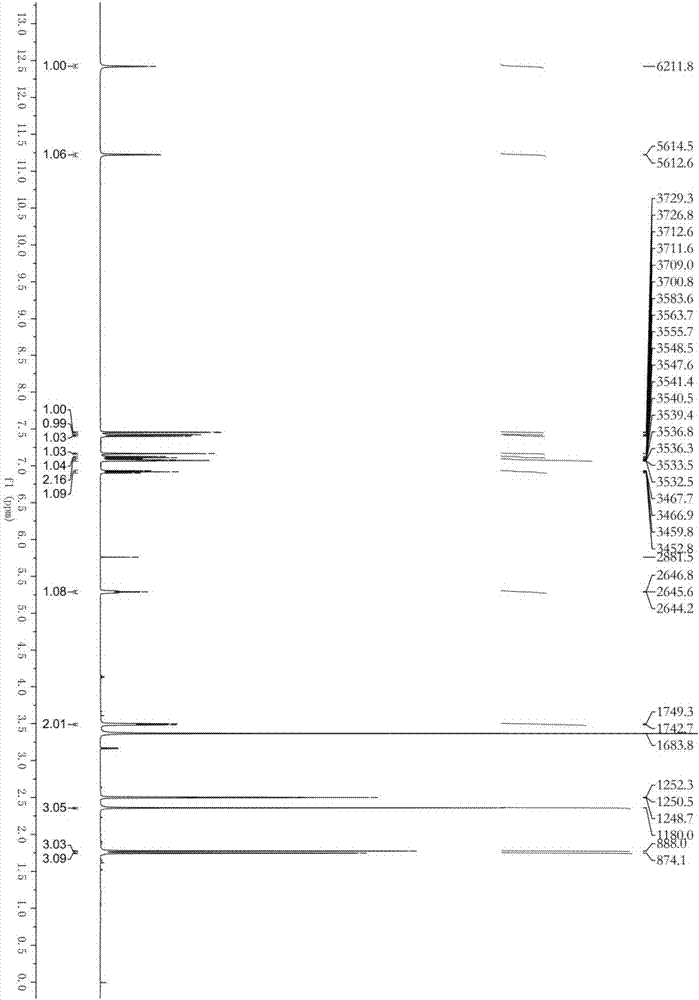
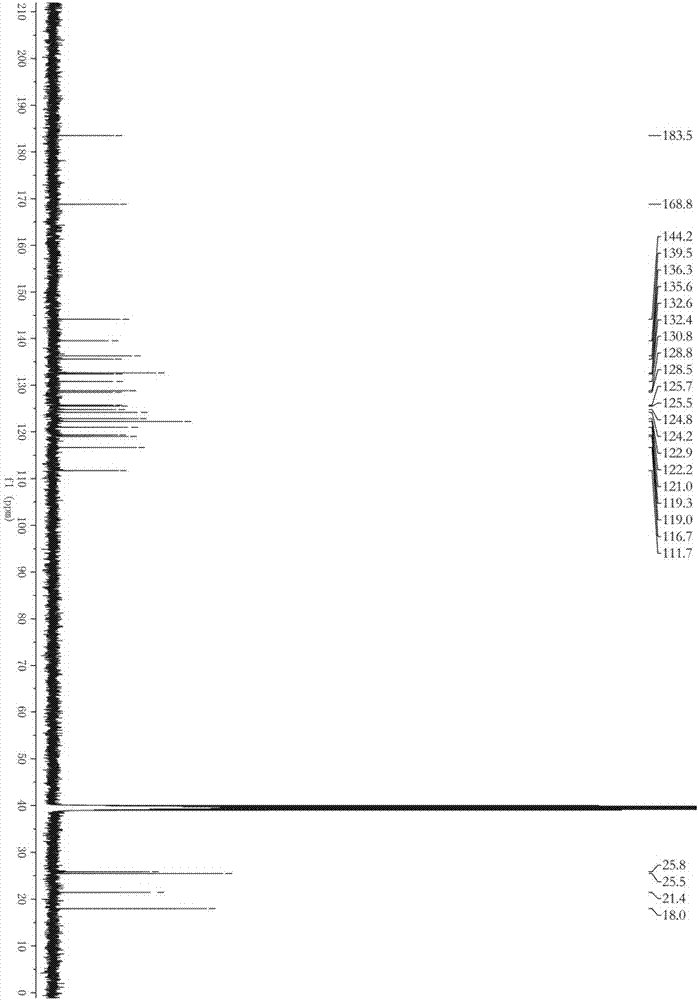
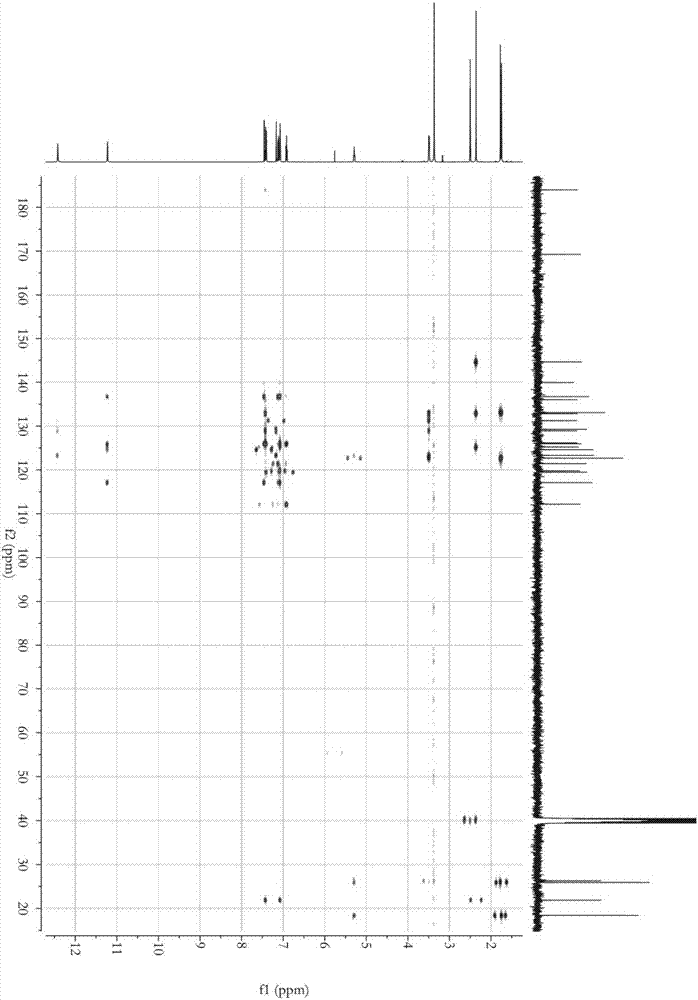
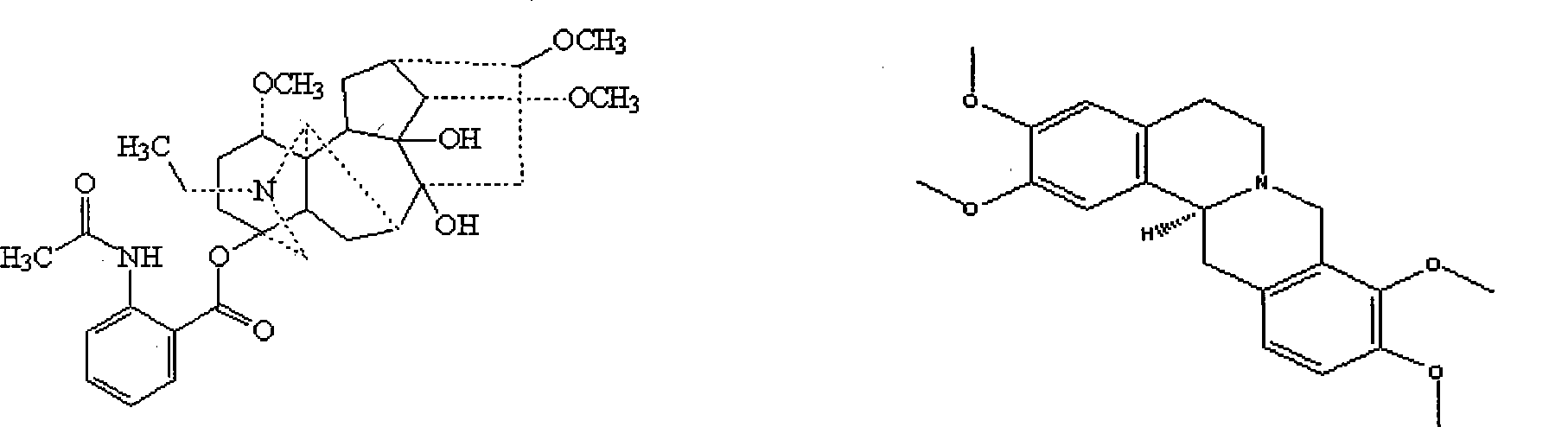Patents
Literature
33 results about "Median lethal dose" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In toxicology, the median lethal dose, LD₅₀ (abbreviation for "lethal dose, 50%"), LC₅₀ (lethal concentration, 50%) or LCt₅₀ is a measure of the lethal dose of a toxin, radiation, or pathogen. The value of LD₅₀ for a substance is the dose required to kill half the members of a tested population after a specified test duration. LD₅₀ figures are frequently used as a general indicator of a substance's acute toxicity. A lower LD₅₀ is indicative of increased toxicity.
