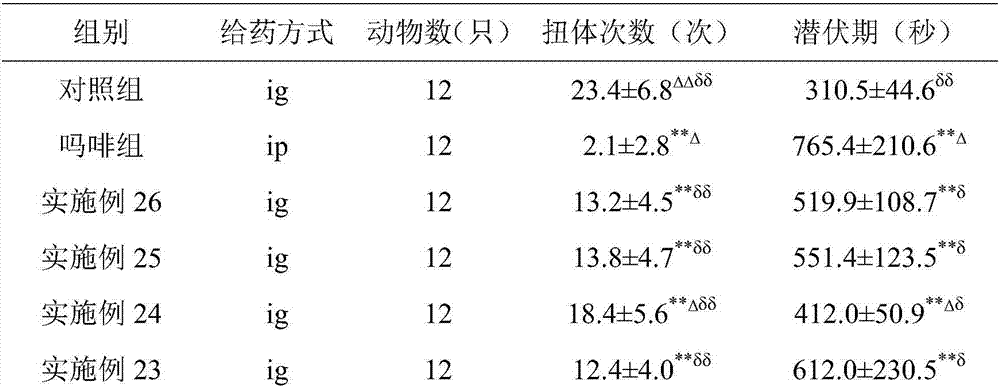
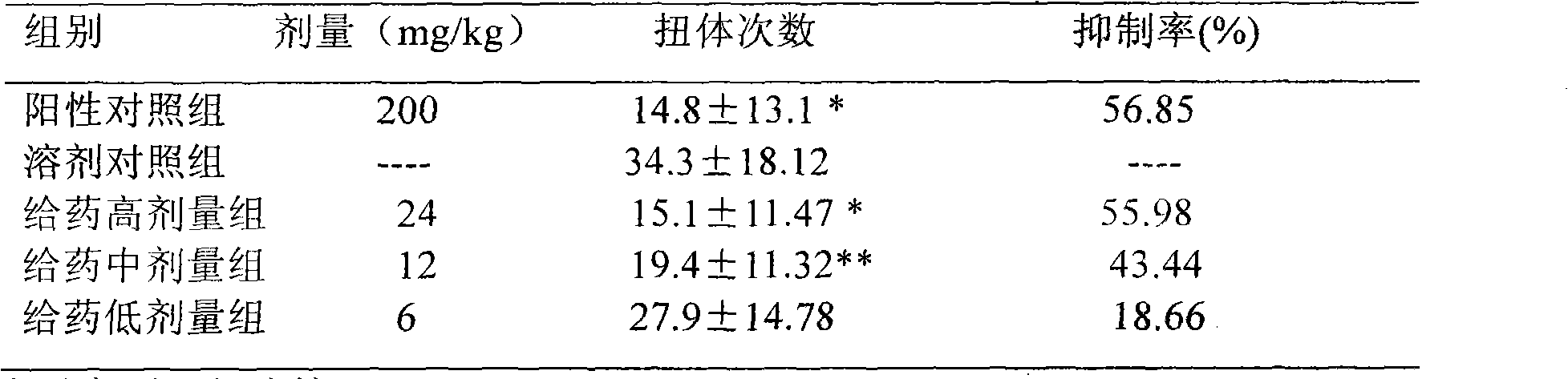
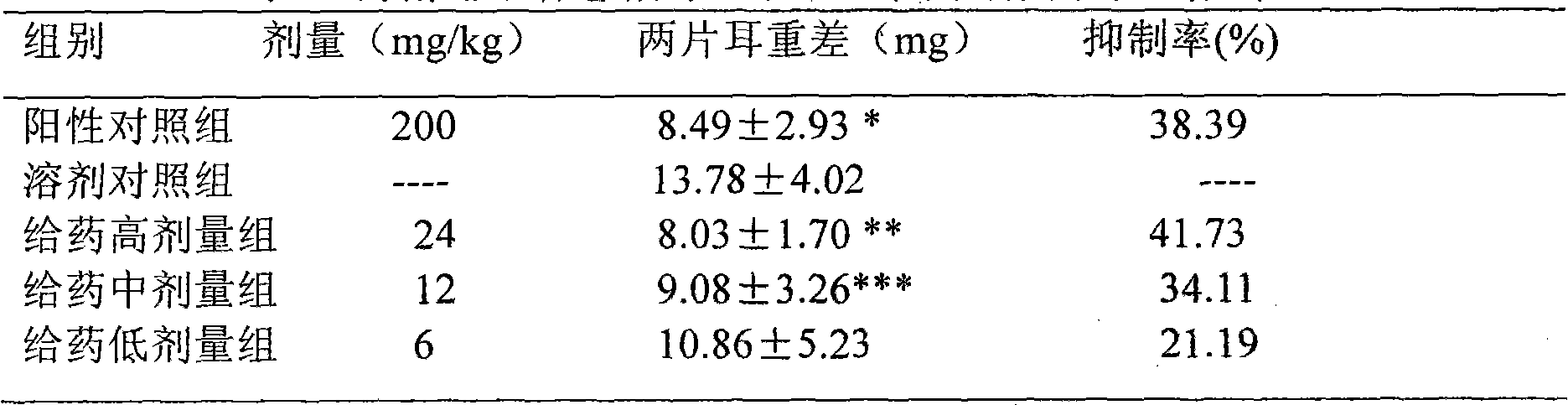
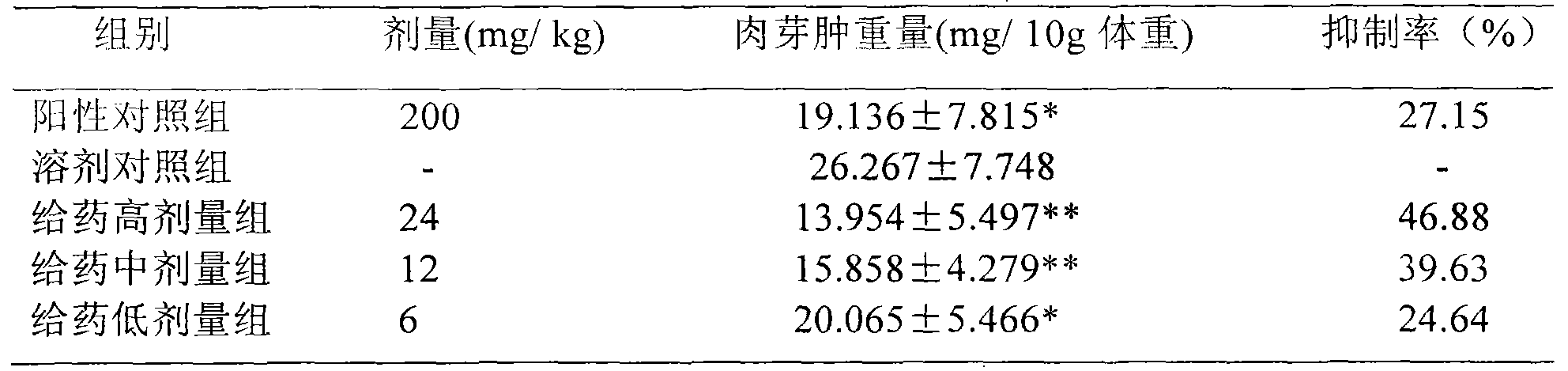
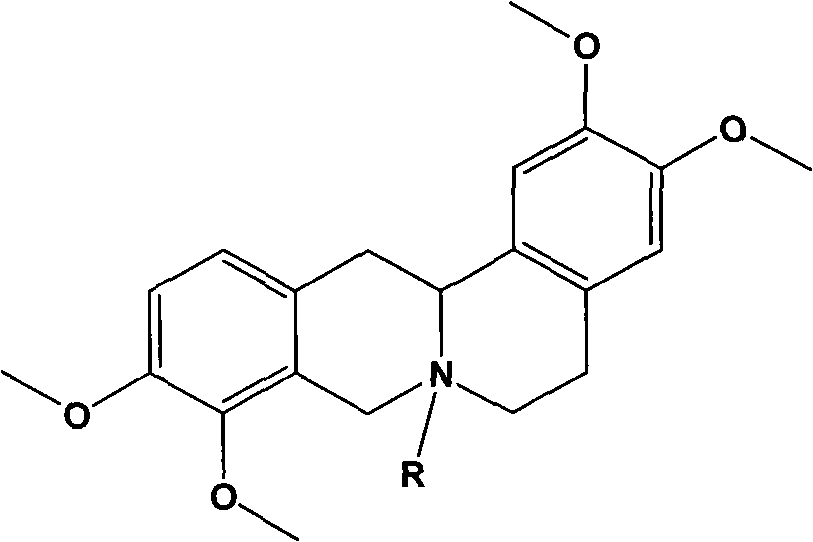
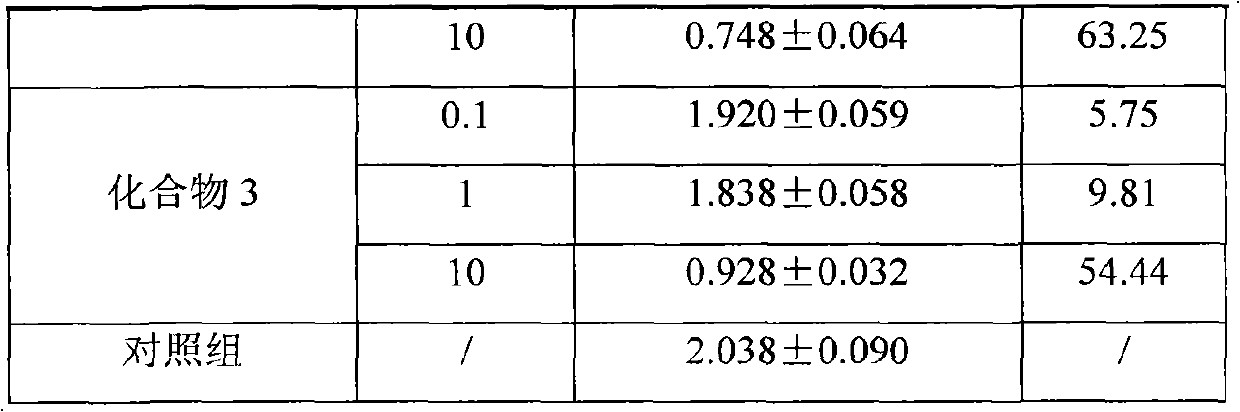
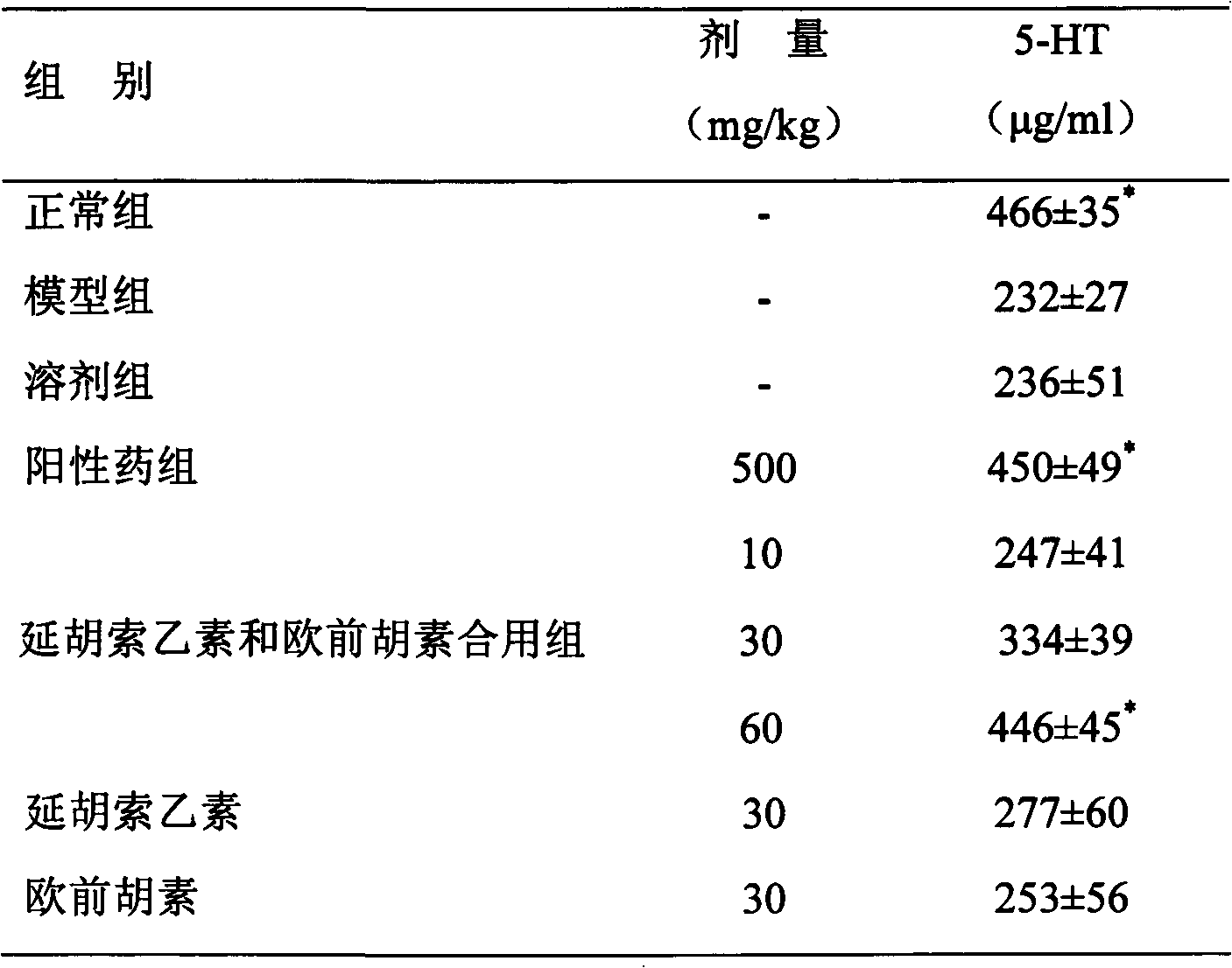
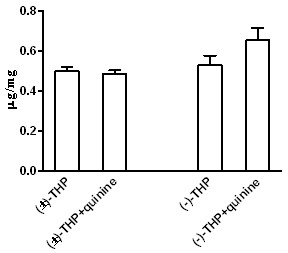
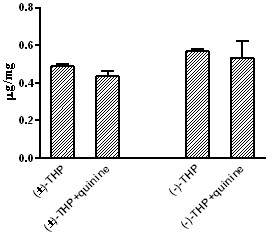
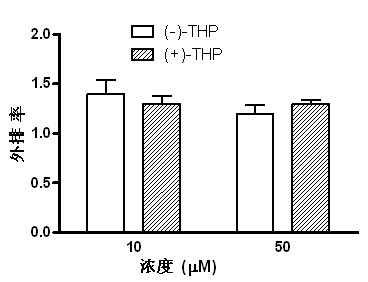
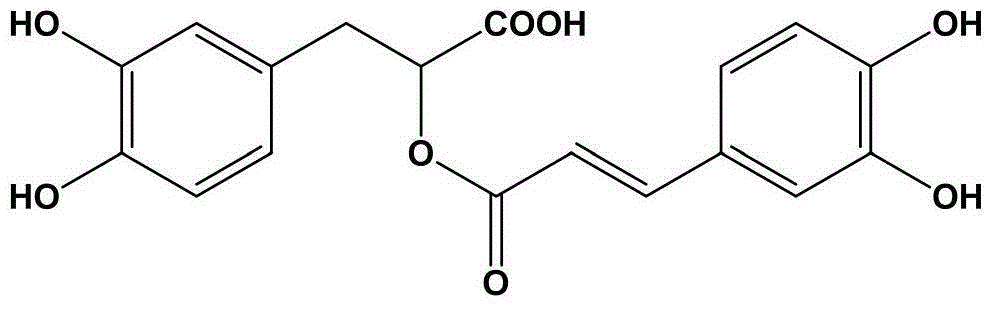
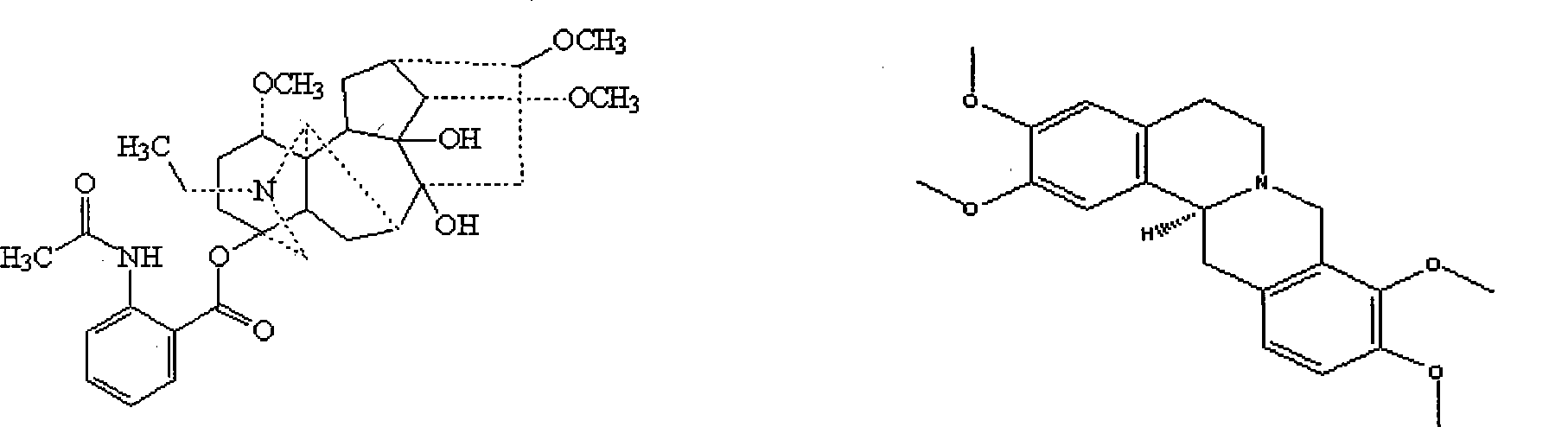Patents
Literature
166 results about "Tetrahydropalmatine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
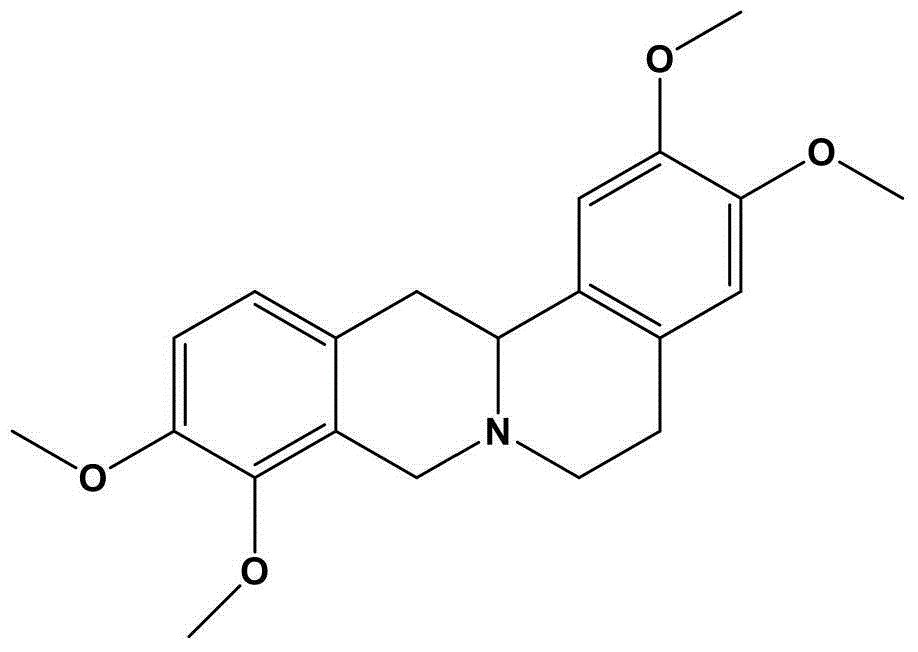
Tetrahydropalmatine (THP) is an isoquinoline alkaloid found in several different plant species, mainly in the genus Corydalis (Yan Hu Suo), but also in other plants such as Stephania rotunda. These plants have traditional uses in Chinese herbal medicine. The pharmaceutical industry has synthetically produced the more potent enantiomer Levo-tetrahydropalmatine (Levo-THP), which has been marketed worldwide under different brand names as an alternative to anxiolytic and sedative drugs of the benzodiazepine group and analgesics such as opiates. It is also sold as a dietary supplement.
Total alkaloids extraction of corydalis, its preparation method, medicine composition containing the total alkaloids extraction and application thereof
InactiveCN101054377AEfficient preparation methodIn line with the proportion of natural occurrencePowder deliveryAlkaloids chemistryHarmineFreeze-drying
The present invention discloses a Rhizoma Corydalis Decumbentis total alkaloids extract, its preparation method, pharmaceutical composition containing same. The total alkaloids mainly comprise : macleyine, tetrahydropalmatine, bicucalline, palmatine hydrochloride, 'xiawuning' alkaloid, corydaline harmine, other alkaloid and extract. The preparation method is : using Rhizoma Corydalis Decumbentis as material, adding right amount of polar solvent for leaching, merging the leachate, carrying out large pore adsorption resin chromatography, eluting impurity with diluted acid and alkaline aqueous solution orderly, eluting using polar organic solvent, collecting elution liquor, eliminating impurity further by alumina column adsorption, Collecting after-column liquid, concentrating liquor to obtain the Rhizoma Corydalis Decumbentis total alkaloids extract. The present invention also discloses a pharmaceutical composition containing the total alkaloids extract and uses of the pharmaceutical composition in preparing tablet, capsule, soft capsule, suppository, granula, transdermal absorption agent, drop pills, oral disintegrating agent, slow release agent, freeze-drying powder injection etc.
