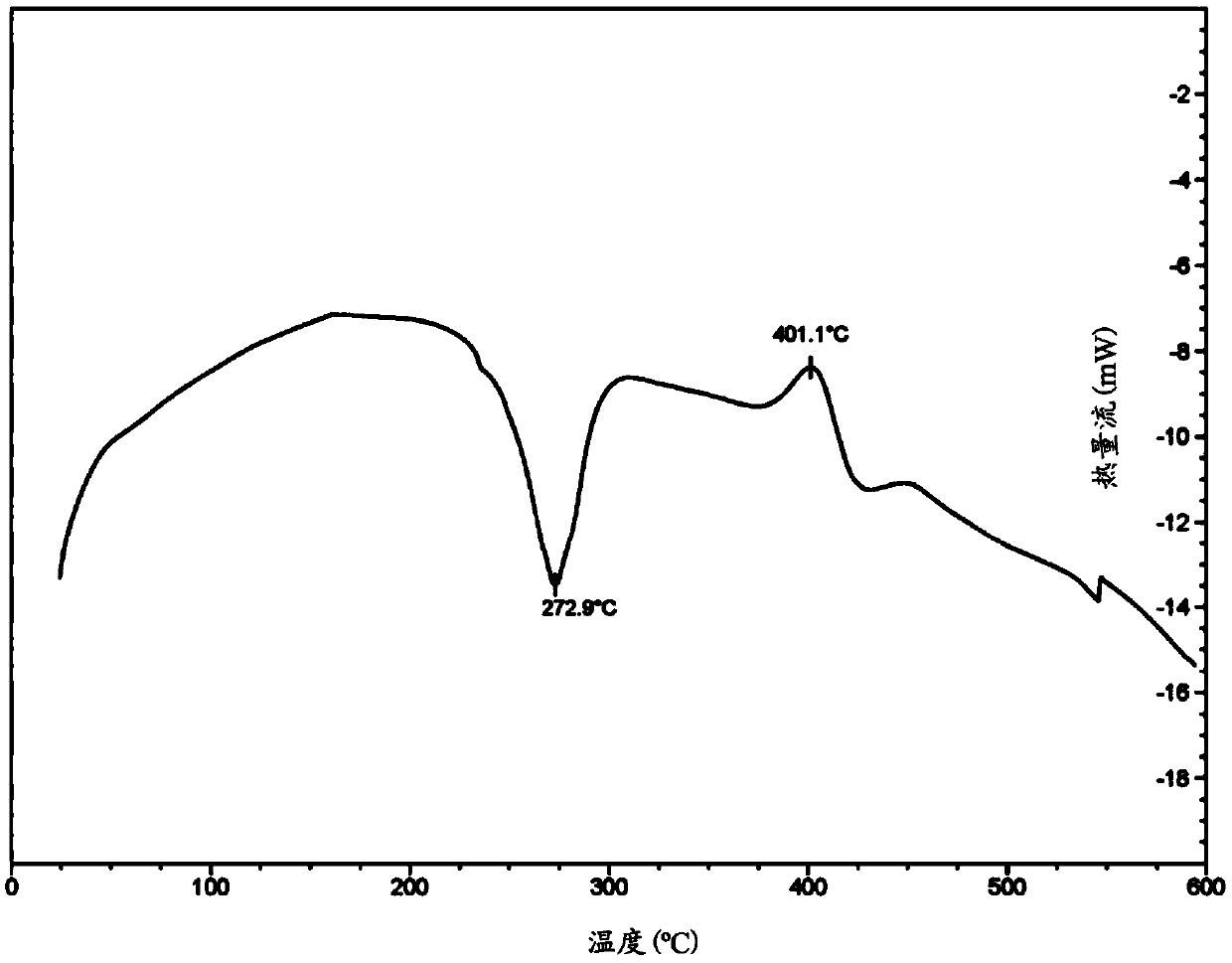
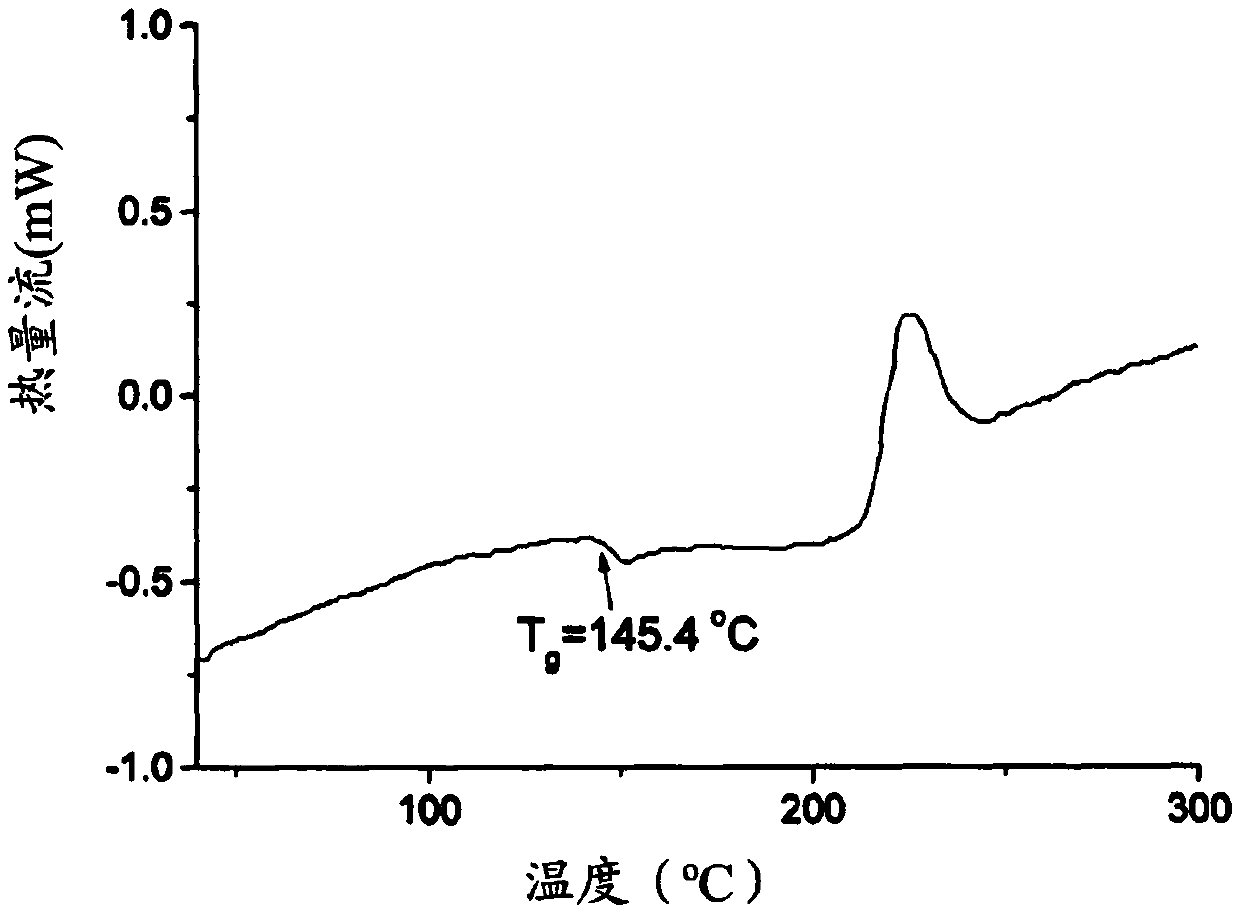
Transdermal delivery based pharmaceutical composition and preparation method and application thereof
A technology for transdermal drug delivery and composition, applied in the field of transdermal drug delivery pharmaceutical composition and preparation thereof, can solve the problems of inconvenient medication for patients, adverse psychological effects of patients, poor compliance and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] The transdermal patch preparation of compound (dexketamine) hydrochloride shown in embodiment 1 formula (I)
[0070] (1) 5 g of compound (dexketamine) hydrochloride shown in formula (I), 2 g of povidone (PVP-S630), 0.12 g of magnesium stearate are micronized, and polyethylene glycol (molecular weight 2000) 2.88 g is added , mixed evenly to obtain a physical mixture;
[0071] (2) Set the extrusion temperature of the twin-screw extruder to 120°C, start the screw after rising to the set temperature, add the physical mixture in step (1) to the extruder, heat-melt and extrude, Extrude in the form of spherical particles to obtain amorphous particles, and then micronize them to obtain micronized amorphous particles, the particle size is controlled at about 100-150nm.
[0072] (3) Weigh 5 g of micronized amorphous particles prepared in step (2), 0.2 g of soybean lecithin, 0.03 g of cholesterol, 3 g of absolute ethanol, and 1.77 g of water;
[0073] (4) dissolving soybean leci...
Embodiment 2
[0077] Example 2 Preparation of transdermal patch of compound (ketamine) represented by formula (I)
[0078] (1) Micronize compound (ketamine) 6g and povidone (PVP-S630) 1g, magnesium stearate 0.18g shown in formula (I), add polyethylene glycol (molecular weight 3000) 2.82g, mix well, produce a physical mixture;
