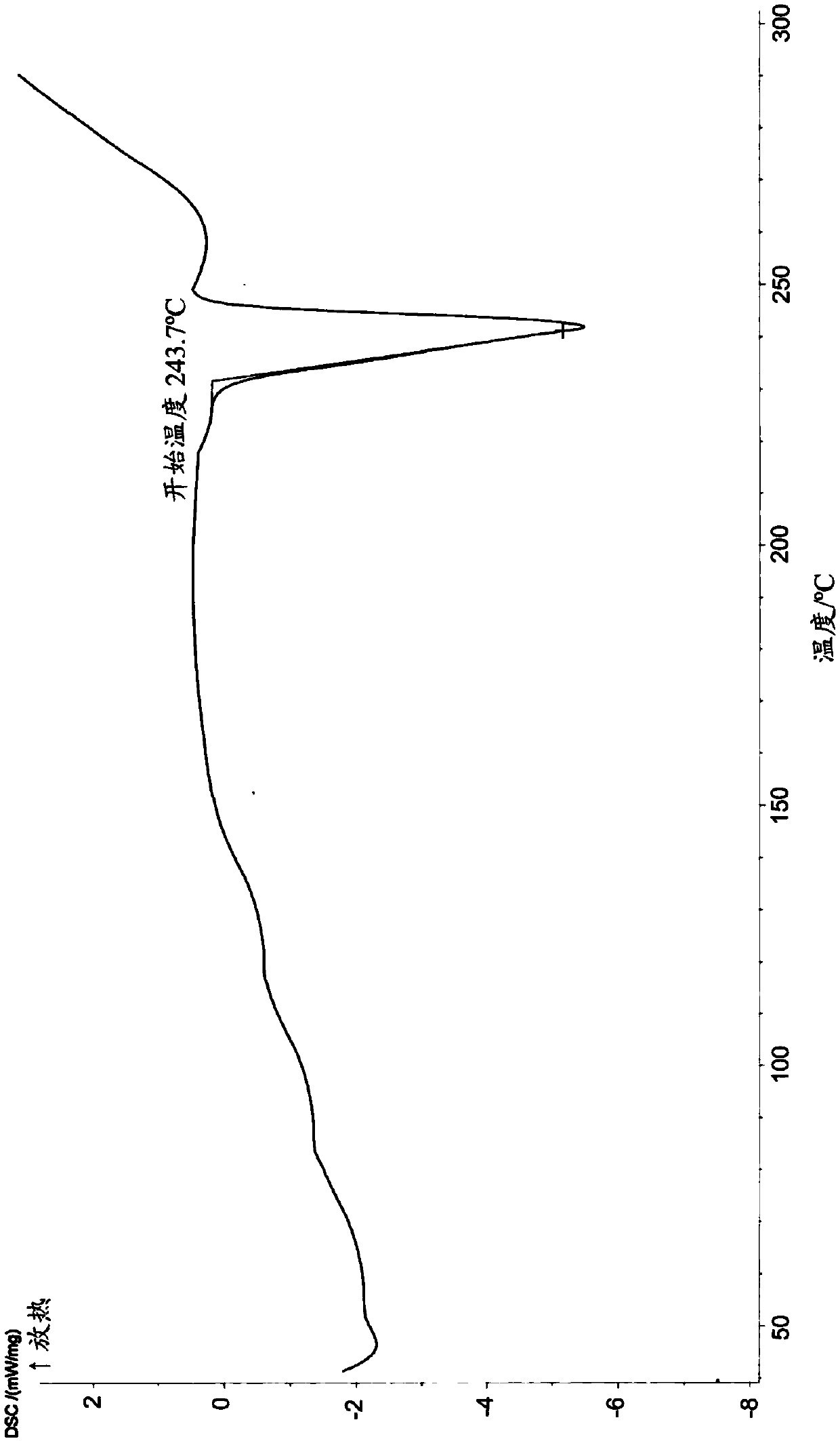
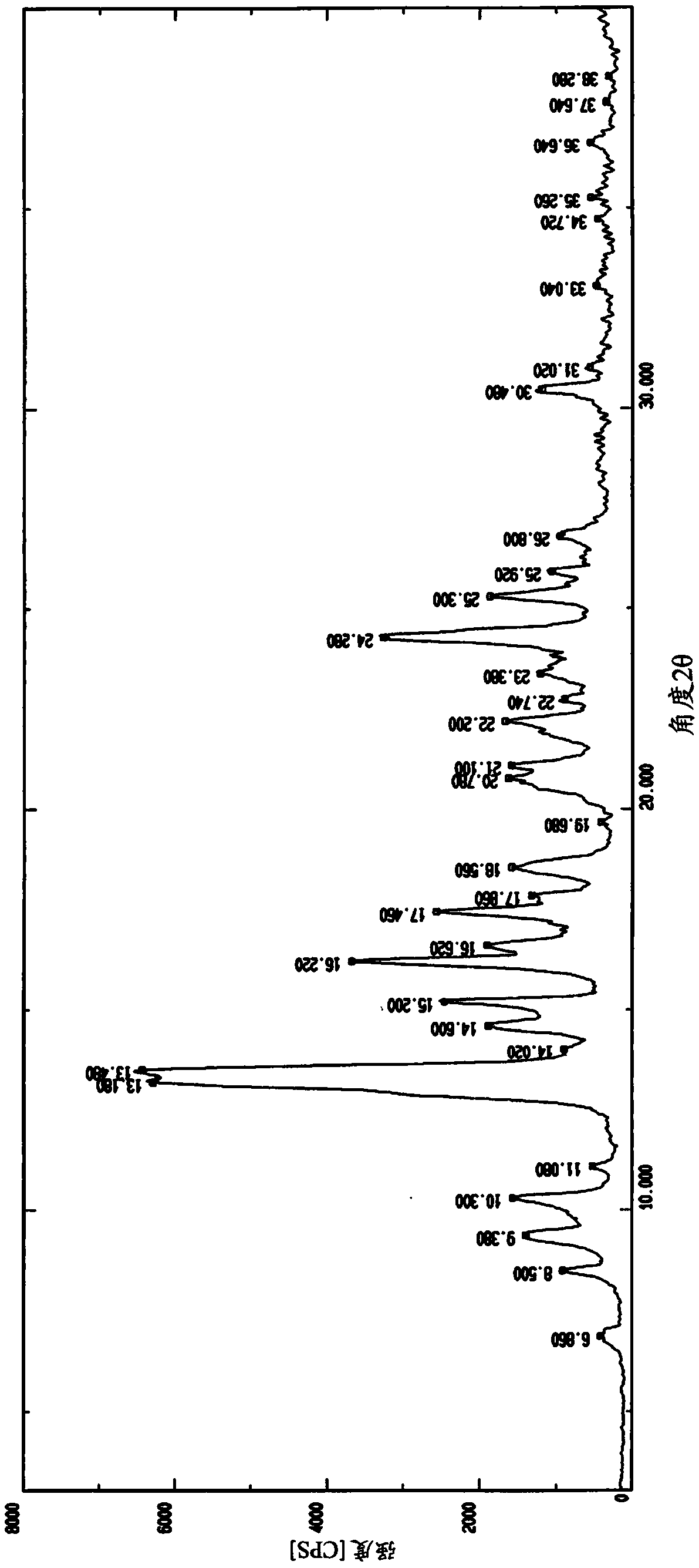
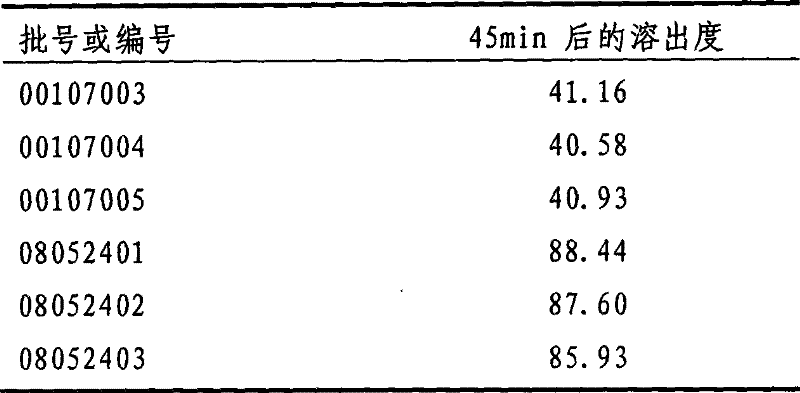
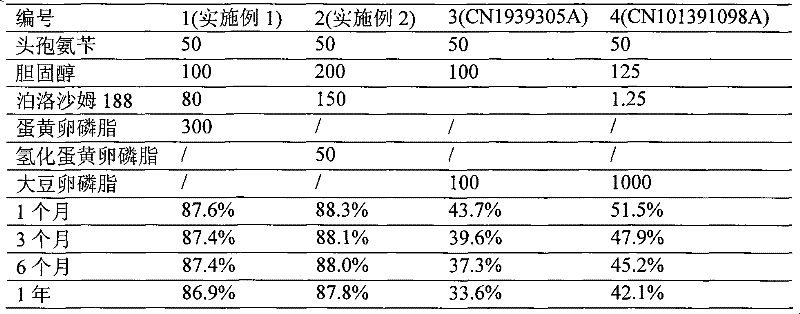
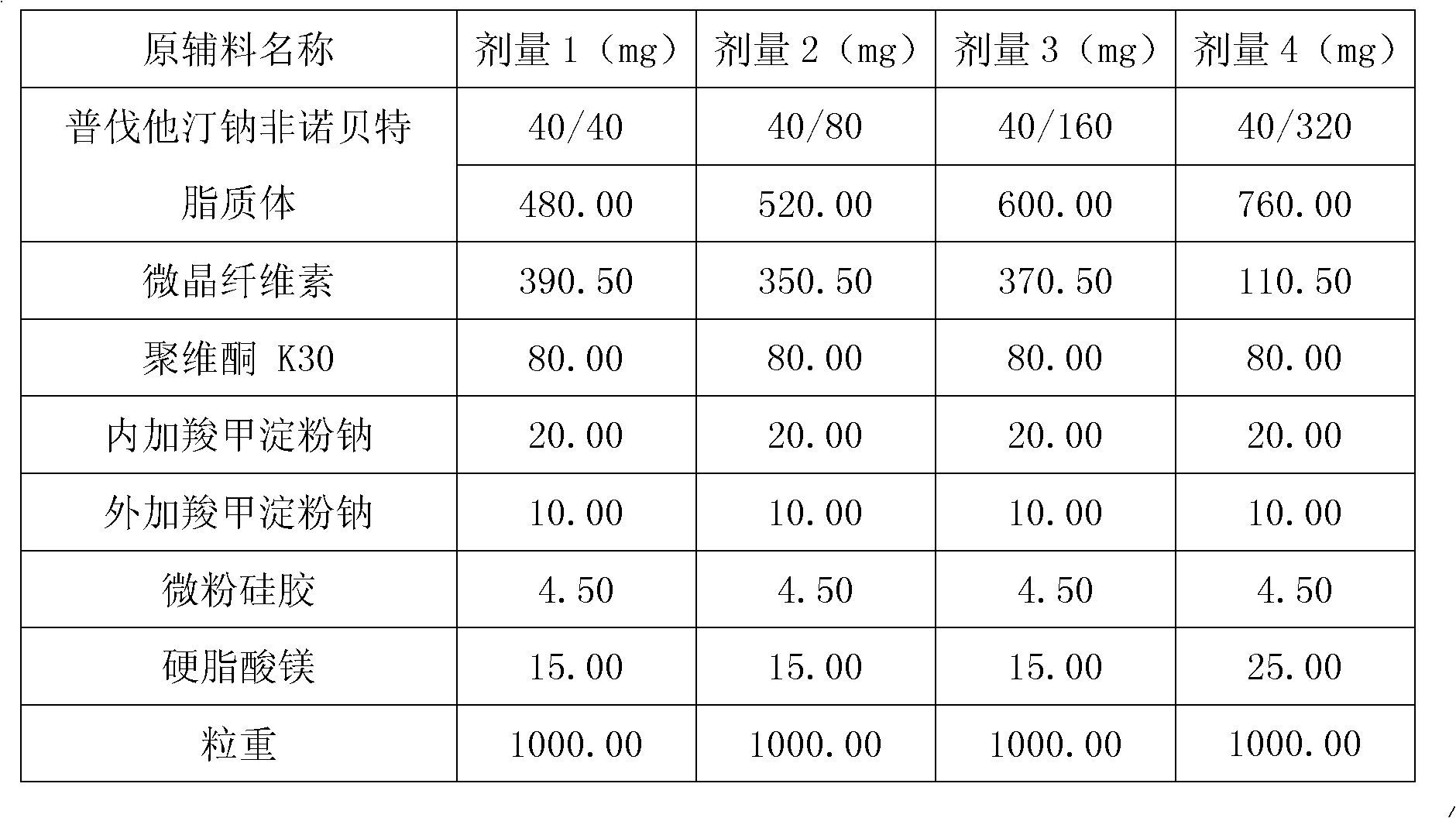Patents
Literature
37results about How to "Give full play to the medicinal effect" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
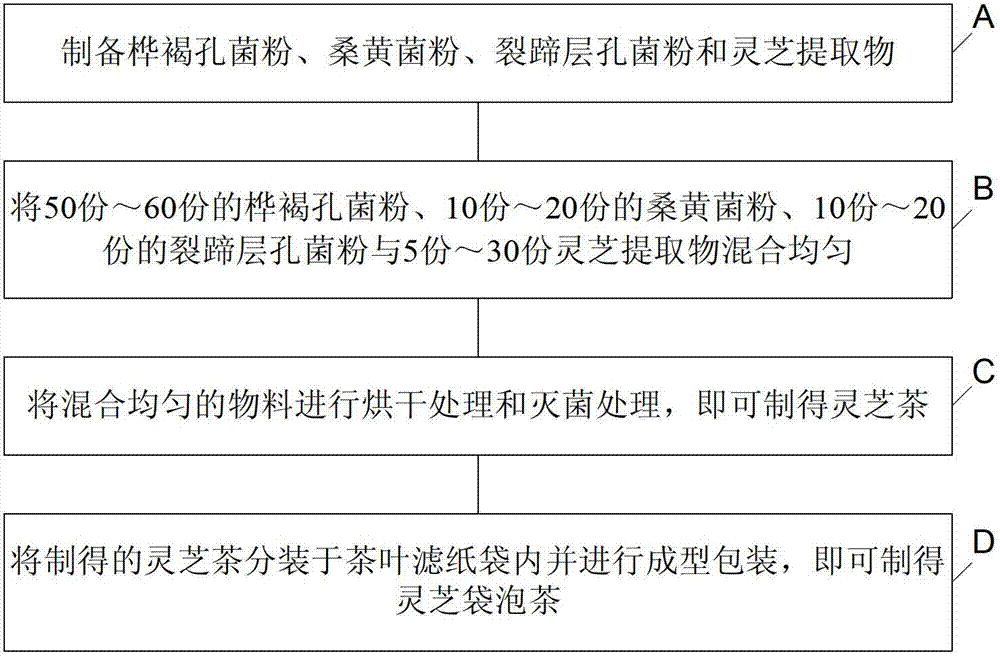
Lucid ganoderma tea, lucid ganoderma teabag and processing technology thereof
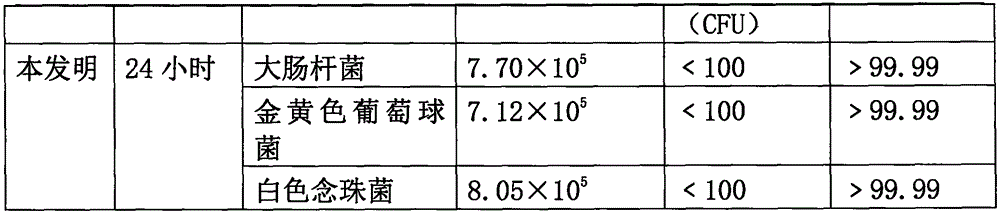
ActiveCN102726580AGive full play to the medicinal effectGive full play to the health benefitsTea substituesPhellinus igniariusInonotus obliquus
The invention discloses lucid ganoderma tea, lucid ganoderma teabag and a processing technology thereof. The lucid ganoderma tea comprises the following four components in parts by weight: 50-60 parts of inonotus obliquus powder, 10-20 parts of phellinus igniarius powder, 10-20 parts of phellinus linteus powder and 5-30 parts of lucid ganoderma extract. Thus, the lucid ganoderma tea disclosed by the embodiment of the invention has perfect mouthfeel, can give full play to the medicinal effect and healthcare effect of lucid ganoderma, improves the body immunity, and realizes an effect of adjusting and protecting the central nervous system, digestive system, endocrine system and respiratory system and the organs such as liver, prostate and the like.
