Patents
Literature
76results about How to "Promote percutaneous absorption" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
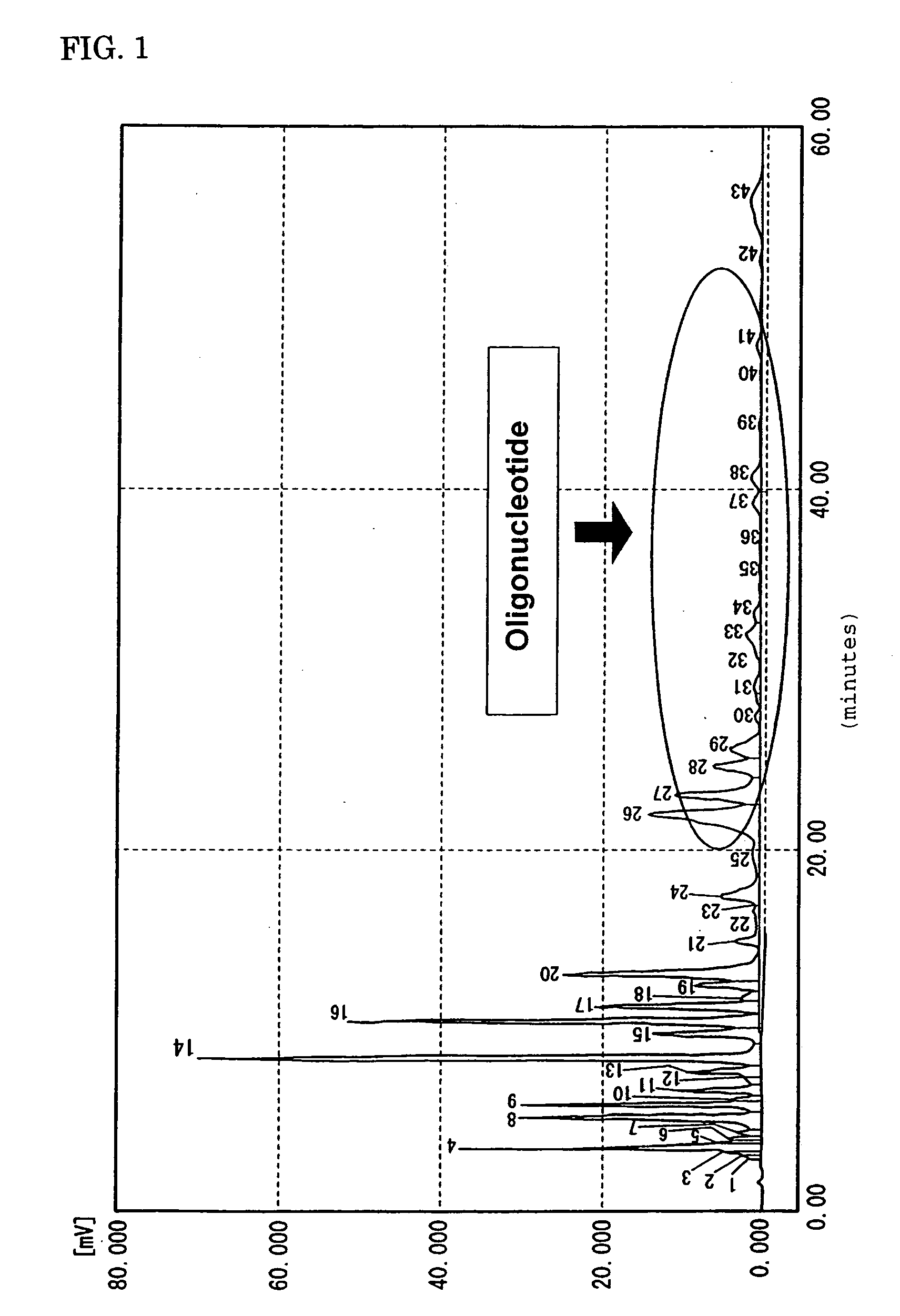
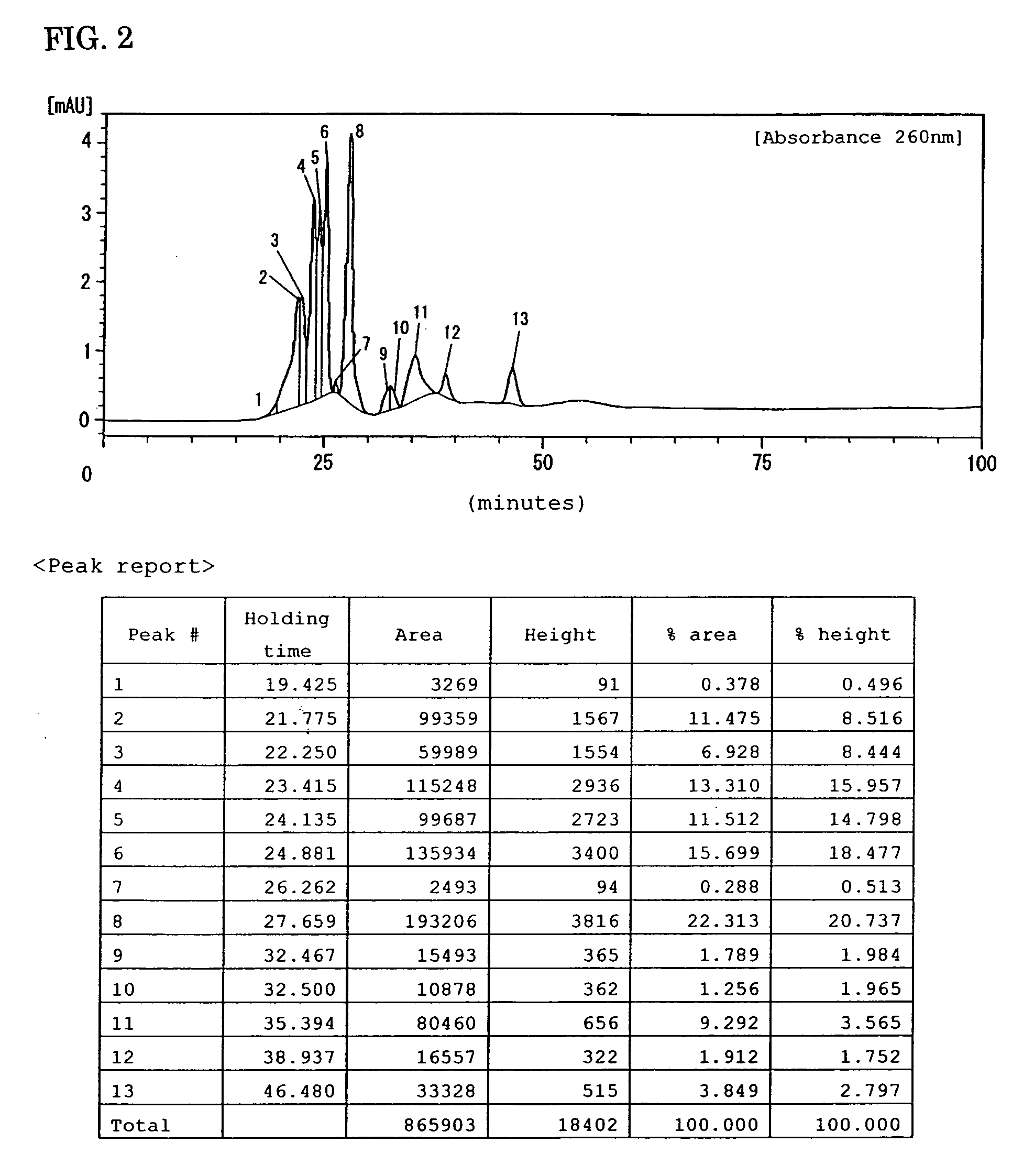
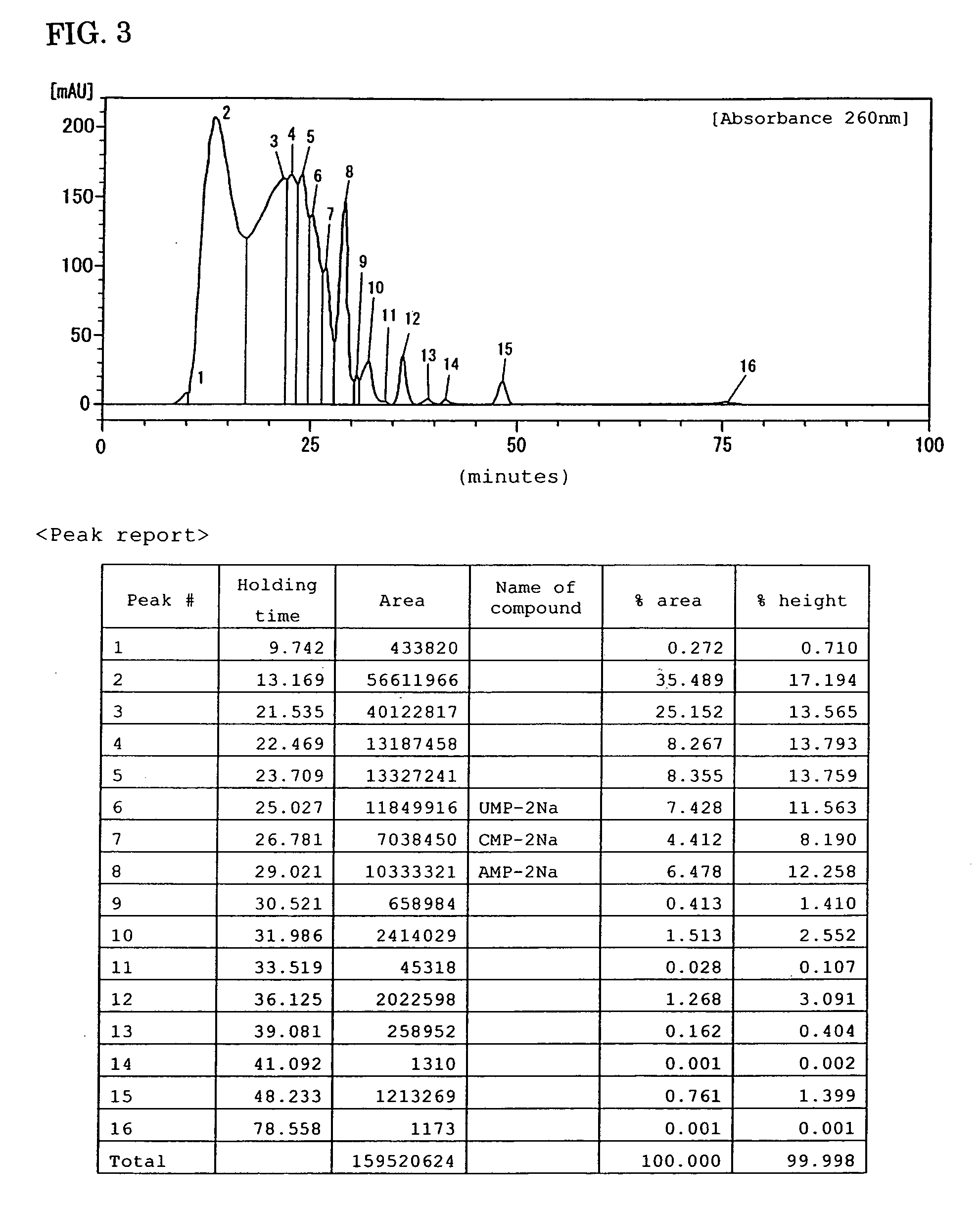
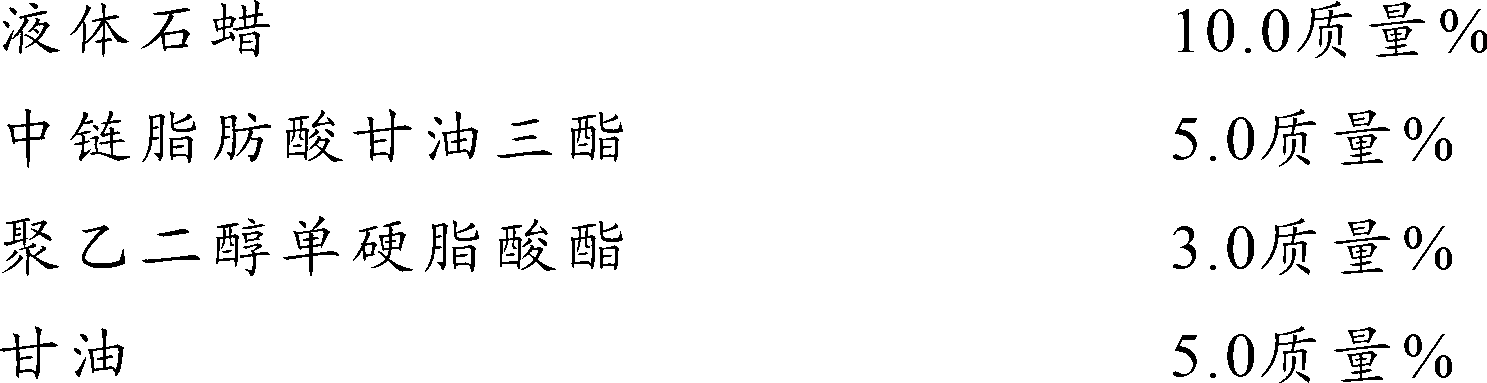
Preparations for percutaneous absorption
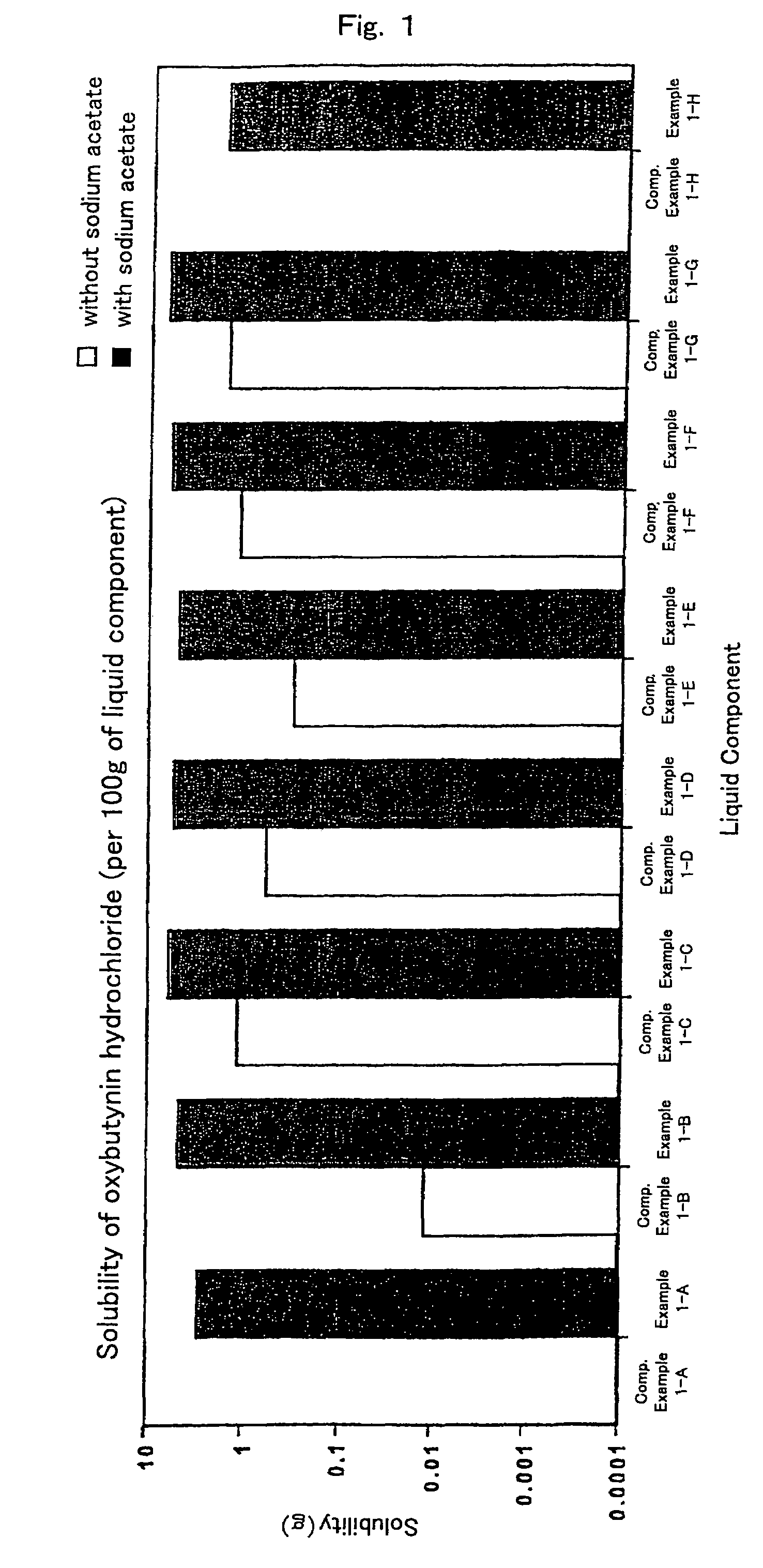
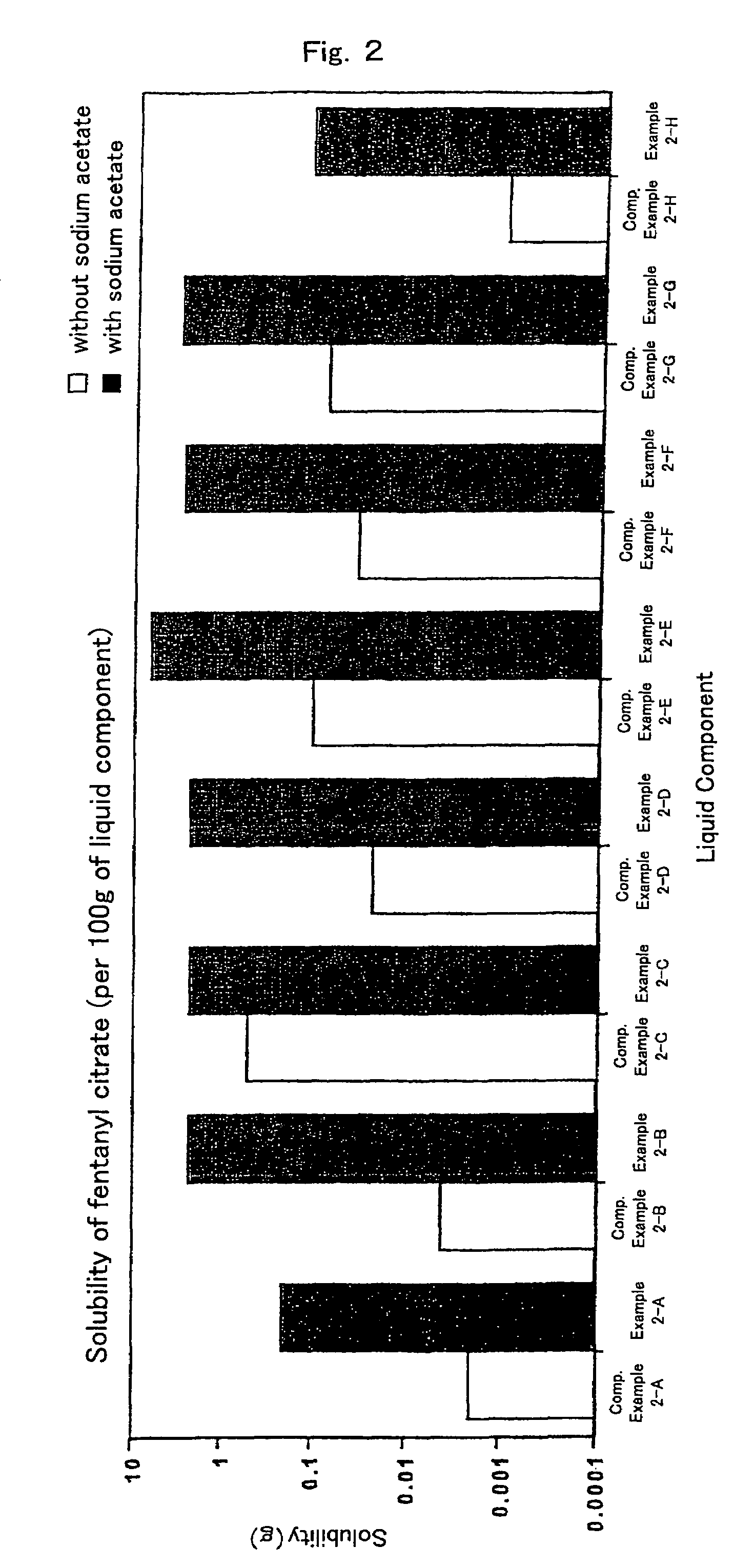
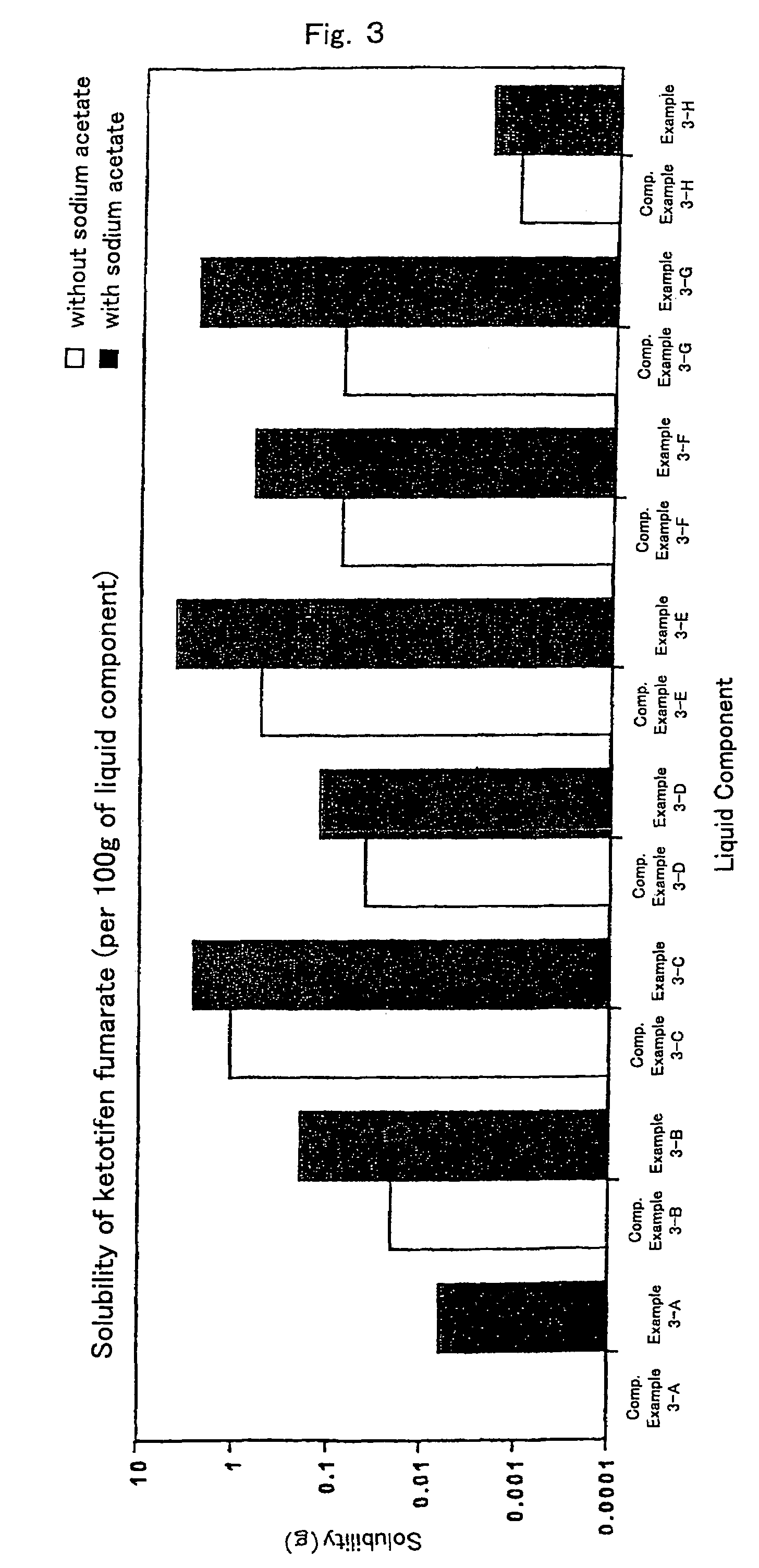
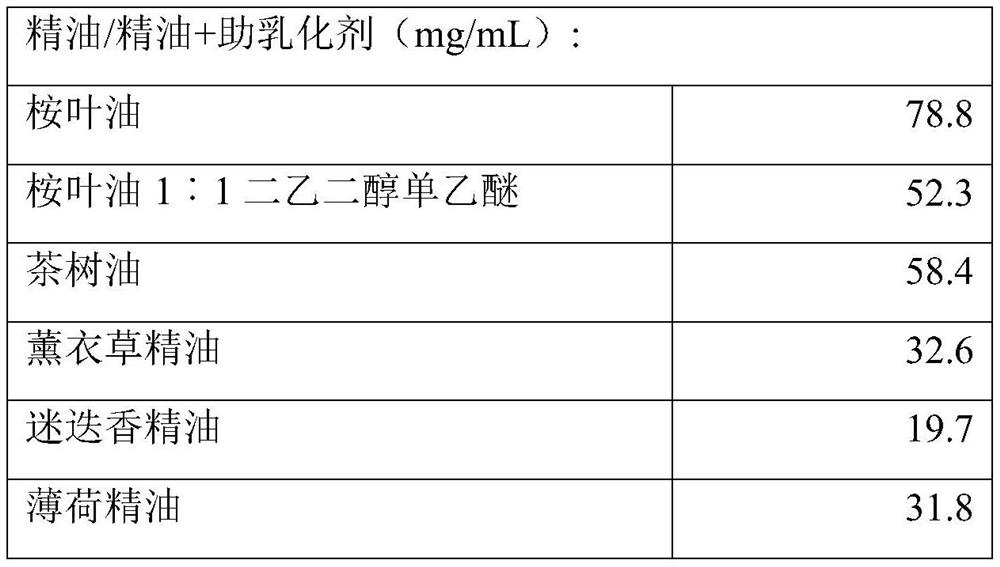
InactiveUS7504114B1Promote percutaneous absorptionSkin safeAntiinfectivesPharmaceutical non-active ingredientsOrganic acidSolubility
Preparations for percutaneous absorption comprising a basic drug or its salt dissolved in a liquid component and having an enhanced percutaneous absorbability and a safety to the skin, i.e., the administration site. The preparations for percutaneous absorption, preferably patches, contain a basic drug or its salt, an organic acid or its salt and a liquid component having a solubility parameter of from 7 to 13 (cal / cm3)1 / 2 and a have a very excellent skin permeability of the drug.
