Compound lidocaine medicine composition and preparation method thereof
A lidocaine and composition technology, applied in the field of medicine, can solve problems such as unsatisfactory stability, influence on product quality, decreased stability, etc., and achieve the effects of enhancing percutaneous penetration effect, shortening operation time, and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
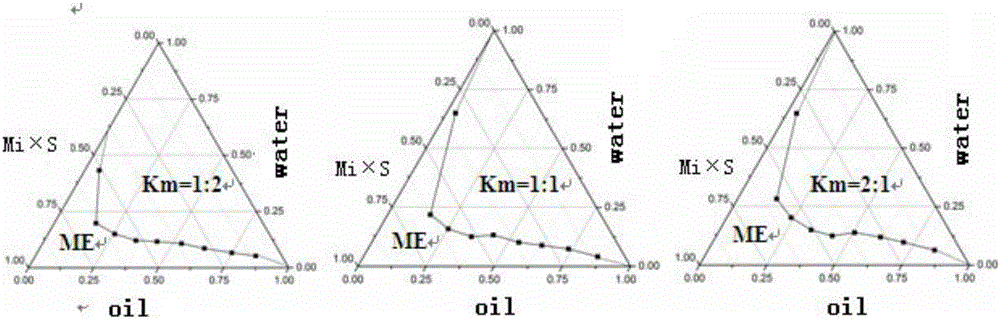
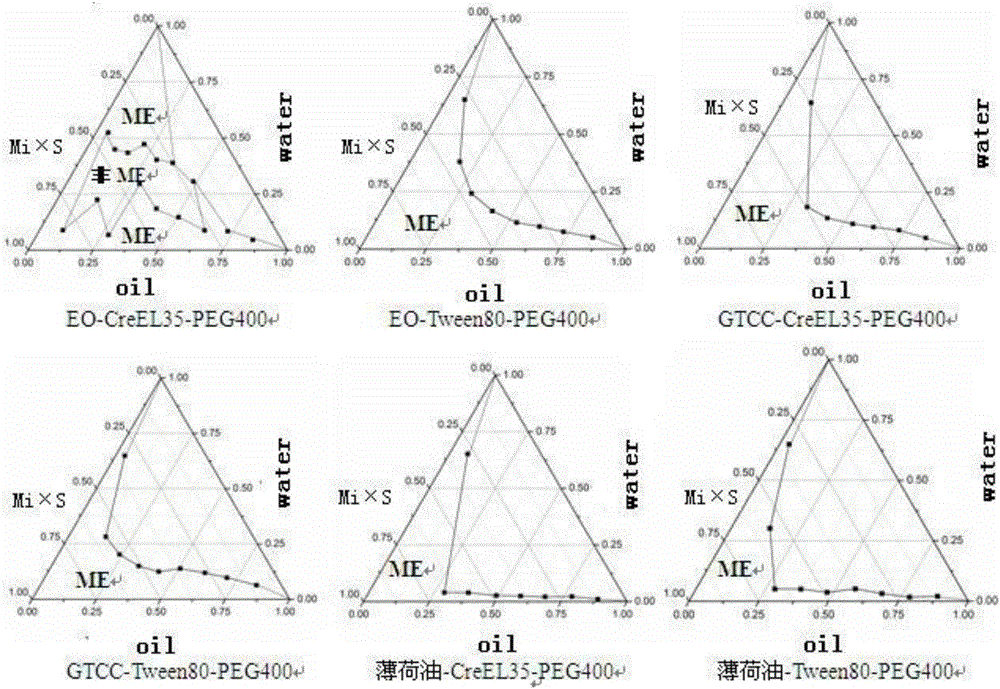
[0033] [Recipe] Lidocaine 2g, prilocaine 3g, peppermint oil 2g, caprylic capric macrogolglyceride 18g, polyethylene glycol 4006g and water 80g.
[0034] [Preparation method] Weigh the oil phase, surfactant, co-surfactant, lidocaine and prilocaine according to the formula and stir to form a mixed drug solution, then add dropwise the water weighed according to the formula, using The pharmaceutical composition is prepared by adding water dropwise.
Embodiment 2
[0036] [Recipe] Lidocaine 3g, prilocaine 2g, oleic acid 9g, Tween-8013g, ethanol 9g and water 60g.
[0037] [Preparation method] Weigh the oil phase, surfactant, co-surfactant, lidocaine and prilocaine according to the formula and stir to form a mixed drug solution, then add dropwise the water weighed according to the formula, using The pharmaceutical composition is prepared by adding water dropwise.
Embodiment 3
[0039] [Recipe] Lidocaine 2.5g, prilocaine 2.5g, ethyl oleate 5.5g, polyoxyethylene castor oil 15.5g, polyethylene glycol 4007.5g and water 70g.
[0040] [Preparation method] Weigh the oil phase, surfactant, co-surfactant, lidocaine and prilocaine according to the formula and stir to form a mixed drug solution, then add dropwise the water weighed according to the formula, using The pharmaceutical composition is prepared by adding water dropwise.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com