Owner:王力
Immunity enhancing Dendrobium officinale jelly and production method thereof
InactiveCN106072216AGive full play to the medicinal effectFit for consumptionSugar food ingredientsFood ingredient as taste affecting agentFlavorLigustri lucidi
The invention relates to an immunity enhancing Dendrobium officinale jelly and a production method thereof. The jelly is produced from a Dendrobium officinale normal juice, Ligustri Lucidi Fructus powder and Polygonatum sibiricum paste. The jelly has good immunity enhancing and nutrition and health functions, the Polygonatum sibiricum paste and the Ligustri Lucidi Fructus powder are used as auxiliary materials to produce the jelly with unique flavor, and the jelly becomes people's favorite nutrition and health instant food; and the jelly has the health functions of Dendrobium officinale, has extremely good mouthfeel, is suitable for all people to eat, and fully performs the medicinal efficacy of the Dendrobium officinale.
Owner:杨文明
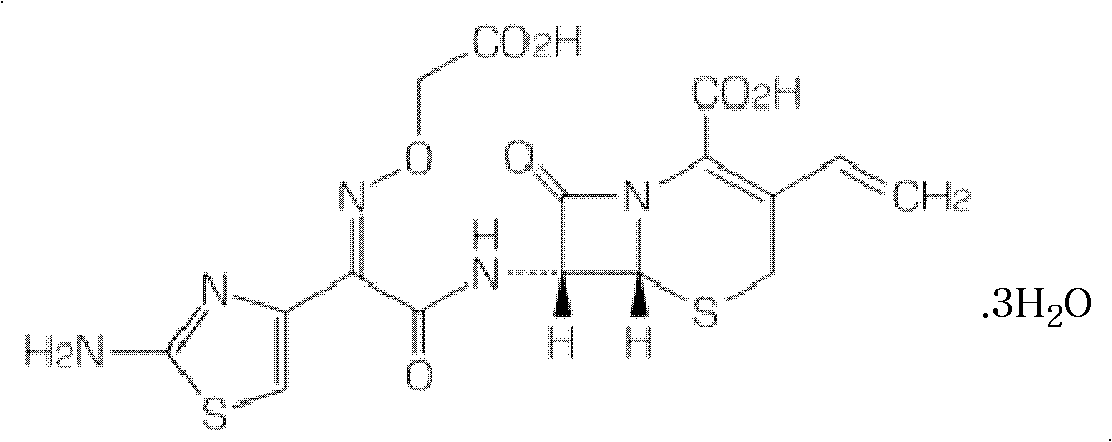
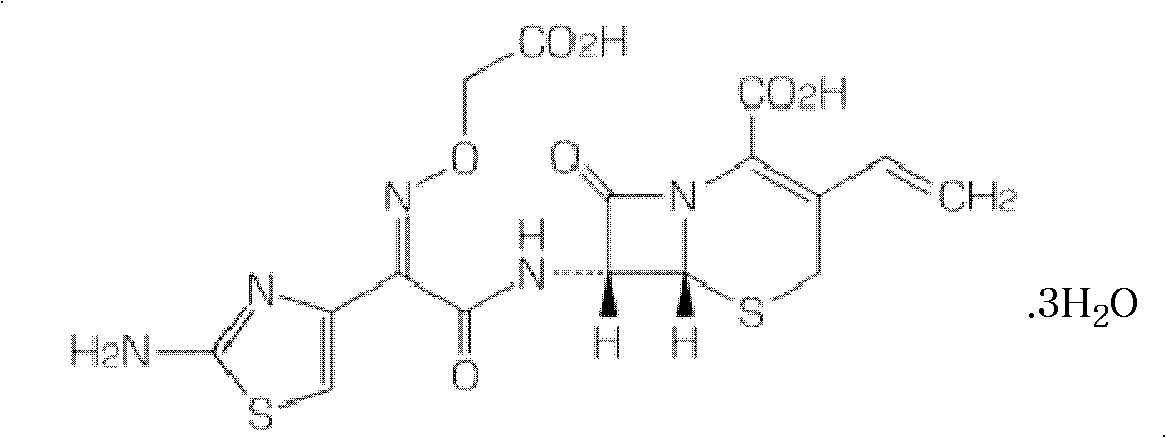
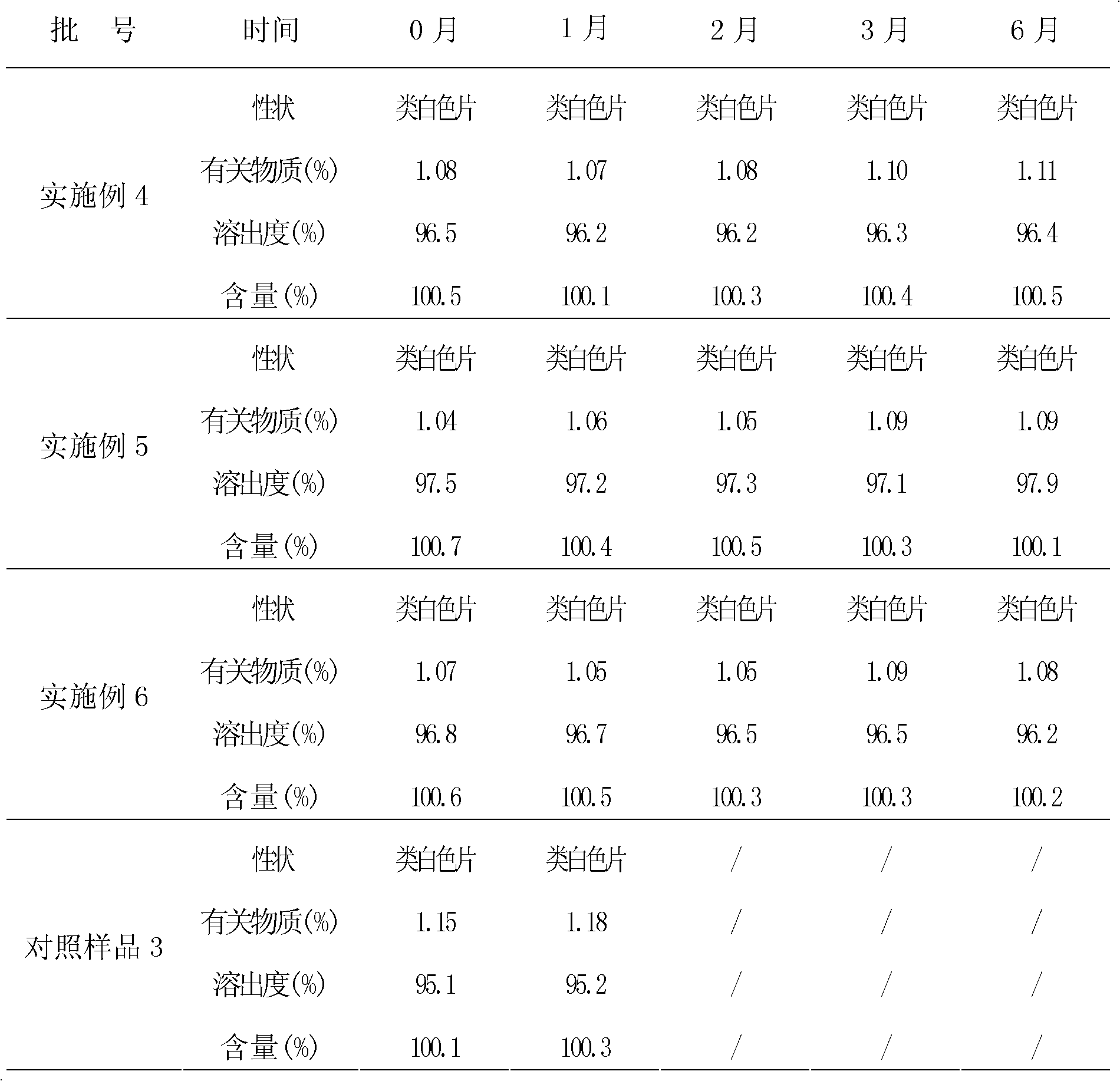
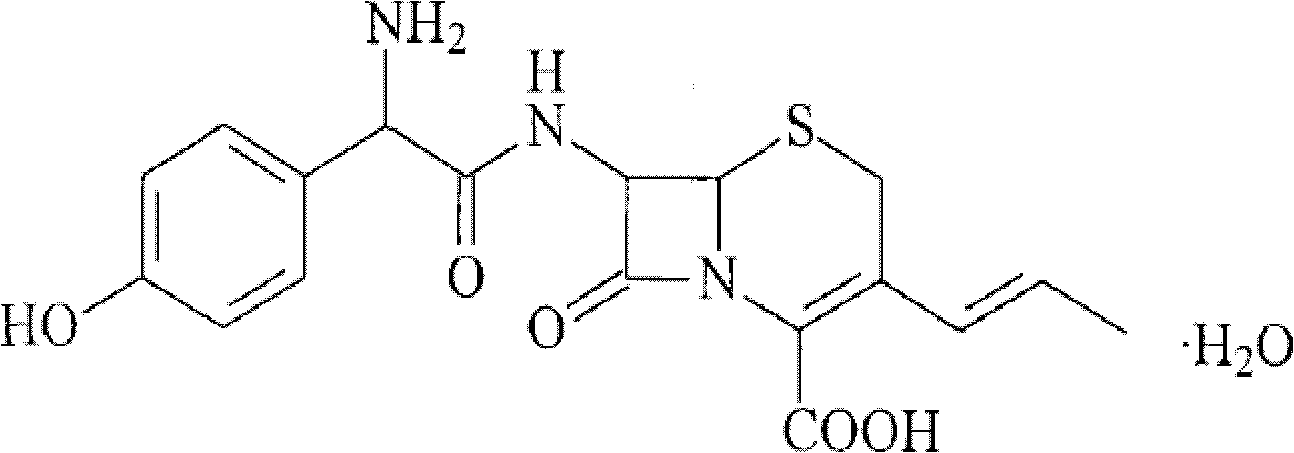
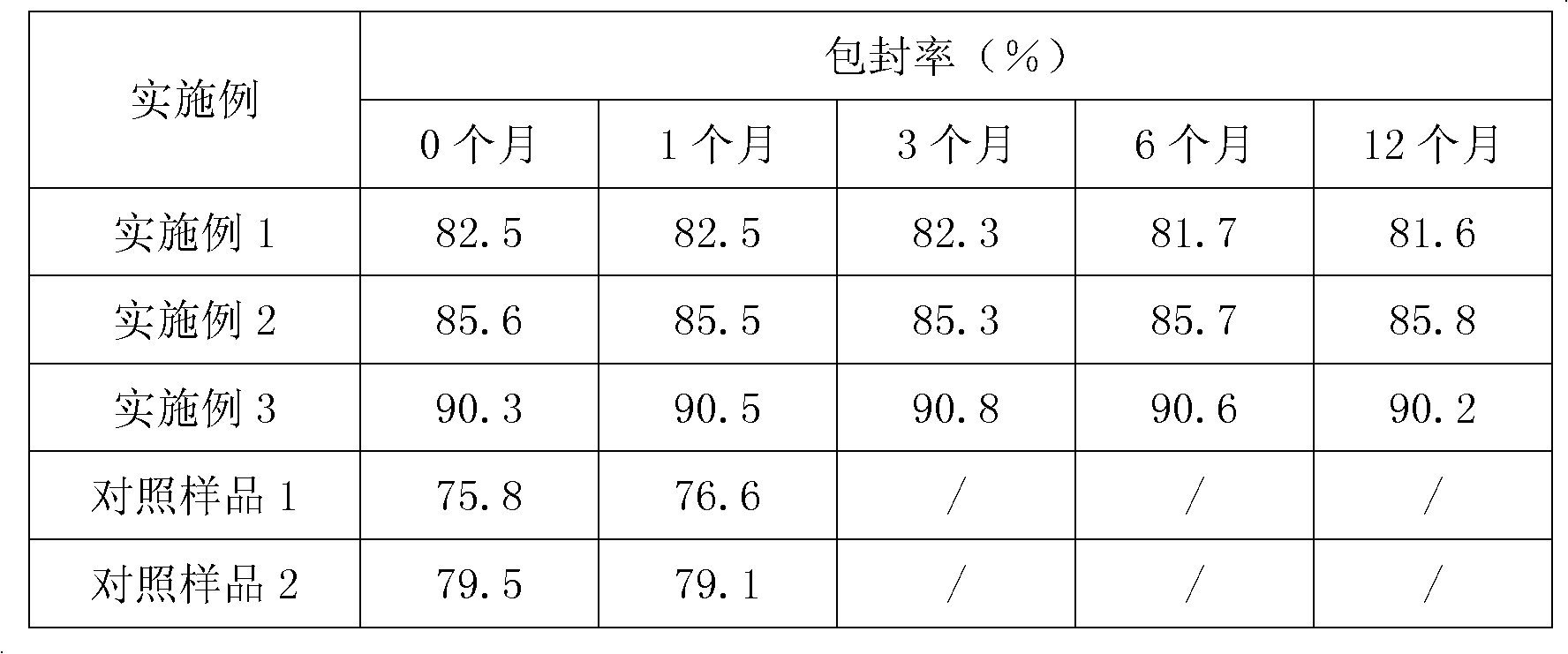
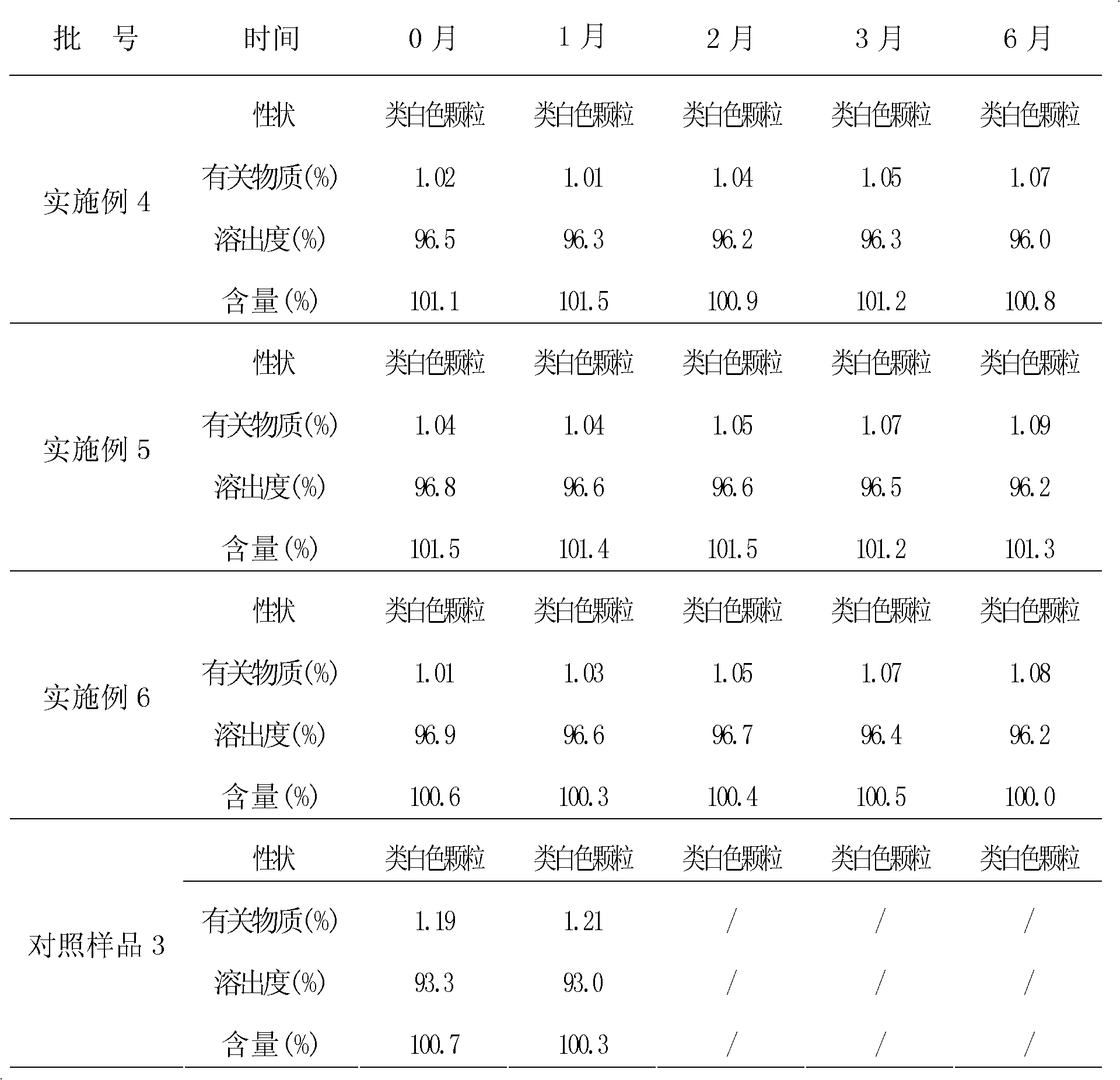
Granular formulation containing cefixime liposomes and preparation method thereof
InactiveCN101966154AHigh encapsulation efficiencyReduce leak rateOrganic active ingredientsAntiinfectivesYolkCholesterol
The invention relates to a cefixime liposome, a preparation method and a granular formulation containing the cefixime liposome. The granular formulation comprises the cefixime liposome and a pharmaceutically acceptable vector, wherein the cefixime liposome is prepared from the following components in parts by weight: 1 part of cefixime, 1.25-5 parts of hydrogenated soybean lecithin, 1.25-5 parts of hydrogenated egg yolk lecithin, 2.5-10 parts of cholesterol and 0.1-4.5 parts of polysorbate 80. The granular formulation not only accords with the requirement on Chinese pharmacopoeia, but also has the advantages of stabler storage and rapider drug effect exertion and remarkably improved bioavailability compared with the common pharmaceutical cefixime composition at a room temperature.
Owner:王丽燕
Herbal modified material based on PU foam as well as preparation method and application of herb modified material
The invention provides a herbal modified material based on PU foam as well as a preparation method and an application of the herb modified material. The material is prepared from the following raw materials in parts by weight: 80 parts of traditional Chinese medicines, 320 parts of sponge, 14-23 parts of a diluent, 42.8-51.5 parts of gum and 0.6-1 part of a catalyst. The herbal modified material has the advantages that secondary pollution is avoided, the production efficiency is high, long-term bacteriostat and deodorant effects can be realized, the bacteriostat and deodorant properties can be significantly improved when the material is applied to shoe soles or insoles, foot odor is prevented, and accordingly, the life quality of people is improved.
Owner:HENAN BANGNI BIOLOGICAL ENG CO LTD
White gourd beverage for clearing away heat and toxic substances and increasing urine excretion and preparation method thereof
The invention relates to a white gourd beverage for clearing away heat and toxic substances and increasing urine excretion and a preparation method thereof. The white gourd beverage is prepared from the following components according to parts by weight: 100-150 parts of white gourd, 6-10 parts of mung bean, 1-3 parts of lotus seed and 100-360 parts of water. The preparation method of the white gourd beverage sequentially comprises the steps of water treatment; preparation of white gourd pulp; preparation of mung bean juice and lotus seed juice; and preparation of composite white gourd juice. The white gourd beverage for clearing away heat and toxic substances and increasing urine excretion disclosed by the invention is convenient to make, low in cost and good in mouth feel, and the white gourd beverage has the effects of clearing away heat, increasing urine excretion, dissolving phlegm, clearing away toxic substances, and the like so as to bring medicinal efficacy of white gourd into full play. Moreover, the composite white gourd juice is mixed with pulp, so that various requirements of vast consumers are met, and the implementation effect is good.