Mode creature method for medicament toxicity research
A method for studying toxicity of drugs by using model organism zebra fish comprises the following steps of: selecting 100 to 200 adult zebra fishes, respectively disposing in bottles containing a solution of 30 to 50 mL, keeping the temperature in the bottles substantially constant at 22 to 25 DEG C, randomly grouping, each group containing 10 to 20 fishes, wherein the solution in one of the groups is pure water (blank), the solutions in other groups are medicinal solutions with different concentrations, and 0.5% to 2% dimethylsulfoxide (DMSO) can be added to assist dissolving a drug that has poor water solubility, when an additional solvent comparison group prepared by adding 0.5% to 2% DMSO into the pure water is needed; recording the death number of zebra fishes in the medicinal solutions of different concentrations within 24 h, and calculating the death rate (%); calculating median lethal dose-LD50 by Bliss method according to the experiment result; and representing the acute toxicity by the value of LD50. According to the method, the LD50s of zebra fish of triptolide, matrine and emodin are respectively 5.39*10<-3>, 112.3 and 1.08*10<3> mug / mL. The method can objectively reflect the toxicity of drugs.
Owner:CHINA PHARM UNIV
High performance biodegradation type metal machining protection liquid
InactiveCN102618372APromote degradationImprove the lubrication effectLubricant compositionCarboxylic acidLubrication
The invention discloses high performance biodegradation type metal machining protection liquid, which is composed of high polymer carboxylic acid, organic amine, higher alcohol, a surfactant, plant oil or synthetic lubrication grease, pure water, an antifoaming agent and preservative or bacteriacide. The high performance biodegradation type metal machining protection liquid is good in biodegradable performance. A formula of the high performance biodegradation type metal machining protection liquid can be designed by choosing the plant oil or synthetic ester and other additives. Biochemical oxygen demand (BOD) / chemical oxygen demand (COD) of 3.3% of diluent is larger than or equal to 1.0, so that the high performance biodegradation type metal machining protection liquid is excellent in lubrication performance. The frictional factor mu of the 3.3% of diluent is smaller than or equal to 0.03, and tapping torque of the 3.3% of diluent is smaller than or equal to 400N*cm, so that the high performance biodegradation type metal machining protection liquid is safe, hygienic and reliable in quality. The biological median lethal dose LD50 of the high performance biodegradation type metal machining protection liquid is larger than or equal to 5,000mg / kg, the medical lethal concentration LC50 is larger than or equal to 5,000mg / m<3>, respiratory tract mucous membrane irritation is smaller than or equal to 1 level, and the high performance biodegradation type metal machining protection liquid is friendly to human and environment.
Owner:SHANGHAI YUSHIRO CHEM IND +1
Acrylate combined casting filling material as well as preparation method and application thereof
ActiveCN106927719AReduce the impactImprove fill curing strengthBuilding constructionsOrganic fertilisersCross-linkPolymer science
The invention discloses an acrylate combined casting filling material as well as a preparation method and application thereof. The acrylate combined casting filling material comprises the following components: a main agent and a cross-linking agent, wherein the main agent is a mixture of acrylate; the cross-linking agent is one or a mixture of two of polyethylene glycol diallyl ether and oxidized bisphenol A diallyl ether. As the non-toxic polyethylene glycol diallyl ether and / or oxidized bisphenol A diallyl ether are adopted as the cross-linking agent, the rat oral half lethal dose of the casting material is greater than or equal to 6500mg / kg, the material is actually non-toxic, then a safe operation environment can be provided for construction operators, and the influence on the environment can be reduced to the maximum extent.
Owner:SHANDONG UNIV
Anti-tumor activating fuling scloerotium glucan sulfate and its preparation and use
InactiveCN1583800AGood antitumor activitySimple production processOrganic active ingredientsAntineoplastic agentsSolubilityChlorosulfuric acid
An anti-tumour activated Tuckahoe sclerotium glucan sulfuric is prepared by: taking fresh Tuckahoe sclerotium, extracting non-water soluble (1->3)-beta- D- glucan with NaOH, solving them in anhydrous dimethylsulfoxide, adding pyridine and chloro-sulfonic acid to react to get glucan sulfuric ester. The glucan sulfuric ester's substituted reaction is of non-selective substitution, occurred at C-6 position of hydroxy, the substitution value of sulfuric ester is 0.66- 1.21, weight-average molecular weight is 0.8X104-16.4X104, and has well water solubility. Testing in vivo and in vitro showed that, the glucan sulfuric ester has obvious inhibit effect for Sarcoma 180 and proliferation of adenocarcinoma of stomach. The toxic testing in small rat prove that half lethal dose of glucan sulfuric ester (LD50)>1600 mg / kg, and without toxic or side effects. The Tuckahoe sclerotium glucan sulfuric ester is capable of use in preparing anti-tumour pharmaceuticals or health food with function of increasing body immunological competence.
Owner:WUHAN UNIV
Superfine oil for abrasive machining of high-lubrication and high-water-separability bearing
ActiveCN102660360AImprove the lubrication effectImprove water separation effectLubricant compositionAntioxidantHazardous substance
The invention discloses superfine oil for abrasive machining of a high-lubrication and high-water-separability bearing. The superfine oil consists of mineral oil, polymeric carboxylic acid, organic amine, a surfactant, a synthetic lubricant, an anticorrosive agent, an extreme-pressure additive and an antioxidant. The superfine oil has an excellent lubricating property, a Bouton tester is used fortesting, and a friction coefficient value of raw oil is less than or equal to 0.1; the superfine oil has excellent water separability, and the separation time of oil and water is less than or equal to 10 min (50 ml of sample is added into 50 ml of water, and the mixture is turned for 10 times); and raw materials in a formula are not toxic and harmful substances which influence the environmental safety and human health, the biological median lethal dose LD50 of the product is more than or equal to 5,000 mg / kg, the median lethal concentration LC50 is more than or equal to 5,000 mg / m<3>, the respiratory mucosa irritation is less than or equal to class 1, and the superfine oil has reliable safety and sanitation quality.
Owner:QIDONG YUSHIRO CHEMICAL INDUSTRY CO LTD +1
Nano aqueous completely-synthetic environmental-friendly metal working fluid
InactiveCN104762127AImprove the lubrication effectHigh safety and health qualityLubricant compositionHazardous substanceMetal working fluid
The invention discloses a nano aqueous completely-synthetic environmental-friendly metal working fluid being composed of an organic acid, an organic amine, a corrosion inhibitor, a synthetic lubricant, a surfactant, water, a nano material and a defoaming agent. In the invention, a nano high-molecular lubricating raw material is employed for synthesizing the metal working fluid, so that the metal working fluid is greatly improved in lubricating performance (a laboratory test proves that a 5% diluted solution of the metal working fluid is not more than 0.03 in friction coefficient). The raw materials are free of harmful substances harming body health and environment safety so that the metal working fluid is greatly improved in safety and sanitation quality (a test proves that the metal working fluid is not less than 5000 g / kg in biological median lethal dose LD50, is not less than 5000 mg / m<3> in medial lethal concentration LC50 and is not higher than 1 grade in respiratory tract mucous membrane irritation).
Owner:QIDONG YUSHIRO CHEMICAL INDUSTRY CO LTD +1
Screening method for glyphosate-resisting medicago sativa plants
InactiveCN104255403AEasy and fastCultivating equipmentsPlant genotype modificationGermplasmScreening method
The invention mainly provides a medicago sativa plant resistant to herbicide and a method, particularly relates to a method for screening medicago sativa plants resistant to the herbicide by utilizing chemical mutagen EMS (ethyl methane sulfonate) for mutagenesis of medicago sativa, and discloses a culture medium screening method for glyphosate-resisting medicago sativa plants. The method is characterized by including the following steps: (1) germinating seeds by an MS culture medium; (2) performing dosage screening; (3) subjecting medicago sativa seeds to mutagenesis by median lethal dose of the EMS; (4) screening the glyphosate-resisting medicago sativa plants; (5) rescreening the resistant plants. The method has the advantages of being simple and easy to implement, taking effect fast and capable of allowing single plants of herbicide-resistant medicago sativa to be obtained within a short time. A mutagenesis way is adopted. The chemical mutagen EMS is utilized to mutate the medicago sativa seeds to generate point mutation, the single plants of the herbicide-resistant medicago sativa are screened by two ways of the culture medium and soil screening, new germplasm resources are provided for conventional breeding, and certain references are provided for herbicide-resistant breeding of other plants.
Owner:LANZHOU UNIVERSITY
Analgesic anti-inflammatory composite medicament and method for preparing same
ActiveCN101518531AImprove bioavailabilityLasting effectOrganic active ingredientsAntipyreticSide effectHepatic first pass effect
The invention discloses an analgesic anti-inflammatory composite medicament made from lappaconitine and tetrahydropalmatine, a patch made from the composite medicament and a method for preparing the patch. The patch comprises a back lining layer, a medicament storage layer and a protection layer, wherein the medicament storage layer is made from raw materials including the lappaconitine, the tetrahydropalmatine and pressure-sensitive adhesive; and the patch is made by adding a proper amount of transdermal enhancerfrom in to the same three raw materials. Experiments and clinical research prove that the composite medicament made from raw medicament materials of lappaconitine and tetrahydropalmatine according to a certain proportion can reduce the toxicity of the lappaconitine and improve the median lethal dose (LD 50). Due to the use of the patch made from the composite medicament, the blood concentration can be kept stable for a long time, the first-pass effect on liver and the damage to gastrointestinal tracts are avoided, the toxicity and the side effect of the composite medicament are reduced, and the bioavailability and the safe curative effect of the composite medicament are improved. The composite medicament is convenient to use without causing patients to be afflicted by pain and is very effective in relieving pain and diminishing inflammation.