Owner:SHANGHAI INST OF PHARMA IND
Quadruple long-acting medicine composition and application thereof to pain treatment
ActiveCN107260732AGood analgesic effectImprove solubilityOrganic active ingredientsNervous disorderSolubilityDisease
The invention relates to a quadruple long-acting medicine composition and application thereof to pain treatment, in particular to the quadruple long-acting medicine composition. The medicine composition contains lappaconitine, tetrahydropalmatine, fumaric acid and succinic acid of the treatment and / or effective quantity as active ingredients. Compared with a composition formed by single ingredients or two ingredients or three ingredients, the quadruple long-acting medicine composition has the characteristics of good water solubility, good curative effect, low toxic and side effects and the like. The quadruple long-acting medicine composition can be used for preventing and / or treating diseases relevant to the pain; the pain includes tumor pain, pain of cancer patients after chemotherapy, joint pain, trauma pain, postoperative pain, inflammatory pain, pain caused by drug detoxification or drug addicition and intractable pain due to unknown reasons. The quadruple long-acting medicine composition can also be used for sedation, hypnosis, collaborative anesthesia, antiarrhythmia and the like.
Owner:林嗣松 +1
Rhizoma corydalis decumbentis extract, preparation method thereof, medicament composition containing the rhizoma corydalis decumbentis and application thereof
InactiveCN101058576AConsistent with multi-pathway mode of actionVarious ingredientsPowder deliveryAlkaloids chemistryFreeze-dryingPalmatine
The invention discloses a total alkaloid extract and making method of eye drip, which comprises the following parts: opium alkaline, tetrahydropalmatine, cumic acid, alcaine palmatine, corydaline, summer alkaline and other biological alkalines. The preparing method comprises the following steps: adopting summer non as raw material; optimizing hypercritical CO2 flow to extract; collecting extract; drying; obtaining the total alkalilne extract; fitting for tablet, pill, suppository, percutaneous absorption agent, oral rapid-breaking agent, release-control agent and freeze dried.
