Tetrahydropalmatine derivative and application thereof
A technology of compounds and medicinal salts, applied to tetrahydropalmatine derivatives and their application fields, can solve problems such as visual impairment, allergic reactions, skin diseases, itching and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0212] The general method of the present invention is further explained below, the compound of the present invention can be prepared by methods known in the art, and the preparation method of the preferred compound of the present invention is used as an example to illustrate in detail below, but the preparation method of the compound of the present invention is not limited thereto .
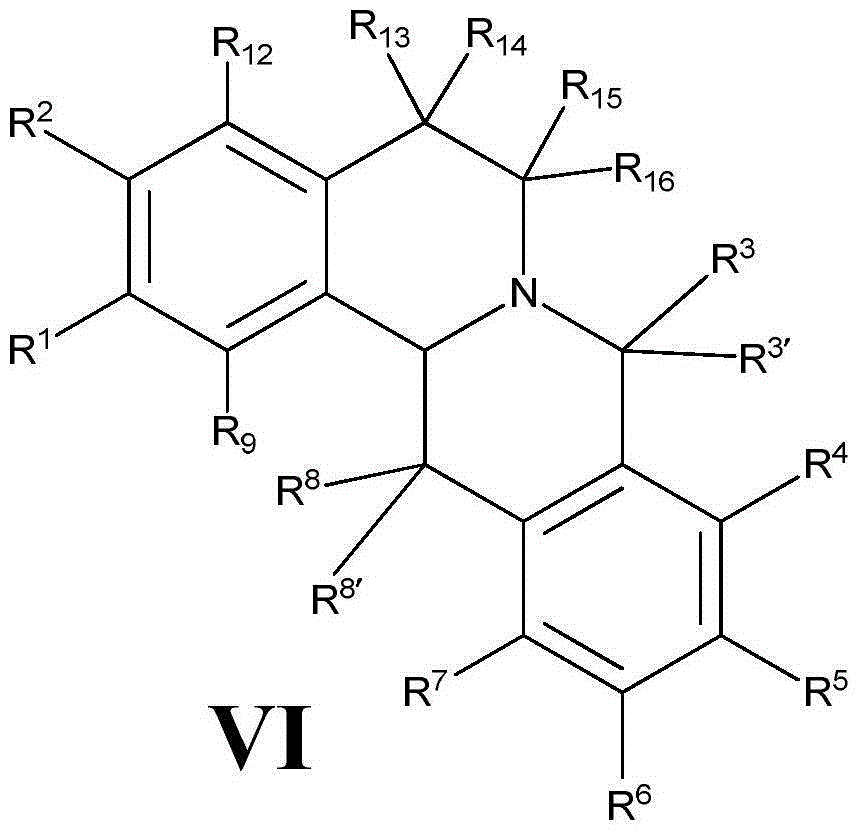
[0213] The preparation of the general formula compound (VI) disclosed in the present invention is mainly prepared according to the following scheme:
[0214]
[0215]
[0216] The above preparation route is described as follows: V1 and V2 are the starting materials of this scheme, which can be obtained from commercially available products, or prepared according to methods reported in literature. First, V1 and V2 are dehydrated under a certain reaction temperature to obtain an imine compound V3, and the compound V3 is reduced to an "amine" compound V4. Under certain reaction conditions, V4 r...