Owner:GANSU CHEEZHENG TIBETAN MEDICINE CO LTD
Algae-lysing aeromonas sp. and application thereof in controlling cyanobacterial blooms
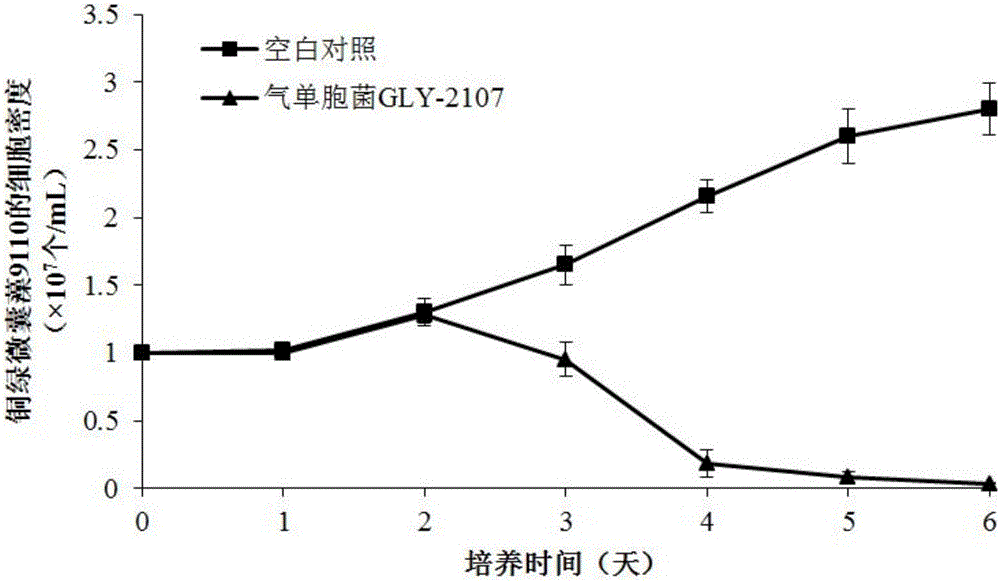
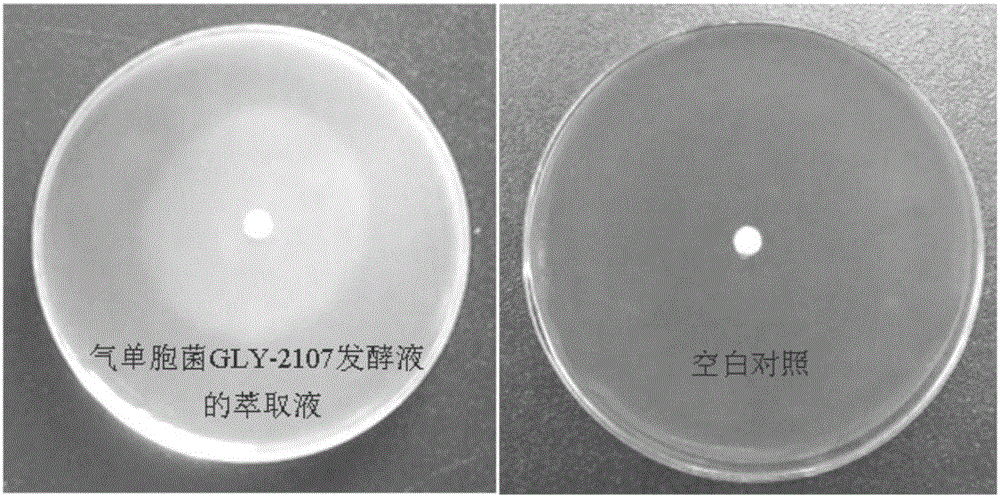
The invention discloses algae-lysing aeromonas sp. and an application thereof in controlling cyanobacterial blooms. The aeromonas sp. GLY-2107, which has a significant algae-lysing activity, is separated from a water body from Lake Taihu, and the preservation number of the aeromonas sp. is CGMCC No.8979; and active algae-lysing ingredients, namely 3-phenmethyl-piperazine-2,5-dione and 3-methylindole, are separated, purified and identified from a metabolic product of the aeromonas sp., wherein the median lethal dose LD50 of the 3-phenmethyl-piperazine-2,5-dione on microcystis aeruginosa 9110 is 4.72 [mu]g / mL and the median lethal dose LD50 of the 3-methylindole on the microcystis aeruginosa 9110 is 1.10 [mu]g / mL. The algae-lysing aeromonas sp. is applicable to research, development and production of novel biological algicides, and is finally applied to the control of the cyanobacterial blooms in lakes.
Owner:SHANGHAI JIAO TONG UNIV
Algicidal Shewanella and application thereof in controlling blue algae water bloom
InactiveCN103509744AEasy to prepareShort preparation cycleBacteriaMicroorganism based processesSynechococcusMetabolite
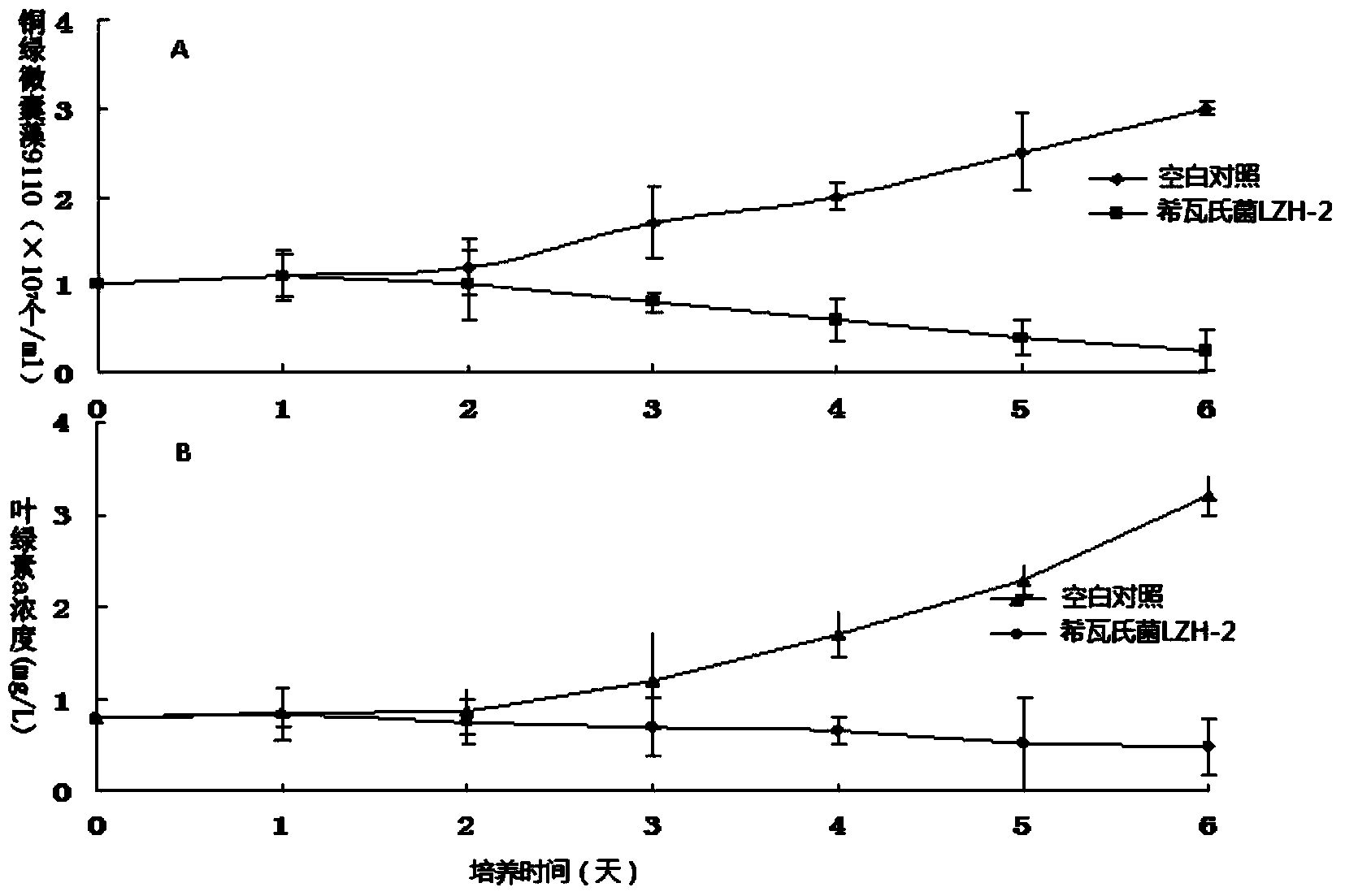
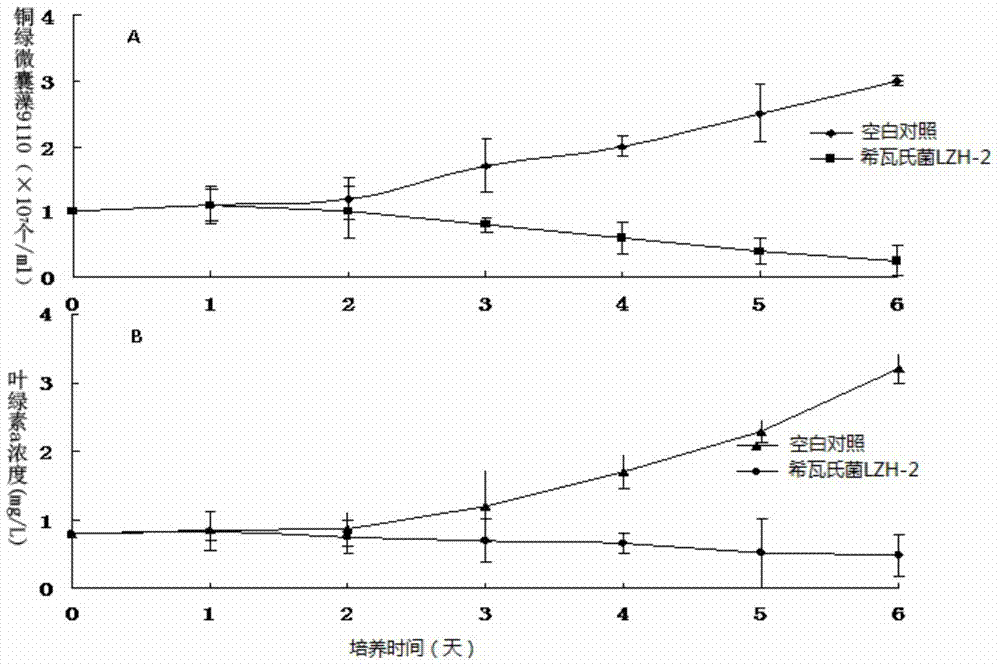
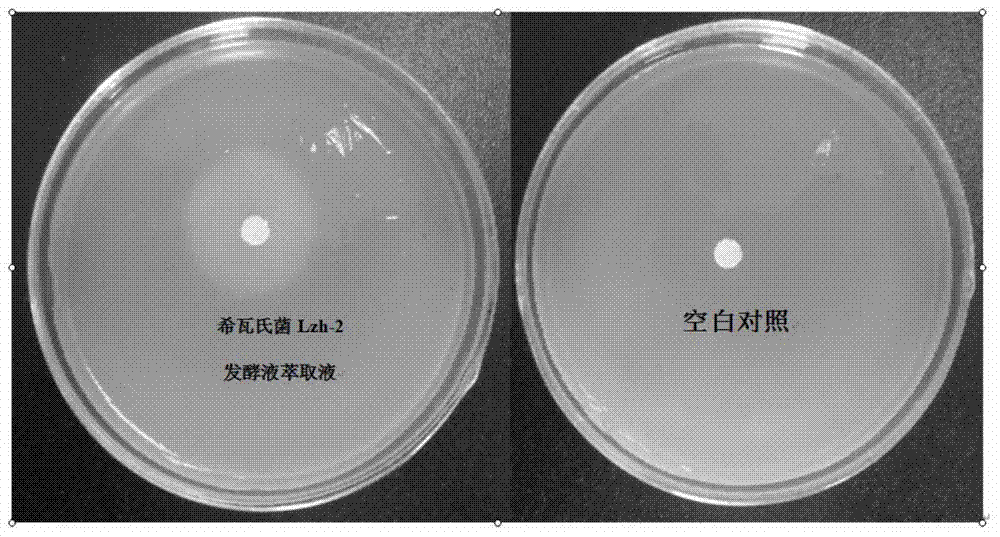
The invention discloses an algicidal Shewanella and application thereof in controlling blue algae water bloom. The Shewanella sp. Lzh-2 (CGMCC No.6549) with significant algae-lysed activity is isolated from Tai Hu lake water, and effective algicidal components hexahydro-pyrrolo[1,2-A]pyrazine-1,4-dione and indoline-2,3-dione are isolated, purified and identified from the metabolites of the Shewanella, wherein hexahydro-pyrrolo[1,2-A]pyrazine-1,4-dione has median lethal dose LD50 to Microcystis aeruginosa 9110 of 5.7 Mug / mL and has no lethal effect to Synechococcus BN60, and indoline-2,3-dione has median lethal doses to Microcystis aeruginosa 9110 and Synechococcus BN60 of 12.5 Mug / mL and 34.2 Mug / mL respectively. The Shewanella sp. Lzh-2 can be used for developing and producing novel biological algicides and ultimately used for controlling lake blue algae bloom.
Owner:SHANGHAI JIAO TONG UNIV
Neovison vison klebsiella peneumoniae
InactiveCN105199991AStrong toxicityIncreased toxicityBacteriaMicroorganism based processesBiotechnologyMicroorganism
The invention relates to a neovison vison klebsiella peneumoniae, and belongs to the technical field of microorganisms. The neovison vison klebsiella peneumoniae is named as a WMOK strain with the preservation number of CGMCC NO.11222, belongs to the K2 type, and is gram-negative (G-), facultative anaerobic, atrichous and asporous, has an obvious capsule, and besides, most strains have fimbriae and are rod-shaped with finely rounded ends; the WMOK strain has relatively high toxicity, has a half lethal dose (HLD) of 1.8 CFU to mice, and further has an HLD of 15 CFU to neovison vison. According to the neovison vison klebsiella peneumoniae, provided by the invention, separation and identification of the strain provide a certain foundation for further deep development of correlational studies on and control over neovison vison klebsiella peneumoniae.
Owner:王贵升
Culture medium for anti-glufosinate-ammonium alfalfa plants and soil screening method
InactiveCN104186308AEasy and fastHorticulture methodsPlant tissue cultureEthylmethane SulfonateGermplasm