Owner:SHANGHAI INST OF PHARMA IND CO LTD
Stephania hainanensis total alkaloid extract and preparation method thereof
The invention belongs to the technical field of pharmacy, and particularly discloses a stephania hainanensis total alkaloid extract extracted from stephania hainanensis tubers and a preparation method thereof. The preparation process is as follows: material (stephania hainanensis dry tubers); grinding; soaking in a moderate amount of 0.01 to 10 percent of hydrochloric acid or sulfuric acid; 1 to 4 times of ultrasonic extraction; filtration; storng-acid cation exchange resin purification; solvent recovery for drying; and stephania hainanensis total alkaloid extract. The extract mainly comprises L-tetrahydropalmatine, palmatine, fangchinoline, hanfangchin and other active ingredients, the percentage by weight of stephania hainanensis total alkaloid is greater than or equal to 70 percent, the percentage by weight of the palmatine is greater than or equal to 20 percent, and the percentage by weight of the fangchinoline is greater than or equal to 40 percent. Reports on the stephania hainanensis total alkaloid extraction process are not found in related literatures so far, the operation flow of the method is simple, the purity of the product is high, the efficacy is remarkable, and the method disclosed by the invention is applicable to industrial production and the exploitation and utilization of local stephania hainanensis resources.
Owner:朱毅
Improved rail lubricating grease
The invention discloses improved rail lubricating grease prepared from the following raw materials in parts by weight: 3-9 parts of tetrahydropalmatine, 1-3 parts of potassium dihydrogen phosphate, 2-6 parts of isodehydrocostus lactone, 3-7 parts of oleanonic acid, 1.5-4 parts of oleic acid, 2.5-6 parts of dialkyl calcium benzene sulfonate, 2-4 parts of imidazoline, 4-6 parts of alkylphenol sulfide, 1-5 parts of sodium citrate, 1.5-6 parts of polyacrylate copolymerization emulsion, 1-4 parts of alkyl naphthalene, 6-10 parts of thickening agent and 2-6 parts of extreme-pressure additive. The improved rail lubricating grease disclosed by the invention can form a lubricating layer between a rail and an object to reduce the friction between the rail and the object and prolong the service life of the rail, and has a certain cooling effect at the same time.
Owner:QINGDAO KANGTAIXIN ENVIRONMENTAL PROTECTION TECH
Special lubricating oil for mechanical gears
InactiveCN106635323AExtended service lifeImprove adhesionLubricant compositionGlycerolPhosphoric acid
The invention discloses a special lubricating oil for mechanical gears. The special lubricating oil for mechanical gears is composed of phosphite, talcum powder, phosphoric acid, sorbitol, p-hydroxy benzyl aldehyde, methyl citrate, monomethyl citrate, tetrahydropalmatine, mesembryanthemoidigenic acid, isodehydrocostus lactone, lupeol palmitate, oleanonic acid, naphthenic oil, butter, tetrahydroxyethyl ammonium swelling oily agent, aluminum disulfide, aromatic hydrocarbon, paraffin base, succimide succinate, boron nitride, fish gallbladder, fatty soap, glycerol, polyalkenyl succinimide, graphite, amino monothioester and molybdenum dialkyldithiocarbamate. Compared with the prior art, the special lubricating oil for mechanical gears can lower the loss of engine oil, increases the environment-friendly effect of the original special engine oil, enhances the adhesion of lubricating oil, increases the lubricating effect of the engine oil, and lowers the friction, so that the mechanical gears can well operate, thereby enhancing the service life of the machine. Thus, the special lubricating oil for mechanical gears has popularization and application value.
Owner:HUAIYIN INSTITUTE OF TECHNOLOGY
Total alkaloid of stephania delavayi and preparation and application thereof
ActiveCN101234141AReduce shrinkageNo dysmenorrheaOrganic active ingredientsDigestive systemStrong acidsSteroidal alkaloid
The invention discloses a total alkaloid of Stephania delavayi Diels, the content of which is measured by tetrahydropalmatine. Tetrahydropalmatine occupies 55-95 percent of the total alkaloid extract of Stephania delavayi Diels, wherein the weight percentage of tetrahydropalmatine contained is no less than 2 percent, while the weight percentage of palmatine hydrochloride contained is no less than 0.8 percent. The invention also discloses a preparation method of the total alkaloid of Stephania delavayi Diels, which relates to the steps as follows: adding the Stephania delavayi Diels meal into ethanol for diacolation or reflux to acquire alcohol extract, decompressing and recycling the ethanol and concentrating into a condensed ointment for dissolving and filtrating with acid to acquire a filtrate, passing the filtrate through a macroporous resin column or a strong acid cation exchange resin column for adsorption, washing with water to remove the impurities and extracting with a eluent, collecting the eluent and decompressing and recycling the extract and then concentrating and drying, thus acquiring the total alkaloid of Stephania delavayi Diels; the steps can also be decompressing a percolating liquid to recycle the ethanol, concentrating, dissolving with acid and then filtrating, adjusting the pH value of the filtrate with weak base to produce deposition, filtrating the deposition to acquire a filter residue, adding ethanol into the filter residue to resolve and decompress, recycle the ethanol and then drying, thus acquiring the total alkaloid. The results of a pharmacological study on the acquired total alkaloid show that the total alkaloid of Stephania delavayi Diels has good effect on spasmolysis, pain relieving, antiphlogosis and antibiosis, blood activating and so on, and can combine with the acceptable components in pharmacy to prepare various sustained release or controlled release formulation or other formulations.
Owner:GUANGZHOU BOJI MEDICINE SERVICES
Novel isoquinoline alkaloid derivatives and preparation method and application thereof
InactiveCN101830897AEnhanced inhibitory effectOrganic active ingredientsOrganic chemistryNasopharyngeal carcinomaStructural formula
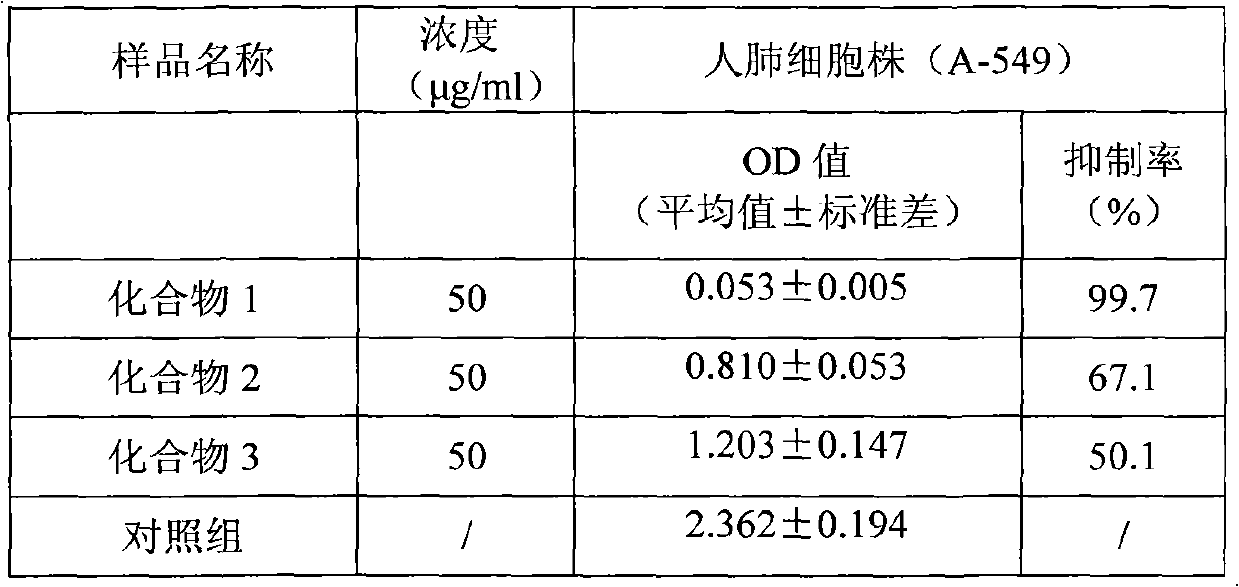
The invention discloses tetrahydropalmatine derivatives and a preparation method and application thereof. The structural formula of the tetrahydropalmatine derivatives is shown as a formula II, wherein R is octyl, isopropyl or 3-methybutyl. The provided application is the application of the tetrahydropalmatine derivatives shown as the formula II or pharmaceutically acceptable salts thereof in the preparation of eukaryotic tumor cell antiblastics and medicaments for preventing and / or treating tumors. The results of in-vitro anti-tumor activity experiments prove that each tetrahydropalmatine derivative has the effect of inhibiting human lung cancer cells A-549, KB human nasopharyngeal carcinoma cells, and HL-60 leukemia cell strains to a certain extent. The results show that the tetrahydropalmatine derivatives shown as the formula II can be applied to preparing the medicaments for treating the tumors, and the pharmaceutical application of the tetrahydropalmatine derivatives is widened.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Anaesthesia possessing lubricant for alimentary tract and respiratory tract endoscope
The invention relates to a lubricant with paresis used in enteron endoscope and respiratory tract endoscope. Components of said lubricant for realizing paresis comprises: phosphate methylmorphine and levogyration tetrahydropalmatine; the components of said lubricant for realizing lubricate effect comprises: disilyloxy, methyl ethylene glycol, chloromycetin in glycerin and ammonium bithiolicum glycerin; and other components of said lubricant comprises: adhesion promoter of Kapo 934, preservative agent of gluconic acid chlorhexidine and pH value adjuster of triethanolamine. And the weight proportion of said lubricant comprises: 1-10% phosphate methylmorphine, 1-10% levogyration tetrahydropalmatine, 20-50% disilyloxy, 5-35% methyl ethylene glycol, 3-30% chloromycetin in glycerin, 3-30% ammonium bithiolicum glycerin, 1-10% adhesion promoter of Kapo 934, 1-10% preservative agent of gluconic acid chlorhexidine, and 1-10% pH value adjuster of triethanolamine.
Owner:陈嘉农
Corydalis decumbens extract, and its preparing method and use
The present invention discloses a Chinese medicinal material Jilong corydalis extract, its preparation method and application. Said extract contains protopine and L-tetrahydropalmatine, their summation is not less than 50% (W / W), in which the protopine content is 24%-50% (W / W), and the L-tetrahydropalmatine content is 26%-50% (W / W). The pharmological tests show that it can be used as N-methyl-D asparaginic acid acceptor antagonist and acetylcholine esterase inhibibor. Besides, said invention also provides the application of said extract in the preparation of medicine for preventing and curing the hypomnesis due to vascular dementia and senile dementia.
Owner:SHANGHAI HUATUO MEDICAL SCI CO LTD
Analgesic anti-inflammatory composite medicament and method for preparing same
ActiveCN101518531AImprove bioavailabilityLasting effectOrganic active ingredientsAntipyreticSide effectHepatic first pass effect
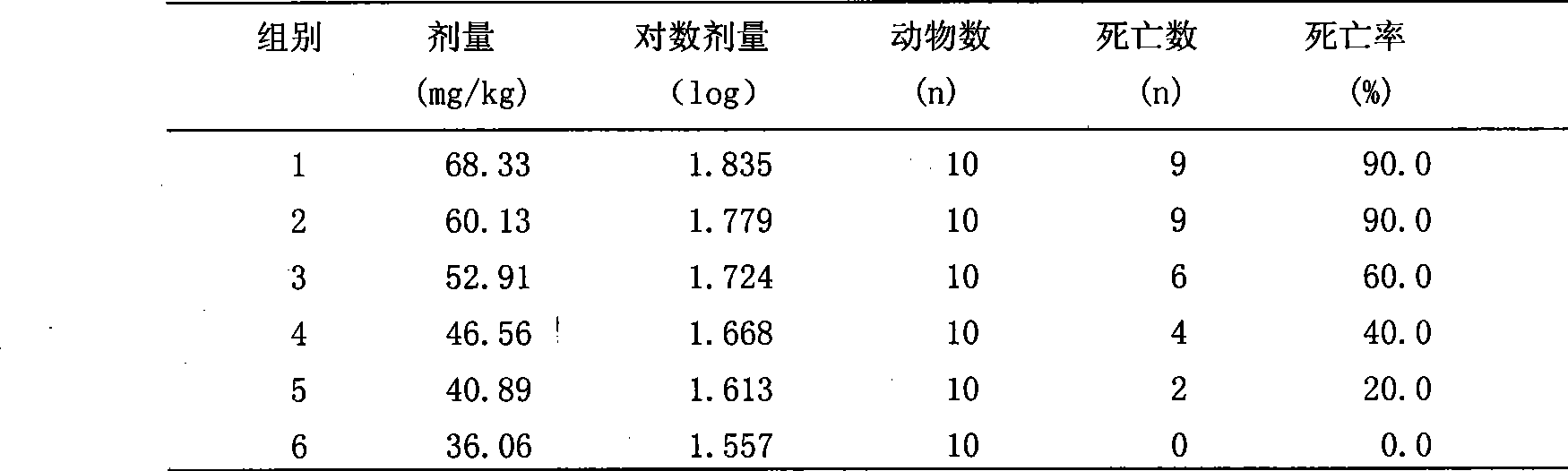
The invention discloses an analgesic anti-inflammatory composite medicament made from lappaconitine and tetrahydropalmatine, a patch made from the composite medicament and a method for preparing the patch. The patch comprises a back lining layer, a medicament storage layer and a protection layer, wherein the medicament storage layer is made from raw materials including the lappaconitine, the tetrahydropalmatine and pressure-sensitive adhesive; and the patch is made by adding a proper amount of transdermal enhancerfrom in to the same three raw materials. Experiments and clinical research prove that the composite medicament made from raw medicament materials of lappaconitine and tetrahydropalmatine according to a certain proportion can reduce the toxicity of the lappaconitine and improve the median lethal dose (LD 50). Due to the use of the patch made from the composite medicament, the blood concentration can be kept stable for a long time, the first-pass effect on liver and the damage to gastrointestinal tracts are avoided, the toxicity and the side effect of the composite medicament are reduced, and the bioavailability and the safe curative effect of the composite medicament are improved. The composite medicament is convenient to use without causing patients to be afflicted by pain and is very effective in relieving pain and diminishing inflammation.
