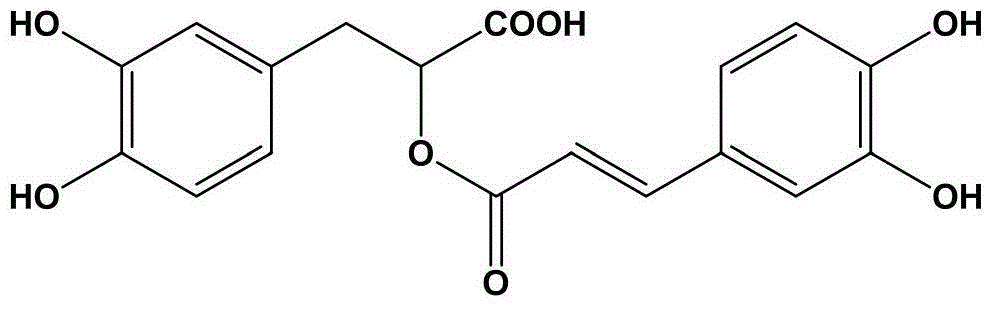
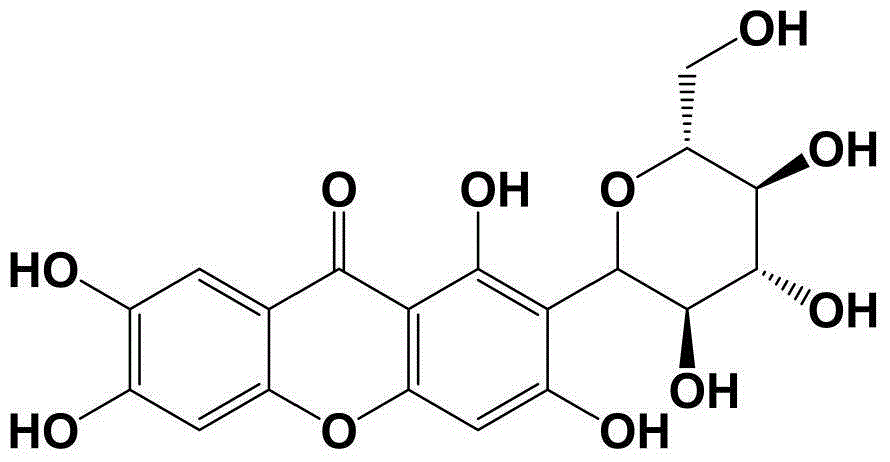
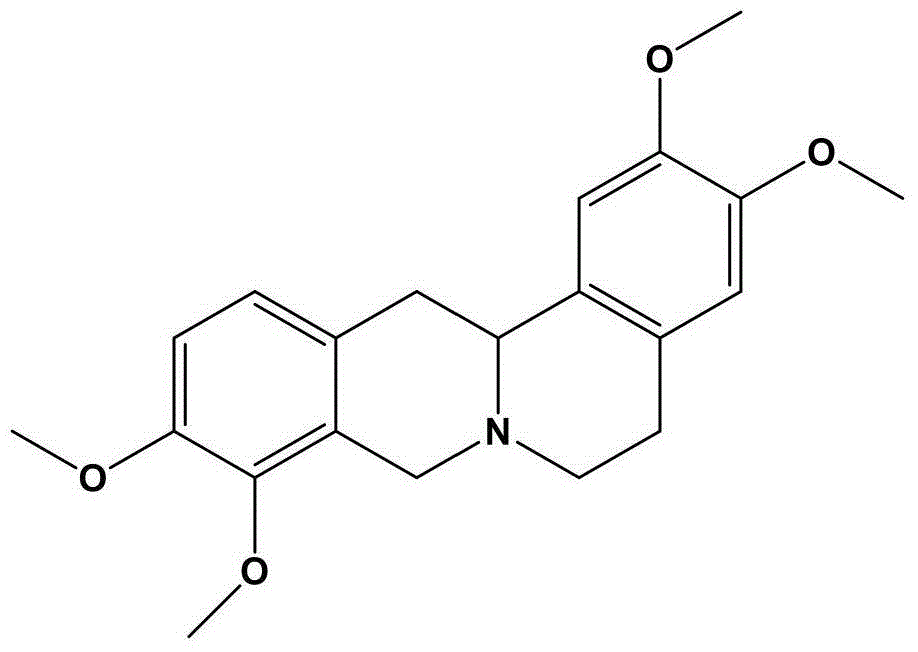
Pharmaceutical composition for relieving pain and application thereof
A composition and drug technology, applied in the direction of drug combination, antipyretics, pharmaceutical formulas, etc., can solve serious problems, gastrointestinal reactions, addiction, etc., to reduce dosage, reduce side effects, and good therapeutic effect Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] 实施例1 药物组合物对醋酸引起的小鼠扭体次数的影响
[0022] 雄性ICR小鼠(20±2)g,按体重随机分组,每组10只,各组分别通过灌胃给予治疗药物。 模型组灌胃给予等体积生理盐水。 小鼠腹腔注射醋酸,引起腹腔深部大面积而较持久的疼痛刺激,致使小鼠产生扭体反应。 各剂量组给药1h后,腹腔注射0.7%醋酸生理盐水溶液0.1ml / 10g,记录自注射醋酸致痛后每只小鼠在20min内出现的扭体反应次数,计算各给药组的扭体 Inhibition rate.
[0023] 扭体抑制率=[(对照组扭体次数-药物组扭体次数) / 对照组扭体次数]×100%
[0024] 给药组中单体药物之间重量比参见下表:
[0025]
Dosing group 1
3
6
5
3
Dosing group 2
4
7
4
2
Dosing group 3
1
5
10
10
[0026] test results
[0027] 药物组合物对醋酸引起的小鼠扭体次数的影响
[0028] group
Embodiment 2
[0029] 实施例2 药物组合物对小鼠热板致痛反应的影响
[0030] 将雌性ICR小鼠(20±2)g,置55±0.5℃的智能热板仪上,记录小鼠足底接触热板至出现舔后足反应的潜伏期(s)为痛阈指标,剔除反应潜伏期30s或跳跃的小鼠。选取140只反应潜伏期在10~30s内的合格小鼠,根据药前痛阈和体重随机分组,灌胃给药;
[0031] 模型组:等体积的生理盐水,
[0032] 组合物组:50mg / kg,ig
[0033] 连续灌胃给药5天,于末次给药后30、60、90、120min时分别测定各给药组小鼠的痛阈1次,痛阈值超过60s者以60s计算。
[0034] 数据以 表示,采用SPSS15.0软件进行方差分析。
[0035] The specific results are as follows:
[0036] 药物组合物对小鼠热板致痛反应的影响
[0037]
[0038]
Embodiment 3
[0039] 实施例3 药物组合物对癌症疼痛的临床治疗效果
[0040] 一般资料:选择癌症疼痛患者200例,年龄32~65岁,体重45~75kg,平均年龄为43.5岁,平均体重为64.3kg。经辅助检查明确诊断,就诊前未进行镇痛治疗,无并发症。200例患者随机分为2组,每组100例。
[0041] 各组分别按下述方式服药:
[0042] 本发明药物组合物组:每次口服使用实施例1中的给药组2(60mg / 次),每日三次;
[0043] 双氯芬酸钠组:口服双氯芬酸钠60mg,每日三次;
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight | aaaaa | aaaaa |
| Average weight | aaaaa | aaaaa |
| Average age | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com