Owner:INST AGRO PROD PROCESSING ANHUI ACADEMY AGRI SCI
Dispersible tablet containing cefixime liposome and preparation method thereof
InactiveCN101966160AHigh encapsulation efficiencyReduce leak rateAntibacterial agentsOrganic active ingredientsCholesterolNiosome
The invention relates to a cefixime liposome, a preparation method thereof and a dispersible tablet containing the cefixime liposome. The dispersible tablet comprises the cefixime liposome and a pharmaceutically acceptable carrier, wherein the cefixime liposome comprises the following components in parts by weight: 1 part of cefixime, 1.25-5 parts of hydrogenated soybean lecithin, 1.25-5 parts of hydrogenated egg yolk lecithin, 1.25-10 parts of cholesterol and 0.2-3.5 parts of polysorbate 80. The dispersible tablet not only conforms to the requirements of a Chinese pharmacopoeia, but also has the advantages of more stable storage under normal temperature and remarkable improvement of bioavailability compared with the ordinary cefixime medicament composition, and can take effect more rapidly.
Owner:王丽燕
Medical biological hydrogel functional dressing for hemorrhoid and preparation method thereof
ActiveCN105169456APromote repairGood regeneration performanceOrganic active ingredientsAbsorbent padsIrritationPolyhexamethylene guanidine
The invention relates to the technical field of medical dressing, and specifically relates to a medical biological hydrogel functional dressing for hemorrhoid and a preparation method thereof. The dressing is composed of the following raw materials: glycerin, chitosan quaternary ammonium salt, Chinese lobelia extract, scutellariae barbatae extract, sedum verticallatum extract, smilax glabra extract, stephanotis extract, rabdosia rubescens extract, mint extract, polyhexamethylene guanidine, and the balance being purified water. The dressing other than the auxiliary materials is made of pure traditional Chinese herbals, thus has little irritation, does not have any side or toxic effect, is safe to use, and has the advantages of good permeability and curative effect, fully-exerted effect, long-lasting effect, and difficult relapse. The dressing can be applied to anus to relieve the symptoms of internal hemorrhoid, external hemorrhoid, and mixed hemorrhoid, and symptoms caused by anal fissure / anal fistula surgery such as bleeding, aches, anal pendant expansion, etc. The dressing can also promote hemorrhoid shrinkage, prevent hemorrhoid prolapse, and relieve hyperemia and edema of hemorrhoid mucous membrane, and is capable of being applied to antibacterial nursing of anus diseases such as hemorrhoid.
Owner:DONGGUAN DAQING MEDICAL DEVICES CO LTD +2
Dry suspension containing cefixime liposome and preparation method thereof
InactiveCN101972231AStorage moreStorage stableOrganic active ingredientsAntiinfectivesPhysical ExertionsYolk
The invention relates to cefixime liposome and preparation method therefore as well as dry suspension containing the cefixime liposome. The dry suspension is composed of the cefixime liposome and pharmaceutically acceptable carriers, wherein the cefixime liposome comprises the following components in parts by weight: 1 part of cefixime, 1.25-5 parts of hydrogenated soybean lecithin, 1.25-5 parts of hydrogenated yolk lecithin, 2.5-10 parts of cholesterol and 0.1-5 parts of polysorbate 80. The dry suspension not only accords with the requirements of Chinese pharmacopoeia, but also has the advantages of better storage stability in normal temperature, faster effect exertion and obviously improved bioavailability in comparison with the common cefixime medicaments.