Owner:GANSU CHEEZHENG TIBETAN MEDICINE CO LTD
Improved lubricating oil
The invention discloses an improved lubricating oil. The lubricating oil is prepared from the following raw materials in parts by weight: 7-14 parts of hydroxy benzyl aldehyde, 5-10 parts of dodecyl benzene sodium sulfonate, 5-10 parts of methyl ciTCMLIBate, 9-12 parts of dichloromethane, 4-10 parts of citric acid mono-methyl ester, 5-8 parts of potassium dihydrogen phosphate, 6-9 parts of tetrahydropalmatine, 3-5 parts of hydrochloric acid, 16-24 parts of sulfur phosphorus primary-secondary alkyl zinc salt, 9-17 parts of sodium citrate, 10-15 parts of sulfureted isobutene, 8-17 parts of sulfureted alkyl phenol calcium, 10-20 parts of methyl acrylate and 30-50 parts of lubricating base oil. The improved lubricating oil disclosed by the invention has the advantages of a better frictional property, a better abrasion-proof property, a better antirust property and stable nature, and the lubrication quality of the lubricating oil is effectively improved.
Owner:QINGDAO TONGSHENGTONG RUBBER & PLASTIC
Medicine for treating coronary heart disease
ActiveCN102228493ARaise the level of creativityImprove international competitivenessOrganic active ingredientsCardiovascular disorderCoronary artery diseasePanax quinquefolium saponin
The invention discloses a medicine for treating coronary heart disease. In the product of the invention for preventing and / or treating coronary heart disease, the active ingredients thereof are composed of panax quinquefolium saponin and tetrahydropalmatine. The experiment proves that compatible American ginseng and rhizoma corydalis can be used for efficiently treating myocardial ischemia; therefore, American ginseng and rhizoma corydalis can be used for preventing and / or treating coronary heart disease. The technique method for preparing the alkaloidal compatible preparation of panax quinquefolium saponin and rhizoma corydalis is stable, the extraction rate of active ingredients is high, and the medicine is suitable for industrial production. The medicine of the invention establishes a technical platform for the research and development of innovative traditional Chinese medicines for coronary heart disease in the aspects of the safety, efficacy evaluation, pharmacokinetics and the like of compound traditional Chinese medicine, which is good for developing new traditional Chinese medicines with good clinical application prospect and market competitiveness, and further increases the innovation level and international competitiveness of new domestic medicines.
Owner:PEKING UNIV FIRST HOSPITAL
Medicinal composition containing tetrahydropalmatine and imperatorin
InactiveCN102429905AClear ingredientsQuality is easy to controlNervous disorderAntipyreticMenstrual painsTetrahydropalmatine
The invention discloses a medicinal composition containing tetrahydropalmatine and imperatorin and application. The medicinal composition is prepared into various pharmaceutically acceptable solid oral formulations, such as granules, powder, tablets, pills and capsules, from the tetrahydropalmatine and the imperatorin in a weight ratio of (1-8):(1-5). Pharmacological tests show that the composition has the effects of suppressing pain and discomforts, including headache, migraine, stomachache, neuralgia, lumbago and menstrual pain; and the composition can be used for treating the pains.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Tetrahydropalmatine intra-gastric floating oral microsphere
ActiveCN103751115ASimple preparation processLow costOrganic active ingredientsDigestive systemTetrahydropalmatineOrganic solvent
The invention provides a tetrahydropalmatine intra-gastric floating oral microsphere. The microsphere is prepared by the method comprising the following steps: uniformly mixing the tetrahydropalmatine and pharmaceutically acceptable carrier materials, dissolving in a pharmaceutically acceptable organic solvent to form an organic phase; preparing a pharmaceutically acceptable hydrophilic emulsifier aqueous solution as an aqueous solution; slowly dripping the organic phase into the aqueous phase under a stirring state, constantly stirring until the organic solvent is volatized, standing, filtering, removing the filtrate, washing the filter cake with distilled water, and drying in vacuum to obtain the tetrahydropalmatine intra-gastric floating oral microsphere. The tetrahydropalmatine intra-gastric floating oral microsphere is simple in preparation process, good in reproducibility, high in drug-loading capacity and encapsulation efficiency, good in intra-gastric floating and sustained-releasing performance and can constantly float and slowly release drugs at constant speed in 12 hours, and the drug can be released over 90 percent in accumulation of 12 hours, so that the drug can be released stably, slowly and directionally.
Owner:ZHEJIANG CHINESE MEDICAL UNIVERSITY
New applications of tetrahydropalmatine
InactiveCN101773499ALow toxicityExpand the scope of medicinal useOrganic active ingredientsOrganic chemistryTumour volumePharmacy
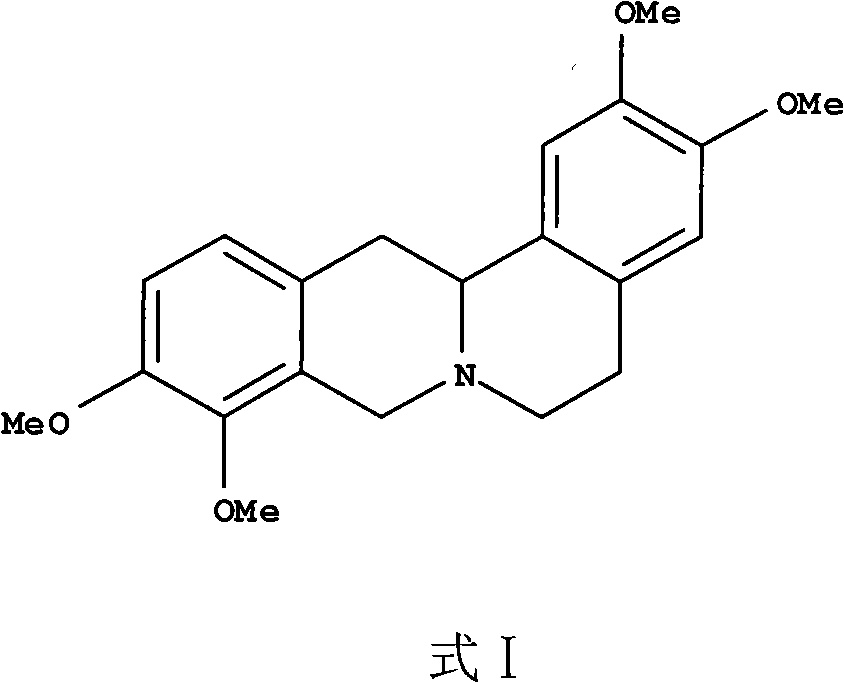
The invention discloses new applications f tetrahydropalmatine. The new application is an application that tetrahydropalmatine shown in formula I or salts thereof which are acceptable in pharmacy are used to prepare medicines used for inhibiting the proliferation of tumor cells and an application for preparing medicines used for preventing and / or curing tumors. The result of the in vivo experiment using nude mice shows that tetrahydropalmatine can effectively inhibit the growth of tumors, and the tumor volume is much smaller than the volume of the negative control group. Therefore, tetrahydropalmatine which is widely used for analgesia has good antitumor activity, and the result shows that tetrahydropalmatine can be used to prepare medicines for curing cancers, in particular to prepare medicines for curing the lung cancer. The invention broadens the medical application field of tetrahydropalmatine.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Preparation method and application of middle calming tablet
ActiveCN102836381AReduce dosageAntineoplastic agentsMolluscs material medical ingredientsCassiaTetrahydropalmatine
The invention provides a preparation method of a middle calming tablet. The middle calming tablet is prepared from 180g of cassia twig, 180g of vinegar-treated corydalis tuber, 180g of calcined oyster, 120g of common fennel, 120g of amomum fruit, 60g of Chinese ginger and 120g of liquoric root serving as bulk pharmaceuticals. The middle calming tablet is prepared through ultrafine extraction and microwave extraction, so that the convent of tetrahydropalmatine is increased greatly. The invention further provides an application of the middle calming tablet to preparation of a medicament for inhibiting cell proliferation of rat breast cancer cells SHZ-88.
Owner:SHIJIAZHUANG DONGFANG PHARMA
Anti-oxidant lubricating grease
The invention discloses anti-oxidant lubricating grease, belonging to the technical field of lubricants. The technical scheme of the invention is that the anti-oxidant lubricating grease is prepared from the following raw materials in parts by weight: 10-30 parts of an oligomer of trimethyl dihydroquinazolone, 2-10 parts of isodehydrocostus lactone, 1-6 parts of tetrahydropalmatine, 2-7 parts of sodium dipenta acid, 2-7 parts of bissalicylidene propane diamine, 3-9 parts of p-Hydroxybenzaldehyde, 1-3 parts of methyl citrate, 2-7 parts of chlorinated paraffin, 2-10 parts of diphosphorothioate, 1-3 parts of monoglvceride citrate, 2-5 parts of alkyl succinimide, 1-3 parts of viburnum themoidigenic acid, 1-5 parts of boron nitride, 1-3 parts of oleanonic acid, 2-8 parts of lupeol palmitate and 2-5 parts of polyisobutene. Compared with the prior art, the anti-oxidant lubricating grease disclosed by the invention is simple to prepare, economical and practical and low in cost, can effectively prevent instruments from being damaged, and is high in anti-oxidant performance, long in service cycle, free from any damages to mechanical equipment, and meanwhile has good frictional characteristic, wear resistance and anti-corrosion performance.
Owner:青州市东能润滑油脂有限公司
Application of tetrahydropalmatine and naringin in preparing oral care products
InactiveCN103520010ARaise pain thresholdGood analgesic effectOrganic active ingredientsCosmetic preparationsNaringinTetrahydropalmatine
The invention discloses application of tetrahydropalmatine and naringin in preparing oral care products, i.e., tetrahydropalmatine and naringin are added into an oral care health-care product as active ingredients with analgesic effect in form of a compound in a process of preparing oral care products. Compared with the prior art, the oral care products provided by the invention not only are safe to use, but also have remarkable analgesic effects.
Owner:LIUZHOU LIANGMIANZHEN +2
Method for preparing Chinese medicinal stomach medicament preparation and quality test method thereof
ActiveCN101708220AEasy to prepareEasy to operateComponent separationDigestive systemLiver stomachEggshell
The invention belongs to the technical field of Chinese medicaments, in particular relates to a method for preparing a Chinese medicinal stomach medicament preparation and a quality test method thereof. The conventional preparation method is fussy, needs to prepare particles in four different colours, and has complicated granulation process and inconvenient operation; and the conventional test method does not have very high accuracy and reliability, and cannot determine the source of contained tetrahydropalmatine. The method for preparing the Chinese medicinal stomach medicament and the quality test method thereof, the Chinese medicinal stomach medicament consists of rhizoma corydalis (vinegar processed), drift cuttlebone, elecampane, calcined alum, fried chicken eggshell and calcined nacre. The Chinese medicament treats ache, stops pain, and is used for treating gastric cavity ache, acid stomach, and noisy acid regurgitation caused by liver-stomach disharmony. The method for preparing the Chinese medicinal stomach medicament preparation of the invention is simple and easy to operate, simplifies preparation processes, and ensures the uniformity of a product better; and the quality test method has simple operation, and accurate and reliable testing result.