Owner:HISAMITSU PHARM CO INC
Skin External Preparation
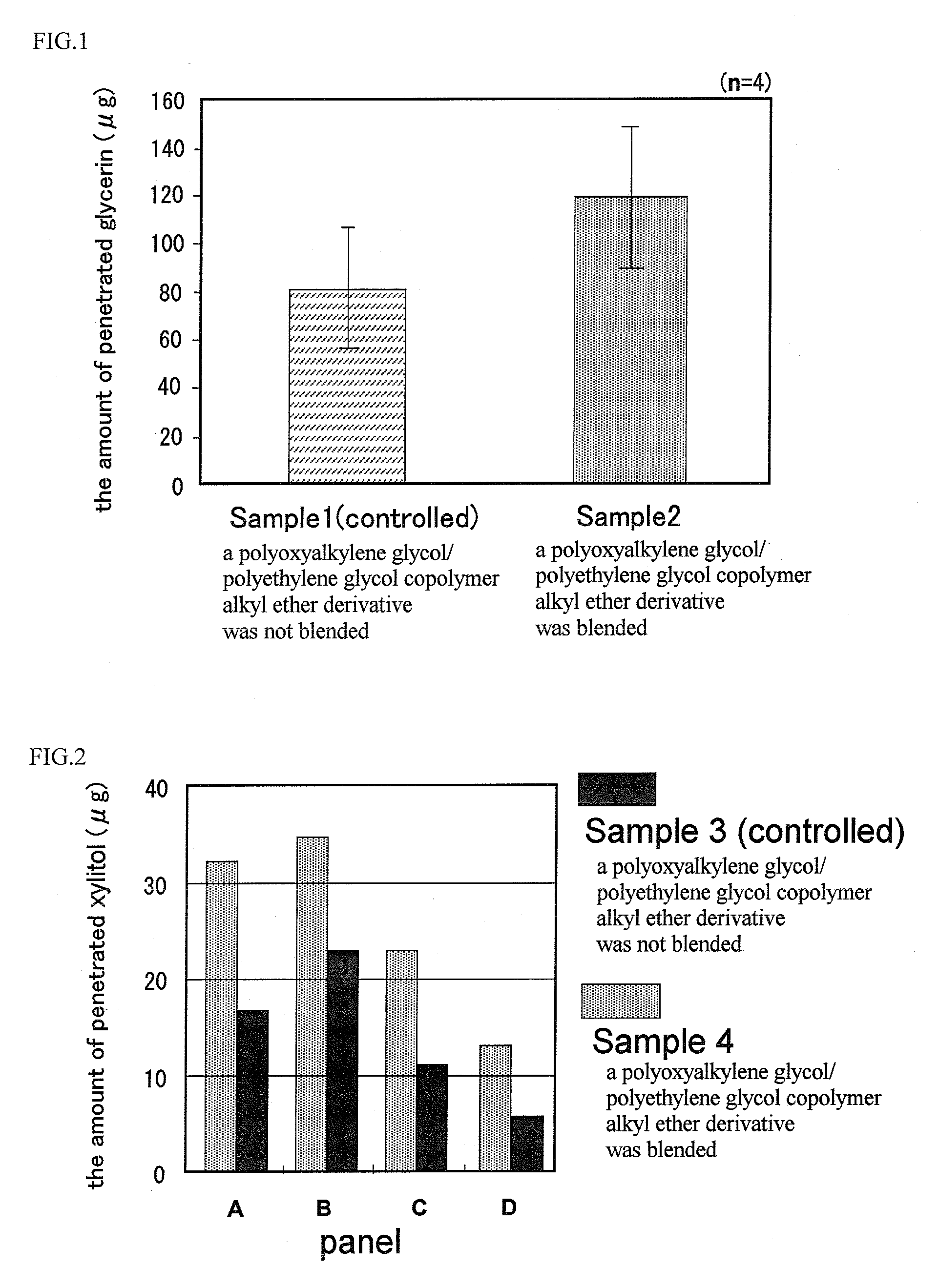
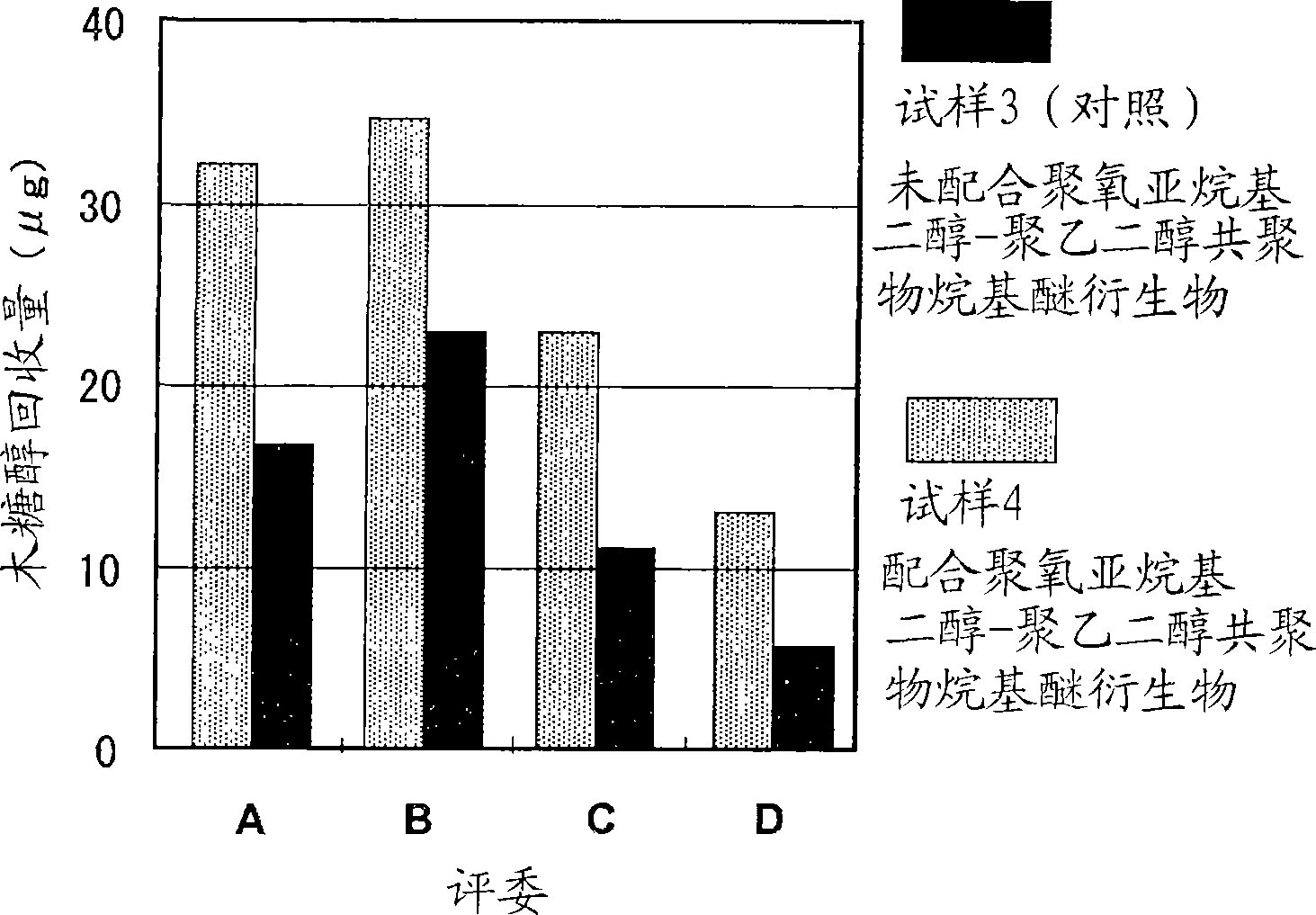
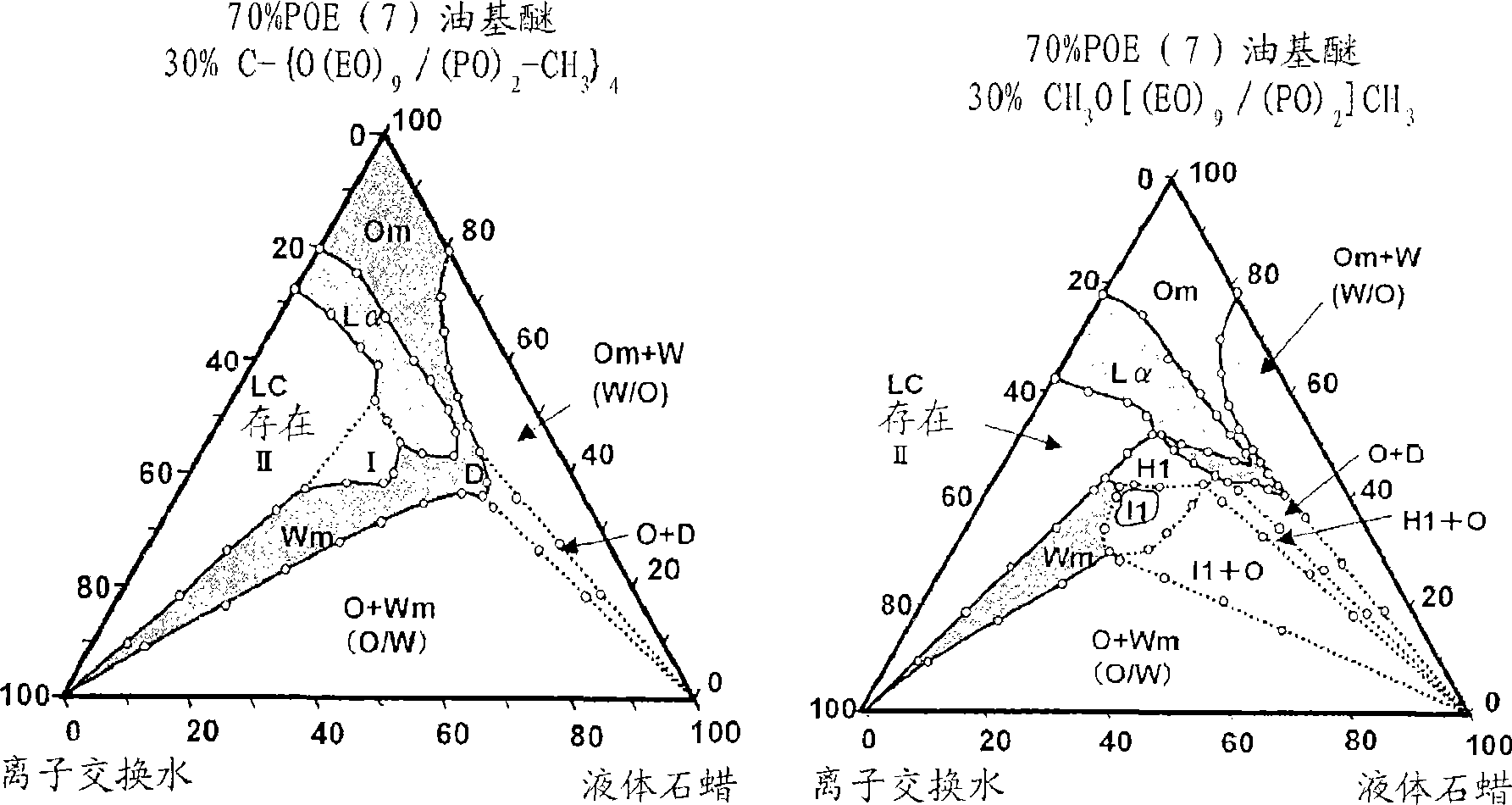
InactiveUS20090197948A1Improve texturePromotes stratum corneum penetrationBiocideCosmetic preparationsPolyethylene glycolRough skin
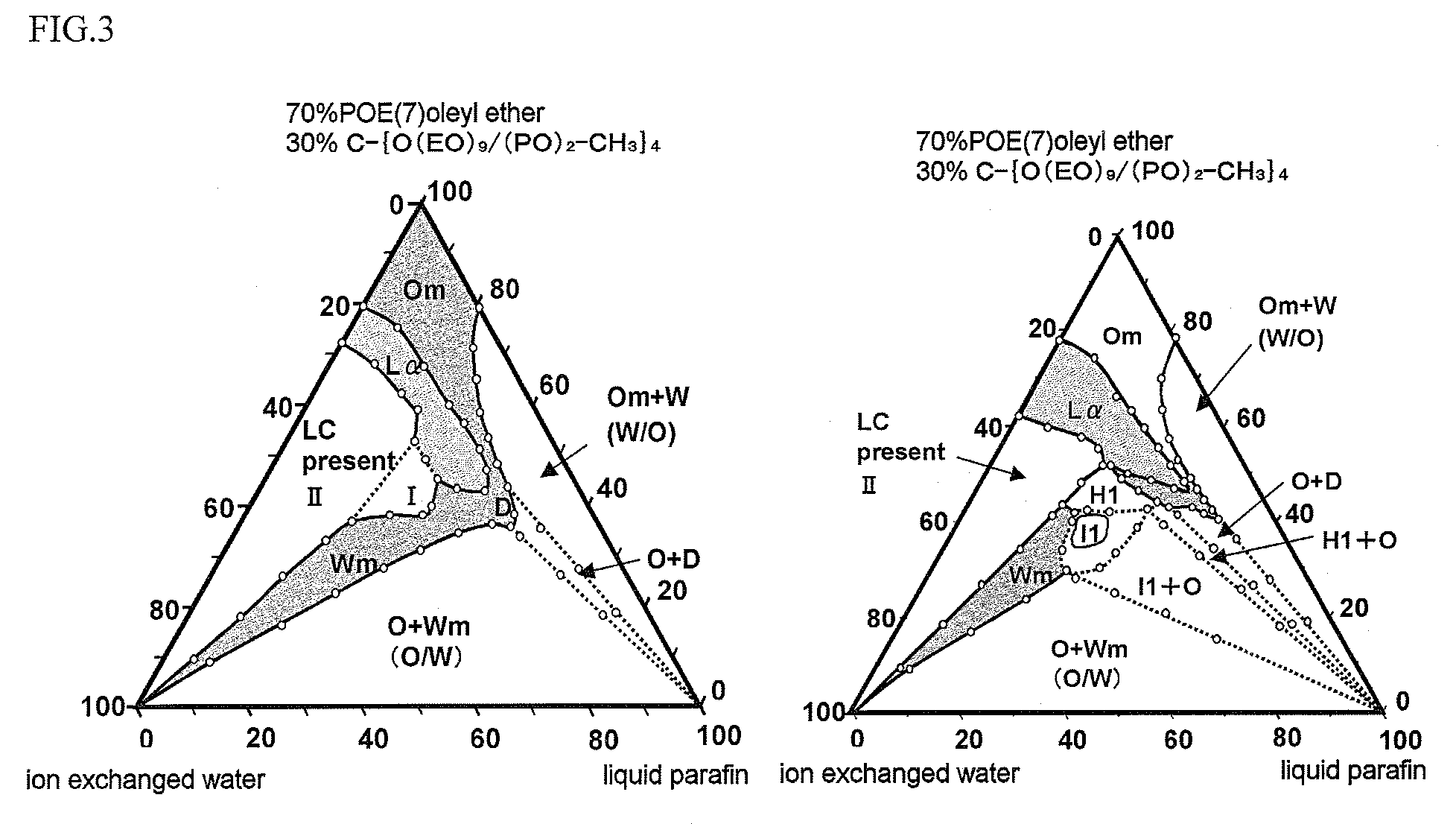
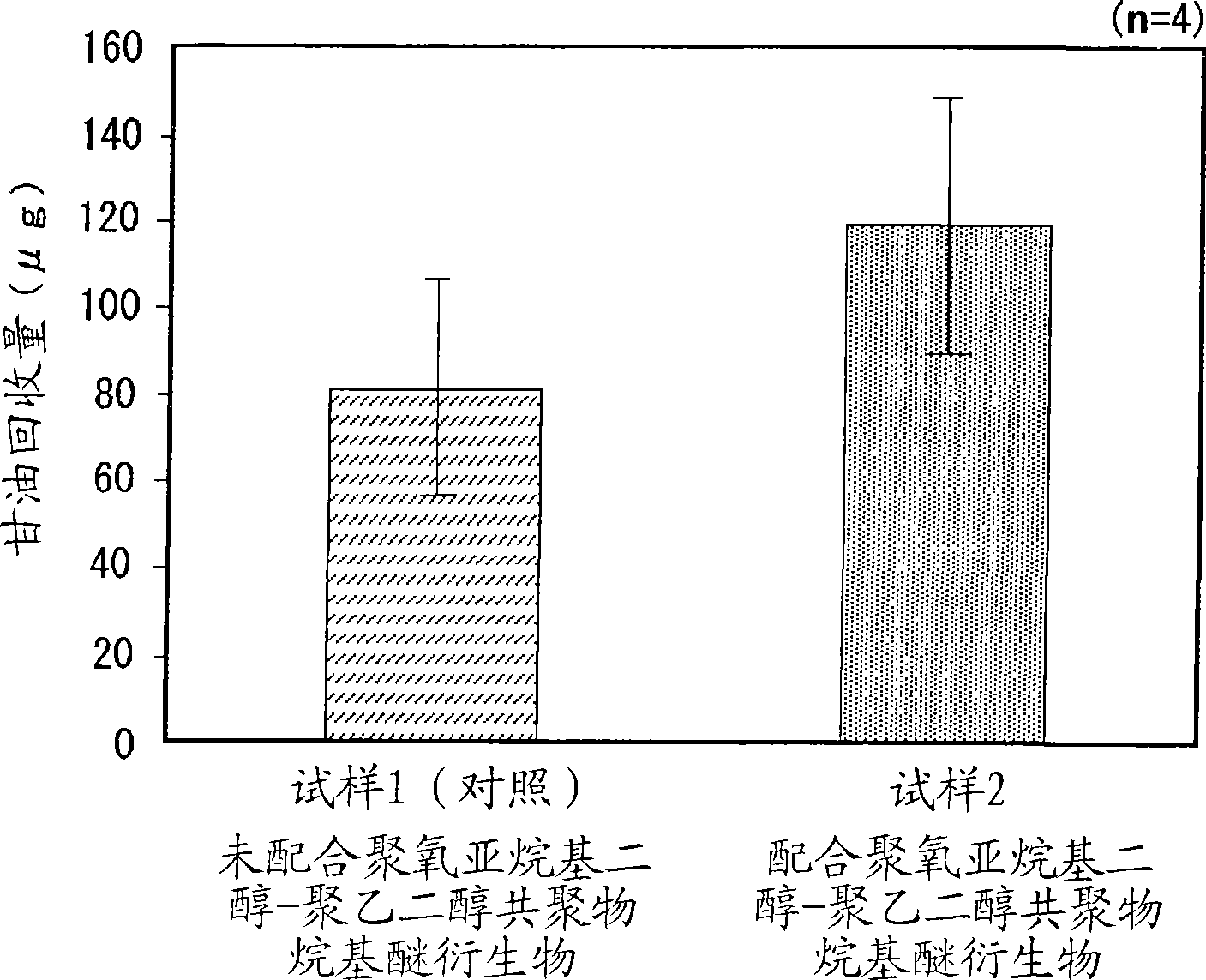
The present invention is to provide a skin external preparation with good moisture retention, a rough skin improving effect, and an improved feeling in use. A skin external preparation comprising a polyoxyalkylene glycol / polyethylene glycol copolymer alkyl ether derivative represented by the below-described general formula (I):(formula I)Y—{O(EO)a / (AO)b—R}k (I)(In the formula, Y is the residue given by removing hydroxyl groups from a polyhydric alcohol having 3 to 6 hydroxyl groups, and k is the number of hydroxyl groups of the polyhydric alcohol. EO is an oxyethylene group; AO is an oxyalkylene group having 3 to 4 carbon atoms, and 1≦a≦70, 1≦b≦70. The percentage of the oxyethylene groups with respect to the sum of the oxyalkylene groups having 3 to 4 carbon atoms and the oxyethylene groups is 20 to 100 mass %. The oxyalkylene group having 3 to 4 carbon atoms and the oxyethylene group are added randomly. Rs are either identical to or different from each other, and they are hydrocarbon groups having 1 to 4 carbon atoms.)
