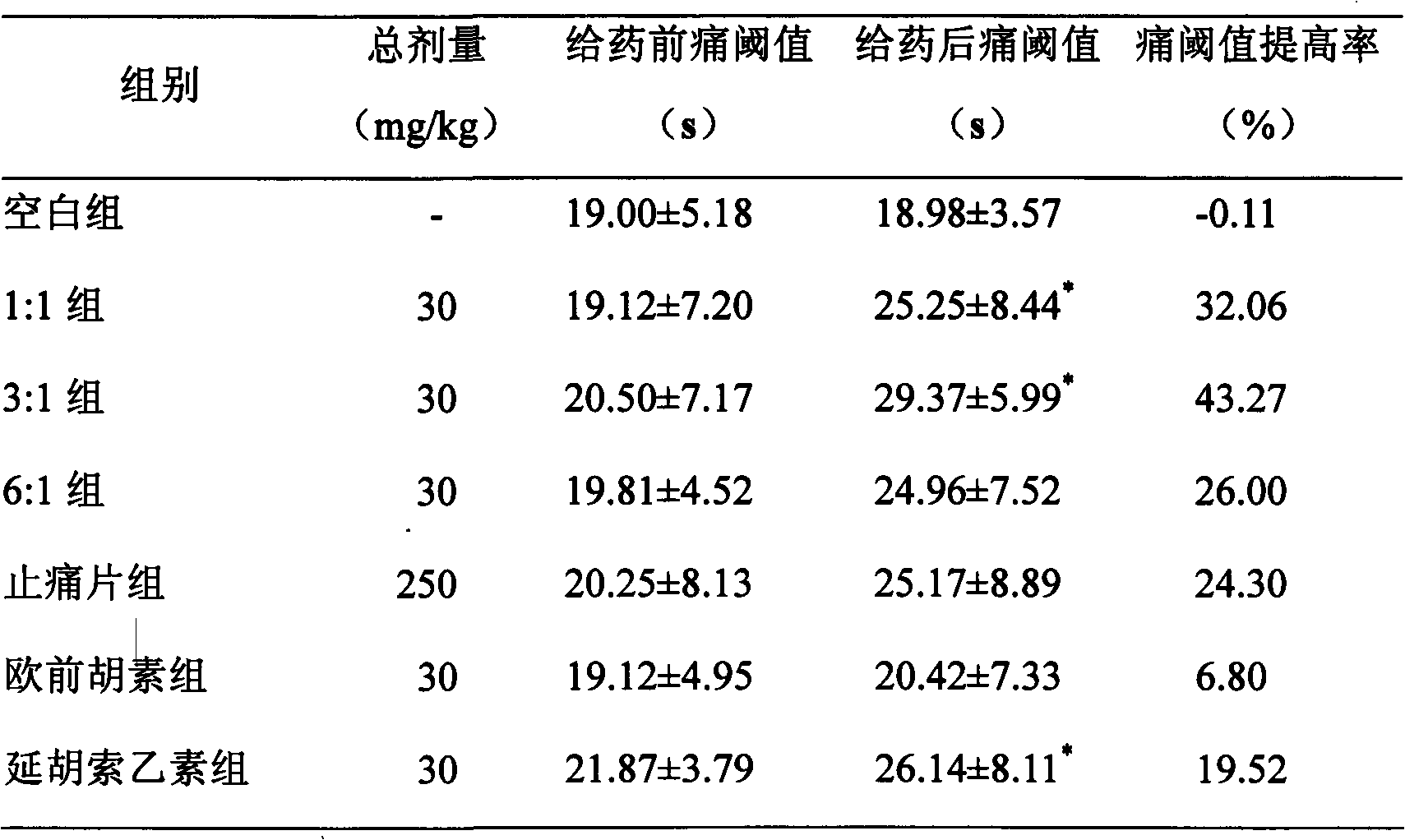
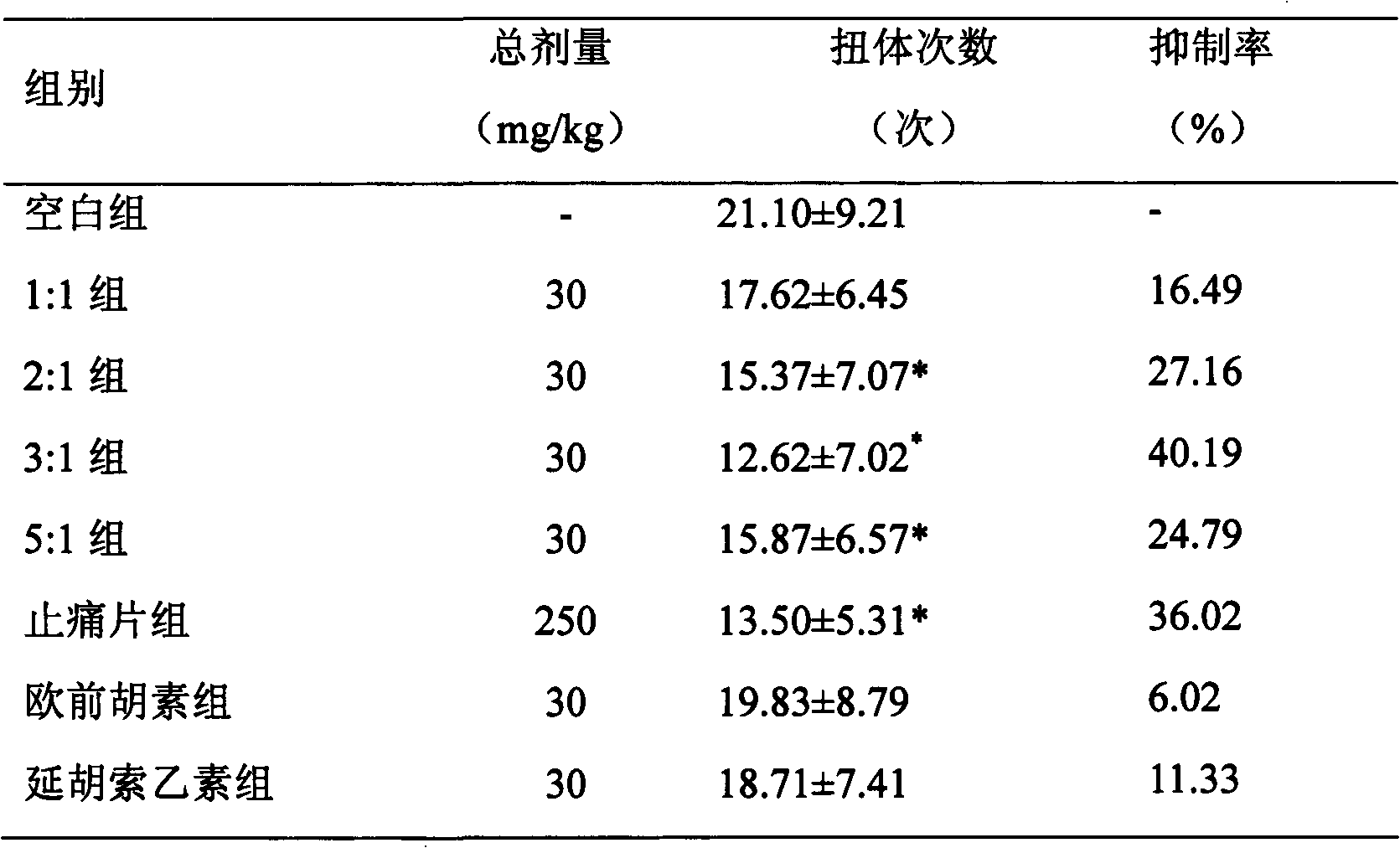
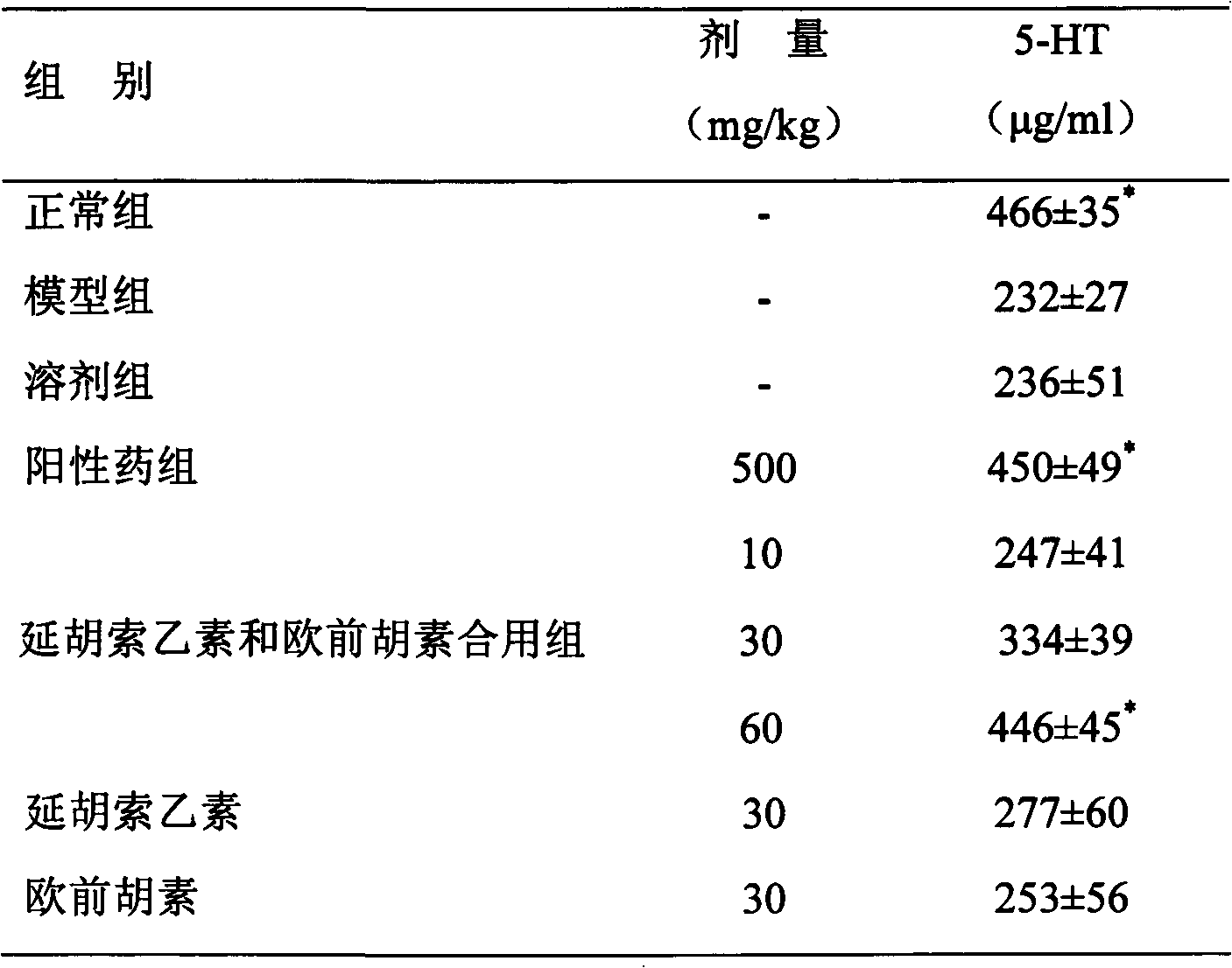
Medicinal composition containing tetrahydropalmatine and imperatorin
A technology of tetrahydropalmatine and imperatorin, which is applied in the field of medicine and can solve the problems of large dosage and single effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] (1) Prescription
[0065]
[0066] (2) Preparation method
[0067] Weigh the prescribed amount of tetrahydropalmatine, imperatorin, cross-linked polyvinylpyrrolidone, and microcrystalline cellulose, mix evenly, add 2% hypromellose aqueous solution as a binder soft material, and granulate with a 20-mesh sieve , dried at 50°C for 3 hours, sieved with a 20-mesh sieve, added the prescribed amount of talc powder and magnesium stearate, mixed evenly, and pressed into tablets. Each tablet contains a total of 100mg of the main drug.
[0068] (3) Determination of disintegration time and dispersion uniformity