Owner:王丽燕
Cynanchum otophyllum schneid dispersible tablets and preparation method thereof
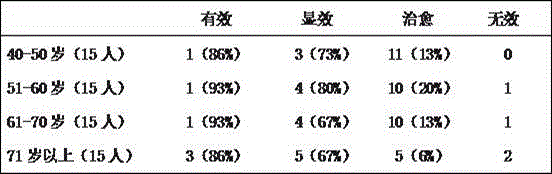
InactiveCN101357149AImprove bioavailabilityGive full play to the medicinal effectNervous disorderPill deliveryDissolutionMagnesium stearate
The invention discloses a cynanchum otophyllum dispersible tablet and a preparation method thereof, relating to medicines. The cynanchum otophyllum dispersible tablet is made of the following raw materials by weight portions: 50-200 portions of an extract of cynanchum otophyllum total glucoside, 20-100 portions of an disintegrating agent, 1-10 portions of a lubricant and 1-20 portions of a surfactant, wherein, the disintegrating agent is CMS-Na, CCNa, PVPP, L-HPC and dry starch, the lubricant is magnesium stearate or talcum powder or superfine silica gel powder, the surfactant is SDS, sodium hexadecyl sulfate and sodium stearyl sulfate. The preparation method consists of the following steps: 20-200 portions of total glucoside of the cynanchum otophyllum by weight is taken out and sieved with a 100-mesh sieve; 20-100 portions of the disintegrating agent by weight and 1-10 portions of the lubricant by weight are taken out and respectively sieved with the 100-mesh sieve; 1-20 portions of the surfactant by weight is taken out and sieved with a 80-mesh sieve; then the sieved extract, the sieved disintegrating agent, the sieved lubricant and the sieved surfactant are ground and evenly mixed, and then the mixture is sieved with the 80-mesh sieve, and direct compression and sieving are carried out on the sieved mixture to obtain the dispersible tablet. The dispersible tablet has high dissolution and high bioavailability, can fully exert the drug action of the cynanchum otophyllum, and the preparation method is simple and feasible while suitable for industrialized production.
Owner:GUIZHOU UNIV
Preparation method of glycyrrhiza sweetening and moistening essence for mouth stick
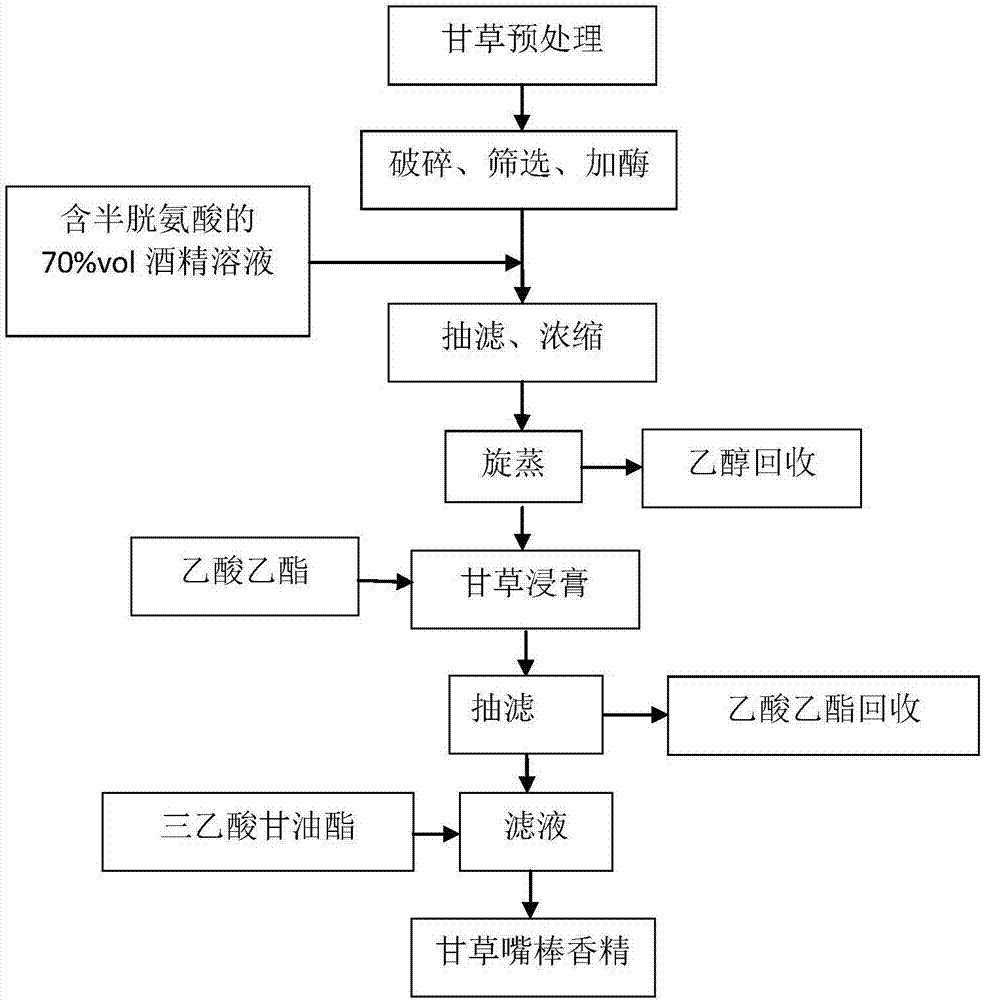
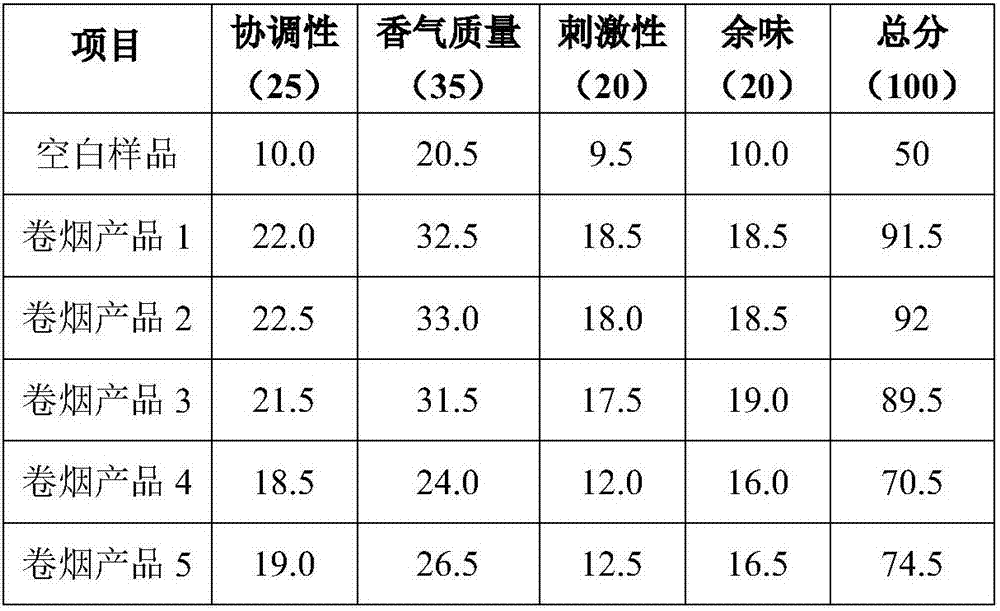
ActiveCN107057856AAvoid lossAvoid pyrolysisTobacco smoke filtersEssential-oils/perfumesAlcoholIrritation
The invention belongs to the technical field of tobacco flavors and in particular relates to a method of preparing essence of a mouth stick for a cigarette by taking glycyrrhiza as extract. The essence is used for improving sucking sweet feel of tobacco and enhancing the sensing comfort, so that the sucking quality of tobacco is effectively enhanced. The preparation method of glycyrrhiza sweetening and moistening essence for the mouth stick comprises the following steps: enzymolysis, alcohol extraction, extraction and the like. The prepared essence is directly added in a mouth stick processing step, so that escape and pyrolysis of essence in the storage and ignition and suction processes of tobacco are avoided, the loss of flavors in static igniting period of the tobacco can be also avoided, throttling of cut tobacco and filter tip on the flavors can be reduced, and the transfer efficiency is increased. The glycyrrhiza sweetening and moistening essence for the mouth stick prepared by the invention can remarkably improve the quality of fragrance of the cigarette, reduce the irritation and improve the quality of the cigarette.
Owner:CHINA TOBACCO JIANGXI IND CO LTD
Transdermal delivery based pharmaceutical composition and preparation method and application thereof
ActiveCN111346073AImproves transdermal penetrationLittle side effectsOrganic active ingredientsAntipyreticIntramuscular injectionChemical compound
The present invention discloses a transdermal delivery based pharmaceutical composition. The composition comprises a compound as shown in a formula (I) or pharmaceutically acceptable salt thereof, a macromolecular dispersing carrier material, a hot melting protecting agent and an optional fluxing agent. A preparation method of the transdermal delivery based pharmaceutical composition comprises thesteps of micronizing the compound as shown in the formula (I) or the pharmaceutically acceptable salt thereof, the macromolecular dispersing carrier material and the hot melting protecting agent, optionally adding the fluxing agent, mixing the components uniformly, and performing hot melting extrusion and micronizing to obtain microparticles of the compound as shown in the formula (I) or the pharmaceutically acceptable salt thereof. In addition, the invention provides an application of the pharmaceutical composition. The transdermal delivery based pharmaceutical composition can enable the compound as shown in the formula (I) to be quickly absorbed, so that the purpose of calming before anesthesia is achieved, breathing is not affected, and the adverse psychological effects on children dueto intramuscular injection or intravenous injection are avoided. Meanwhile, the pharmaceutical composition also can be used for preventing and / or treating hyperactivity.
Owner:YICHANG HUMANWELL PHARMA
Medical biological dressing suppository for treating hemorrhoid
InactiveCN104043089ALess irritatingNo side effectsHydroxy compound active ingredientsSuppositories deliverySide effectBiological dressing
The invention discloses a medical biological dressing suppository for treating hemorrhoid. The medical biological dressing suppository is prepared from a medicinal component and a suppository matrix, wherein the medicinal component is prepared from the following components in parts by weight: 18-20 parts of golden cypress, 12-15 parts of radix sophorae flavescentis, 12-15 parts of purslane, 5-8 parts of gleditsia sinensis, 6-8 parts of dried ginger, 8-12 parts of rheum officinale, 5-8 parts of verdigris, 8-10 parts of borneol, 3-6 parts of realgar, 15-18 parts of bidens pilosa L, 12-15 parts of rhizoma smilacis glabrae, 8-10 parts of common cnidium fruits, 5-8 parts of cortex dictamni, 5-8 parts of curcuma zedoary, 8-10 parts of folium artemisiae argyi, 6-8 parts of dried alum, 12-15 parts of blue salt and 6-8 parts of Sichuan pepper; the suppository matrix is prepared from the following components in percentage by weight: 22% of chitosan, 8% of carbopol and 70% of polyethylene glycol. The medical biological dressing suppository is prepared according to a traditional Chinese medicine formula so as to be free of toxic or side effect and capable of ensuring that hemorrhoid is not easy to recur; the suppository is used as the dosage form so as to have the advantages of high disintegration speed, absorption completeness, no injury to mucous membranes and cleanness, and effects are rapid to take; in addition, the medical biological dressing suppository is good in curative effect, long in acting time and favorable in curative effect for hemorrhage, swell, pain, astriction and the like caused by internal hemorrhoid, external hemorrhoid and mixed hemorrhoid.
Owner:安徽承庆堂国药股份有限公司
Pomelo flavedo fresh-product lozenge and preparation method thereof
The invention relates to a pomelo flavedo fresh-product lozenge and a preparation method thereof. The pomelo flavedo fresh-product lozenge is composed of the following components by mass fraction: 25-30% of a dried fresh pomelo flavedo peel product, 0.7-1.5% of Arabic gum, 1-5%of an adhesive, 61-72% of a filler, 0.1-0.6% of menthol, 0.7-1% of magnesium stearate, and 0.5-1.3% of a sweetener. The preparation method adopts Arabic gum as an emulsifier and a binder, and can emulsify the volatile oil in the pomelo flavedo, so that the active ingredient is not destroyed and lost, and the efficacy ofthe pomelo flavedo can be fully exerted; The addition of the binder can promote the tableting; and the addition of a sweetener can alleviate the bitterness of the pomelo flavedo and the taste is good.The lozenge has good stability through the cooperation of the components, and can fully protect the active ingredient of the pomelo flavedo to fully exert the pharmacological effect.