Owner:SHISEIDO CO LTD +1
Marine biological functional cosmetic for removing acne
ActiveCN102525864APromote absorptionDoes not cause allergiesCosmetic preparationsToilet preparationsIrritationVitamin B6 synthesis
The invention discloses marine biological functional cosmetics for removing acne. The marine biological functional cosmetics contain marine shellfish active peptides, algal polysaccharides and pearl powder as major active ingredients, in addition of linoleic acid, allantoin, vitamin A palmitate, vitamin B6 and aloe juice, and have a remarkable and highly-effective anti-acne effect. The invention solves the problems in the prior art that the existing anti-acne cosmetics have serious irritation and possibly causes skin allergy, the Chinese medicinal anti-acne cosmetics possibly causes dark acnescars and have unpleasant odor, and the use of marine shellfish active peptides in anti-acne cosmetics is unknown. The anti-acne functional cosmetics are safe, mild and sustained, and can significantly kill microbes, relieve inflammations, keep oil secretion in balance, promote wound healing, heal scars, remove acne scars and inhibit pigmentation. The anti-acne functional cosmetics are available in various forms such as creams, emulsions, lotions, gels, sprays or facial masks, have remarkable and long-lasting anti-acne effects, prevent acne recurrence after withdrawal, and are suitable for various populations suffering from acnes, scars or acne scars.
Owner:FOSHAN UNIVERSITY
Adhesive composition for dermal patch and production process thereof
InactiveUS20060079640A1Improve adhesionLess irritatingOrganic active ingredientsAntipyreticAlcoholHydrogen atom
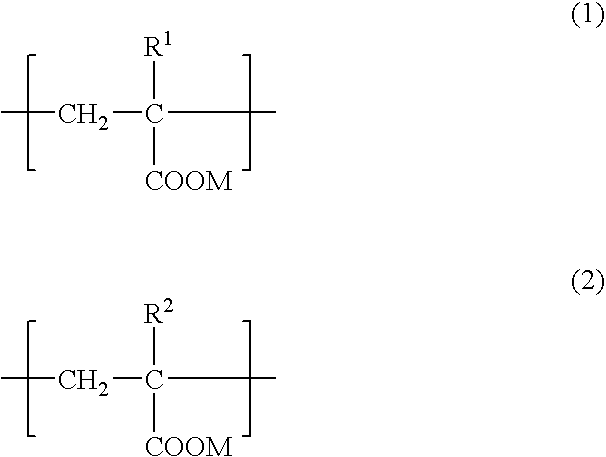
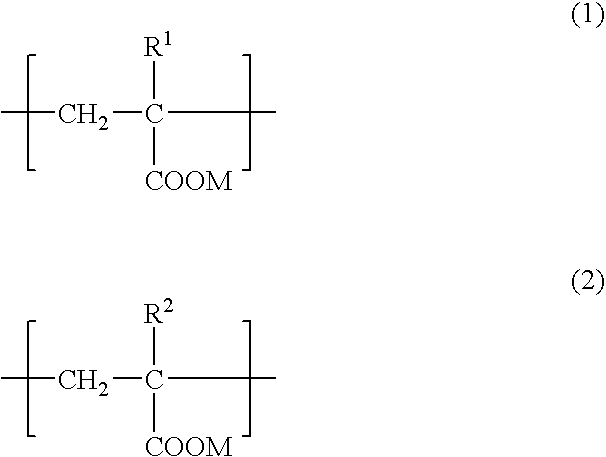
An adhesive composition for dermal patches, comprising (A) a (meth)acrylic acid-base polymer having repeating units represented by formulae (1) and (2): (wherein R1 and R2 each independently represents a hydrogen atom or a methyl group and M represents NH4+ or an alkali metal) with the ratio of (1) / (2) being in a range of 100 / 0 to 90 / 10 (by mol), (B) water, (C) a polyhydric alcohol and (D) an aluminum compound, with the content of (B) water being from 5 to 30 mass %; and a production process thereof. The adhesive composition for dermal patch of the present invention can contain a large amount of polyhydric alcohol between skeletons of the adhesive layer and forms a stable between skeletons of the adhesive layer and forms a stable base material free from syneresis of the polyhydric alcohol. Furthermore, the dermal patch using the adhesive composition for dermal patch of the present invention is excellent in the release property and adherence and has high safety.
Owner:SHOWA DENKO KK
Method of enhanced benzoyl peroxide application
InactiveUS20070003504A1Promote percutaneous absorptionImprove permeabilityCosmetic preparationsBiocideBenzoyl peroxideBacillus acnes
Methods of treating acne are disclosed comprising applying a benzoyl peroxide solution in one or more solvents to the skin of a user and applying a sealer thereto. The methods are useful to improve the penetration of active drug into the skin in need thereof, and are highly effective in treating P. Acne.
Owner:JR CHEM LLC
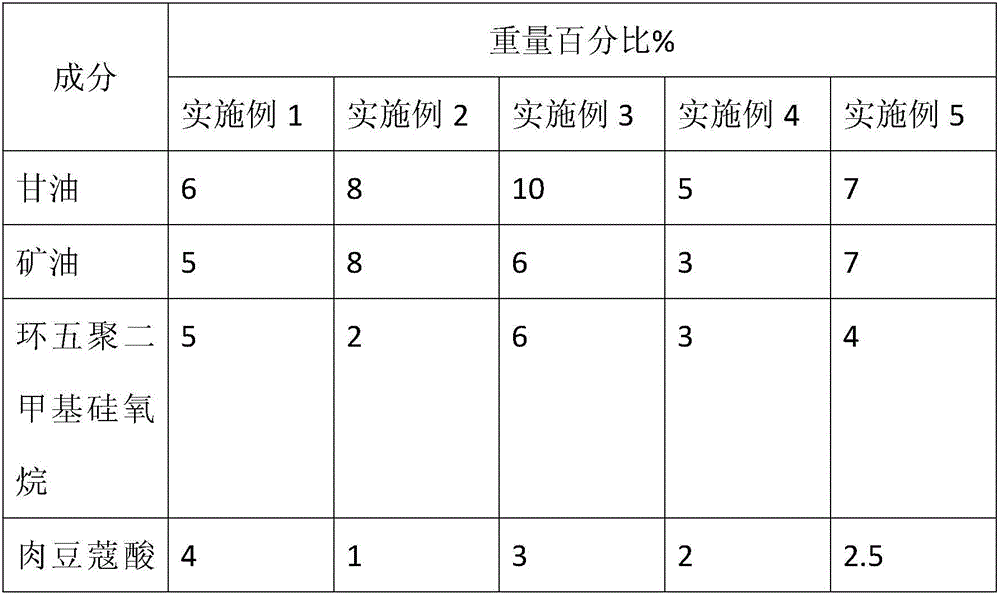
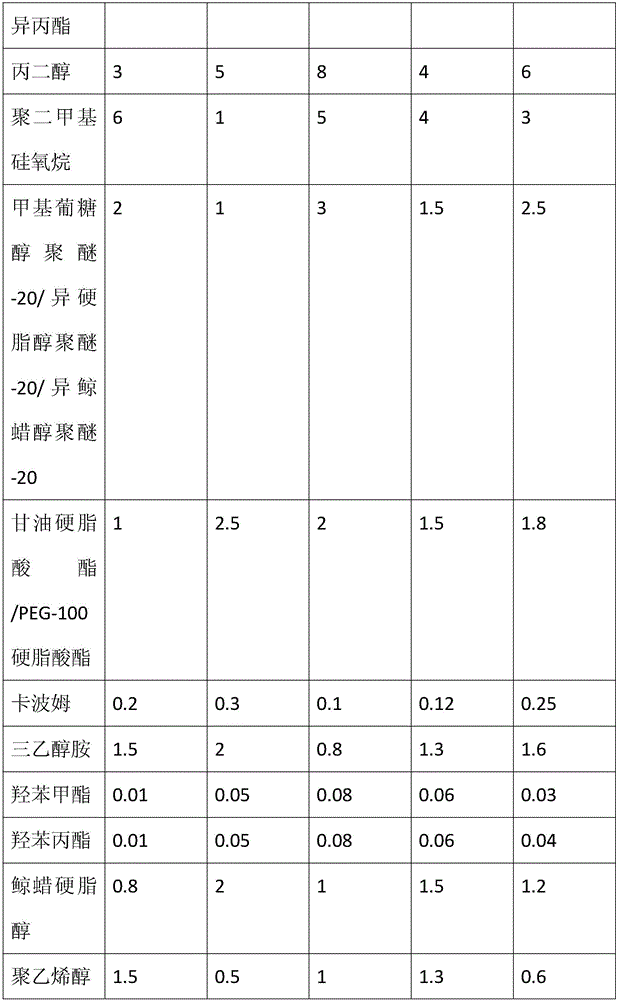
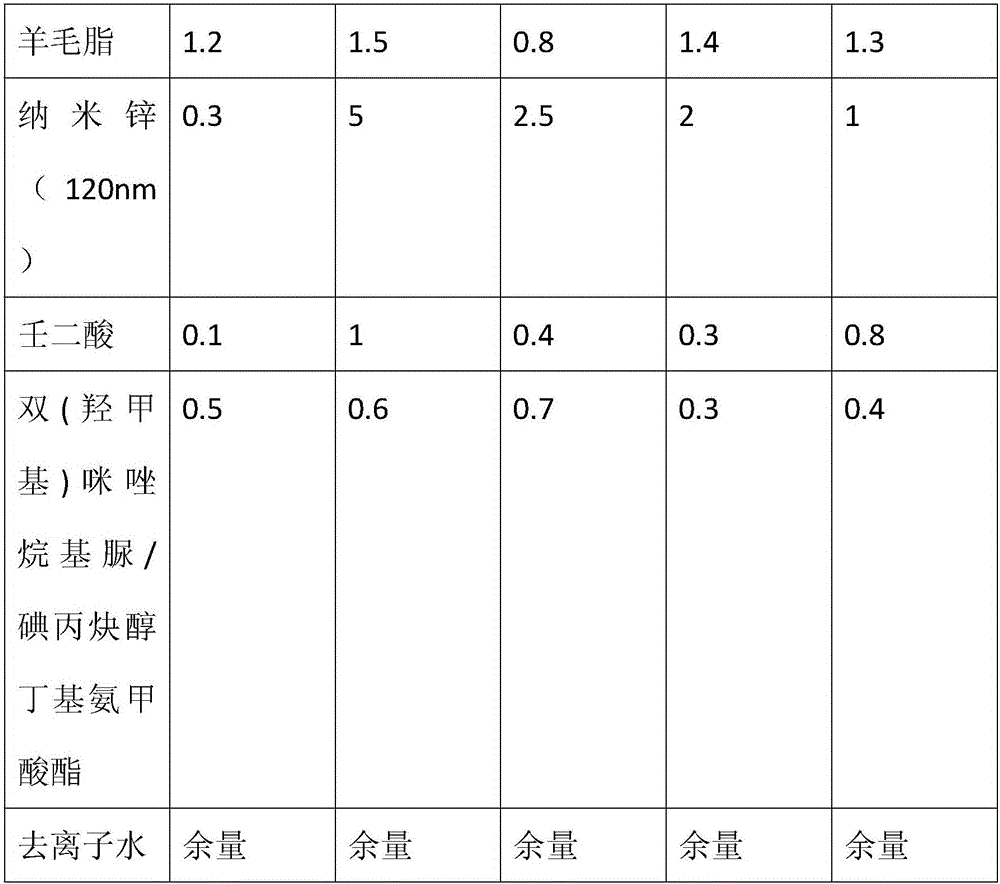
Acne-removal cosmetic composition containing nano-zinc
InactiveCN105708718AInhibitionInhibit hyperkeratosisCosmetic preparationsToilet preparationsMedicineSecretion
The invention discloses acne-removal cosmetic composition containing nano-zinc. The acne-removal cosmetic composition comprises nano-zinc and other cosmetic matrix components. Through synergistic interaction of nano-zinc and azelaic acid, the acne-removal cosmetic composition can perform the efficacy of pore clearing, balancing of grease secretion, sterilization, inflammation eliminating, acne mark removal, moisturization, sun blocking and the like, comprehensively repairs and improves the barrier function of skin and thoroughly removes acne.
Owner:CANTON DAMEKISS DAILY CHEM FACTORY LIMITED
Ivermectin transdermal liniment for animals
InactiveCN102440944APromote percutaneous absorptionReduce decomposition rateOrganic active ingredientsPharmaceutical delivery mechanismIrritationEthyl acetate
The invention discloses an ivermectin transdermal liniment for animals, which is prepared from medicinal liquor and ivermectin, wherein the medicinal liquor is prepared from the following components in percentage by volume: 20-40% of ethyl acetate, 2-5% of azone and 55-75% of propylene glycol; and the ratio of ivermectin to medicinal liquor is (0.5-1g):100mL. The novel transdermal accelerator selected in the formula can enhance the absorption of skin for medicines, does not have toxicity or irritation on the skin and body, does not have pharmacological action, and does not react with the medicines and other additives. The invention is simple to prepare and convenient to use, has high stability, and can achieve favorable anthelminthic effect.
Owner:INNER MONGOLIA AGRICULTURAL UNIVERSITY
Cosmetic or dermatological composition as well as preparation method and application thereof
ActiveCN113230172AGood safety and efficacyImprove dispersibility and stabilityCosmetic preparationsHydroxy compound active ingredientsChemistryTriterpenoid Compound
The invention discloses a cosmetic or dermatological composition as well as a preparation method and application thereof, and belongs to medicines and cosmetics. The composition comprises 0.001%-10% of triterpene acid, 0%-10% of triterpenoid saponin, 0%-10% of vitamin A or vitamin A derivatives, 0.1%-20% of terpene-containing essential oil, 1%-40% of an emulsifier, 1%-40% of a co-emulsifier and a proper amount of water and additives. The preparation method comprises the following steps: dissolving the triterpenic acid, the triterpenoid saponin and the vitamin A or the derivatives thereof in the emulsifier, the co-emulsifier and the terpene-containing plant essential oil, performing dispersing in a water phase while stirring, and carrying out high-pressure homogenization. The prepared nano-emulsion is in an oil-in-water type, is excellent in water solubility, clear, transparent, good in stability and high in absorption efficiency, the effects of resisting aging, reducing wrinkles, whitening, inhibiting acnes, inhibiting sebum overflow, inhibiting scar formation, promoting skin wound repair and the like are greatly improved, the preparation method is simple, and the application value of the composition in the fields of medicines and cosmetics is improved.