Embodiment 1
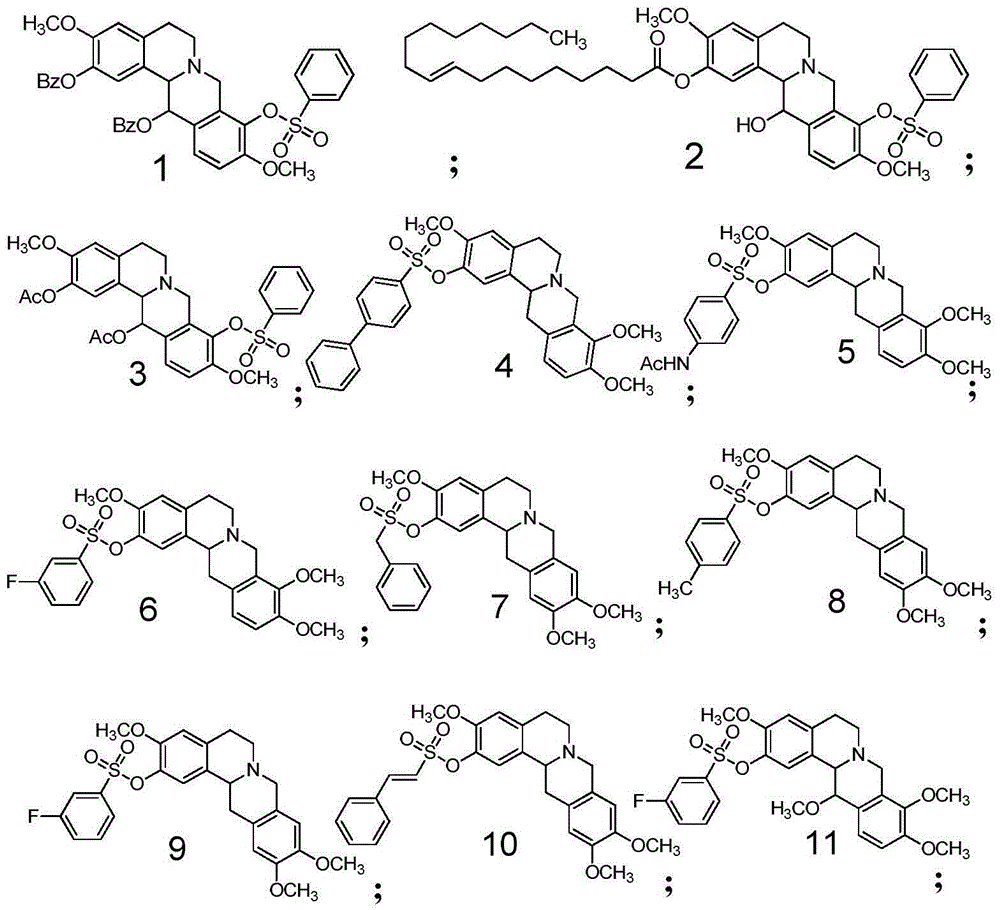
[0251] Compound 1 of the present invention is implemented according to the following two-step scheme:
[0252] Step 1: Preparation of key intermediate D (3-methoxy-4-hydroxyphenethylamine hydrochloride)
[0253]
[0254] Put vanillin A (30.4, 200mol) and nitromethane (16.1ml, 300mmol) in methanol successively, and add 33% methanol solution (1.5ml). The reaction was stirred at 50°C for 2 hours, the solution was homogeneous, and gradually changed from colorless to dark brown, and a large amount of yellow solid appeared after 1.5 hours. After the reaction was complete by TLC, let it cool and filter with suction. Yellow solid B (33.6 g, 172.3 mmol) washed with a small amount of methanol.
[0255] Add B (33.6 g, 172.3 mmol) obtained in the previous step to 1,4-dioxane (500 mL), stir vigorously, and make it a cloudy liquid for later use. Add ethanol (75mL), 1,4-dioxane (300mL), NaBH 4 (32.0g, 845.9mmol), add the reserved suspension dropwise under stirring, and finish adding wi...
Embodiment 2
[0266] Compound 2 of the present invention was first prepared with reference to the steps of Example 1 to obtain Compound J, and then the following reactions were carried out:
[0267]
[0268] Compound J (300mg, 0.621mmol) was dissolved in 10ml of dichloromethane, trans-9-octadecanoic acid (208mg, 0.74mmol), DCC (164mg, 0.80mmol), DMAP (98mg, 0.80mmol), Stir the reaction at room temperature for 8 h, remove DCU by filtration, wash the filter cake with dry dichloromethane, combine the organic layers, and concentrate under reduced pressure to obtain the crude product of compound 2, and obtain 126 mg of compound 2 (yield 27.2%) by silica gel column chromatography. 1 HNMR (400MHz, CDCl 3)δ8.03–7.97(m,2H),7.72–7.66(m,1H),7.60–7.55(m,2H),7.31(d,J=8.5Hz,1H),6.96(s,1H),6.80 (d, J=8.5Hz, 1H), 6.71(s, 1H), 5.46–5.33(m, 2H), 4.76(d, J=9.8Hz, 1H), 4.24(d, J=16.1Hz, 1H) ,3.81(s,3H),3.71(s,1H),3.60(d,J=16.2Hz,1H),3.45(s,3H),3.19–3.07(m,2H),2.78–2.49(m,5H ),2.02–1.89(m,2H),1.82–1.70(m,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com