Owner:GUANGDONG PHARMA UNIV
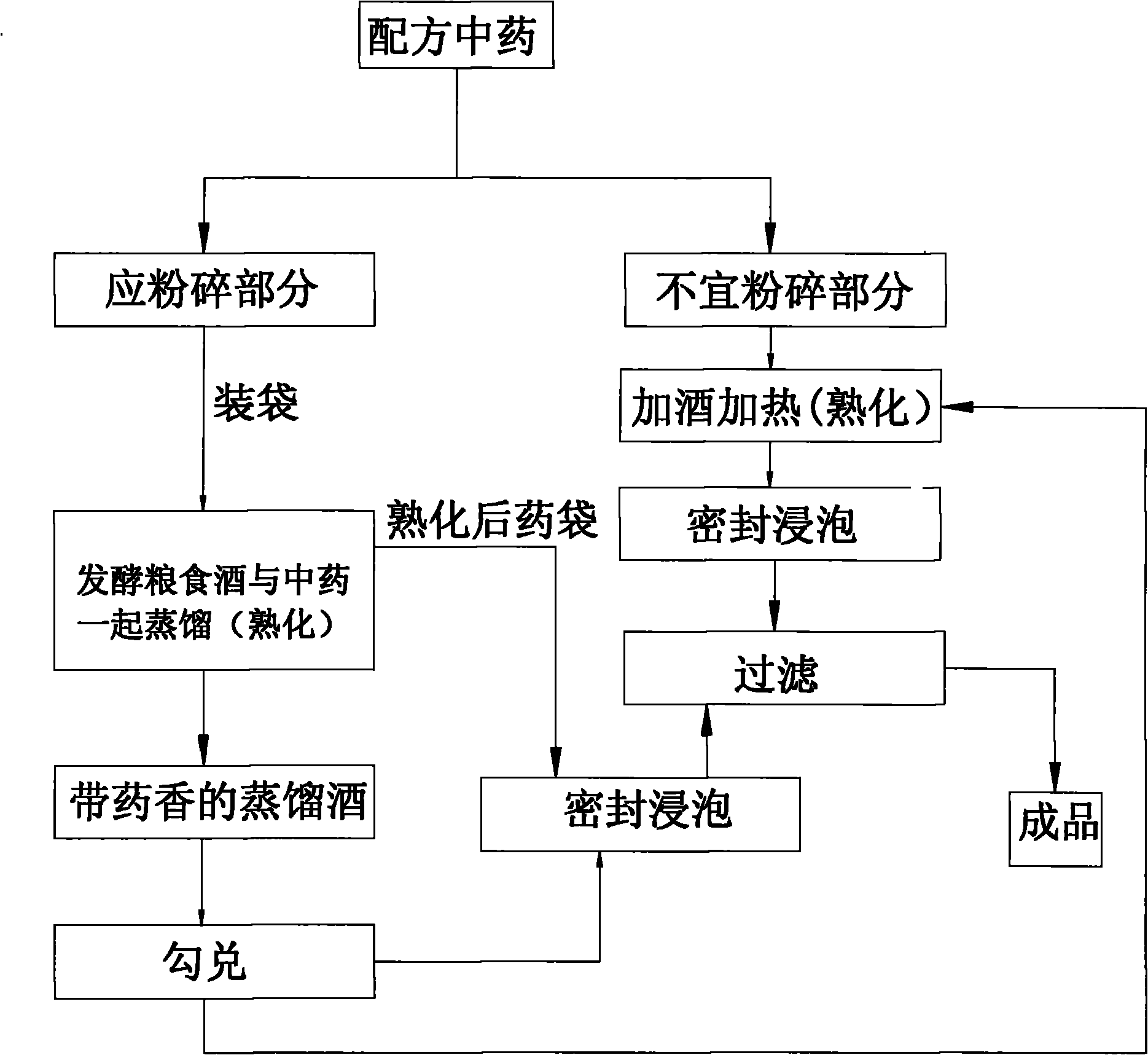
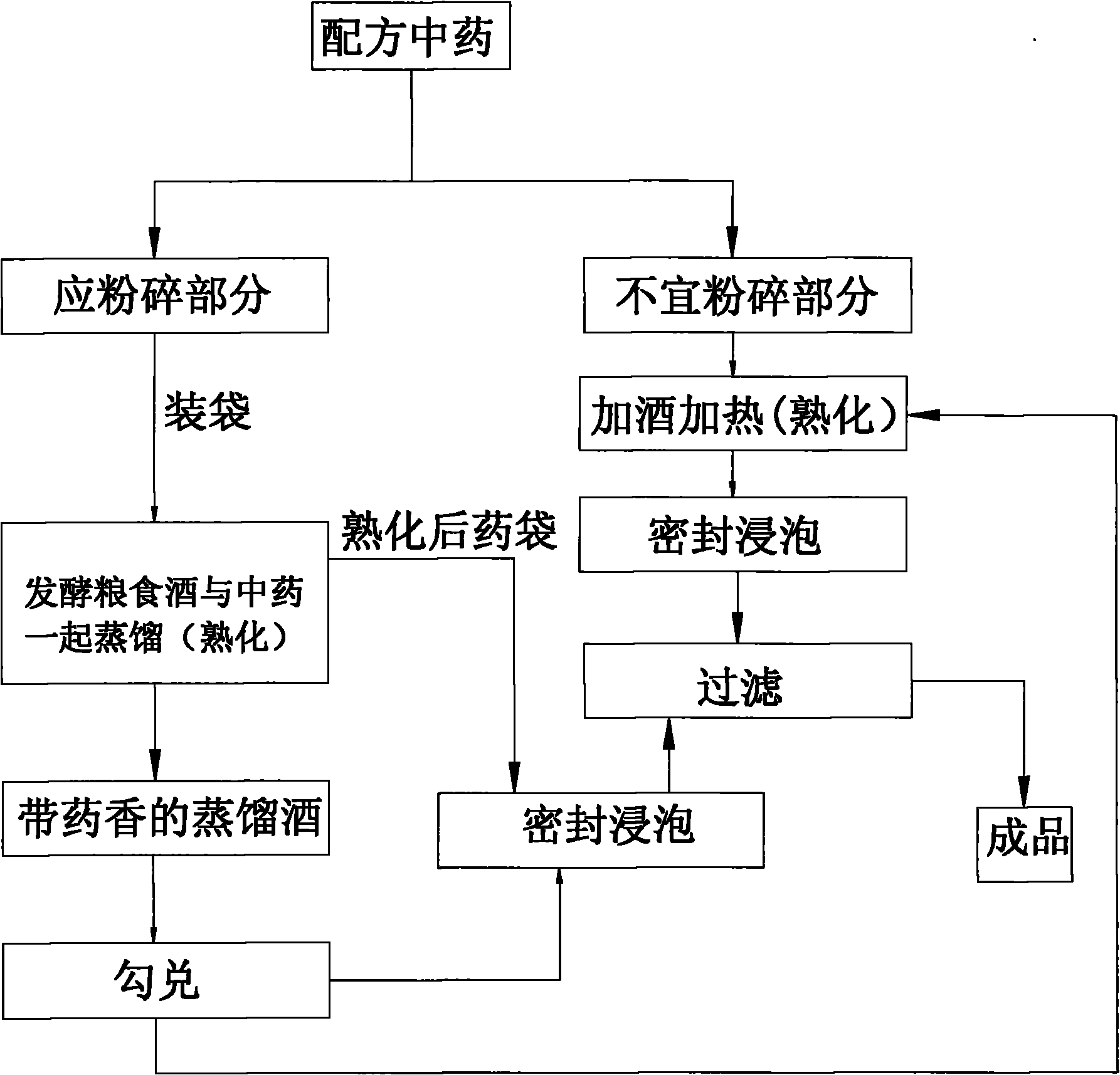
Preparation method for medicinal liquor
InactiveCN101768537AImprove medicinal propertiesGreat tasteAlcoholic beverage preparationChinese herbologyAdditive ingredient
The invention relates to a preparation method for medicinal liquor. The preparation method includes the following steps: A. traditional Chinese medicine in a formula is taken and crushed; B. the crushed traditional Chinese medicine is put into a bag and is placed in a distiller for distilling and curing together with fermented liquor grain till the baking of the liquor is finished so as to obtain distilled liquor with medicine scent, and then the traditional Chinese medicine bag is taken out for stand-by use; C. the distilled liquor obtained in the step B is blended to 38 to 50 DEG; D. the traditional Chinese medicine bag taken out in the step B is soaked in blended liquor obtained in the step C for 60 to 90 days in a sealed manner; E. the traditional Chinese medicine bag is taken out and filtered to obtain the traditional Chinese medicinal liquor in the invention. The medicinal liquor prepared by the method in the invention can lead effective ingredients of the traditional Chinese medicine to be dissolved in the liquor to the maximum degree. Moreover, the medicinal liquor has the advantages of being mellow as well as palatable and has good taste.
Owner:王建平
Tablet containing cefprozi liposome and preparation method thereof
InactiveCN101953787AHigh dissolution rateThe drug works quicklyAntibacterial agentsOrganic active ingredientsYolkCholesterol
The invention relates to a cefprozi liposome, a preparation method thereof and a tablet containing the cefprozi liposome. The cefprozi liposome comprises cefprozi, hydrogenated soybean phosphatidylcholine, hydrogenated egg yolk lecithin and cholesterol, wherein the weight ratio of the cefprozi, the hydrogenated soybean phosphatidylcholine, the hydrogenated egg yolk lecithin and the cholesterol is 1:1.25-5:1.25-5:2.5-10. The cefprozi tablet meets the requirements of Chinese Pharmacopoeia and also has the advantages of higher dissolution rate, faster effect and greatly enhanced bioavailability compared with the ordinary cefprozi drug composite.
Owner:王丽燕
Capsule containing cefprozil liposome and preparation method thereof
InactiveCN101953791AHigh dissolution rateThe drug works quicklyAntibacterial agentsOrganic active ingredientsYolkCholesterol
The invention relates to a cefprozil liposome and a preparation method thereof as well as a capsule containing the cefprozil liposome. The cefprozil liposome comprises cefprozil, hydrogenated yolk lecithin, soyabean lecithin, cholesterol and vitamin E, wherein the weight ratio of the cefprozil to the hydrogenated yolk lecithin to the soyabean lecithin to the cholesterol and to the vitamin E is 1:(1.25-5):(1.25-5):(2.5-10):(0.1-3). The cefprozil capsule not only meets the Chinese pharmacopoeia requirement but also has the advantages of higher dissolution rate, faster action of medicament effect and obvious improvement of bioavailability compared with those of a common cefprozil pharmaceutical composition.
Owner:王丽燕
Tianma food additive and preparing method thereof
InactiveCN101032301AHigh medicinal valueLow costFood preparationPlant ingredientsFood additiveGastrodia
The present invention discloses one kind of gastrodia tuber food additive and its preparation process. The gastrodia tuber food additive is prepared with gastrodia tuber starch gastrodia tuber dregs and gastrodia tuber, and through washing, crushing, grinding, centrifugal separating and drying. The present invention features the comprehensive gastrodia tuber utilizing process including separating fresh gastrodia tuber juice in a centrifuge to obtain fresh gastrodia tuber juice, fresh gastrodia tuber starch and gastrodia tuber dregs; producing gastrodia tuber beverage with the fresh gastrodia tuber juice; and producing food additive with gastrodia tuber starch, gastrodia tuber dregs and partial gastrodia tuber juice. The gastrodia tuber food additive has high medicinal value, low cost and simple production process.
Owner:熊桐培
Lumbricus protein health-care product and preparation technology thereof
InactiveCN107348484AGive full play to the medicinal effectImprove efficacyFood scienceDiseaseVitamin C
The invention discloses a lumbricus protein health-care product. The lumbricus protein health-care product consists of the following raw materials according to the formula by weight: lumbricus protein, a radix salviae miltiorrhizae extract, a glossy ganoderma extract, a cinnamon extract, lipoic acid, cornu saigae tataricae, semen cassiae, Chinese wolfberry fruits, coix seeds, ramuli umcariae cum uncis, semen platycladi, radix hemerocallis, vegetable protein, vitamin C, vitamin E and vitamin B. The invention further provides a preparation technology of the lumbricus protein health-care product. According to the lumbricus protein health-care product disclosed by the invention, the raw materials are mutually synergistic, so that the efficacy of the lumbricus protein health-care product is greatly improved, and the medical effects of lumbricus protein are fully exerted; various maintenance products are combined, so that the lumbricus protein health-care product has favorable treatment effects on diseases of heart and cerebral vessel systems and blood systems; and besides, the health-care product is prepared through combination of pure plants or extracts, free from toxins, free from side effects, simple in formula, low in cost and excellent in effects.
Owner:南京世界村天然保健品有限公司
Tapentadol pharmaceutical composition for transdermal administration as well as preparation method and application of pharmaceutical composition
ActiveCN111346077AImproves transdermal penetrationGuaranteed continuous performanceOrganic active ingredientsNervous disorderAcupuncturePharmaceutical medicine
The invention discloses a pharmaceutical composition for transdermal administration. The pharmaceutical composition contains tapentadol or a pharmaceutically acceptable salt thereof, a polymer dispersion carrier material, a hot-melt protective agent and an optional solubilizer. A preparation method of the pharmaceutical composition includes the following steps: mixing the tapentadol or the pharmaceutically acceptable salt thereof, the polymer dispersion carrier material, the hot-melt protective agent and the optional solubilizer, performing hot-melt extrusion, and performing micronization to obtain the microparticles of the tapentadol or the pharmaceutically acceptable salt thereof. The pharmaceutical composition for the transdermal administration can make the tapentadol or the pharmaceutically acceptable salt thereof rapidly absorbed, and achieve the purpose of sedation before anesthesia without affecting breathing, thereby avoiding the adverse psychological effects of intramuscular injection or intravenous injection acupuncture on children; and at the same time, the pharmaceutical composition can also be used to prevent and / or treat and delay the development of Parkinson disease.