Owner:XIAN BEILIN PHARMA
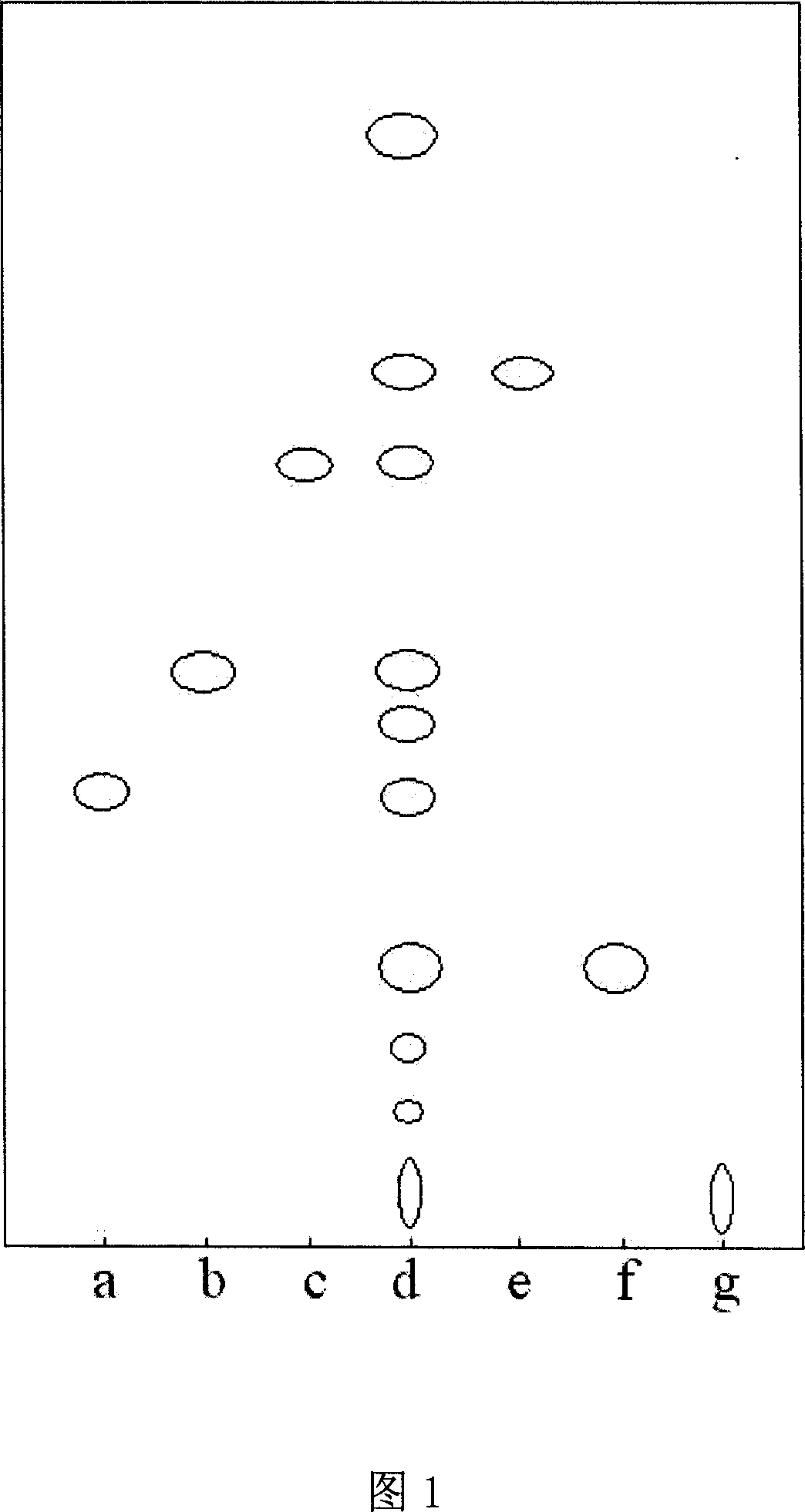
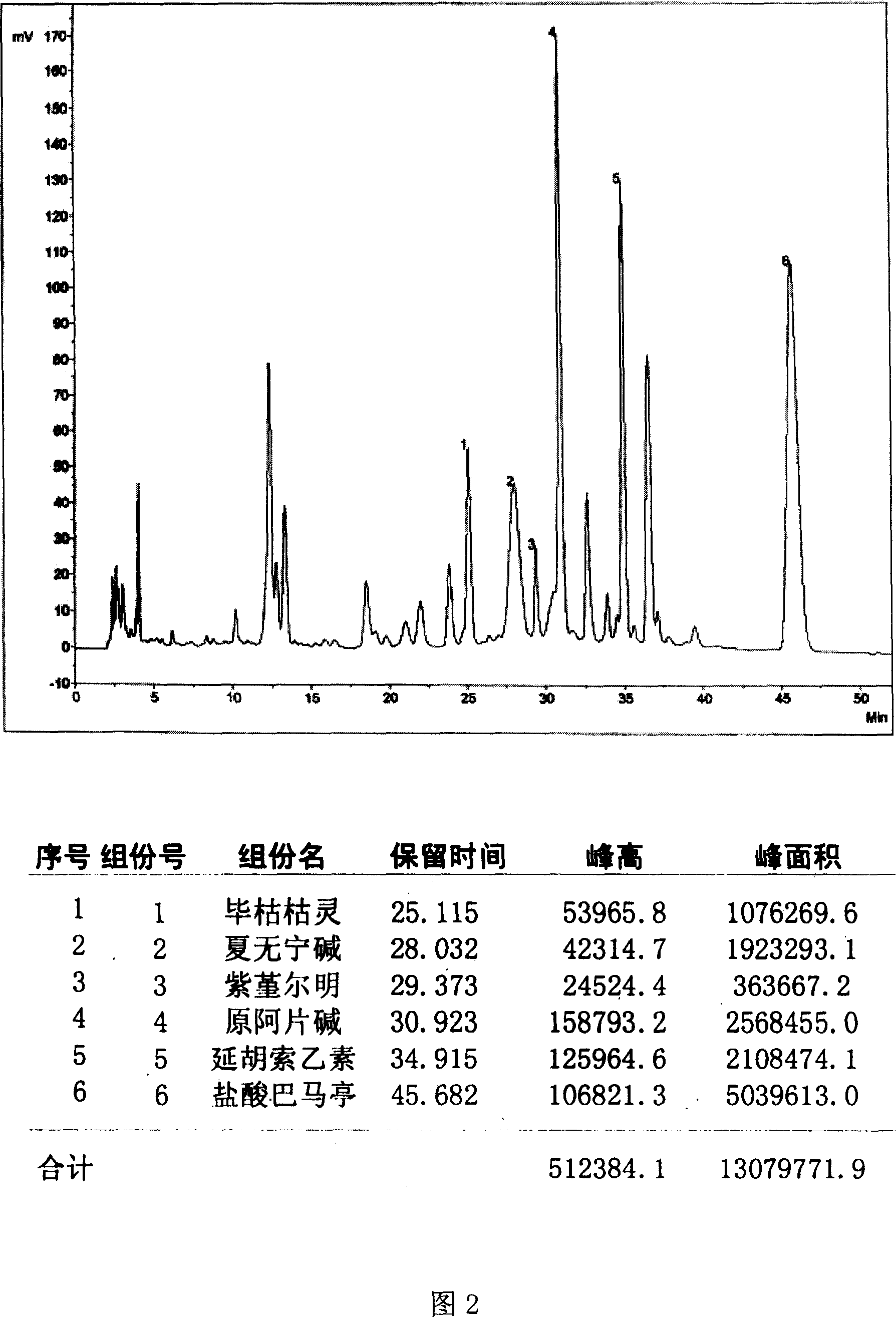
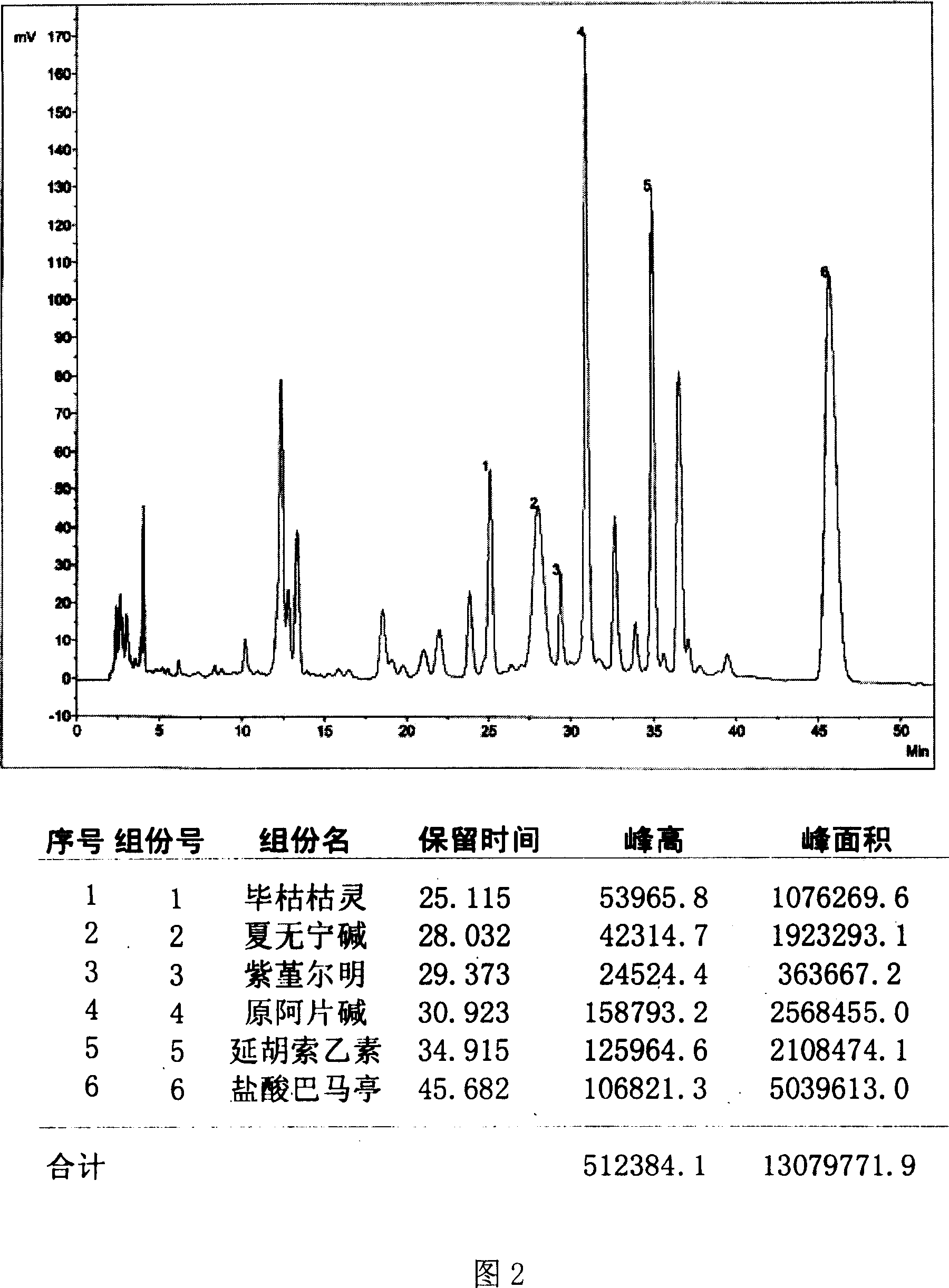
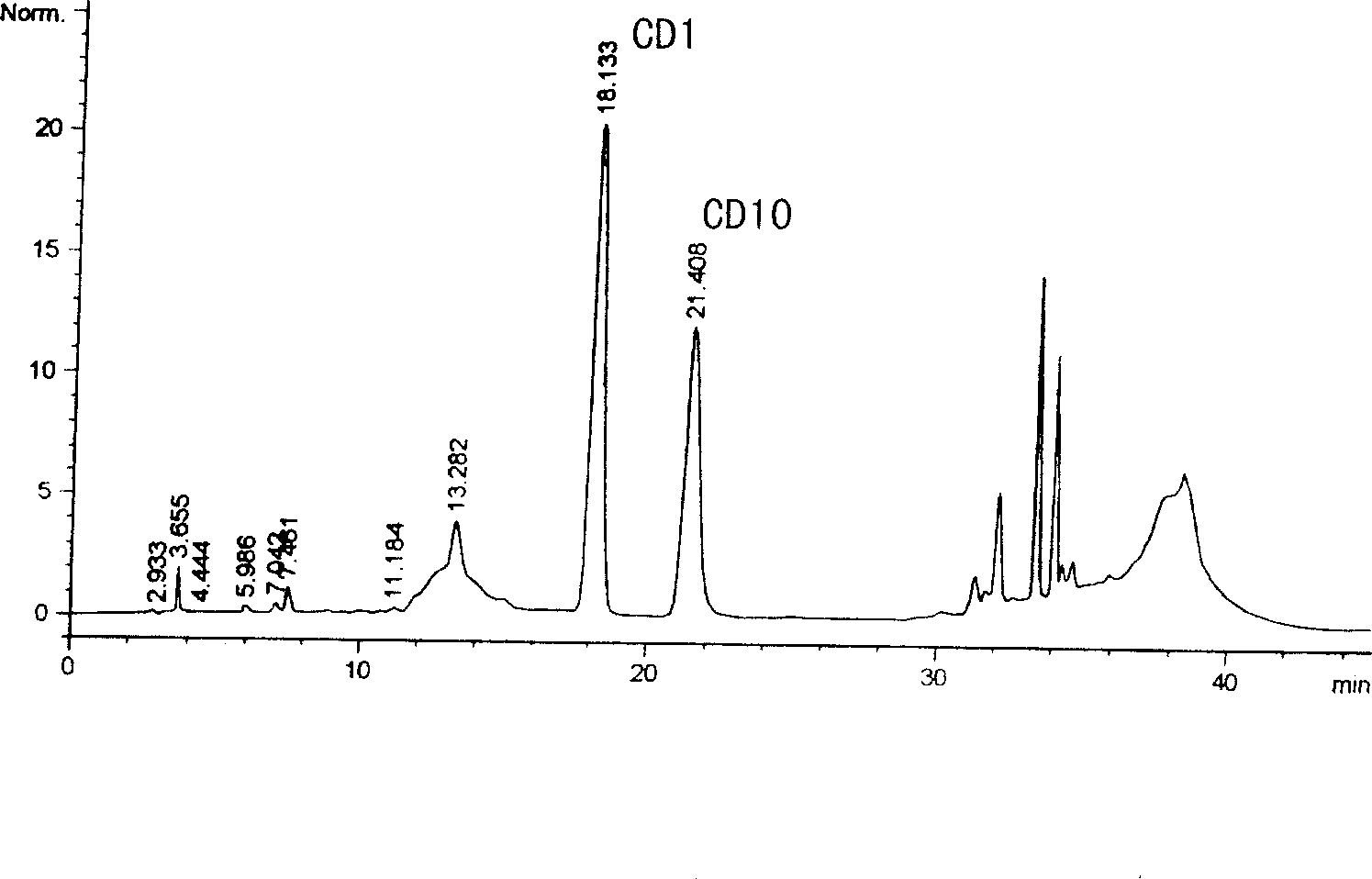
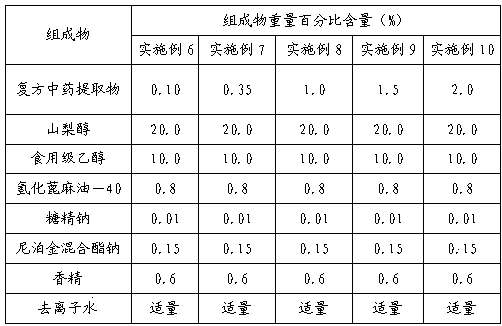
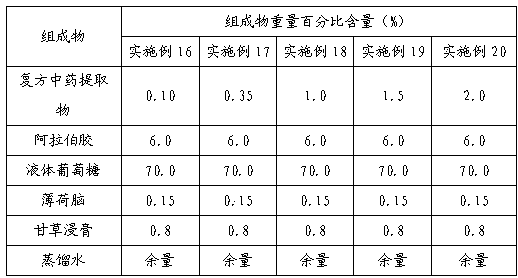
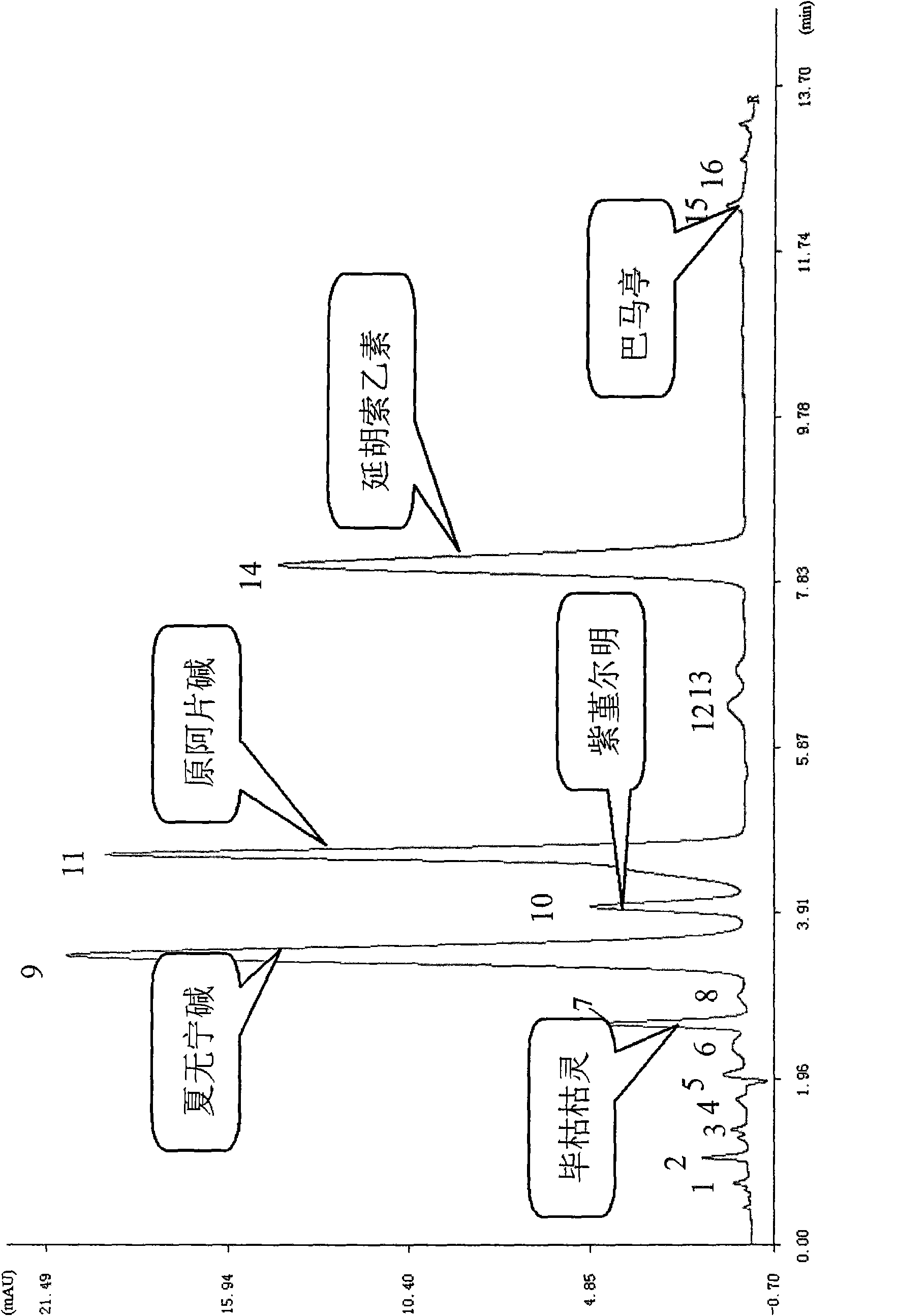
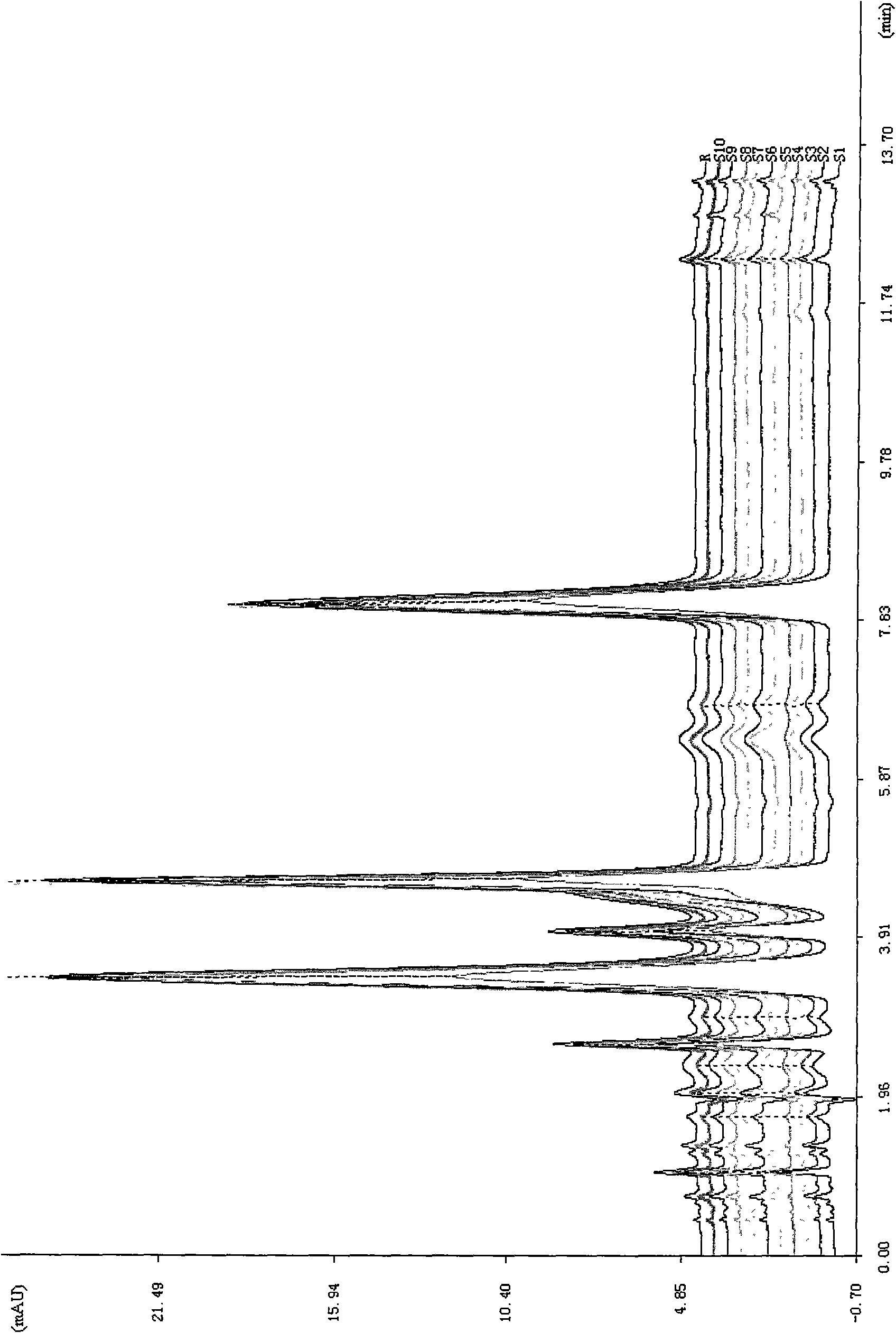
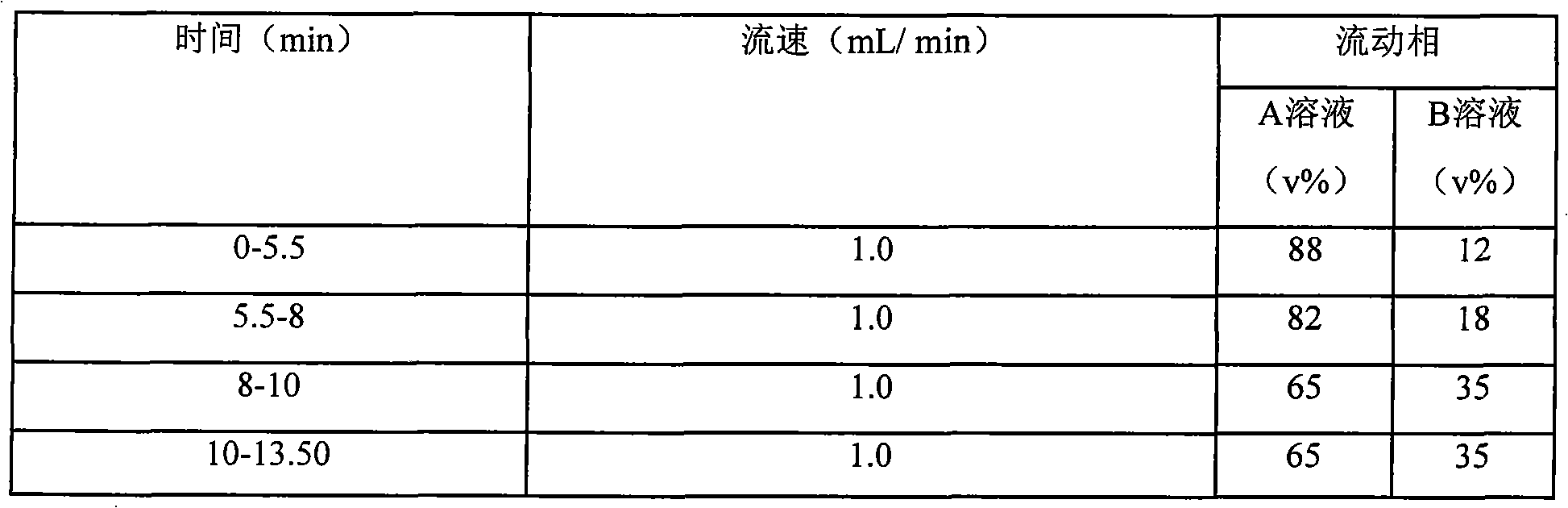
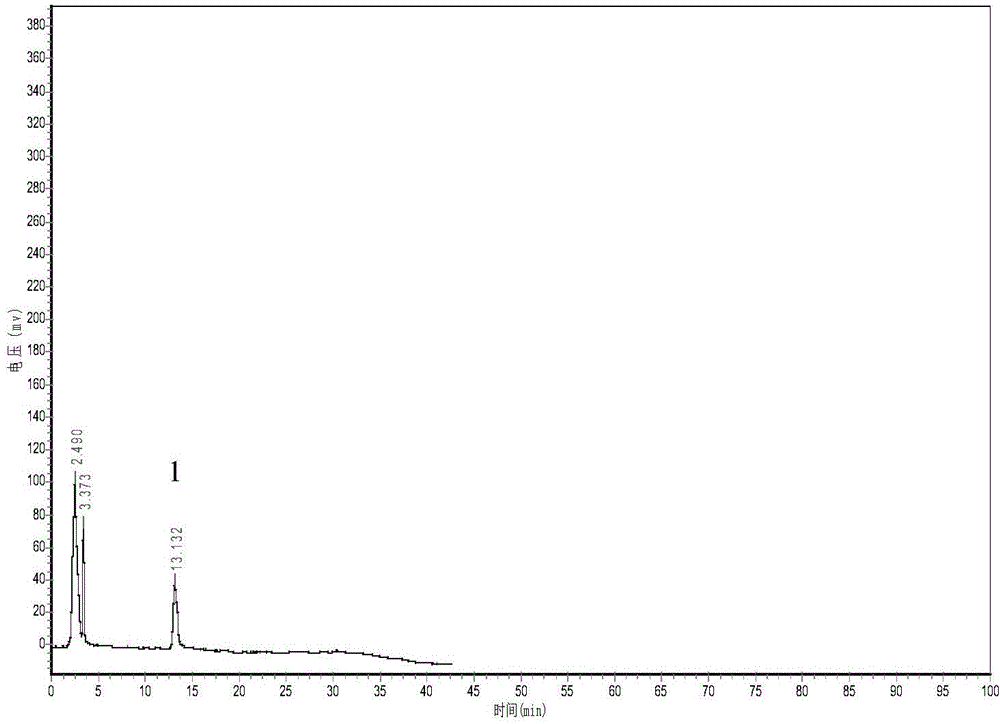
Method for determining decumbent corydalis tuber total alkaloid extractive content with UFLC and method for establishing UFLC finger print thereof
InactiveCN101893607AHigh precisionGood reproducibilityComponent separationTetrahydropalmatineHarmine
The invention relates to determination of alkaloid content in decumbent corydalis tuber total alkaloid extractive. The determination method comprises the following steps: (1) preparing a decumbent corydalis tuber total alkaloid extractive solution; (2) preparing a standard solution; (3) respectively sucking the extractive solution and the