Owner:泉州达浔生物科技有限公司
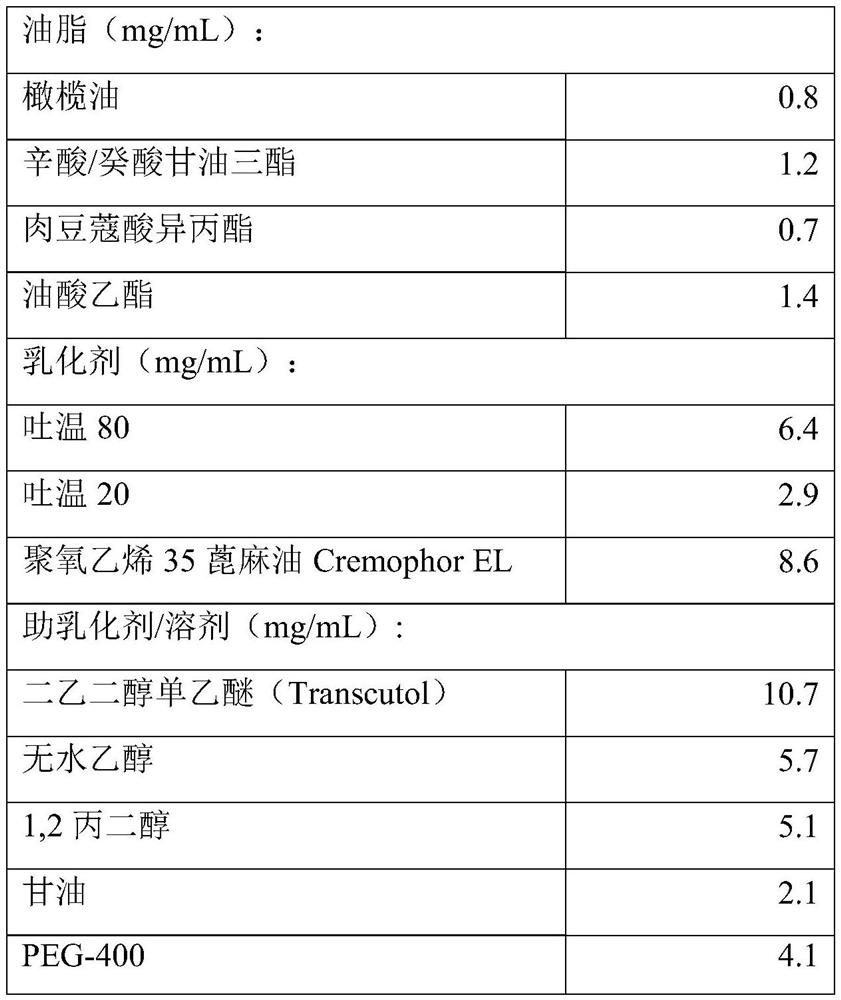
Alprostadil nano granule formulation and preparation thereof
InactiveCN101322712AReduce chance of regroupingOvercoming chemistryPowder deliveryOrganic active ingredientsDiseasePulmonary vasculature
The invention relates to an alprostadil nanoparticle preparation and a preparation method thereof. The preparation is essentially used for transdermal administration and used for treating diseases such as diabetic ulcer, ischemia of extremity end, burn and the like, and belongs to the technical field of medicine. The preparation consists of the raw materials by the following weight ratio that alprostadil:lipid component: emulsifier: excipient matrix: water is 0.01-0.1:0.5-10:0.5-5:550-950:5. The alprostadil nanoparticle preparation has the advantages of overcoming the chemical and physiological instability of the alprostadil, strengthening in vitro stability, reducing degradation of the alprostadil caused by inactivation effect of in vivo pulmonary circulation, enhancing concentration of the preparation on the local affected parts, being more easy to aggregate at the affected part, sustained release and long effect, and reducing irritation of the preparation.
Owner:SHENYANG WANJIA INST OF BIOLOGICAL TECH RES
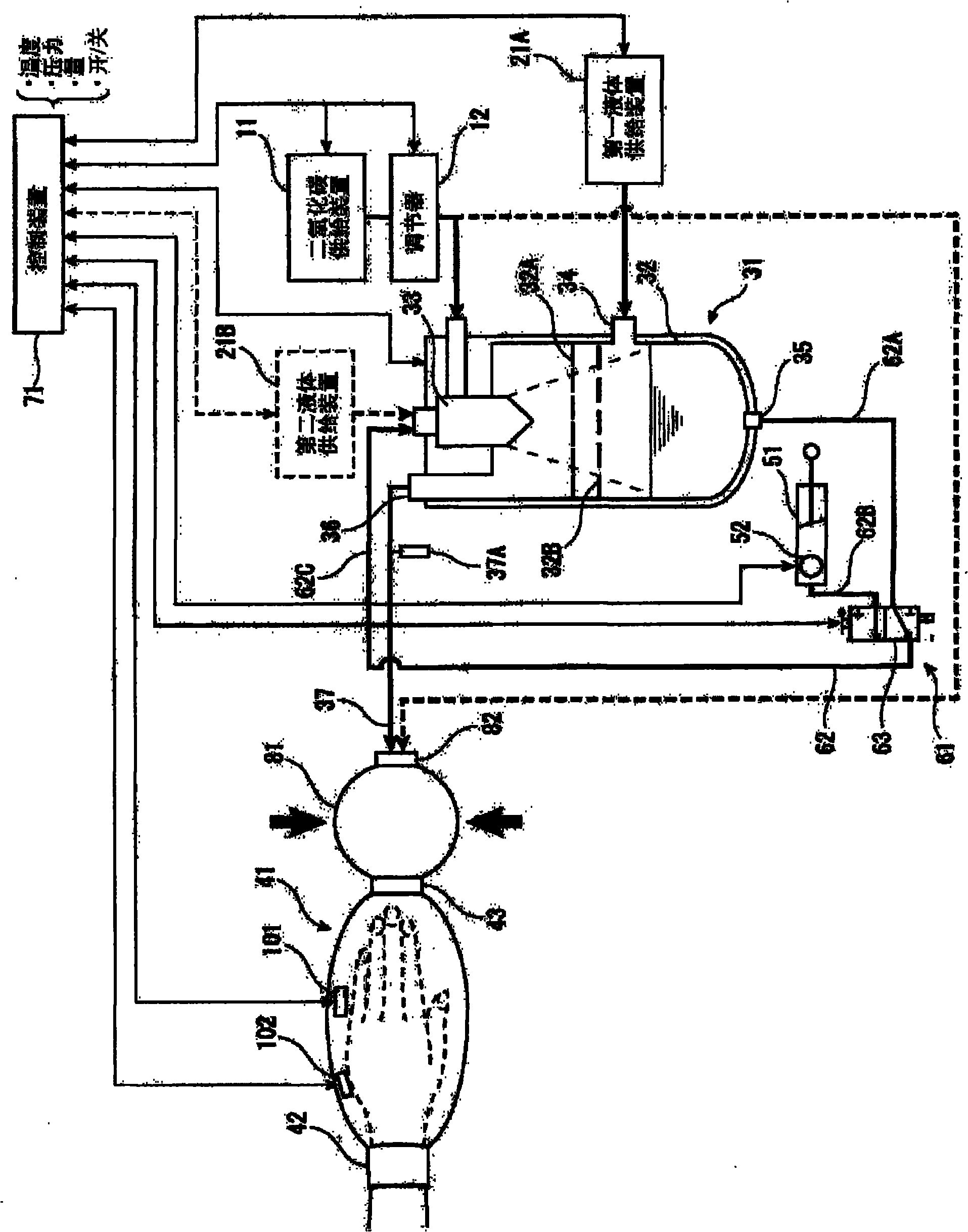
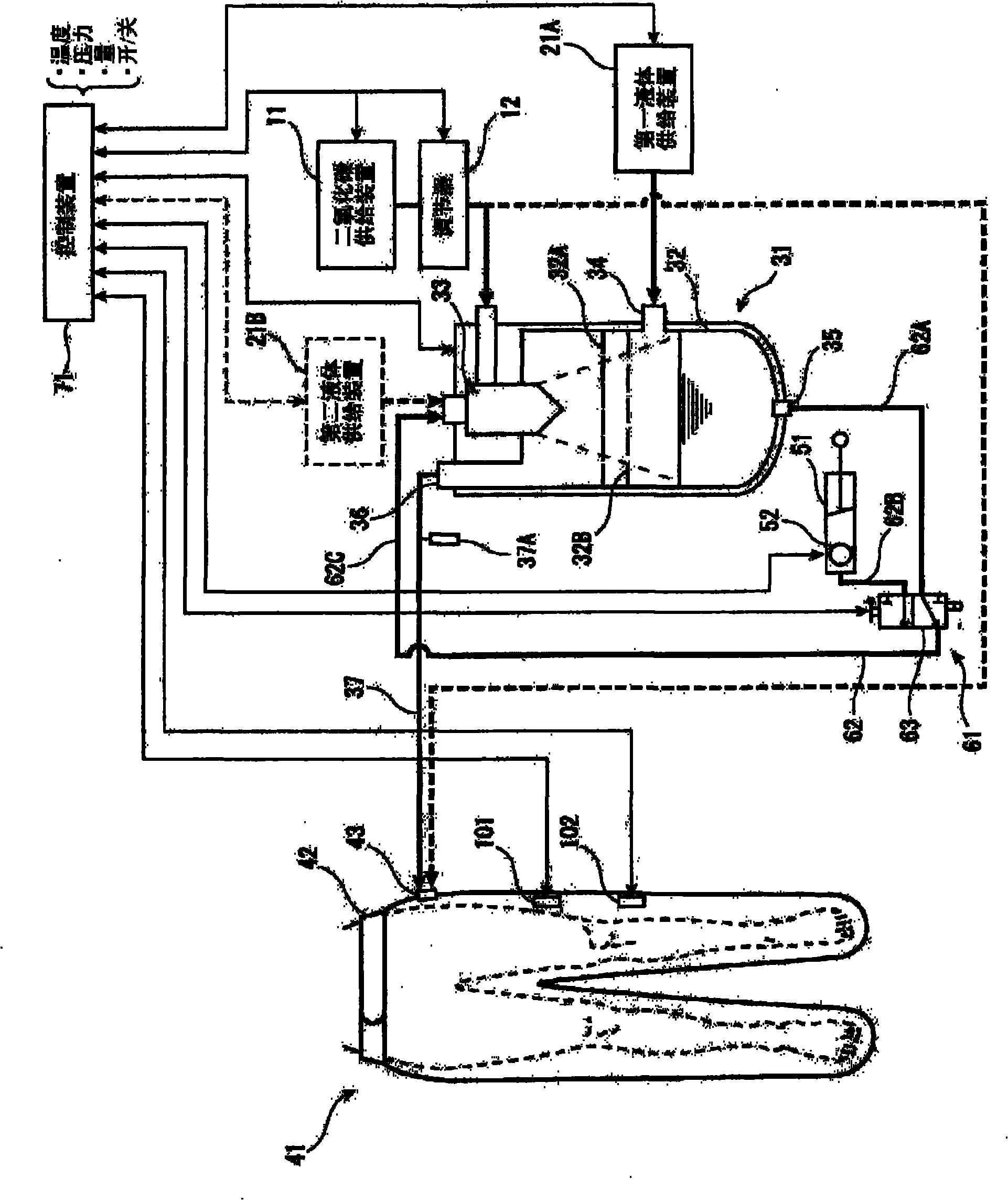
Carbon dioxide mist bathing system
InactiveCN101917954APromote percutaneous absorptionIncrease concentrationBathing devicesChemistryHigh carbon dioxide
Provided is a pressurized carbon dioxide mist bathing system whereby a carbon dioxide mist having a high carbon dioxide concentration can be efficiently absorbed through the skin and mucosa of a living body. A pressurized carbon dioxide mist bathing system comprising a carbon dioxide gas-supplying means (11), a liquid-supplying means (21), a carbon dioxide mist-forming means (31) in which the carbon dioxide gas and the liquid are ground and dissolved to give a carbon dioxide mist, a body cover member (41) covering the skin and mucosa of the living body and providing a space in which the carbon dioxide gas mist formed by the carbon dioxide mist-forming means (31) is enclosed , a liquid-circulating means (61) for supplying again the liquid pooled in the carbon dioxide mist-forming means (31) into the carbon dioxide mist-forming means (31), and a body cover member-pressurizing means (81) for pressurizing the inside of the body cover member (41).
Owner:中村正一 +1
Compounding ingredients for basic cosmetics and basic cosmetics
InactiveUS20080161229A1Prevention effects of chapping of the face skin, blotches, freckles, wrinklesAvoid crackingCosmetic preparationsPeptide/protein ingredientsWrinkle skinAdditive ingredient
The present invention relates to a compounding ingredients for basic cosmetics and basic cosmetics containing the compounding ingredients which contain enzymes or hydrolysis products of nucleoprotein and / or DNA or RNA, or deoxy oligonucleotide, deoxy mononucleotide, oligopeptide, oligonucleotide mononucleotide separated from the hydrolysis products, or at least two kinds of mixtures selected from the hydrolysis products or compounds as active ingredients. Because the active ingredients such as deoxy oligonucleotide and others have comparatively small molecular weights, they are easy to be absorbed percutaneously, and when they are percutaneously absorbed, they exhibit cellular stimulating effects and blood circulation promoting effects, and therefore, in the event that they are applied to the facial skin, they marvelously prevent skin roughness, blotches, freckles, wrinkles, etc. and exhibit superb skin-lightening effects.
Owner:NISSEI BIO
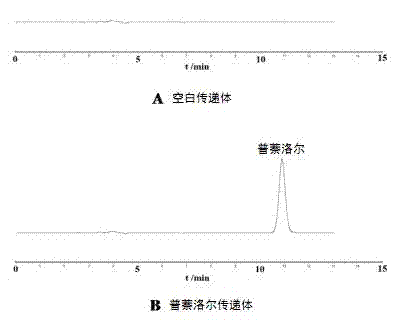
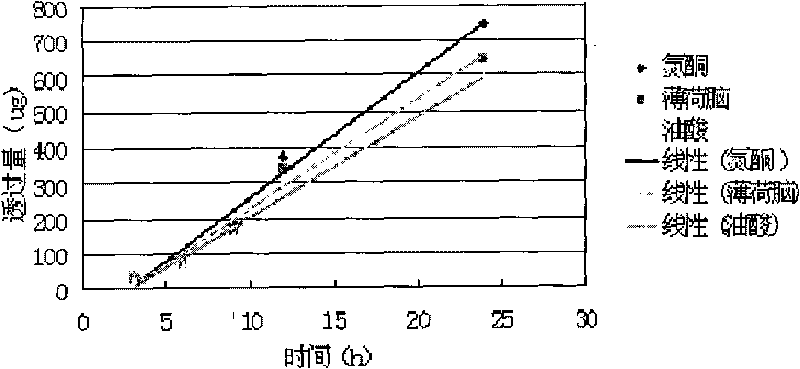
Percutaneous-absorption-promoting propranolol composite phospholipid transfersome, and prepartion method and application thereof
InactiveCN102846546APromote percutaneous absorptionImprove bioavailabilityOrganic active ingredientsPharmaceutical non-active ingredientsInfantile haemangiomaBlood plasma
The invention provides a composite phospholipid transfersome which promotes propranolol percutaneous absorption, and a preparation method thereof. According to the invention, two phospholipid materials with different phase-change temperatures, which are dipalmitoyl phosphatidyl choline and soybean lecithin, are adopted as a composite phospholipid material. Compared with a transfersome with a single phospholipid material in prior art, the propranolol composite phospholipid transfersome prepared with the phospholipid material provided by the invention has substantially improved encapsulation efficiency, reduced leakage, improved stability in rat plasma, and substantially improved bioavailability after percutaneous administration. The propranolol composite phospholipid transfersome provided by the invention is especially suitable to be used for treating infantile hemangioma. With the transfersome, propranolol percutaneous administration can be realized, and propranolol can directly act upon a hemangioma affected part. The treatment effect is improved, toxic and side effects are reduced, and children medication compliance can be improved. Also, the invention provides a preparation method of the propranolol composite phospholipid transfersome.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
Acne-removing skin-care emulsion and preparation method thereof
InactiveCN107137243AUnique skin protection effectElasticCosmetic preparationsToilet preparationsDicaprylyl carbonateMonoglyceride
The invention relates to an acne-removing skin-care emulsion and a preparation method thereof. The acne-removing skin-care emulsion consists of the following components in percentage by weight: 1 to 5 percent of cetearyl glucoside, 1 to 6 percent of self-emulsifying monoglyceride, 2 to 8 percent of dimethyl silicone, 1 to 10 percent of squalane, 1 to 10 percent of dicaprylyl carbonate, 0.5 to 5 percent of water-soluble vitamin E, 1 to 10 percent of 1,3-butanediol, 0.1 to 1 percent of sodium hyaluronate, 1 to 10 percent of an amino acid wetting agent, 0.1 to 0.5 percent of dipotassium glycyrrhizinate, 0.01 to 1 percent of xanthan gum, 0.3 to 1.1 percent of a preservative, 1 to 10 percent of nanometer zinc, 0.1 to 1 percent of a pH regulator, 0.01 to 0.3 percent of essence and the balance of water, wherein the sum of the components is 100 percent. The preparation process of the acne-removing skin-care emulsion is simple; the prepared acne-removing skin-care emulsion has an excellent acne-removing effect; the product is mild and non-irritant, and has a good skin-care effect.