[0079] (2) Set the extrusion temperature of the twin-screw extruder to be 100°C, start the screw after rising to the set temperature, add the physical mixture in the step (1) into the extruder, and then heat-melt and extrude, It is extruded into spherical particles to obtain amorphous particles, which are then micronized to obtain micronized amorphous particles, and the particle size is controlled at about 150-200 nm.
[0080] (3) Weigh 5 g of micronized amorphous particles prepared in step (2), 0.2 g of phosphatidylcholine, 0.08 g of cholesterol, 3 g of propylene glycol, and 1.72 g of water;
[0081] (4) dissolving phosphatidylcholine, cholesterol, and the compoun...
Embodiment 3
[0085] Example 3 Preparation of the transdermal patch of the compound represented by formula (I) (levoketamine)
[0086] (1) Micronize 7 g of the compound represented by formula (I) (levoketamine), 1.5 g of povidone (PVP-VA64) and 0.2 of talc, add 1.3 g of polyethylene glycol (molecular weight 4000), mix well, and prepare a physical mixture;
[0087] (2) Set the extrusion temperature of the twin-screw extruder to be 140°C, start the screw after rising to the set temperature, add the physical mixture in the step (1) to the extruder, and then heat-melt and extrude, It is extruded into spherical particles to obtain amorphous particles, which are then micronized to obtain micronized amorphous particles, and the particle size is controlled at about 250-300 nm.
[0088] (3) Weigh 7g of micronized amorphous particles prepared in step (2), 2g of dipalmitoyl phosphatidylcholine, 0.3g of cholesterol, 30g of dehydrated alcohol, and 60.7g of water;
[0089] (4) dissolving dipalmitoyl ph...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com