standard solution of an equal amount, and carrying out UFLC determination to obtain a UFLC chromatogram; and (4) analyzing chromatographic peaks of protopine, bicuculline, xiawuning alkaloid, corydaline harmine, tetrahydropalmatine and palmatine in the chromatogram, and calculating contents thereof by an external standard method. Besides, the invention also provides a method for establishing a UFLC finger print of decumbent corydalis tuber total alkaloidextractive. The determination method can quickly and accurately determine contents of six main alkaloids in decumbent corydalis tuber total alkaloid extractive, and the UFLC finger print of decumbentcorydalis tuber total alkaloid extractive can be used for the overall control on substance groups in decumbent corydalis tuber total alkaloid extractive, thereby controlling the quality of extractivefrom the macro analysis perspective.
Owner:SHANGHAI INST OF PHARMA IND
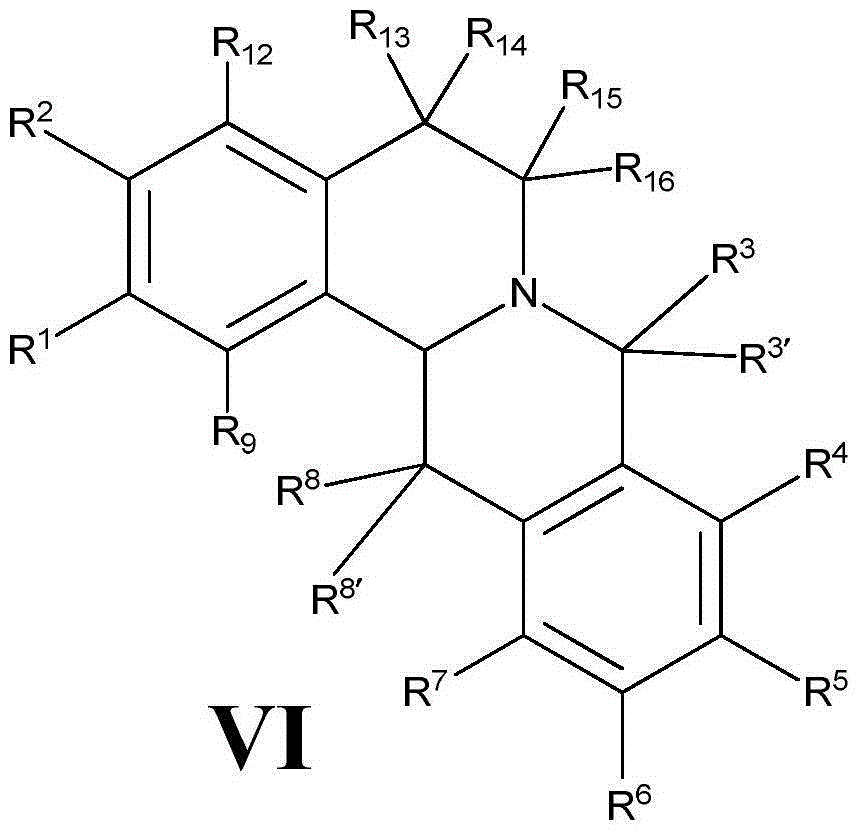
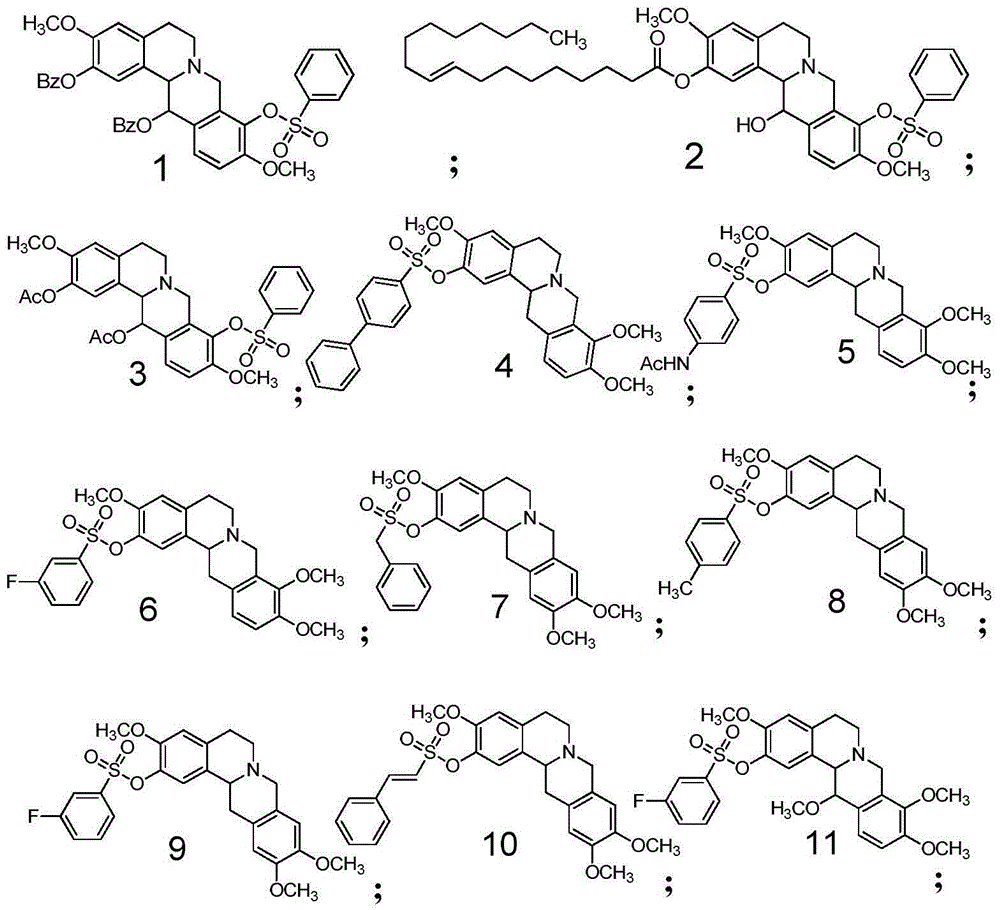
Tetrahydropalmatine derivative and application thereof
InactiveCN105481849AOrganic active ingredientsMetabolism disorderAcute hyperglycaemiaInsulin resistance
The invention relates to a tetrahydropalmatine derivative and application thereof, a compound as shown in a formula (VI) and a preparation method thereof, and an application of the compound in medicine. Specifically, the invention relates to a derivative of the compound as shown in the general formula (VI) and a preparation method thereof, and an application of the derivative as a therapeutic agent in prevention and treatment of hyperlipidemia, hypercholesterolemia, hypertriglyceridemia, hepatic steatosis, diabetes type II, hyperglycemia, adiposis, or insulin resistance and metabolic syndrome. The compound disclosed by the invention also can reduce total cholesterol, low density lipoprotein (LDL)-cholesterol and triglycerides, and increases the expression of a liver LDL receptor and inhibits the expression of proprotein convertase subtilisin / kexin type 9 (PCSK9).