Owner:南京凯创协同纳米技术有限公司
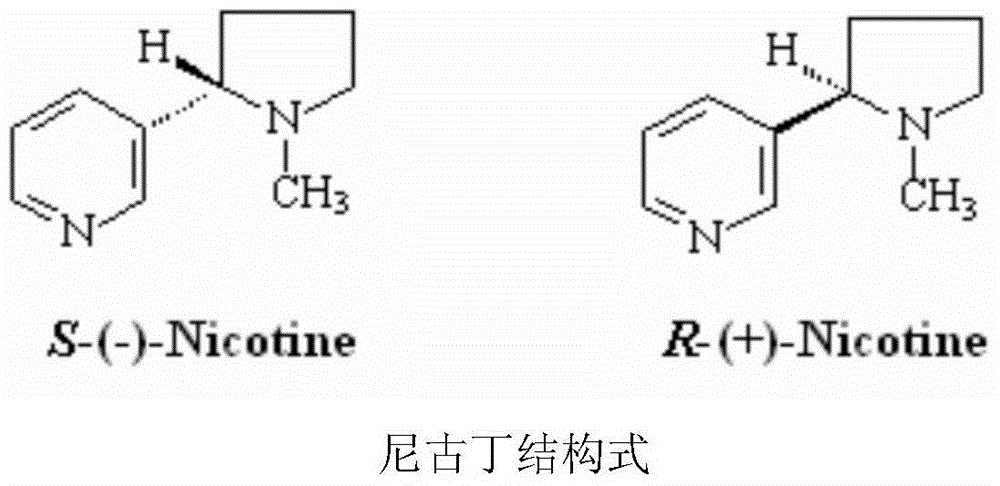
Nicotine slow-release patch
InactiveCN104013603AExtended use timeReduce releaseOrganic active ingredientsNervous disorderExternal applicationAdhesive
The invention relates to a slow-release patch used for external application and prepared from natural tobacco extracts (nicotine as the main component). The slow-release patch is prepared from the natural tobacco extracts and proper auxiliary materials and is used for achieving the aim of slowly releasing nicotine according to a particle slow-release principle. The nicotine slow-release patch provided by the invention comprises a back lining layer, a medicine-contained paste body and a cover lining layer, wherein the medicine-contained paste body is prepared from auxiliary materials such as nicotine micelle, an adhesive, a filling agent, a transdermal enhancer, a humectant and the like according to a certain ratio; each patch contains 20-40mg of nicotine. The nicotine slow-release patch has the characteristics of slow release, rapidness in taking effects, good moisture retention and air permeability, comfort in use and the like.
Owner:SHANGHAI TOBACCO GRP CO LTD
Pressurized carbon dioxide-containing mist bathing system
InactiveCN101917953APromote percutaneous absorptionIncrease concentrationBathing devicesChemistryHigh carbon dioxide
Provided is a pressurized carbon dioxide -containing mist bathing system which enables effective absorption of a carbon dioxide-containing mist having a high carbon dioxide concentration through the skin and mucosa of living body. A pressurized carbon dioxide -containing mist bathing system which comprises a carbon dioxide-supplying means (11), a liquid-supplying means (21), a carbon dioxide-containing mist-forming means (31) by which the carbon dioxide and the liquid are ground and dissolved to form a carbon dioxide-containing mist, a body-covering member (41) which covers the skin and mucosa of living body so as to provide a space wherein the carbon dioxide-containing mist formed by the carbon dioxide-containing mist-forming means (31) is enclosed, and a liquid circulation means (61) for supplying the liquid having been pooled in the carbon dioxide-containing mist-forming means (31) into the carbon dioxide-containing mist-forming means (31) again.
Owner:中村正一 +1
Traditional Chinese medicine composition with bacteriostatic, anti-inflammatory, hemostatic and analgesic effects and preparation method thereof
InactiveCN111000891AImprove anti-inflammatory and antibacterial effectAntibacterial and anti-inflammatory fastAntipyreticAerosol deliveryMedicinal herbsPolyethylene glycol
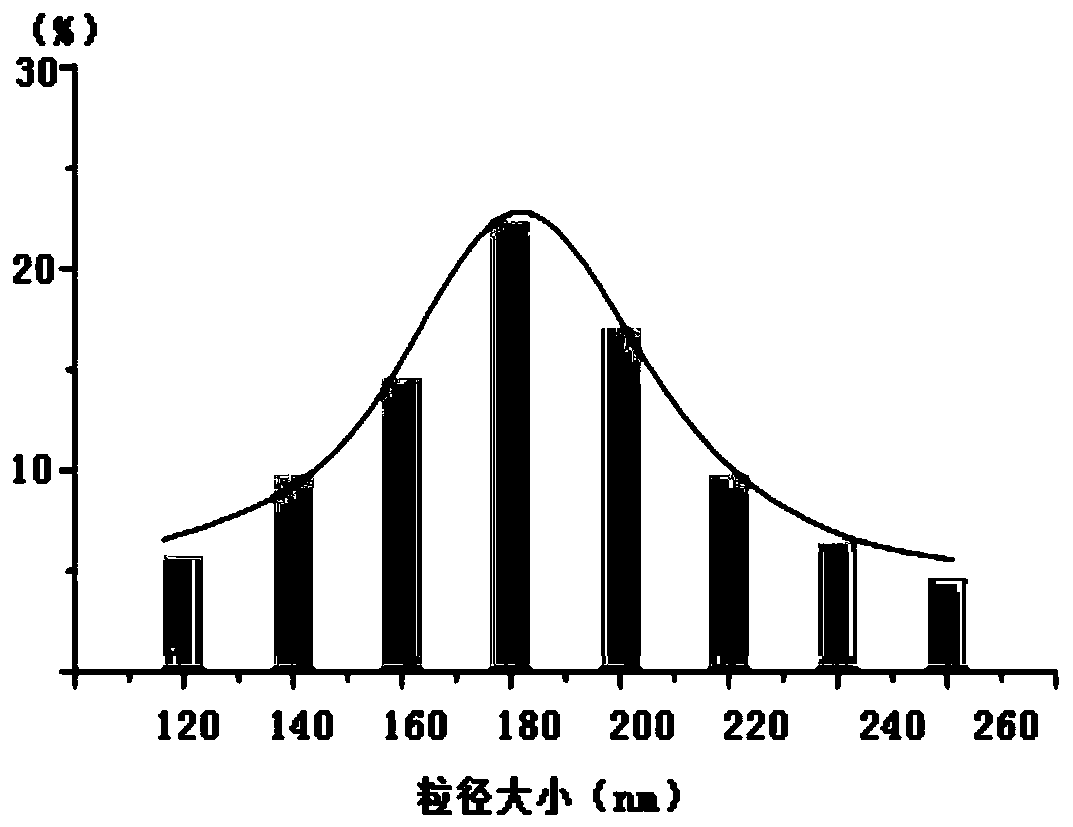
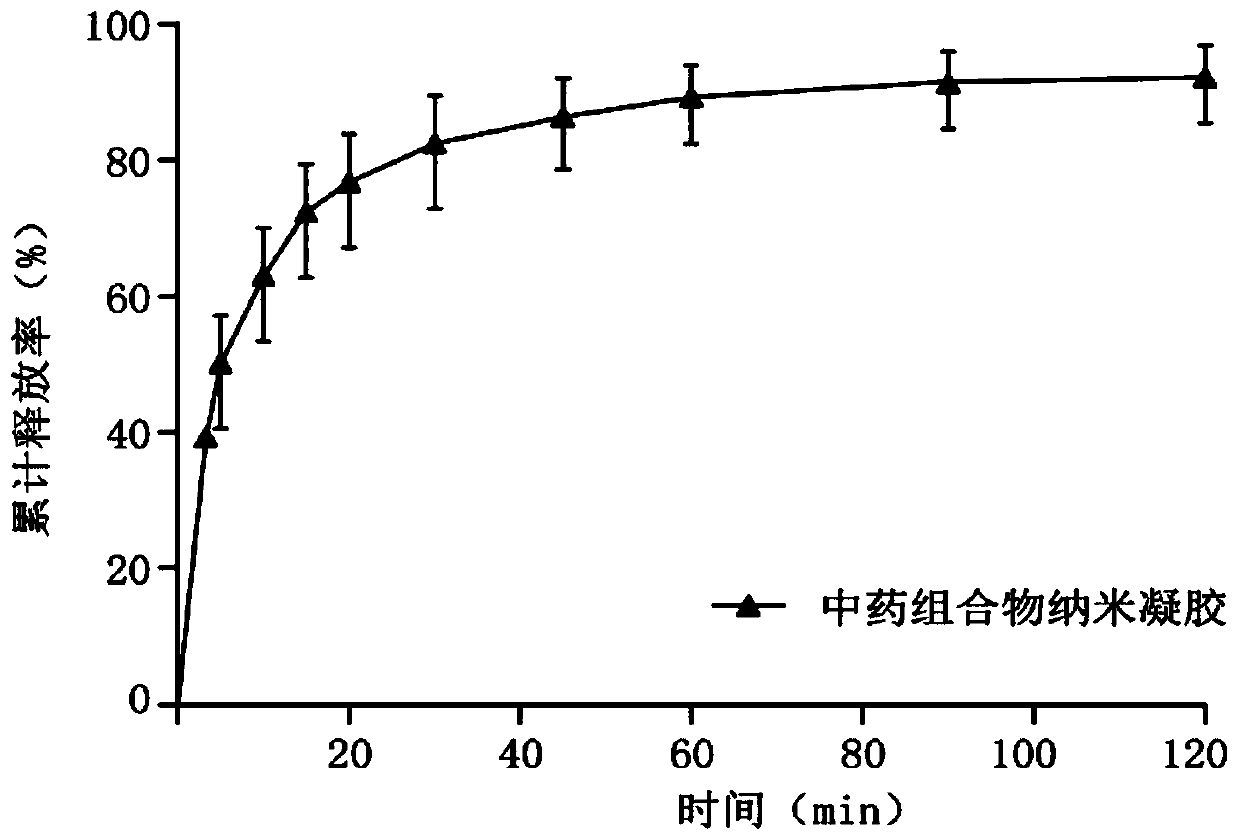
The invention discloses a traditional Chinese medicine composition with bacteriostatic, anti-inflammatory, hemostatic and analgesic effects and a preparation method thereof. According to the method, apithecellobium clypearia extract and a lamiophlomis rotata extract are used as raw materials; poly(lactic-co-glycolic acid) PLGA, vitamin E polyethylene glycol succinate TPGS and poloxamer are used as a drug-loaded matrix. The preparation method comprises the following steps: mixing raw medicinal materials with the drug-loaded matrix to form an oil phase and an oil-in-water phase, performing probe ultrasonic emulsification to obtain a nano-suspension, and adding carbomer, collagen tripeptide, oat beta-glucan and propylene glycol as a gel matrix to obtain traditional Chinese medicine composition nanogel. The prepared traditional Chinese medicine composition nanogel has the particle size of 120 nm-260 nm, has good drug loading capacity, obviously improves the solubility and bioavailabilityof hydrophobic drugs, inhibits bacteria, diminishes inflammation, stops bleeding and relieves pain, and is definite in curative effect.
Owner:江西杏林白马药业股份有限公司
Acne-removing skin cream and preparation method thereof
InactiveCN107213038AGood compatibilitySimple preparation processCosmetic preparationsToilet preparationsMonoglycerideGlycerol
The invention relates to acne-removing skin cream and a preparation method thereof. The acne-removing skin cream is prepared from the following components in percentage by weight: an oil phase, including 1-10% of white oil, 1-10% of C16-18 alcohol, 2-8% of dimethicone, 2-10% of isopropyl myristate, 2-10% of bleached beeswax, 1-5% of cetearyl glucoside and 1-6% of self-emulsifying monoglyceride, an aqueous phase, including 1-10% of glycerin, 1-10% of propylene glycol, 0.1-1% of a pH modifier and the balance of water, and auxiliary materials, including 0.5-5% of water-soluble vitamin E,1-10% of nanometer zinc, 0.3-1.1% of a preservative, 0.1-1% of sodium hyaluronate, 0.2-1% of carbomer 940 and 0.01-0.3% of an essence, wherein the sum of the components in percentage by weight is 100%. The acne-removing skin cream is simple in preparation technology, the prepared acne-removing skin cream has excellent effects of removing acnes, whitening the skin and increasing the glossiness of the skin, and the product is mild and free of thrill, and has a good skin-protecting effect.
Owner:南京凯创协同纳米技术有限公司
External preparation for skin
ActiveCN101448484AImprove smoothnessPromote cutin permeabilityCosmetic preparationsMake-upPolyethylene glycolEthyl group
The present invention discloses an external preparation for the skin, which is excellent in a moisturizing effect, a skin roughness-ameliorating effect and feeling during use. The external preparation comprises a polyoxyalkylene glycol / polyethylene glycol copolymer alkyl ether derivative represented by the general formula (I): (I) wherein Y represents a residue given by removing hydroxyl groups from a polyhydric alcohol having 3 to 6 hydroxyl groups; k represents the number of hydroxyl groups in the polyhydric alcohol; EO represents an oxyethylene group; AO represents an oxyalkylene group having 3 to 4 carbon atoms; and a and b respectively satisfy the requirements shown by the formulae: 1=a=70 and 1=b=70; the ratio of the amount of the oxyethylene group to the total amount of the oxyalkylene group having 3 to 4 carbon atoms and the oxyethylene group is 20 to 100% by mass; the oxyalkylene group having 3 to 4 carbon atoms and the oxyethylene group are added randomly; and R's independently represent a hydrocarbon group having 1 to 4 carbon atoms.
Owner:SHISEIDO CO LTD +1
Acne-removing essence and preparation method thereof
InactiveCN107028797APromote absorptionInhibition of growth and reproductionCosmetic preparationsToilet preparationsPreservativeJojoba oil
The invention relates to an acne-removing essence and a preparation method thereof. The acne-removing essence is prepared from the following components in percentage by weight: 0.1-1% of xanthan gum, 0.5-3% of water-soluble jojoba oil, 1-10% of glycerin, 1-10% of 1,3-butanediol, 0.5-5% of water-soluble vitamin E, 1-10% of nano zinc, 0.1-1% of sodium hyaluronate, 0.2-1.5% of a preservative, 0.1-1% of a pH modifier, 0.01-0.3% of an essence and the balance of water, wherein the sum of the percentages of the components is 100%. The acne-removing essence is simple in preparation technology, the prepared nano zinc moisturizing and acne-removing essence has a relatively good moisturize effect and an excellent acne-removing effect, and a product is mild and free of thrill and has a good skincare effect.
Owner:南京凯创协同纳米技术有限公司
Transepidermal drug delivery system containing tulobuterol
InactiveUS20110182971A1Easy maintenanceIncrease stickinessBiocideOrganic active ingredientsMedicineAcid value
Disclosed is a pharmaceutical composition containing tulobuterol. More specifically, disclosed is a transepidermal drug delivery system including a drug layer containing tulobuterol and a natural rubber-based adhesive material, and a supporter adhered to one surface of the drug layer to support the drug layer, wherein the natural rubber-based adhesive material comprises 10 to 40 parts by weight of a natural rubber, 54.5 to 85 parts by weight of a rosin ester resin and an acid value controller, and the drug layer has a thickness of 25 μm to 75 μm.
Owner:SINSIN PHARMA
Hydrogel patch for skin care and preparation method thereof
ActiveCN112121032AImprove toughnessHigh strengthCosmetic preparationsToilet preparationsBULK ACTIVE INGREDIENTUlcer care
The invention discloses a hydrogel patch for skin care and a preparation method of the hydrogel patch. A hyaluronic acid substance serves as a main hydrogel matrix to be compounded with a small amountof gel polymers, so that the toughness and the strength of the hydrogel patch are improved. Meanwhile, the hydrogel patch is high in water locking property and low in drainage rate, has the effects of preserving moisture for a long time and promoting percutaneous absorption of active ingredients or medicines, and can be used for skin beauty care, skin trauma and ulcer care and application care ofsoft tissue sprain. Different shapes can be formed by pouring according to different use parts, including the whole face, the nose part, the eyes, the lip, the neck and the body parts such as the abdomen, the hip and the four limbs.