The invention mainly relates to a screening method of anti-glufosinate-ammonium alfalfa plants, in particular to an anti-glufosinate-ammonium germplasm screening method. A screening method for a culture medium for anti-glufosinate-ammonium alfalfa plants is mainly characterized by comprising the following steps: (1) germinating seeds by the MS (Murashige and Skoog) culture medium; (2) screening the dose; (3) inducing alfalfa seeds through ethylmethane sulfonate median lethal dose; (4) screening the anti-glufosinate-ammonium alfalfa plants; and (5) screening resistant plants again. Compared with the prior art, the method has the advantages of being simple and easy to implement, taking effect quickly, and being capable of obtaining the herbicide resistant alfalfa individual plant in a short term. The method adopts a mutation measure, utilizes a chemical mutagen EMS (ethylmethane sulfonate) to induce the alfalfa seeds to cause point mutation, can screen the herbicide resistant alfalfa individual plant by the culture medium and the soil screening method, can provide a new germplasm resource for conventional breeding and can provide a reference for breeding of herbicide resistant agents of other plants.
Owner:LANZHOU UNIVERSITY
Algicidal Shewanella and application thereof in controlling blue algae water bloom
The invention discloses an algicidal Shewanella and application thereof in controlling blue algae water bloom. The Shewanella sp. Lzh-2 (CGMCC No.6549) with significant algae-lysed activity is isolated from Tai Hu lake water, and effective algicidal components hexahydro-pyrrolo[1,2-A]pyrazine-1,4-dione and indoline-2,3-dione are isolated, purified and identified from the metabolites of the Shewanella, wherein hexahydro-pyrrolo[1,2-A]pyrazine-1,4-dione has median lethal dose LD50 to Microcystis aeruginosa 9110 of 5.7 Mug / mL and has no lethal effect to Synechococcus BN60, and indoline-2,3-dione has median lethal doses to Microcystis aeruginosa 9110 and Synechococcus BN60 of 12.5 Mug / mL and 34.2 Mug / mL respectively. The Shewanella sp. Lzh-2 can be used for developing and producing novel biological algicides and ultimately used for controlling lake blue algae bloom.
Owner:SHANGHAI JIAO TONG UNIV
B-cell epitope of VP(viral protein)3 of DHAV (duck hepatitis A virus)-1 as well as identification method and application of B-cell epitope
ActiveCN105198969AEasy to identifyRapid identificationSsRNA viruses positive-senseViral antigen ingredientsDuck hepatitis A virusElisa method
Owner:SICHUAN AGRI UNIV
Rhizoma cimicifugae extractive, two kinds of rhizoma cimicifugae ketone alkali, preparation method and application
ActiveCN106860624AThe drug effect is goodOrganic active ingredientsOrganic chemistryHuman leukemiaLethal dose
The invention provides rhizoma cimicifugae extractive and two kinds of rhizoma cimicifugae ketone alkali. The rhizoma cimicifugae extractive and pure products of the two kinds of rhizoma cimicifugae ketone alkali can be obtained by utilizing cimicifuga foetida plants as a raw material, extracting through an organic solvent and extracting through a solvent or separating through a chromatograph, and the two kinds of rhizoma cimicifugae ketone alkali are first rhizoma cimicifugae ketone alkali and second rhizoma cimicifugae ketone alkali. The extractive and the two kinds of rhizoma cimicifugae ketone alkali can effectively restrain proliferation and growth of tumor cell lines like a human lung cancer cell line A549, a human stomach cancer cell line MKN7, GSU, a human melanoma cell line A375, a human lung adenocarcinoma cell line NCI-H1975, a human colon cancer cell line Colo-205, a human leukemia cell line HL-60 and the like; the half lethal dose of the first rhizoma cimicifugae ketone alkali and the second rhizoma cimicifugae ketone alkali is in a concentration range of 1.36 to 21.09 mu M, so that the first rhizoma cimicifugae ketone alkali and the second rhizoma cimicifugae ketone alkali have obvious antitumor activity and can be applied to preparation of antitumor medicine. Structural formulas of the first rhizoma cimicifugae ketone alkali (1) and the second rhizoma cimicifugae ketone alkali (2) are shown in the specification.