Owner:YICHANG HUMANWELL PHARMA
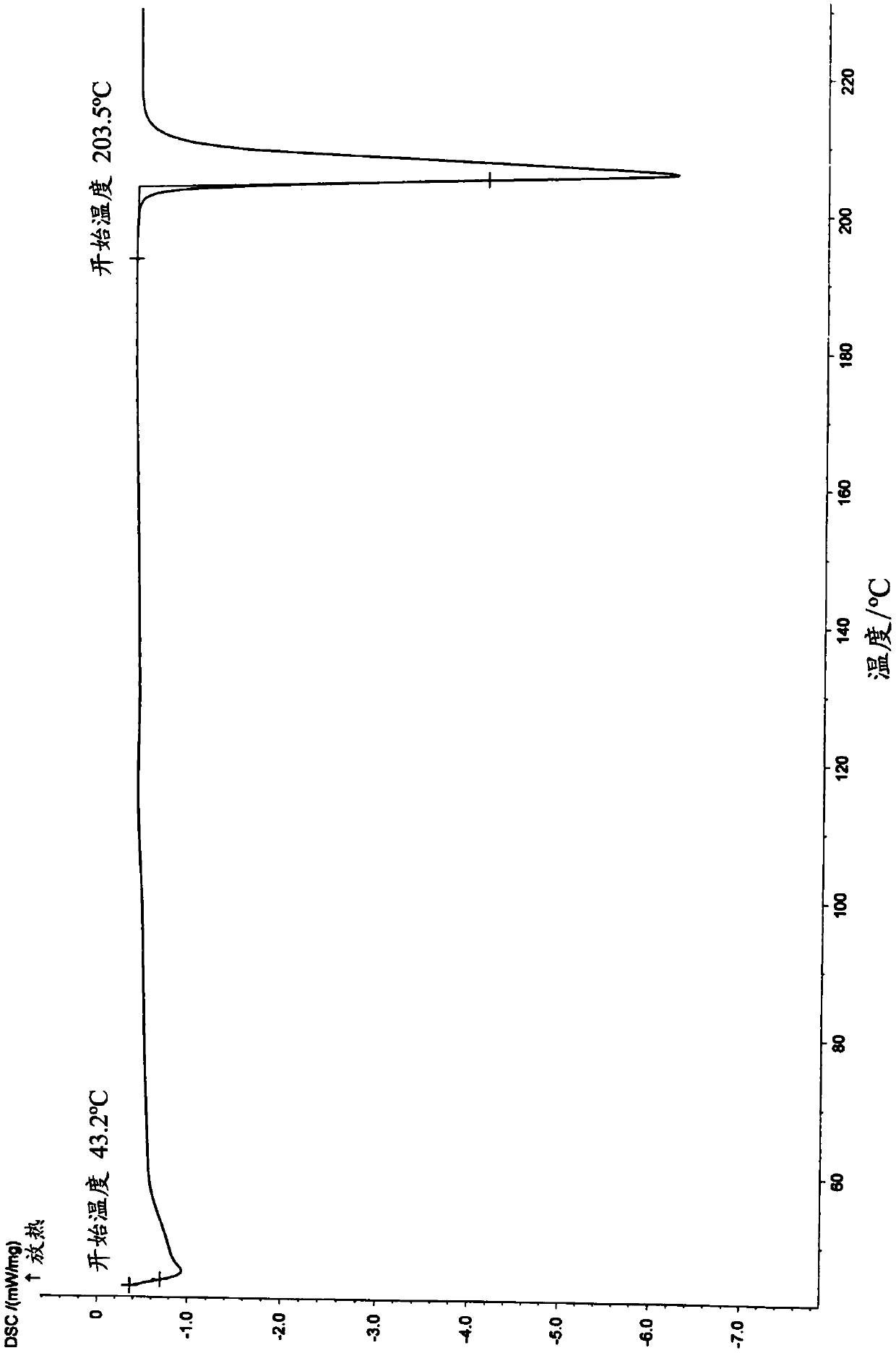
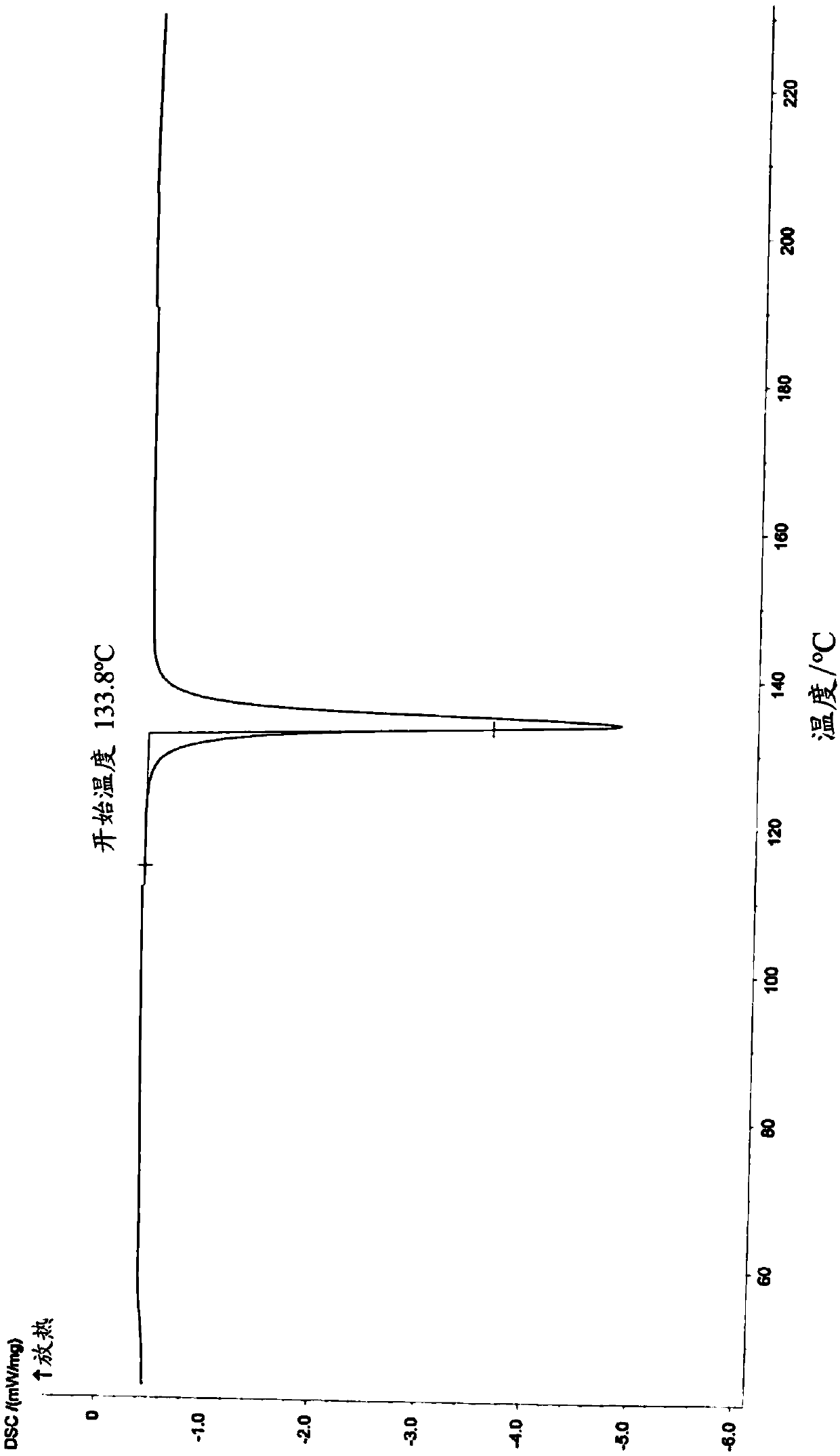
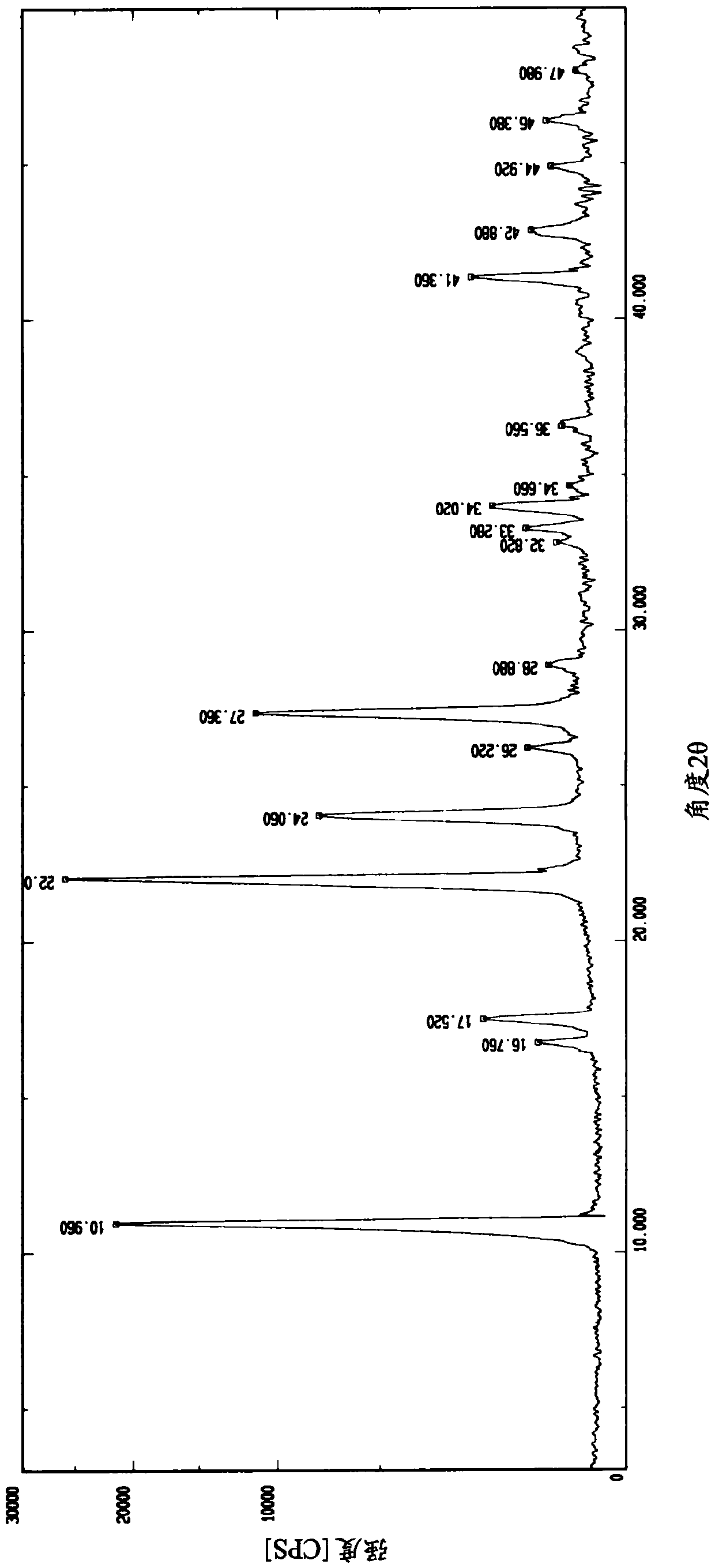
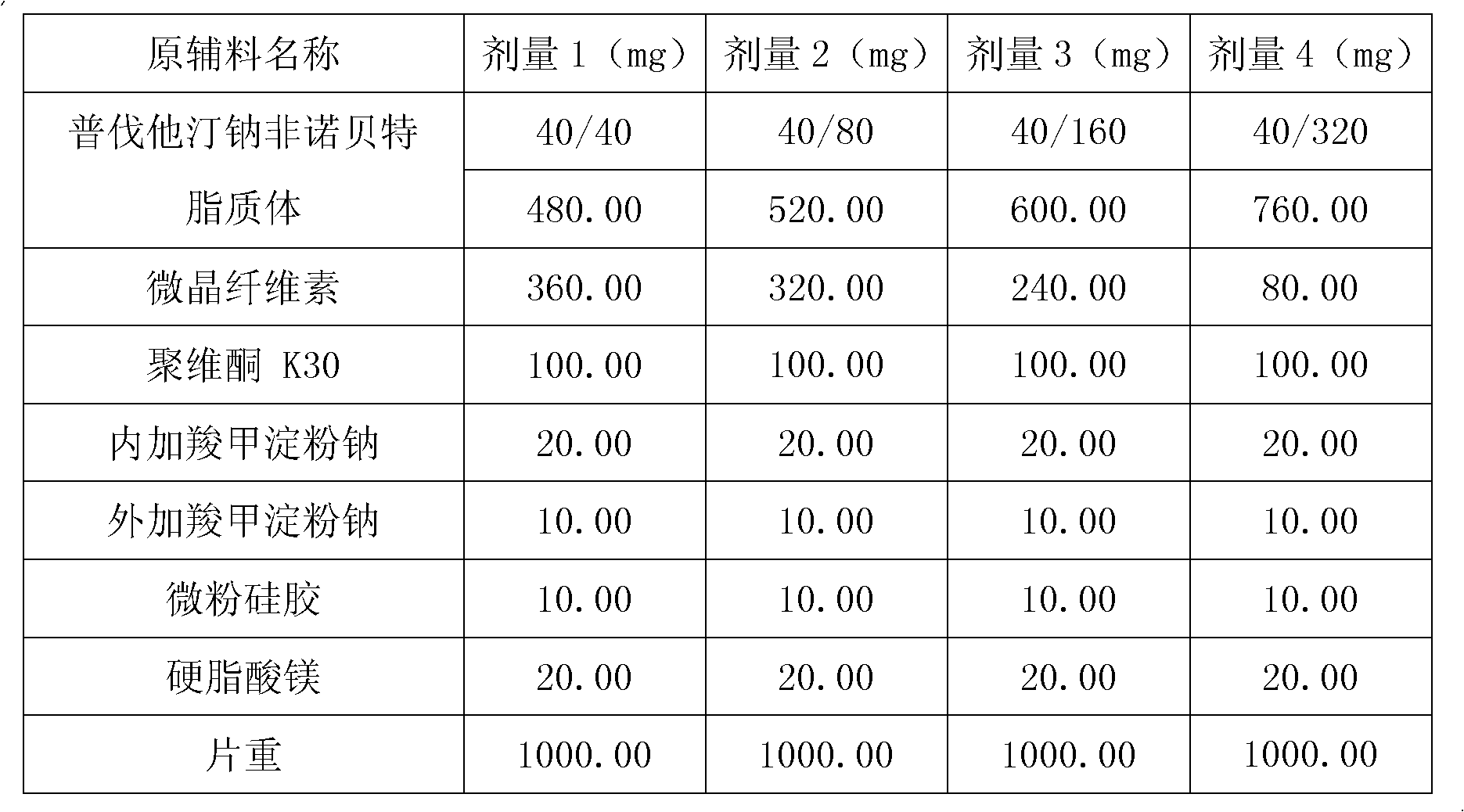
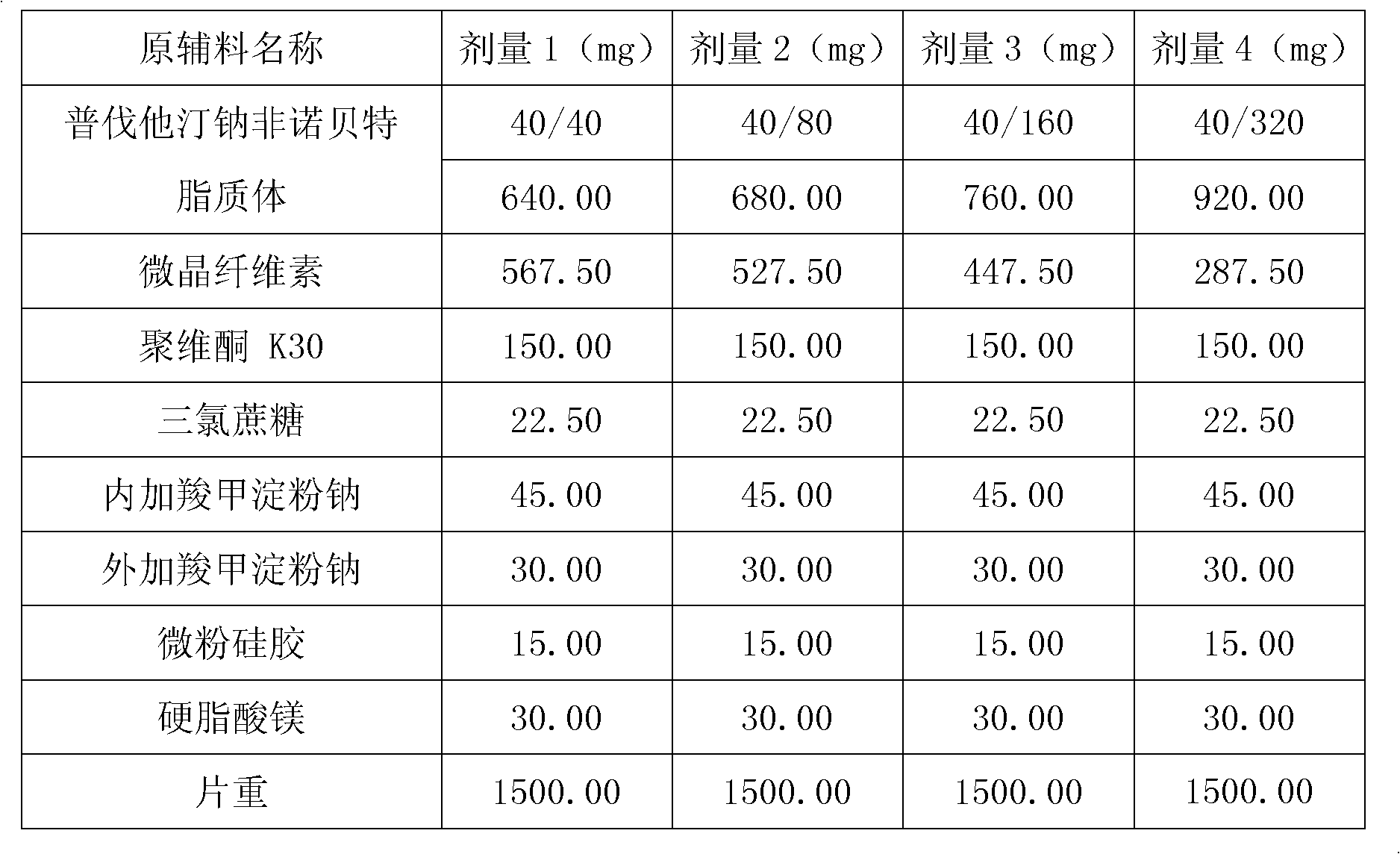
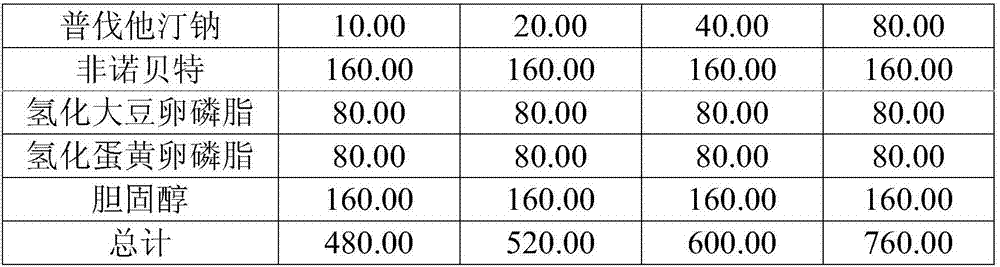
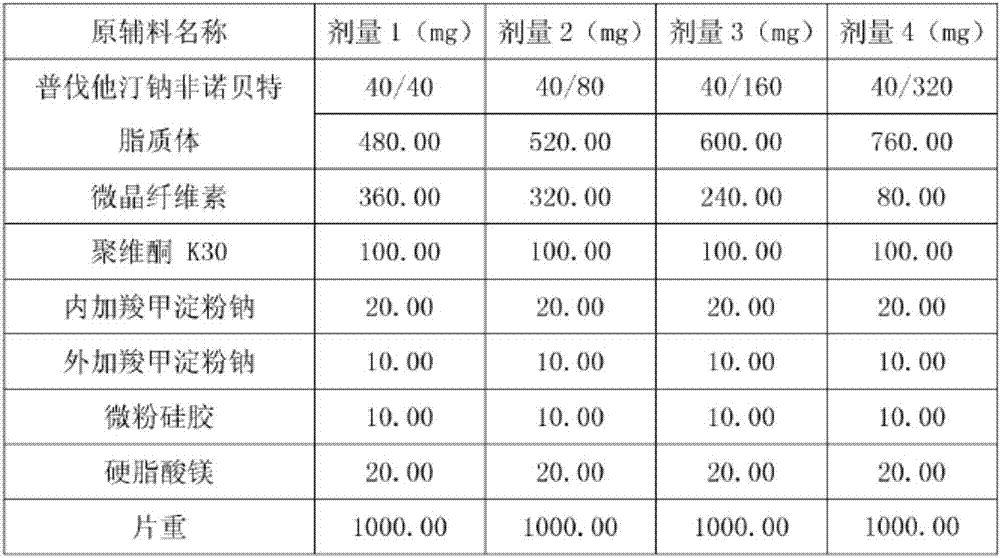
Medicine composition containing pravastatin sodium fenofibrate liposome and preparation method of medicine composition
InactiveCN102552143AHigh content of the main drugHigh dissolution rateMetabolism disorderPharmaceutical non-active ingredientsCholesterolDissolution
The invention relates to a medicine composition containing a pravastatin sodium fenofibrate liposome and a preparation method of the medicine composition. The medicine composition comprises a pravastatin sodium fenofibrate liposome and at least one pharmaceutically acceptable vector, wherein the pravastatin sodium fenofibrate liposome comprises pravastatin sodium, fenofibrate, phospholipid and cholesterol. The medicine composition prepared by the pravastatin sodium fenofibrate liposome not only satisfies the requirements of Chinese Pharmacopoeia and has the advantages of faster dissolution, faster medicine efficacy, high bioavailability, good stability as well as worth of being widely promoted and applied when being compared with the common pravastatin sodium fenofibrate medicine composition.
Owner:海南欣莱医药科技股份有限公司
Sufentanil pharmaceutical composition for transdermal administration as well as preparation method and application of composition
ActiveCN111419826AImproves transdermal penetrationGuaranteed continuous performanceOrganic active ingredientsNervous disorderAcupuncturePharmaceutical medicine
The invention discloses a pharmaceutical composition for transdermal administration. The pharmaceutical composition comprises sufentanil or a pharmaceutically acceptable salt thereof, a polymer dispersion carrier material, a hot-melt protective agent and an optional solubilizer. The pharmaceutical composition for transdermal administration is obtained by the following steps: mixing the sufentanilor the pharmaceutically acceptable salt thereof, the polymer dispersion carrier material, the hot-melt protective agent and the optional solubilizer, performing hot-melt extrusion and micronization toobtain microparticles of the sufentanil or the pharmaceutically acceptable salt of the sufentanil. In addition, the invention provides a preparation method and application of the above pharmaceuticalcomposition. According to the pharmaceutical composition for transdermal administration, the sufentanil can be quickly absorbed, the purpose of treating postoperative pain without affecting breathingcan be achieved, and adverse psychological effects of intramuscular injection or intravenous injection acupuncture on patients can be avoided; and at the same time, the pharmaceutical composition canbe used to prevent and / or treat postherpetic neuralgia.
Owner:YICHANG HUMANWELL PHARMA