Owner:BLOOMAGE BIOTECHNOLOGY CORP LTD
Transdermal delivery based pharmaceutical composition and preparation method and application thereof
ActiveCN111346073AImproves transdermal penetrationLittle side effectsOrganic active ingredientsAntipyreticIntramuscular injectionChemical compound
The present invention discloses a transdermal delivery based pharmaceutical composition. The composition comprises a compound as shown in a formula (I) or pharmaceutically acceptable salt thereof, a macromolecular dispersing carrier material, a hot melting protecting agent and an optional fluxing agent. A preparation method of the transdermal delivery based pharmaceutical composition comprises thesteps of micronizing the compound as shown in the formula (I) or the pharmaceutically acceptable salt thereof, the macromolecular dispersing carrier material and the hot melting protecting agent, optionally adding the fluxing agent, mixing the components uniformly, and performing hot melting extrusion and micronizing to obtain microparticles of the compound as shown in the formula (I) or the pharmaceutically acceptable salt thereof. In addition, the invention provides an application of the pharmaceutical composition. The transdermal delivery based pharmaceutical composition can enable the compound as shown in the formula (I) to be quickly absorbed, so that the purpose of calming before anesthesia is achieved, breathing is not affected, and the adverse psychological effects on children dueto intramuscular injection or intravenous injection are avoided. Meanwhile, the pharmaceutical composition also can be used for preventing and / or treating hyperactivity.
Owner:YICHANG HUMANWELL PHARMA
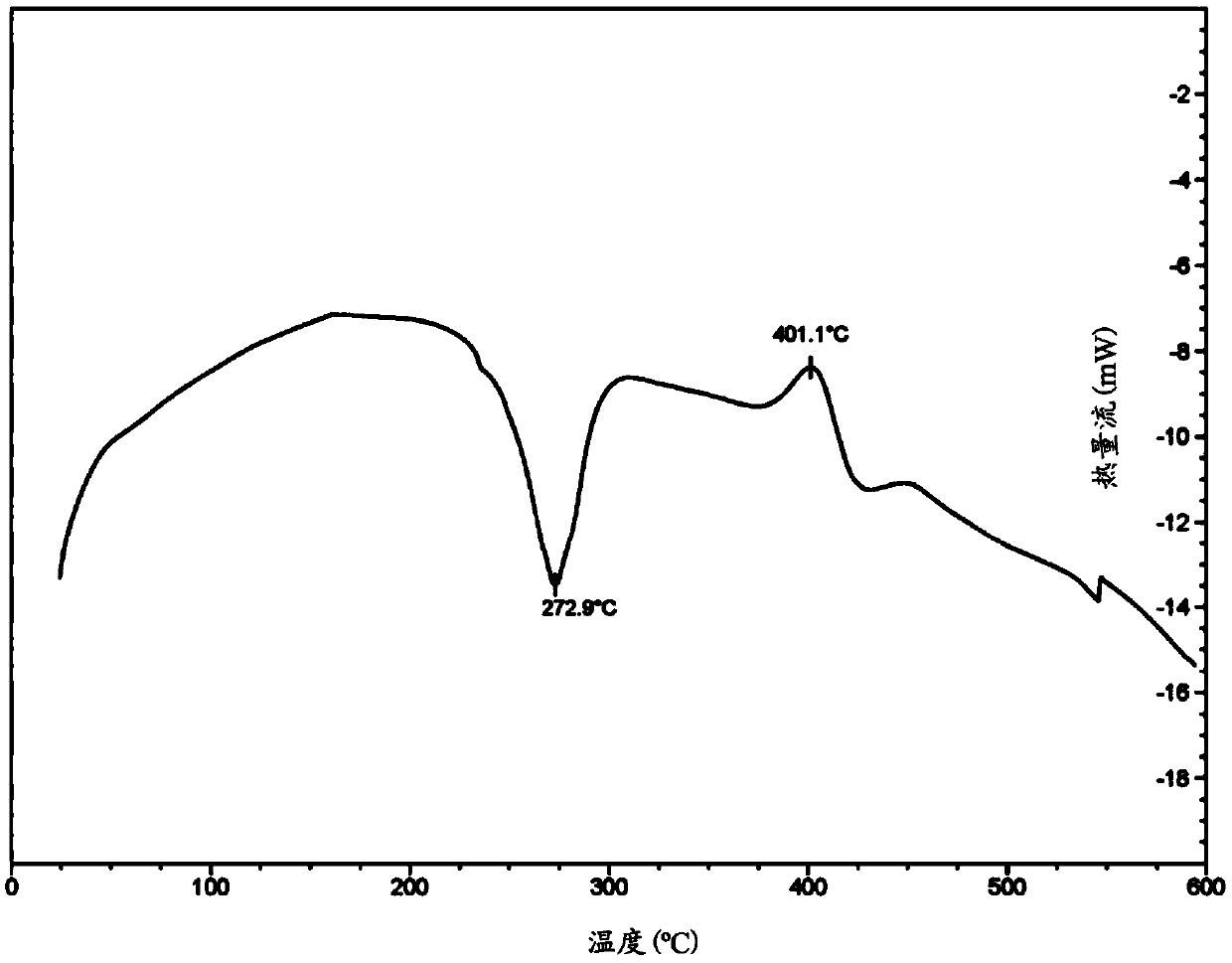
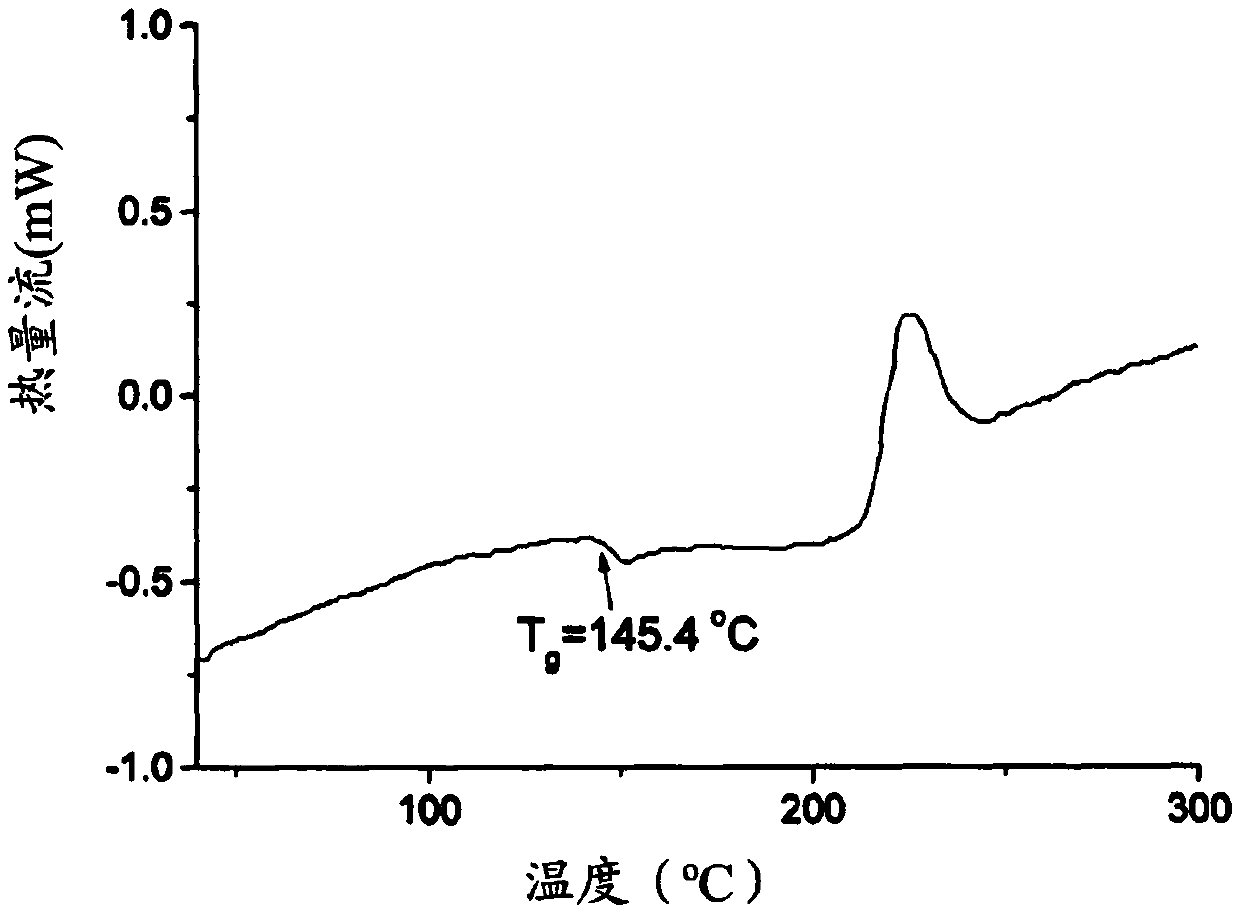
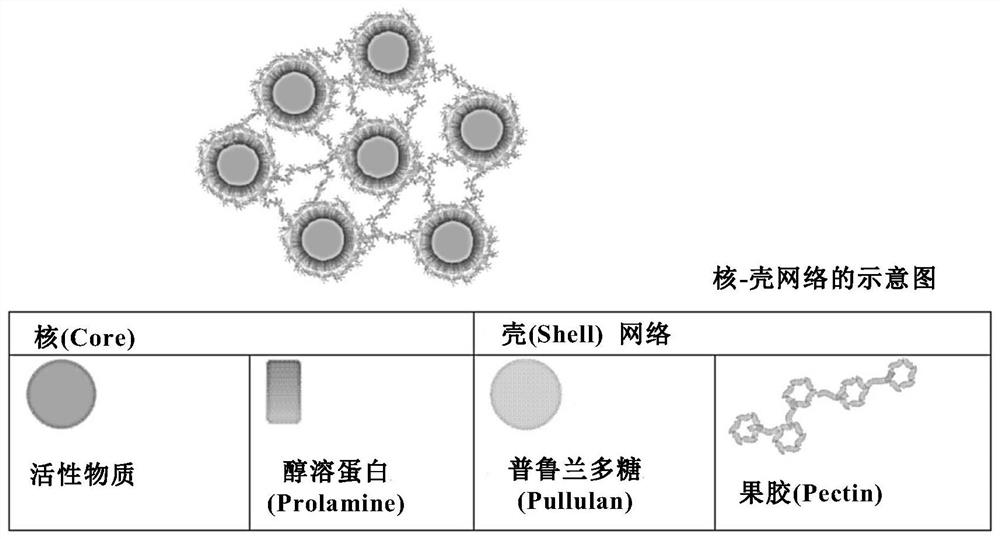
Active substance carrier comprising biopolymer
PendingCN112891244APromote percutaneous absorptionEffective absorptionCosmetic preparationsToilet preparationsPolymer sciencePullulan
The present disclosure relates to an active substance carrier comprising a core-shell network structure formed using a biopolymer. In an aspect of the present disclosure, the core-shell network structure comprises a core-shell particle comprising: a core comprising prolamin; and a shell comprising pullulan and pectin, wherein the pullulan comprised in the shell surrounds the core and the pectin is located in the outermost layer of the shell, so that a network is formed between the core-shell particles. It can effectively facilitate the transdermal absorption of an active substance.
Owner:AMOREPACIFIC CORP
Transdermal absorption promoter, and external skin formulation thereof
InactiveCN102939075ANon-irritating high securityPromote percutaneous absorptionCosmetic preparationsHydroxy compound active ingredientsActive componentDiol
The present invention provides a substance which promotes the transdermal absorption of a pharmacologically active component while little irritating the skin. The present invention relates to a transdermal absorption promoter which comprises, as the active component, at least one member selected from among isopulegol, 2-(menthoxy)ethanol and 2-methyl-3-(menthoxy)propane-1,2-diol; and an external skin formulation which comprises a pharmacologically active component such as a psychotropic component, an anti-inflammatory component, an analgesic component, an antipyretic component, a whitening component or a hair growth-promoting component, together with the aforesaid transdermal absorption promoter.
Owner:TAKASAGO INTERNATIONAL CORPORATION
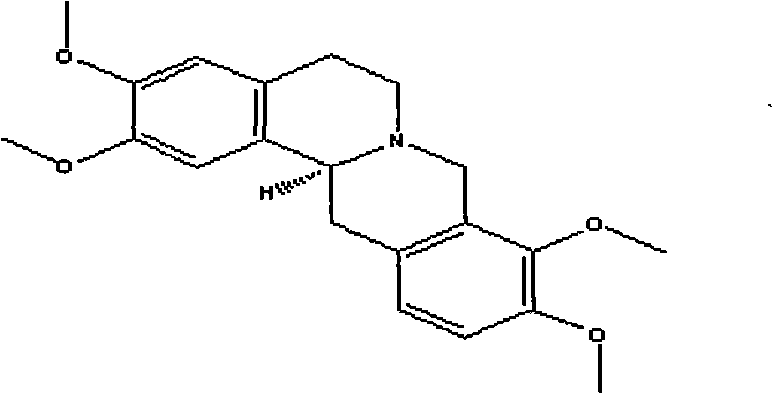
Tetrahydropalmatine transdermal patch and preparation method thereof
ActiveCN101703492AHigh ester solubilityLow toxicityOrganic active ingredientsAntipyreticTransdermal patchSide effect
The invention discloses a tetrahydropalmatine transdermal patch and a preparation method thereof. The patch comprises a back lining layer, a drug-storage layer and a protective layer, wherein the raw materials of the drug-storage layer comprise tetrahydropalmatine and pressure sensitive adhesive, or a moderate amount of transdermal accelerant and the like is added to finally prepare the tetrahydropalmatine patch. The tetrahydropalmatine patch has the advantages that the patch has convenient use, high bioavailability and lasting action and avoids liver 'first pass effect' and damages on gastrointestinal tract; and the patch reduces drug toxicity and side effects and increases drug bioavailability and therapeutic safety, thereby having outstanding anti-inflammatory and analgesic effects.
Owner:GANSU CHEEZHENG TIBETAN MEDICINE CO LTD
Application of composition to preparation of veterinary anti-parasitic drug, transdermal solution of veterinary anti-parasitic drug and preparation method of transdermal solution
InactiveCN111514157ASolve the problem of small range of deworming by single applicationReduce labor costsOrganic active ingredientsPharmaceutical delivery mechanismAntiparasiticGlycerol
The invention belongs to the technical field of an anti-parasitic drug, and particularly relates to an application of a composition containing ivermectin and levamisole hydrochloride to preparation ofa veterinary anti-parasitic drug, a transdermal solution of the veterinary anti-parasitic drug and a preparation method of the transdermal solution. The transdermal solution comprises components in parts by volume as follows: 20-50 parts of isopropanol, 10-40 parts of glycerol, 10-30 parts of absolute ethyl alcohol, 5-20 parts of dimethyl sulfoxide and 2-6 parts of azone; the content of ivermectin is 0.3-1 g per 100 mL of solution, and the content of levamisole hydrochloride is 5-15 g per 100 mL of solution. According to the invention, ivermectin and levamisole hydrochloride are combined, a synergistic effect is realized by simultaneous use of ivermectin and levamisole hydrochloride, and an anti-parasitic spectrum is improved; and meanwhile, with adoption of a transdermal absorption dosage form, toxic and side effects caused by correlation of the drug and the dosage are reduced, and a simultaneous internal and external anti-parasitic effect of the preparation is realized.