Owner:CHENGDU BESTCHIRALBIO LIMITED LIABILITY
Application of tetrahydropalmatine enantiomers in preparation of P-glycoprotein inhibitor
InactiveCN102100694AExpanded indicationsNo reversal effectOrganic active ingredientsAntineoplastic agentsMetabolic enzymesTumor chemotherapy
The invention provides application of tetrahydropalmatine enantiomers in preparation of P-glycoprotein inhibitor. The tetrahydropalmatine is one of main active ingredients of the Chinese herbal medicine rhizoma corydalis and comprises L-tetrahydropalmatine and D-tetrahydropalmatine. The research shows that the tetrahydropalmatine enantiomers can obviously inhibit the P-gp function, but the tetrahydropalmatine enantiomers are not the P-gp substrates; the tetrahydropalmatine enantiomers can obviously increase the concentration of anti-tumor medicine adriamycins in medicament-resistant tumor cells and reverse the multi-medicament resistance of tumor cells and do not have obvious effects of inhibiting and inducing main medicament metabolic enzymes of liver microsomes, so that the racemes, laevo isomers or dextro isomers of the tetrahydropalmatine enantiomers can be used as auxiliary medicaments for tumor chemotherapy, and the range of indications of the tetrahydropalmatine is expanded; and simultaneously, the invention provides the medicament for the assistant treatment for tumors, which alleviates pain, improves curative effect and is not addictive, for tumor patients.
Owner:ZHEJIANG UNIV
Heat-absorbing wear-resisting lubricating oil paste
InactiveCN106433872AImprove high temperature resistanceImprove wear resistanceLubricant compositionBenzaldehydeWear resistant
The invention relates to a heat-absorbing wear-resisting lubricating oil paste which comprises the following components in parts by weight: 5-8 parts of P-hydroxy benzaldehyde, 3-5 parts of mono-methyl citrate, 4-9 parts of tetrahydropalmatine, 3-4 parts of mesembryanthemoidigenic acid, 1-8 parts of m-methoxybenzaldehyde, 2-5 parts of 1-hydroxy-2-acetyl-4-methylbenzene, 1-2 parts of hydrotalcite, 1-2 parts of kaolin, 2-3 parts of graphene, 1-2 parts of tea tree oil and 5-20 parts of boron nitride. The hydrotalcite, the kaolin, the graphene and the tea tree are creatively added into the lubricating oil paste and cooperated with the components of the traditional lubricating oil paste, the very high high-temperature-resistant and wear-resistant performances are obtained, meanwhile the corrosion resistance and the extreme pressure resistance are excellent, and an unexpected technical effect is obtained.
Owner:YANTAI LIHENG ENVIRONMENTAL PROTECTION TECH CO LTD
Pharmaceutical composition for relieving pain and application thereof
ActiveCN104147007AReduce dosageGood treatment effectOrganic active ingredientsNervous disorderTetrahydropalmatineDiclofenac Sodium
The invention relates to the field of medicines, and particularly relates to a pharmaceutical composition for relieving pain. The pharmaceutical composition is prepared from the following components: rosmarinic acid, tetrahydropalmatine, mangiferin and diclofenac sodium in a weight ratio of (1-10):(1-10):(1-10):(1-10), preferably (2-4):(5-8):(3-6):(2-4), more preferably 3:6:5:3, or 4:7:4:2.
Owner:DALIAN UNIV
Oral preparation for analgesia, and preparation method of oral preparation
ActiveCN104095867AGood treatment effectReduce dosageOrganic active ingredientsNervous disorderMANNITOL/SORBITOLTetrahydropalmatine
The invention relates to the field of medicines, in particular to an oral preparation for analgesia, and a preparation method of the oral preparation. The oral preparation comprises a medicine composition and auxiliary materials, wherein the medicine composition comprises rosmarinic acid, tetrahydropalmatine, mangiferin and diclofenac sodium at a weight ratio of (2-4):(5-8):(3-6):(2-4); the auxiliary materials are preferably methyl cellulose, mannitol, aerosil and aspartame at a weight ratio of (200-400):(30-80):(30-60):(1-3); and a weight ratio of the medicine composition to the auxiliary materials is 1:(15-30).
Owner:DALIAN UNIVERSITY
Method for preparing externally-used medicine gel for treating perianal ulcer
InactiveCN105147675APromote healingEliminates ulcer exudateOrganic active ingredientsAerosol deliveryIndometacinTetrahydropalmatine
The invention discloses a method for preparing externally-used medicine gel for treating perianal ulcer. The method comprises the following steps of weighing raw materials, preparing a gel substrate, dissolving L-tetrahydropalmatine and indomethacin in ethanol, then adding the solution into the substrate, performing even stirring, adding propylene glycol, glycerin and purified water to reach total amount and regulating a pH value of triethanolamine to be neutral to obtain the externally-used medicine gel. The externally-used medicine gel prepared by means of the method contains the tetrahydropatine and indomethacin, can remove ulcerative exudate and accelerate healing of ulcerative surface and accordingly achieves the purpose of curing the perianal ulcer.
Owner:崔新明
Detection method for musk Xintongning preparation
ActiveCN104897811AShort manufacturing timeEnhanced quality control toolsComponent separationBiotechnologyMedicinal herbs
The invention relates to a detection method for a musk Xintongning preparation. The detection method comprises identification for artificial musk, identification for storax, ligusticum wallichii and borneol, identification for ginseng, identification for corydalis tuber, detection for tetrahydropalmatine, and analysis for characteristic fingerprints and mass spectrum of the musk Xintongning preparation. According to the invention, all the herbal medicines of the musk Xintongning preparation are identified, and the characteristic fingerprints of the musk Xintongning taking tetrahydropalmatine as the reference substance are determined. The detection method provided by the invention is good in stability, easy to operate, small in loss and high in accuracy; the shape of the chromatographic peak meets the detection requirements well, and is symmetrical; ligusticum wallichii, storax and borneol are identified by using the same thin layer plate, and are identified at the same time, so that the specificity is strong; the identification for ginseng can meet the conventional detection standard; meanwhile, the preparation time of a test sample is greatly shortened, and the detection efficiency is improved; the developing spots during identification for corydalis tuber are abundant, so that environment protection purpose is achieved; the mass spectrum identification adopted in the invention is special technology for the musk Xintongning preparation.
Owner:山东宏济堂制药集团股份有限公司
Application of tetrahydropalmatine and rhizoma imperatae extract in preparing oral care product
InactiveCN103536471ARaise pain thresholdGood analgesic effectCosmetic preparationsToilet preparationsTetrahydropalmatineFood science
The invention discloses application of tetrahydropalmatine and a rhizoma imperatae extract in preparing an oral care product, namely adding the tetrahydropalmatine and rhizoma imperatae extract into an oral care product in a compound manner in a technology of preparing an oral care product, wherein the tetrahydropalmatine and rhizoma imperatae extract are used as active components for easing pain. Compared with the prior art, the tetrahydropalmatine and rhizoma imperatae extract are safe to use and have remarkable pain easing effects.
Owner:LIUZHOU LIANGMIANZHEN +2
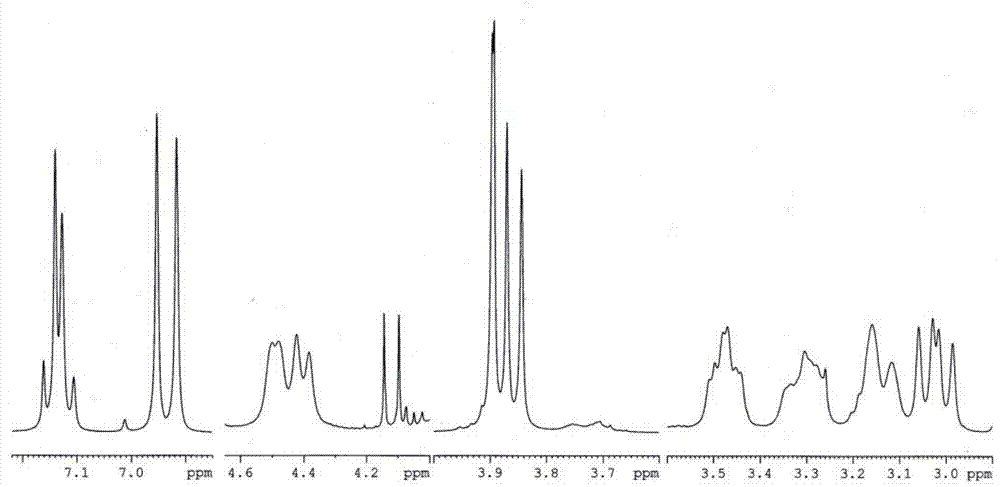
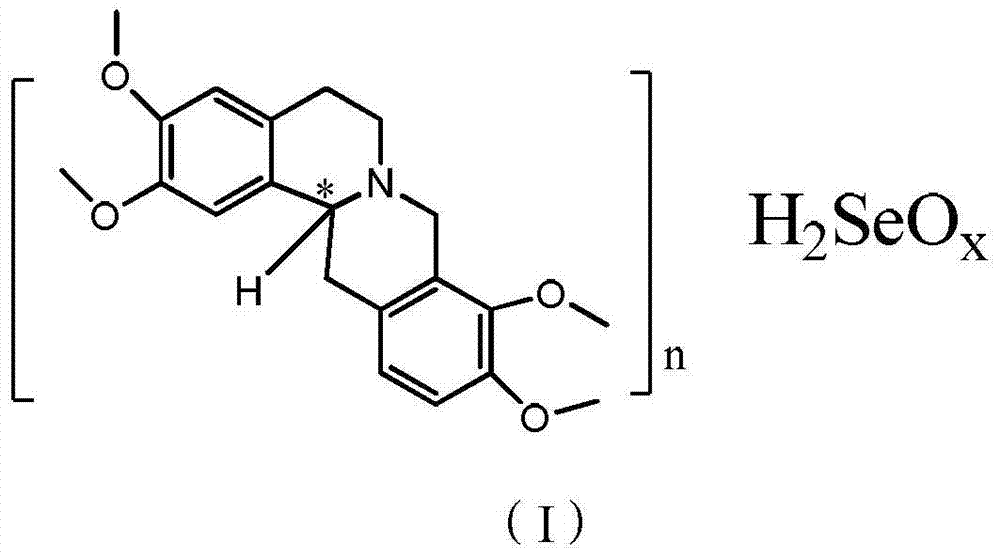
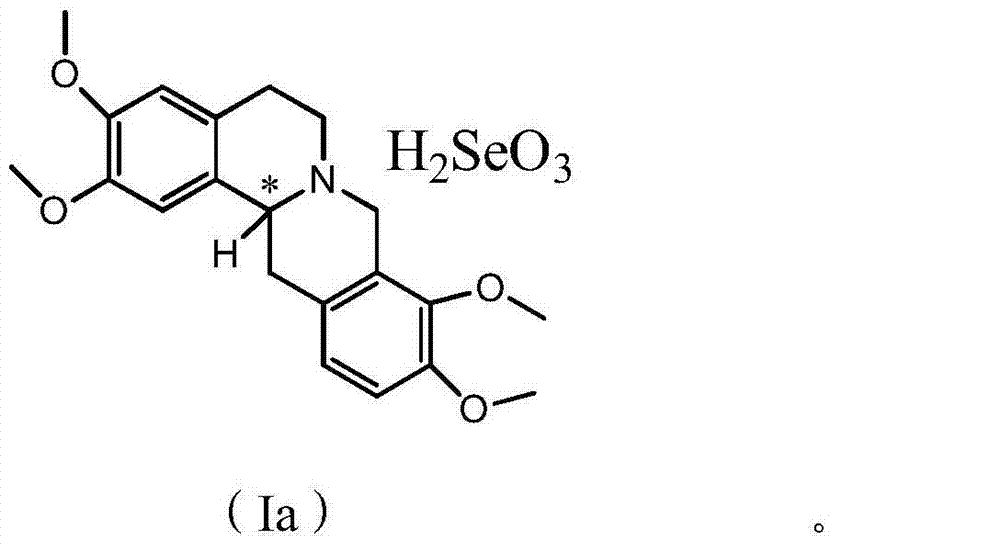
Anticancer analgesic selenium-containing compound and preparation method and application thereof
ActiveCN104327068AImprove the quality of lifeHas analgesic effectOrganic active ingredientsOrganic chemistryLife qualityTetrahydropalmatine
The invention relates to an anticancer analgesic selenium-containing compound and a preparation method and an application thereof. The structure of the selenium-containing compound is as shown in the general formula (I), wherein n=1 or 2, x=2 or 4; and chiral center (*) is R / S-(+ / -) or S-(-) configuration. The selenium-containing compound provided by the invention has anticancer and analgesic functions, has a more excellent analgesic effect than tetrahydropalmatine or rotundine, can be used in cancer pain, even cancer pain in middle and advanced stage, has no addition and can be used to effectively improve life quality of patients.
Owner:SHENZHEN F&S BIO TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com