Owner:ZHEJIANG UNIV
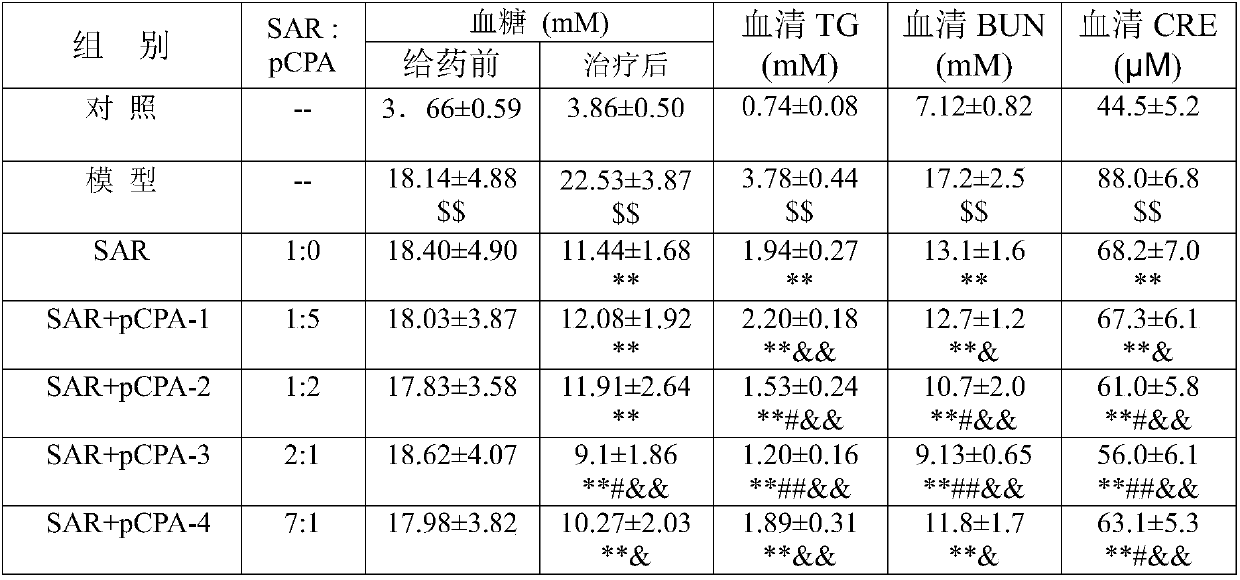
Application of Sinkiang salvia officinalis extract in preparation of medicines for preventing and treating diabetic nephropathy
The invention discloses an application of a Sinkiang salvia officinalis extract in preparation of medicines for preventing and treating diabetic nephropathy. The application has the beneficial effect that the Sinkiang salvia officinalis extract provided by the invention is applied to preparation of the medicines for preventing and treating diabetic nephropathy. An objective and scientific evaluation is made for the safety of total phenolic acid extracts of Sinkiang salvia officinalis through an experiment; a dosage basis is provided for experiment research for preventing and treating DN (diabetic nephropathy); firstly, the median lethal dose (LD50) is determined through an acute peroral toxicity experiment of mice; on this basis, the preventive and therapeutic effect and possible action mechanism of the total phenolic acid extracts of the Sinkiang salvia officinalis on DN are determined through in vivo experiments on animals; an experiment basis is provided for preventing and treating DN employing natural medicines; and technical data are also provided for further development and utilization.
Owner:XINJIANG MEDICAL UNIV
Application of sarpogrelate containing pharmaceutical composition in treatment of diabetic nephropathy
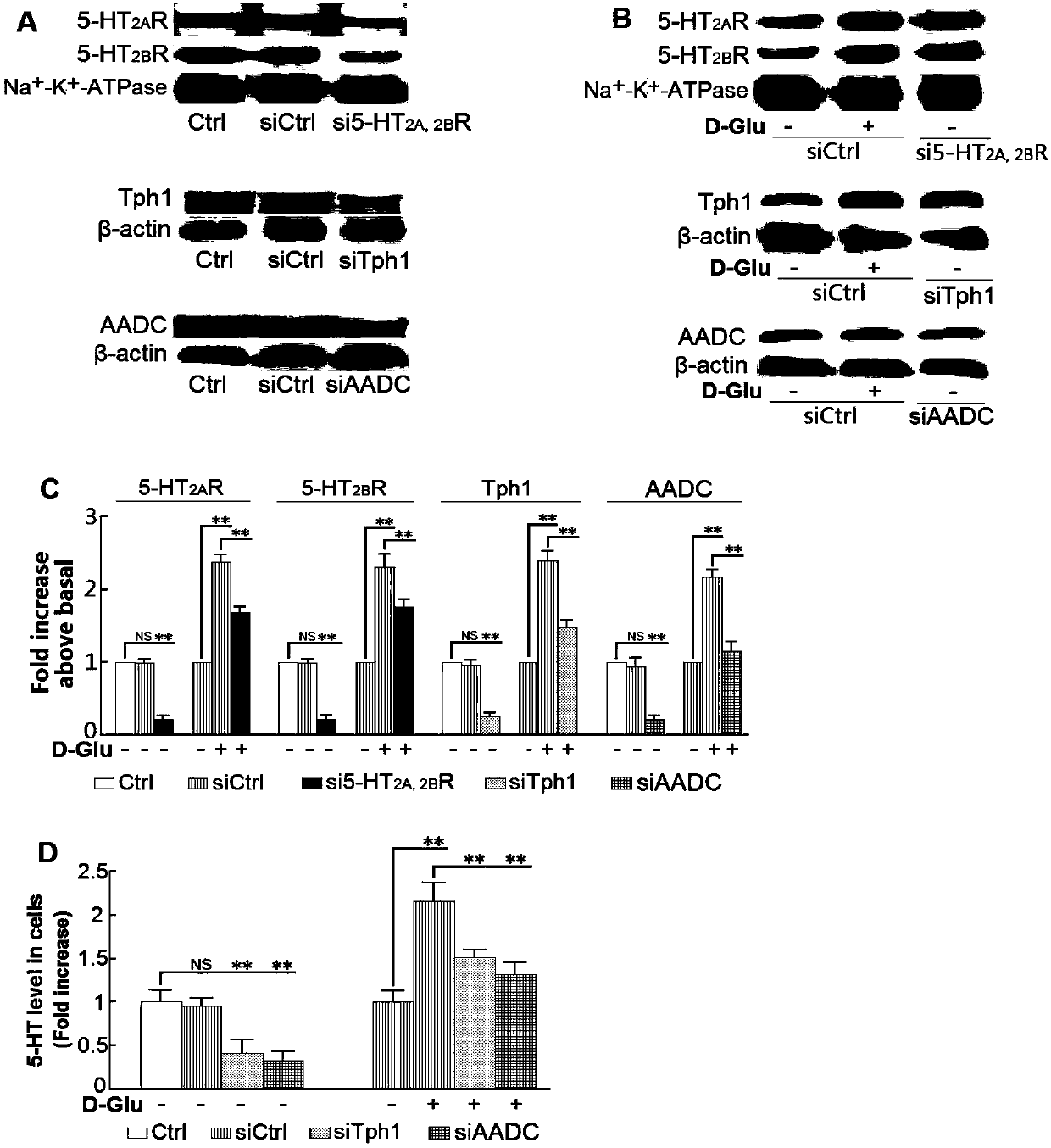
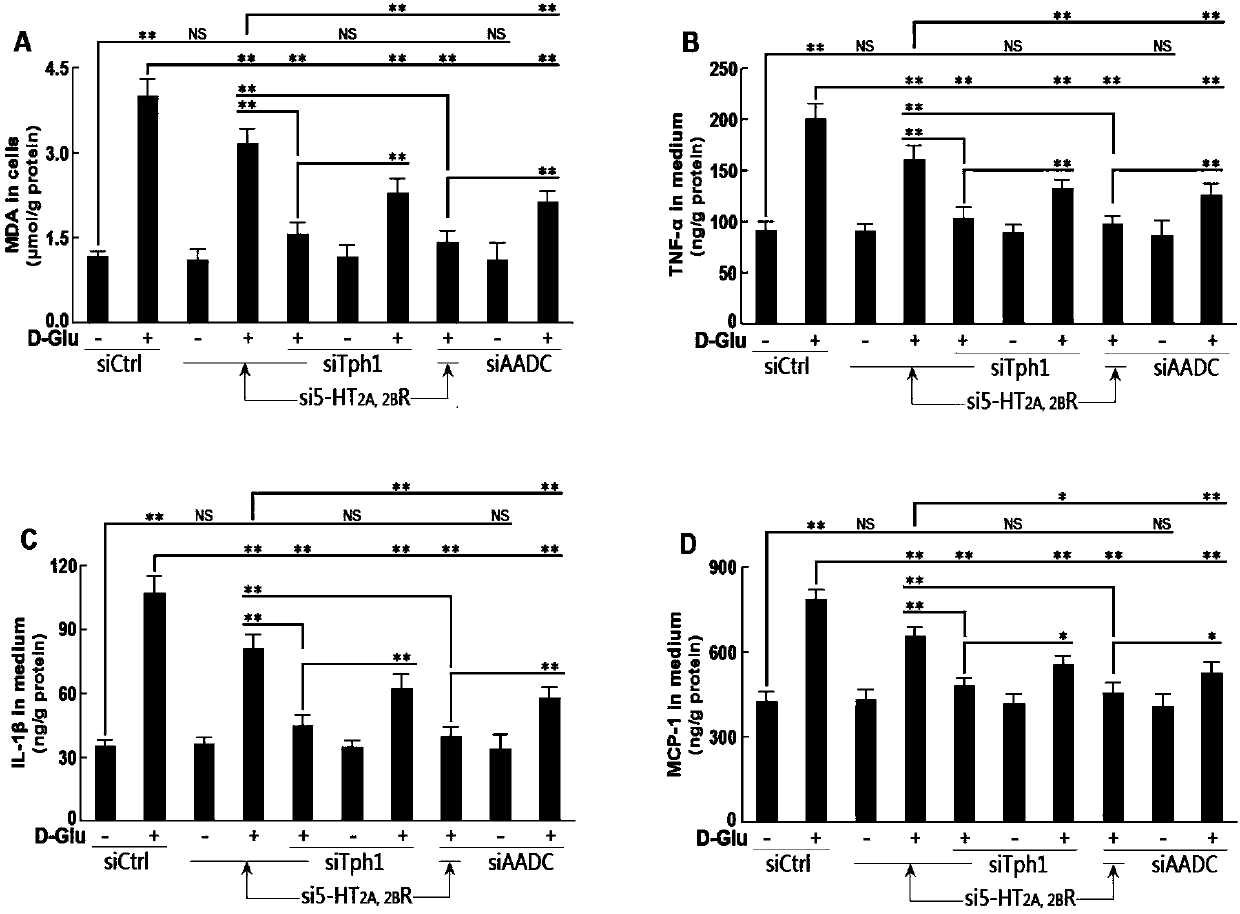
The invention belongs to the technical field of pharmaceuticals, discloses application of a sarpogrelate containing pharmaceutical composition in treatment of diabetic nephropathy, and specifically discloses the application of a compound pharmaceutical composition taking sarpogrelate and 5-hydroxytryptamine synthesizing inhibitor as pharmaceutical active components in preparation of drugs for treating diabetic nephropathy. The test shows that gene silencing 5-HT synthetase and a 5-HT 2 receptor show good effect on inhibiting in-vitro culture and high glucose-induced oxidative stress of human renal mesangial cell strain, proinflammatory factor and chemotactic factor, and meanwhile, the gene silencing 5-HT synthetase system (Tph1 or AADC) and a 5HT 2 receptor are capable of greatly inhibiting high glucose-inducing effect and performing synergistic effect. With the adoption of the pharmaceutical composition, the diabetic nephropathy caused by high-fat feed feeding and streptozotocin induced diabetic nephropathy can be obviously treated; compared with simple prescription, the compound prescription is high in attenuation, and the lethal amount of mice orally taking the drug is obviouslyhalved.