Owner:吉林吉力生物技术研究有限公司 +2
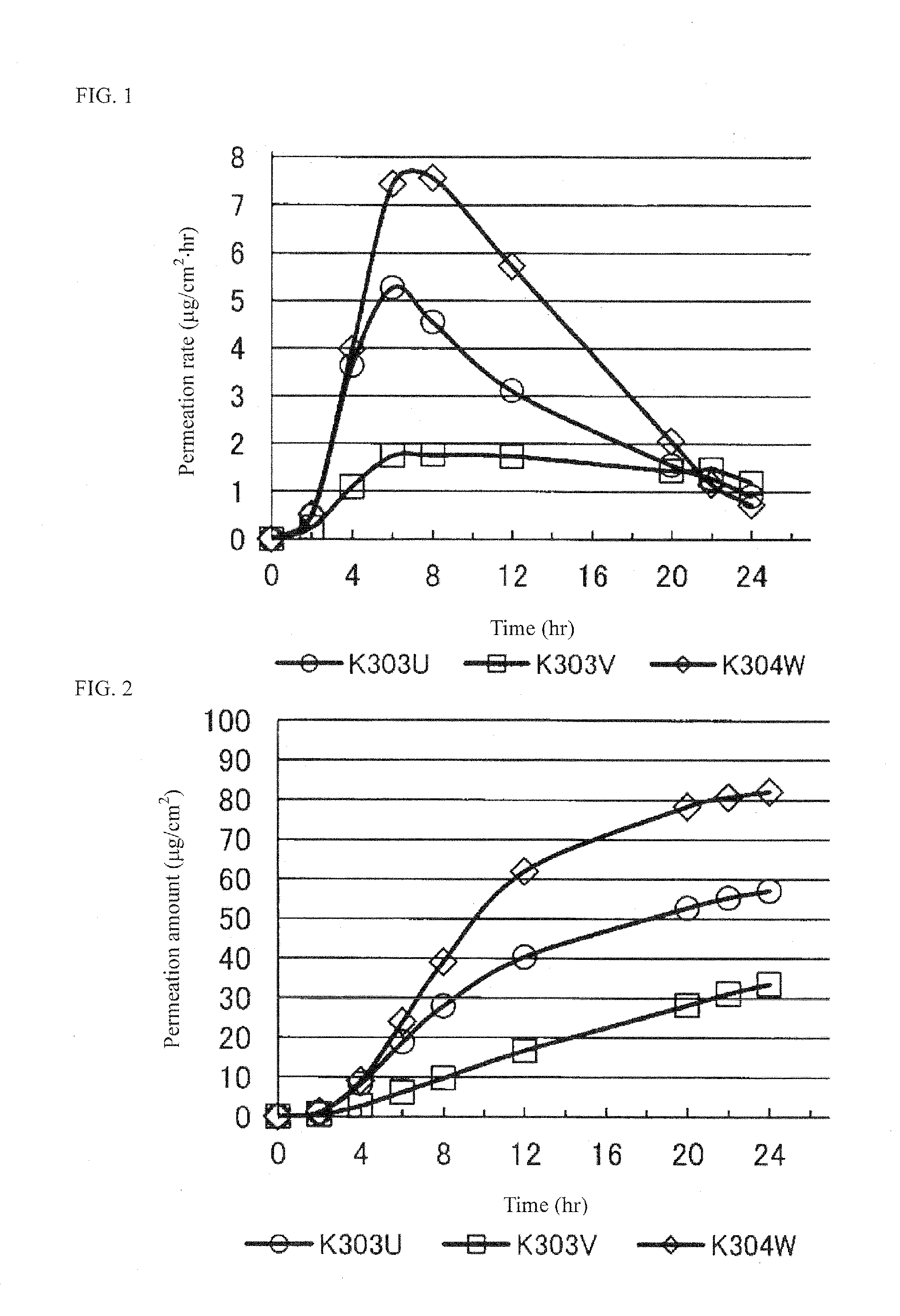
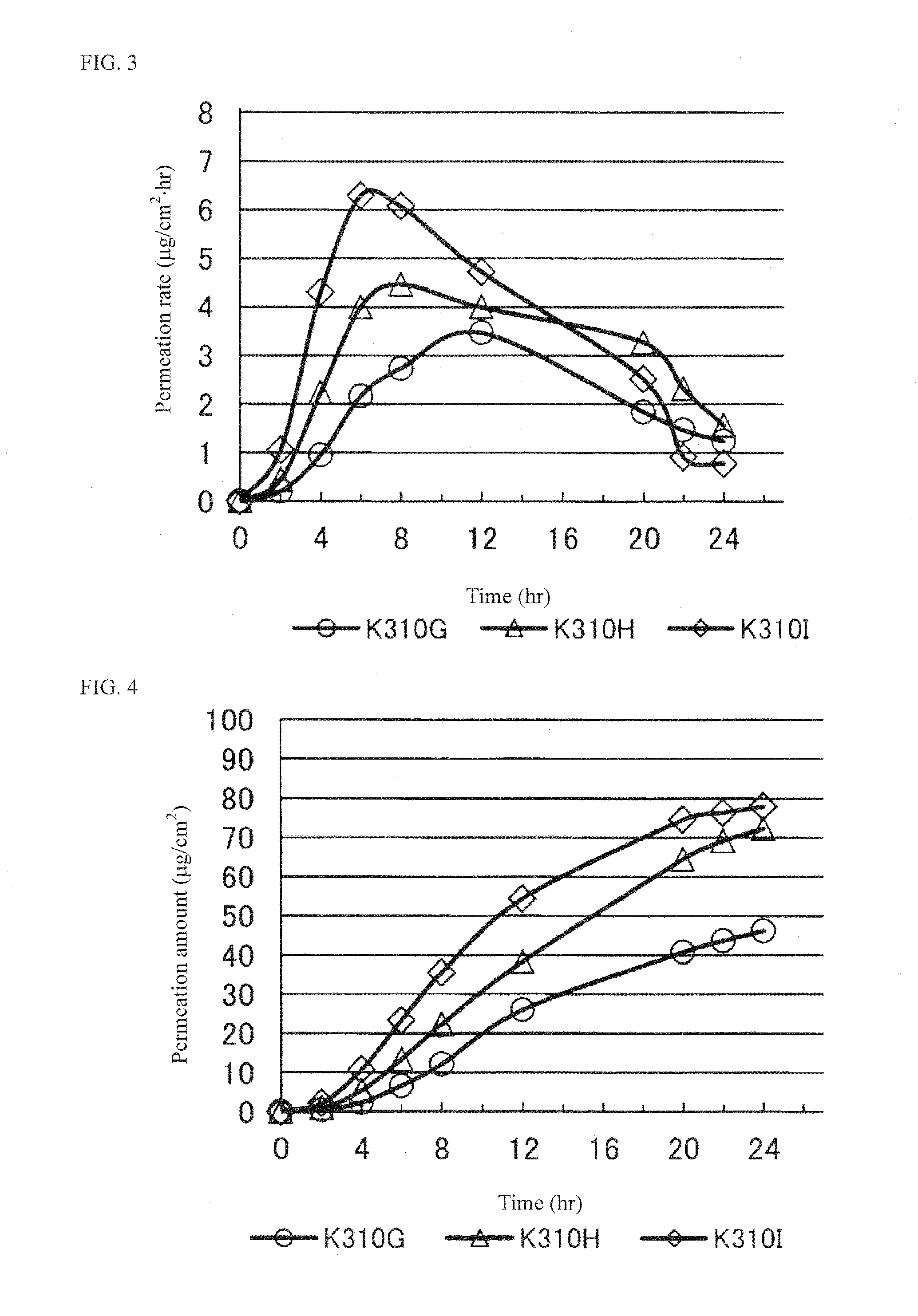
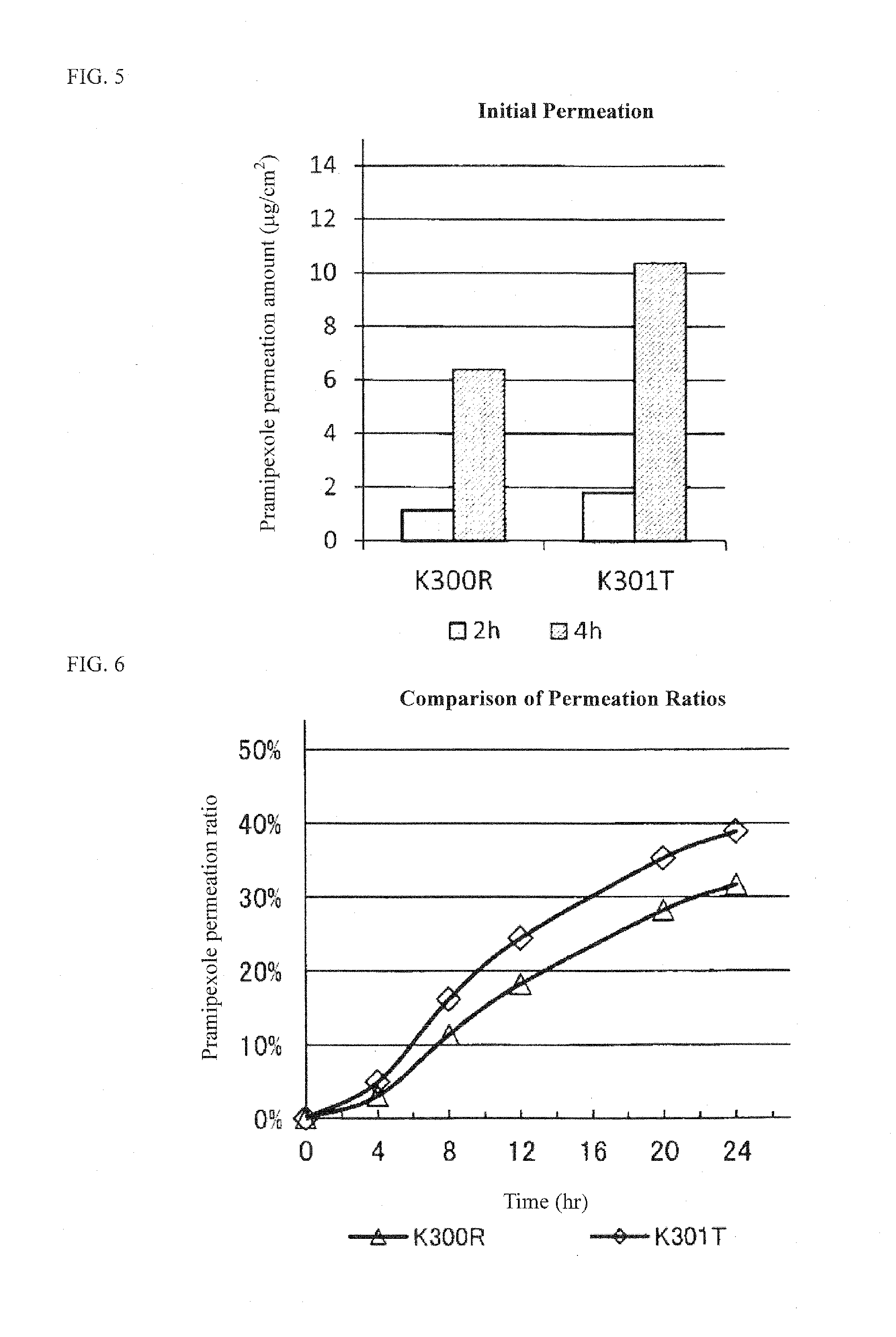
Pramipexole-Containing Transdermal Patch for Treatment of Neurodegenerative Disease
ActiveUS20170014353A1High crystallinityProcess stabilityOrganic active ingredientsNervous disorderTransdermal patchPoultice
The purpose of the present invention is to provide a nonaqueous tape that is stable and has high percutaneous absorption performance, and that contains pramipexole hydrochloride, which is slightly soluble in organic solvents and has high crystallinity. The present invention could produce a nonaqueous tape that is stable and has high percutaneous absorption performance by dissolving pramipexole, using a combination of a fatty acid ionic liquid and a divalent alcohol and a fatty acid ester. As a result, it has become possible to provide a transdermal patch (tape) containing pramipexole for the treatment of Parkinson's disease with which the problems associated with a conventional water-containing poultice of discoloration of the preparation and stability of the pramipexole can be solved.
Owner:MEDRX CO LTD
Patch containing isosorbide dinitrate
InactiveCN1102384CEfficient releaseFacilitated releaseSheet deliveryCardiovascular disorderAcetic acidPolyvinyl acetate
The present invention relates to an isosorbide dinitrate-containing patch, in which an adhesive layer comprising an adhesive composition is formed on a flexible support, and the adhesive composition consists of an acrylic-based adhesive (A), a polyvinyl acetate-based adhesive (B), a plasticizing component (C) and isosorbide dinitrate (D), and satisfies the following weight ratios (1) through (3) of each of the components: (1) A:B = 70:30 to 10:90, (2) the weight ratio of the component C based on the adhesive composition is 10 to 40% by weight, and (3) the weight ratio of the component D based on the adhesive composition is 20 to 35% by weight. Provided is a patch excellent in percutaneous absorption, handleability, resistance to skin irritation, sustained release, adhesivity, etc.
Owner:TEIJIN LTD
Spider freeze-dried powder mosquito repellent liquid and preparation method thereof
InactiveCN104758221AUnique smellGood volatilization effectCosmetic preparationsToilet preparationsFreeze-dryingMosquito bite
The invention relates to the field of mosquito repellent liquids and discloses a spider freeze-dried powder mosquito repellent liquid and a preparation method thereof. The spider freeze-dried powder mosquito repellent liquid utilizes spider freeze-dried powder as a main agent and through compatibility, the spider freeze-dried powder mosquito repellent liquid has substantial mosquito repellent effects. Aiming at symptoms of redness, swelling and itching caused by mosquito bites, the spider freeze-dried powder mosquito repellent liquid has good effects of preventing scar, relieving itching, eliminating swelling and relieving pain. In subtropical zones and tropical rain forest zones, the spider freeze-dried powder mosquito repellent liquid has substantial effects of repelling mosquitoes, eliminating swelling, relieving itching, resisting bacteria and diminishing inflammation.
Owner:HAINAN ZHUWANG PHARMA
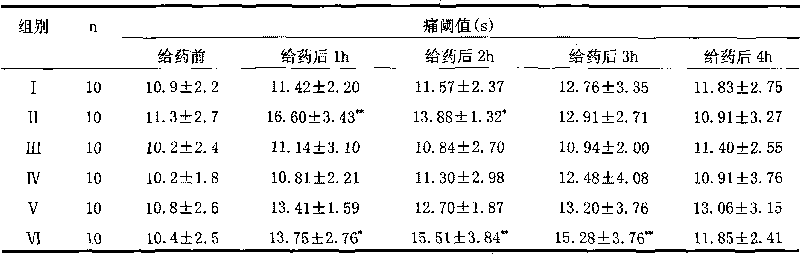
Medical cold application plaster and preparation method thereof
PendingCN110141560AIncrease moisture contentStrong penetrating powerPeptide/protein ingredientsPharmaceutical non-active ingredientsDihydroxyaluminum aminoacetateMedicine
The invention discloses medical cold application plaster. The medical cold application plaster consists of a back lining layer, a gel layer and an antiseizing layer and is characterized in that the gel layer is prepared from the following components in percentage by mass of 4%-5.2% of sodium polyacrylate, 0.08%-0.12% of dihydroxyaluminum aminoacetate, 0.11%-0.12% of ethylenediaminetetraacetic aciddisodium salt, 0.15%-0.22% of tartaric acid, 21%-25% of glycerine, 0.01%-0.1% of a preservative, 0.1%-0.5% of a functional additive, 0.5%-2% of ethanol and the balance water. The invention further discloses a preparation method of the medical cold application plaster. The medical cold application plaster has high molecular gelatin being high in water content, effective components can rapidly penetrate into the fat layer, impregnate into subcutaneous tissue and achieve favorable effect through transdermic absorption.
Owner:广州市白云区大荣精细化工有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com