[0069] Take 6 pieces of samples, place them in the glass tube of the hanging basket of the disintegration apparatus respectively, immerse the hanging basket in a 1000ml beaker, and adjust the position of the hanging basket so that the screen mesh is 25mm away from the bottom of the beaker when it descends, and the temperature of the beaker is ( For water at 37±1)°C, ...
Embodiment 2
[0072] (1) Prescription
[0073]
[0074]
[0075] (2) Preparation method
[0076] 1) Weigh the prescribed amount of tetrahydropalmatine and imperatorin through a 100-mesh sieve, sodium carboxymethyl starch, aspartame, and microcrystalline cellulose through a 80-mesh sieve, fully mix the above raw and auxiliary materials, and set aside .
[0077] 2) Preparation of the adhesive: take the whole amount of polyvinylpyrrolidone K30 to make a 5% aqueous solution, stir until completely dissolved, and set aside.
[0078] 3) The powder in 1) is made of soft material with the binder in 2), granulated through a 20-mesh sieve, dried at 60°C, and the moisture content is controlled to be about 2%. Add the prescribed amount of talcum powder and magnesium stearate, mix well, and press into tablets. Each tablet contains a total of 100mg of the main drug.
[0079] (3) Determination of disintegration time and dispersion uniformity
[0080] Take 6 pieces of samples, and measure the dis...
Embodiment 3
[0083] (1) Prescription
[0084]
[0085] (2) Preparation method
[0086]1) Separately pass the tetrahydropalmatine and imperatorin through a 100-mesh sieve, pulverize the microcrystalline cellulose, sodium carboxymethyl starch, and aspartame, pass through a 80-mesh sieve, fully mix the above-mentioned raw and auxiliary materials, and set aside.
[0087] 2) Preparation of adhesive: take the whole amount of polyvinylpyrrolidone K30 to make a 5% aqueous solution, add surfactant sodium lauryl sulfate, and set aside.
[0088] 3) The powder in 1) is made of soft material with the binder in 2), granulated through a 20-mesh sieve, dried at 60°C, and the moisture content is controlled to be about 2%. Sieve through a 20-mesh sieve for granulation, add magnesium stearate and micropowder silica gel, mix evenly, and press into tablets to obtain the product. Each tablet contains a total of 150mg of the main drug.
[0089] (3) Determination of disintegration time and dispersion unifor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com