Owner:CHINA PHARM UNIV
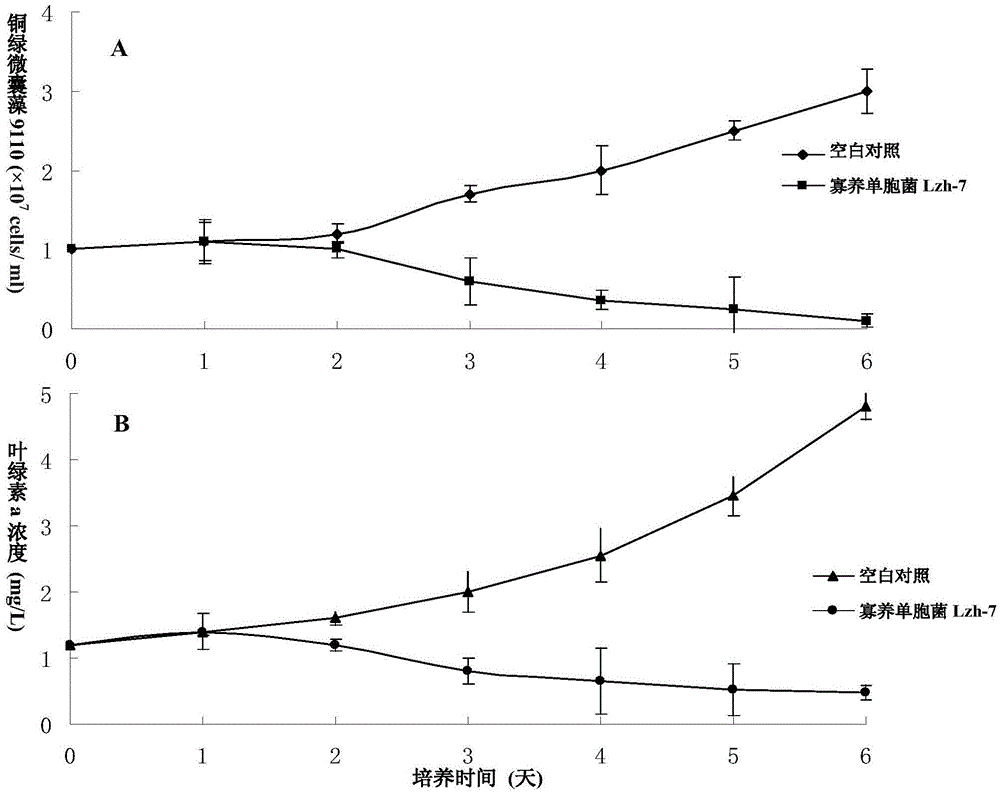
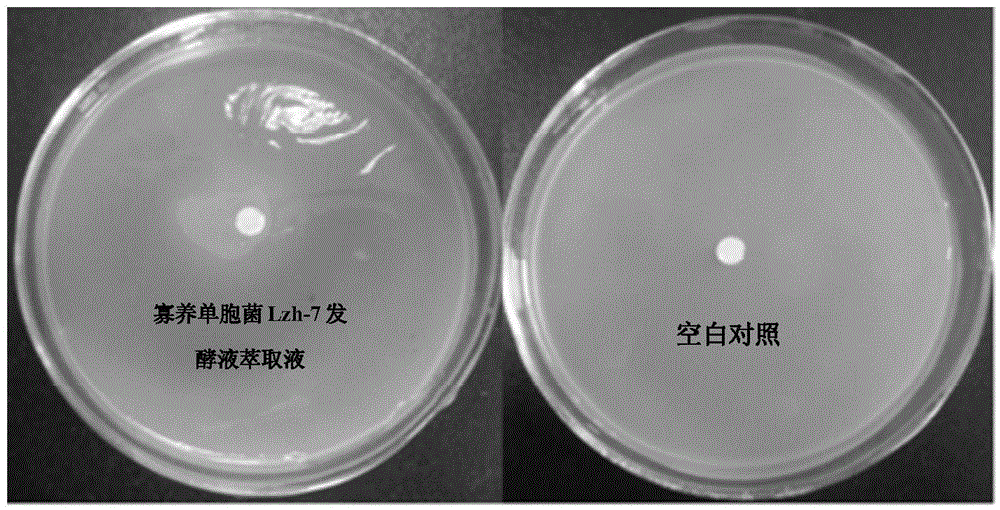
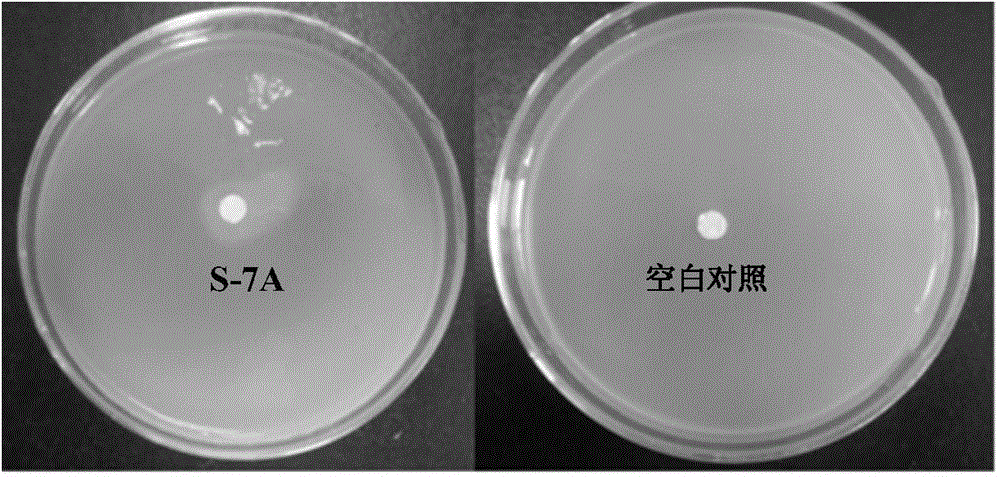
A strain of Stenotrophomonas and its application in the control of cyanobacterial blooms
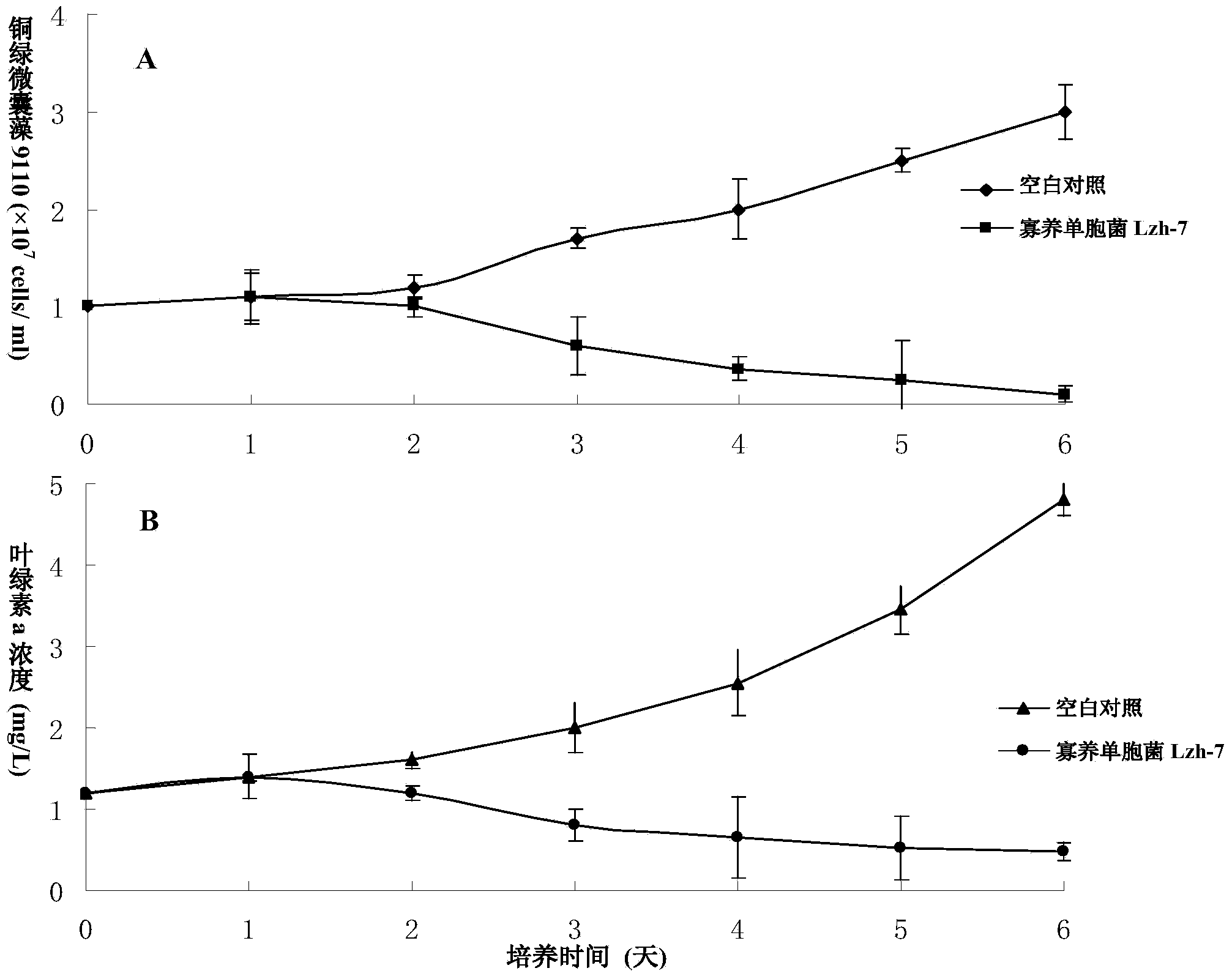
The invention discloses a Stenotrophomonas sp. strain and application thereof in controlling cyanobacterial blooms. A strain of Stenotrophomonas sp. Lzh-7 (CGMCC No.6548) having significant algae-lysing activity is isolated from Taihu water body. The fact is identified that the effective algae-lysing component hydroquinone is isolated and purified from the metabolites of the strain, wherein the median lethal dose (LD50) of hydroquinone to Microcystis aeruginosa 9110 is 3.9 Mug / mL and the median lethal dose (LD50) of hydroquinone to Synechococcus BN60 is 6.7 Mug / mL. The Stenotrophomonas sp. strain can be applied to the research, development and production of novel biological algaecides and eventually applied to the control of lake cyanobacterial blooms.
Owner:SHANGHAI JIAOTONG UNIV
Stenotrophomonas sp. strain and application thereof in controlling Cyanobacteria blooms
The invention discloses a Stenotrophomonas sp. strain and application thereof in controlling cyanobacterial blooms. A strain of Stenotrophomonas sp. Lzh-7 (CGMCC No.6548) having significant algae-lysing activity is isolated from Taihu water body. The fact is identified that the effective algae-lysing component hydroquinone is isolated and purified from the metabolites of the strain, wherein the median lethal dose (LD50) of hydroquinone to Microcystis aeruginosa 9110 is 3.9 Mug / mL and the median lethal dose (LD50) of hydroquinone to Synechococcus BN60 is 6.7 Mug / mL. The Stenotrophomonas sp. strain can be applied to the research, development and production of novel biological algaecides and eventually applied to the control of lake cyanobacterial blooms.
Owner:SHANGHAI JIAO TONG UNIV
Preparation and application of Chinese cobra bite pig model
InactiveCN113016721AIncrease success rateGood repeatabilityCompounds screening/testingAnimal husbandryCobra venomDisease
The invention discloses a manufacturing method of a Chinese cobra bite pig model and half lethal dose (LD50) of cobra venom to pigs and application of the cobra venom to an animal model. Cobra venom freeze-dried powder is dissolved and then injected into pig leg muscles according to 2mg / kg, and a target animal model can be obtained after 6 hours, so that the problem that no Chinese cobra bite model of large animals in the prior art is solved. The model has the advantages of high success rate, strong repeatability, stable pathological change and the like. The cobra bite disease condition is long, the model simulates human being bite by the cobra, the physiological and pathological dynamic process of the human being bite by the cobra can be well close to, and dynamic analysis of the disease clinical manifestation and mechanism of the cobra bite is facilitated. The model can provide a good animal model for researching metabolic change of Chinese cobra venom in a human body and a muscle necrosis mechanism, and provides an innovative technical method support for researching clinical characterization of cobra bite, pathophysiological mechanism, precise treatment, screening of anti-venomous snake bite medicines, evaluation of curative effect of anti-venom serum and the like.
Owner:何冬凌 +6
Application of salvianolic acid K in sage in the preparation of drugs for preventing and treating diabetic nephropathy
InactiveCN105616396BOrganic active ingredientsMetabolism disorderSalvianolic acid KAcute toxicity testing
The invention provides application of salvianolic acid K in salvia japonica thunb to preparation of medicine for preventing and treating diabetic nephropathy.Safety of the salvianolic acid K in the salvia japonica thunb in Sinkiang is evaluated objectively and scientifically through experiments, and a dosage basis is provided for experiment research on prevention and treatment of DN; firstly, the median lethal dose (LD 50) is determined through per os acute toxicity tests of mice, on the basis of the tests, in vivo experiments on animals are conducted, the prevention and treatment functions and possible mechanism of action of the salvianolic acid K on DN are determined, an experiment basis is provided for preventing and treating DN through natural medicine, and technical data are provided for further development and utilization of the salvia japonica thunb in Sinkiang.