Health-care product with function of relaxing bowel and preparation method of health-care product
InactiveCN106511776ASolve distressGive full play to the medicinal effectDigestive systemConiferophyta medical ingredientsCannabisSide effect
The invention provides a health-care product preparation capable of relaxing bowel. The health-care product preparation has the effects of relaxing bowel, and nourishing yin and generating body fluid. The formula is prepared from dendrobium officinale, mulberry fruits, radix polygoni multiflori, semen platycladi, fructus cannabis and Chinese angelica. Research results of traditional Chinese medicine theory and modern pharmacology, and experimental study on pharmacodynamics and clinical practice treatment application prove that the health-care product preparation has an accurate curative effect in the aspect of relaxing bowel and has no untoward effect; and the health-care product preparation has the effects of nourishing yin and generating body fluid, relaxing bowel and purging heat and removing stagnancy, is accurate in curative effect, rapid in effect and high in healing rate, can be used for treating both symptoms and root causes, and is safe and has no toxic or side effect.
Owner:杨文明
Alfentanil pharmaceutical composition for transdermal administration as well as preparation method and application of pharmaceutical composition
ActiveCN111419827AImproves transdermal penetrationGuaranteed continuous performanceOrganic active ingredientsInorganic non-active ingredientsMicroparticleAlfentanil
The invention provides an alfentanil pharmaceutical composition for transdermal administration as well as a preparation method and application of the pharmaceutical composition. The pharmaceutical composition for transdermal administration disclosed by the invention comprises alfentanil or a pharmaceutically acceptable salt thereof, a polymer dispersion carrier material, a hot-melt protective agent and an optional solubilizer, the pharmaceutical composition is obtained by the steps of mixing the alfentanil or the pharmaceutically acceptable salt thereof, the polymer dispersion carrier material, the hot-melt protective agent and the optional solubilizer, and performing hot-melt extrusion and micronization to obtain microparticles of the alfentanil or the pharmaceutically acceptable salt ofthe alfentanil. In addition, the invention provides the preparation method and application of the above pharmaceutical composition. According to the pharmaceutical composition for transdermal administration, the alfentanil or the pharmaceutically acceptable salt thereof can be quickly absorbed to achieve the purpose of pain anesthesia in treating without affecting breathing, and avoids the adversepsychological effects brought to patients by intramuscular injection or intravenous injection; and at the same time, the pharmaceutical composition can also be used to prevent and / or treat skin itching.
