Quadruple long-acting medicine composition and application thereof to pain treatment
A composition and drug technology, applied in the field of medicine, can solve the problems of toxic side effects, poor water solubility, anti-inflammatory and analgesic effects, etc., achieve good analgesic effect, improve solubility, and reduce toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1 Preparation examples of sustained-release preparations, injection preparations, film preparations, spray preparations, tablets, capsules, etc.
[0019] Sustained release preparation
[0020] 【Active ingredients】therapeutic and / or preventive effective doses of urcin, fumaric acid and succinic acid; in parts by weight, preferably 1-5 parts of urcin, 1-5 parts of ketalin, and fumaric acid 2-7 parts, 2-7 parts of succinic acid.
[0021] [Main auxiliary materials] slow-release materials, adhesives, lubricants, commonly used carrier auxiliary materials, etc.
[0022] [Concept and principle] Sustained-release preparations refer to the release of a substance slowly and non-constantly as required in a specified release medium. Compared with the corresponding ordinary preparations, the dosing frequency is doubled or reduced compared with ordinary preparations, and A preparation that can significantly increase patient compliance; use inorganic materials as the wall ...
preparation example
[0023] [Preparation example] According to the content of [active ingredients], weigh each active ingredient separately, mix it with a solubilizer (such as Tween-80, etc.) in an amount below the allowable addition amount in the main excipients, and add an appropriate amount of water to fully dissolve it. , add emulsifier and tetraethyl orthosilicate in turn, fully stir and mix uniformly to form mixed solution A, then add solution A to the reaction device (equipped with electromagnetic stirrer, thermometer, condensing reflux device and dropping funnel), high speed (500 rpm / Minutes) Stir and shear for 10 minutes to form transparent oily colostrum. At room temperature (25°C), gradually drop the gelatin solution into the reaction device, and the main active ingredients form O / W type droplets under stirring. Wrapped in the emulsifier micelles, reacted for 2 hours, after the tetraethyl orthosilicate was fully hydrolyzed, the calcium chloride solution was added dropwise, and the activ...
Embodiment 2
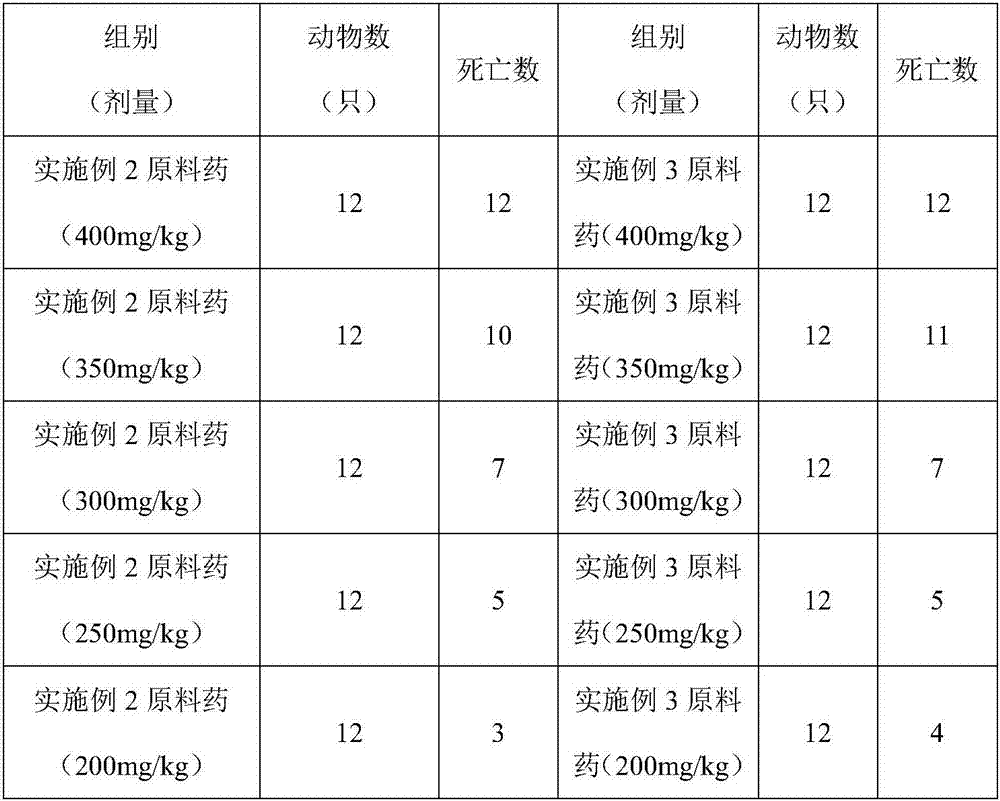
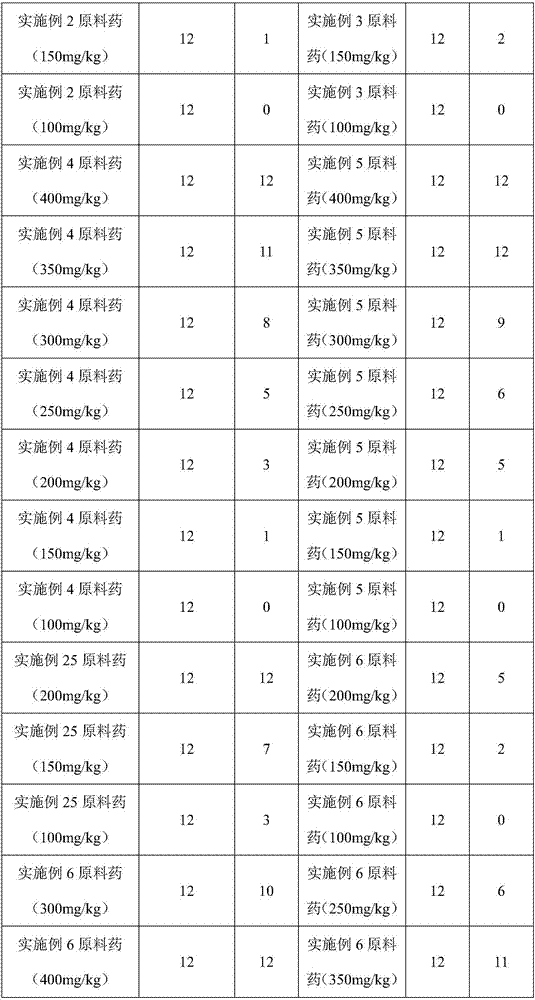
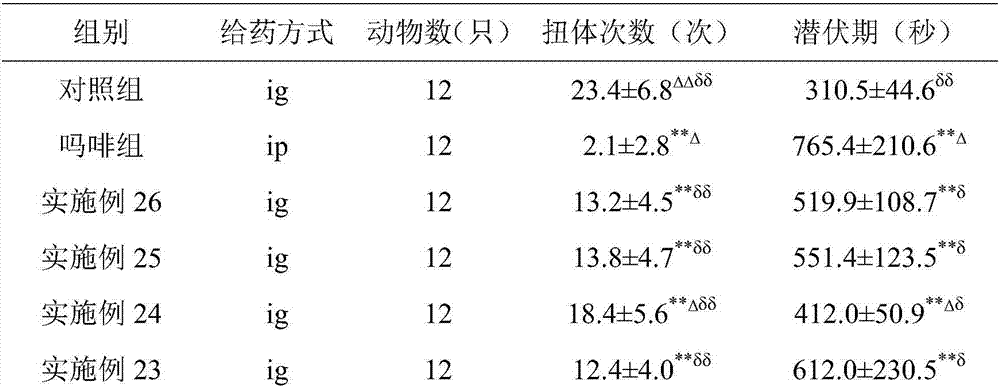
[0049] Raw materials: 1 part of corygone, 1 part of tetrahydropalmatine, 2 parts of fumaric acid, 2 parts of succinic acid
[0050] According to the above parts by weight, take 10 g of homogenate, 10 g of tetrahydropalmatine, 20 g of fumaric acid, and 20 g of succinic acid, and according to the preparation method recorded in Example 1, a nanocapsule sustained-release preparation can be prepared.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com