Owner:XINJIANG MEDICAL UNIV
A kind of acrylate composite grouting filling material and its preparation method and application
ActiveCN106927719BImprove fill curing strengthMeet engineering needsBuilding constructionsOrganic fertilisersCross-linkPolymer science
Owner:SHANDONG UNIV
Extraction, purification method and application of alkaloids in soft-shelled turtle
The invention belongs to the technical field of chemical extraction of traditional Chinese medicine, particularly relates to an extraction method, a purification method and application of alkaloids inground beetles, and specifically provides an extraction method for extracting the alkaloids from the ground beetles, a purification method for the alkaloids obtained according to the extraction method, application of the alkaloids obtained according to the extraction method or the purified alkaloids obtained according to the purification method, and application of the alkaloids obtained accordingto the extraction method or the purified alkaloids obtained according to the purification method in growth inhibition of gastric carcinoma cells. The extraction method has the advantages that GBA18 can be obtained through separation; experimental determination proves that the obtained products have a good inhibiting effect on gastric cancer and bacteria, and the half lethal dose and the minimum lethal dose are within the safety usage range recommended by the Chinese pharmacopoeia; the technical defects that active ingredients in the ground beetles are unclear in the prior art and applicationof the ground beetles is seriously limited are overcome.
Owner:GUANGDONG UNIV OF TECH
Superfine oil for abrasive machining of high-lubrication and high-water-separability bearing
ActiveCN102660360BImprove the lubrication effectImprove water separation effectLubricant compositionAntioxidantHazardous substance
The invention discloses superfine oil for abrasive machining of a high-lubrication and high-water-separability bearing. The superfine oil consists of mineral oil, polymeric carboxylic acid, organic amine, a surfactant, a synthetic lubricant, an anticorrosive agent, an extreme-pressure additive and an antioxidant. The superfine oil has an excellent lubricating property, a Bouton tester is used fortesting, and a friction coefficient value of raw oil is less than or equal to 0.1; the superfine oil has excellent water separability, and the separation time of oil and water is less than or equal to 10 min (50 ml of sample is added into 50 ml of water, and the mixture is turned for 10 times); and raw materials in a formula are not toxic and harmful substances which influence the environmental safety and human health, the biological median lethal dose LD50 of the product is more than or equal to 5,000 mg / kg, the median lethal concentration LC50 is more than or equal to 5,000 mg / m<3>, the respiratory mucosa irritation is less than or equal to class 1, and the superfine oil has reliable safety and sanitation quality.
Owner:QIDONG YUSHIRO CHEMICAL INDUSTRY CO LTD +1
Porcine pseudorabies virus gI/gE double-gene-deleted strain and application thereof
PendingCN114045269AImprove securityImproving immunogenicityViral antigen ingredientsVirus peptidesDiseaseRabies
The invention belongs to the technical field of virology genetic engineering and preventive veterinary medicine, particularly relates to a gI / gE double-gene-deleted strain of a porcine pseudorabies epidemic variation virus, and further discloses a construction method of the gI / gE double-gene-deleted strain and application of the gene-deleted strain. A female parent of the porcine pseudorabies virus gene-deleted strain PRV-E6-delta gE / gI is a wild epidemic variant strain PRV-E6 independently separated from a disease material, and on the basis of the variant strain PRV-E6, a unique and simplified one-step double-knockout technology is adopted to knock out gI / gE double-virulence genes, so the porcine pseudorabies virus gene-deleted strain PRV-E6-gE / gI is obtained. The gB, gC and TK genes of the strain have the same evolutionary characteristics as prevalent variant virulent strains newly appearing after 2012, and have the advantages of high proliferation titer and strong virulence (wherein a median lethal dose to mice is 10<3.3> TCID50, and the morbidity of 2-to-3-weeks-old piglets is 10<5> TCID50).