Owner:YICHANG HUMANWELL PHARMA
Pharmaceutical composition of morphin-6-glucuronid for transdermal administration and preparation method and application of pharmaceutical composition
ActiveCN111346104AImproves transdermal penetrationGuaranteed continuous performanceOrganic active ingredientsSkeletal disorderAnalgesia postoperativeAcupuncture
The invention discloses a pharmaceutical composition for transdermal administration. The pharmaceutical composition comprises morphin-6-glucuronid or a pharmaceutically acceptable salt thereof, a polymer disperse carrier material, a hot melt protecting agent and an optional fluxing agent, wherein the pharmaceutical composition for transdermal administration is prepared by mixing the morphin-6-glucuronid or the pharmaceutically acceptable salt thereof, the polymer disperse carrier material, the hot melt protecting agent and the optional fluxing agent, and then carrying out hot melt extrusion and micronization to obtain particles of the morphin-6-glucuronid or the pharmaceutically acceptable salt thereof. In addition, the invention further provides a preparation method and application of thepharmaceutical composition. According to the pharmaceutical composition for transdermal administration, the morphin-6-glucuronid or the pharmaceutically acceptable salt thereof can be quickly absorbed to achieve the target of postoperative analgesia without affecting breathing, so that an adverse psychological effect to a patient caused by acupuncture of intramuscular injection or intravenous acupuncture is avoided; and meanwhile, the pharmaceutical composition can also be used for preventing and / or treating lumbar and cervical diseases of the patient.
Owner:YICHANG HUMANWELL PHARMA
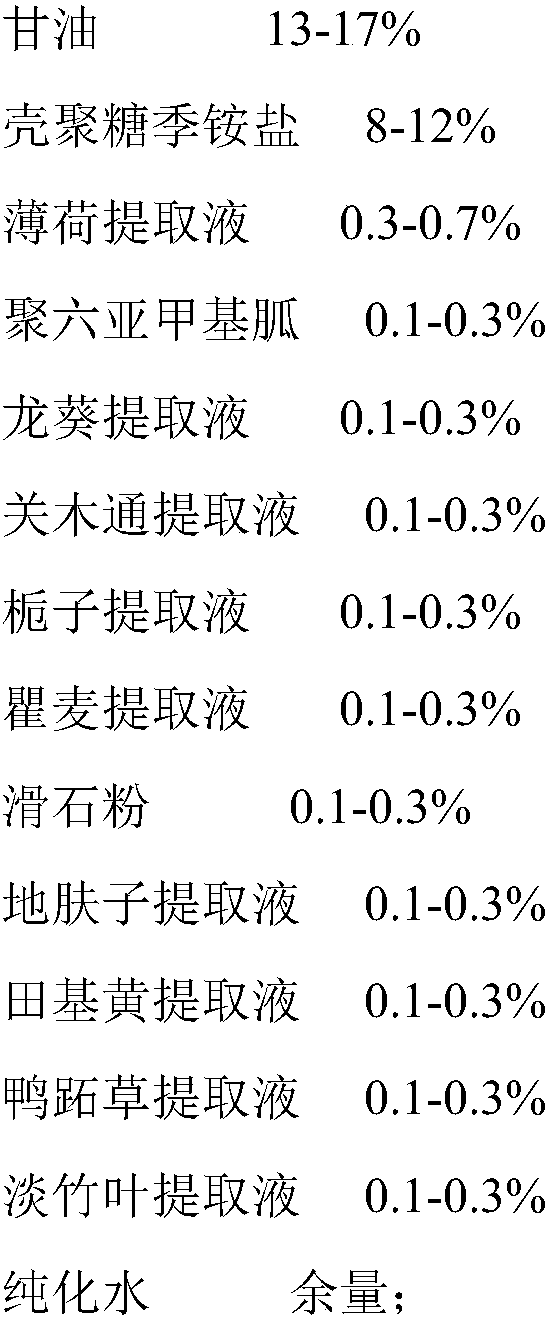
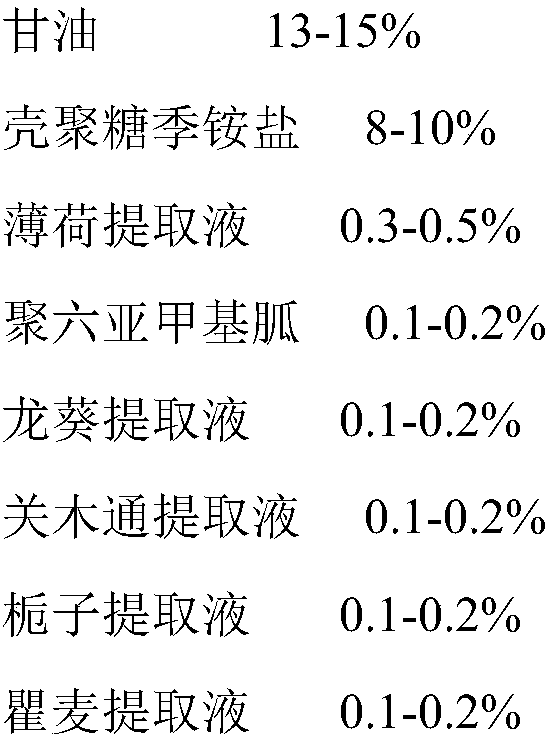
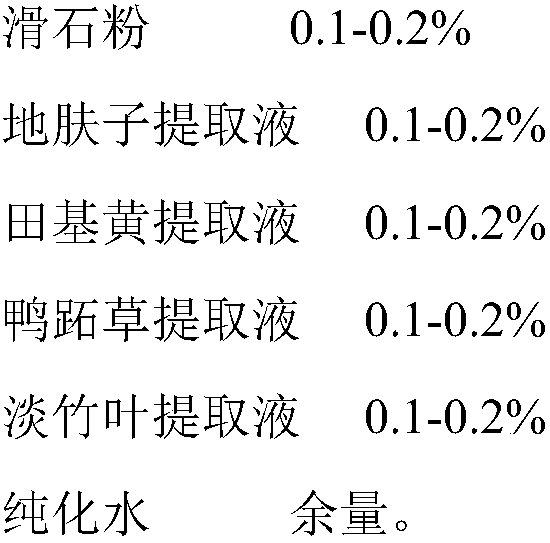
A kind of medical biological urinary hydrogel functional dressing and preparation method thereof
ActiveCN105012994BSimple processRaw materials are cheap and easy to getOrganic active ingredientsAerosol deliveryIrritationPolyhexamethylene guanidine
The present invention relates to the technical field of medical dressings, in particular to a medical biological urinary hydrogel functional dressing and a preparation method thereof. The medical biological urinary hydrogel functional dressing is composed of the following raw materials in percentage by weight: glycerin, chitosan quaternary ammonium Salt, peppermint extract, polyhexamethylene guanidine, Solanum nigrum extract, Akebia extract, gardenia extract, Qumai extract, talc powder, Kochia chinensis extract, Tianjihuang extract, Commelina extract liquid, light bamboo leaf extract, and the balance of purified water. The medical biological urinary hydrogel functional dressing of the present invention has little irritation, no toxic and side effects, safe use, good permeability, good curative effect, can give full play to the drug effect, has a long duration of action, and is not easy to relapse. When used in the anus, it directly penetrates into the prostatic body through the anal wall membrane, and can be widely used in the treatment of prostatitis, chronic benign prostatic hyperplasia, abscess caused by hypertrophy, urgency, frequent urination, and painful urination.
Owner:DONGGUAN DAQING MEDICAL DEVICES CO LTD +2
Heath care product for preventing and healing amnesia and insomnia and preparation method thereof
InactiveCN106421417AGive full play to the medicinal effectSoothe the nerves and benefit the mindNervous disorderPlant ingredientsAmnesiaModern medicine
The invention relates to a heath care product for preventing and healing amnesia and insomnia and preparation method thereof. The main ingredient of the product is dendrobium candidum. The prescription is composed based on the modern medicine and traditional Chinese medicinal theory. The health care product is an oral solution prepared from dendrobium candidum, stir-baked semen ziziphi spinosae, lily, Poria cum Radix Pini, dried rehmannia root, Ophiopogon japonicus and Silktree Albizia Bark. Through the exertion of the functions of compensating Qi, nurturing blood, nourishing heart and calming mind, channeling veins and dispelling wind, nourishing blood and tranquilizing mind, easing heart and restraining sweats, the product can attain the goal of healing the amnesia and insomnia from the 'roots', and solves the anxieties and pains of the people living in a modern life yet inflicted by the amnesia and insomnia.
Owner:杨文明
Pharmaceutical composition containing pravastatin sodium fenofibrate liposome and preparation method thereof
InactiveCN102552143BHigh content of the main drugHigh dissolution rateMetabolism disorderPharmaceutical non-active ingredientsPharmacologyPravastatin Sodium
Owner:海南欣莱医药科技股份有限公司
Cynanchum otophyllum schneid dispersible tablets and preparation method thereof
InactiveCN101357149BImprove bioavailabilityGive full play to the medicinal effectNervous disorderPill deliveryMedicineSodium stearyl
The invention discloses a cynanchum otophyllum dispersible tablet and a preparation method thereof, relating to medicines. The cynanchum otophyllum dispersible tablet is made of the following raw materials by weight portions: 50-200 portions of an extract of cynanchum otophyllum total glucoside, 20-100 portions of an disintegrating agent, 1-10 portions of a lubricant and 1-20 portions of a surfactant, wherein, the disintegrating agent is CMS-Na, CCNa, PVPP, L-HPC and dry starch, the lubricant is magnesium stearate or talcum powder or superfine silica gel powder, the surfactant is SDS, sodium hexadecyl sulfate and sodium stearyl sulfate. The preparation method consists of the following steps: 20-200 portions of total glucoside of the cynanchum otophyllum by weight is taken out and sieved with a 100-mesh sieve; 20-100 portions of the disintegrating agent by weight and 1-10 portions of the lubricant by weight are taken out and respectively sieved with the 100-mesh sieve; 1-20 portions ofthe surfactant by weight is taken out and sieved with a 80-mesh sieve; then the sieved extract, the sieved disintegrating agent, the sieved lubricant and the sieved surfactant are ground and evenly mixed, and then the mixture is sieved with the 80-mesh sieve, and direct compression and sieving are carried out on the sieved mixture to obtain the dispersible tablet. The dispersible tablet has high dissolution and high bioavailability, can fully exert the drug action of the cynanchum otophyllum, and the preparation method is simple and feasible while suitable for industrialized production.
Owner:GUIZHOU UNIV
Medicine for fumigating and washing anal disease
InactiveCN100391502CBlood back to normalBlood circulation returns to normalAnthropod material medical ingredientsAluminium/calcium/magnesium active ingredientsClematisNepeta
The present invention relates to a Chinese medicine for curing anal diseases and its preparation method. The described medicine is made up by using 11 Chinese medicinal materials of clematis root, dahurian angelica root, chrysanthemum, flower, wolfberry bark, rubia root and others through the processes of pulverizing and mixing.
Owner:陈星儒
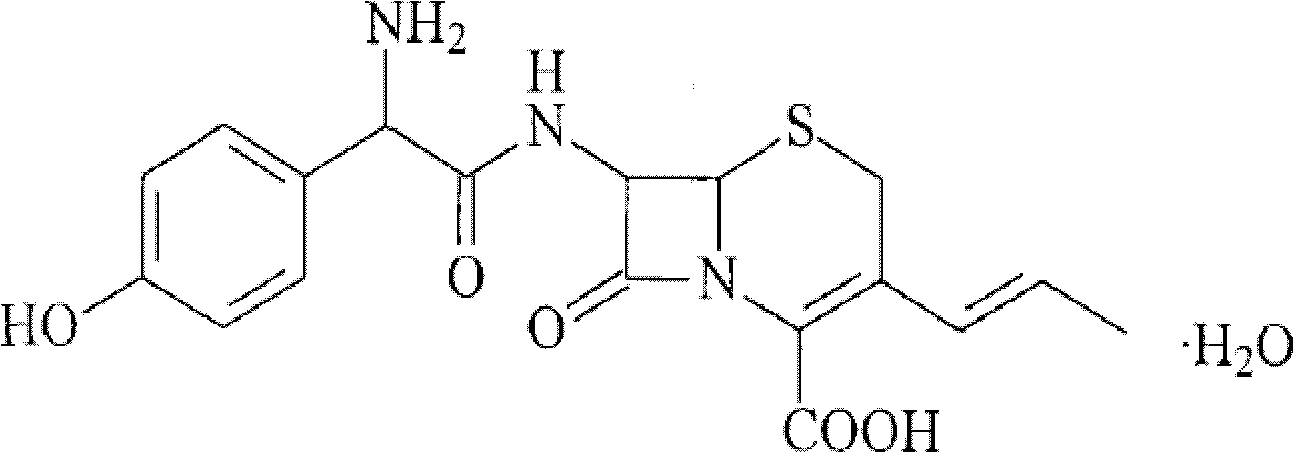
Cefalexin liposome and medicinal composition thereof
InactiveCN101579313BThe encapsulation rate exceedsReduced stabilityAntibacterial agentsOrganic active ingredientsMedicineCholesterol
The invention discloses a cefalexin liposome and a medicinal composition thereof. The cefalexin liposome is mainly prepared from cefalexin, phospholipid, cholesterol and poloxamer 188 in certain proportion.
Owner:HAINAN MEIDA PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com