Owner:BEIJING KEMUFENG BIOLOGICAL PHARMA +3
Analgesic anti-inflammatory composite medicament and method for preparing same
Owner:GANSU CHEEZHENG TIBETAN MEDICINE CO LTD
Anti-tumor activating fuling scloerotium glucan sulfate and its preparation and use
InactiveCN1273495CGood antitumor activitySimple production processOrganic active ingredientsAntineoplastic agentsSolubilityChlorosulfuric acid
An anti-tumour activated Tuckahoe sclerotium glucan sulfuric is prepared by: taking fresh Tuckahoe sclerotium, extracting non-water soluble (1->3)-beta- D- glucan with NaOH, solving them in anhydrous dimethylsulfoxide, adding pyridine and chloro-sulfonic acid to react to get glucan sulfuric ester. The glucan sulfuric ester's substituted reaction is of non-selective substitution, occurred at C-6 position of hydroxy, the substitution value of sulfuric ester is 0.66- 1.21, weight-average molecular weight is 0.8X104-16.4X104, and has well water solubility. Testing in vivo and in vitro showed that, the glucan sulfuric ester has obvious inhibit effect for Sarcoma 180 and proliferation of adenocarcinoma of stomach. The toxic testing in small rat prove that half lethal dose of glucan sulfuric ester (LD50)>1600 mg / kg, and without toxic or side effects. The Tuckahoe sclerotium glucan sulfuric ester is capable of use in preparing anti-tumour pharmaceuticals or health food with function of increasing body immunological competence.
Owner:WUHAN UNIV
A kind of B cell epitope of type 1 duck hepatitis A virus vp3 protein and its identification method and application
ActiveCN105198969BEasy to identifyRapid identificationSsRNA viruses positive-senseViral antigen ingredientsDuck hepatitis A virusElisa method
The invention belongs to the technical field of bioengineering and particularly relates to a B-cell epitope of VP(viral protein)3 of DHAV (duck hepatitis A virus)-1 as well as an identification method and an application of the B-cell epitope. The identification method of the B-cell epitope comprises the following steps: obtaining a target fragment of the VP3; constructing recombinant expression plasmid pG-EX-VP3, preparing VP3 recombinant protein, preparing a polyclonal antibody of the VP3 recombinant protein, determining the median lethal dose ELD50 of the DHAV-1 on chicken embryos or duck embryos, determining the neutralizing titer of the polyclonal antibody of the VP3 recombinant protein and identifying the B-cell epitope on the VP3. The linear B-cell epitope of the VP3 is predicted with comprehensive application of bioinformatics software, epitope peptide is artificially synthesized, identification is performed on the synthetic epitope peptide in combination with an indirect ELISA (enzyme linked immunosorbent assay) method, and reference can be provided for deep development of follow-up DHAV-1 immunological research, construction of novel diagnostic preparations and development of polypeptide vaccines.
Owner:SICHUAN AGRI UNIV
Extraction method, purification method and application of alkaloids in ground beetles
InactiveCN108904540ASolve the technical defects that the active ingredients are not clear and seriously limit its applicationGood effectAntibacterial agentsAntimycoticsPurification methodsGround beetle
The invention belongs to the technical field of chemical extraction of traditional Chinese medicine, particularly relates to an extraction method, a purification method and application of alkaloids inground beetles, and specifically provides an extraction method for extracting the alkaloids from the ground beetles, a purification method for the alkaloids obtained according to the extraction method, application of the alkaloids obtained according to the extraction method or the purified alkaloids obtained according to the purification method, and application of the alkaloids obtained accordingto the extraction method or the purified alkaloids obtained according to the purification method in growth inhibition of gastric carcinoma cells. The extraction method has the advantages that GBA18 can be obtained through separation; experimental determination proves that the obtained products have a good inhibiting effect on gastric cancer and bacteria, and the half lethal dose and the minimum lethal dose are within the safety usage range recommended by the Chinese pharmacopoeia; the technical defects that active ingredients in the ground beetles are unclear in the prior art and applicationof the ground beetles is seriously limited are overcome.
Owner:GUANGDONG UNIV OF TECH
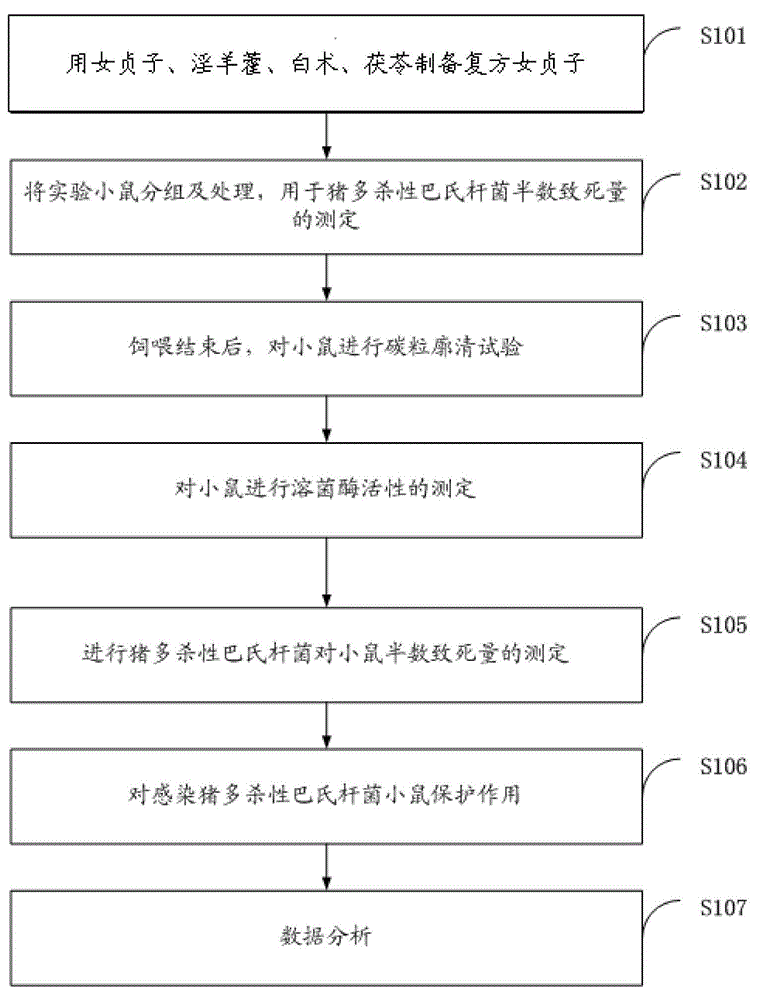
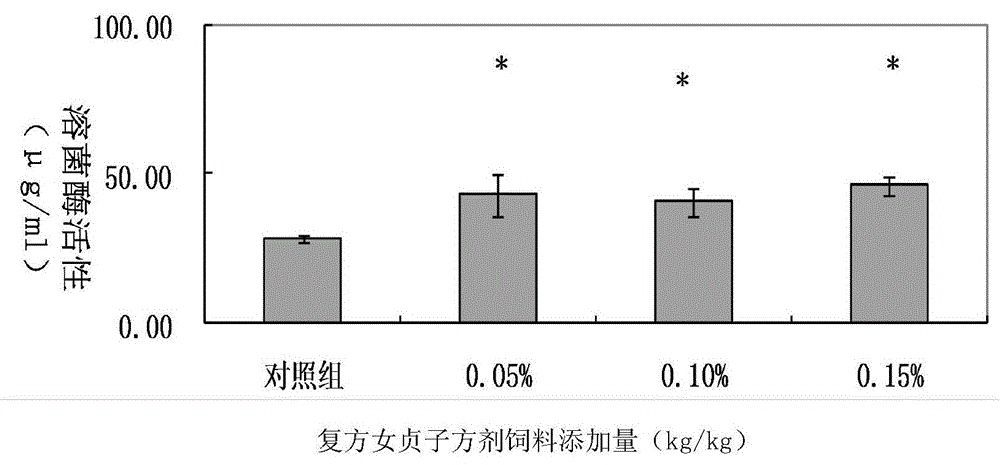
Construction method for compound glossy privet fruit for mouse model infected with pig pasteurella multocida
InactiveCN104095934AHigh activityImprove protectionAntibacterial agentsImmunological disordersMedian lethal doseP. multocida
The invention discloses a construction method for compound glossy privet fruit for a mouse model infected with pig pasteurella multocida. The method comprises the following steps: preparing the compound glossy privet fruit; grouping and treating the experiment mice for measuring the median lethal dose of pig pasteurella multocida; after feeding, conducting the carbon granule clearance test on the mice, and measuring the activity of lysozyme of mice; measuring the median lethal dose of the pig pasteurella multocida on mice, and measuring the protective effect on the mice infected with pig pasteurella multocida; analyzing the data. According to the method, that the compound glossy privet fruit is used as a feed additive and the activity of lysozyme is improved obviously by increasing the immune organ index and the activity of the mononuclear macrophage is verified, thereby reaching the good protective effect on the mice infected with pig pasteurella multocida.
Owner:HUNAN AGRICULTURAL UNIV
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com