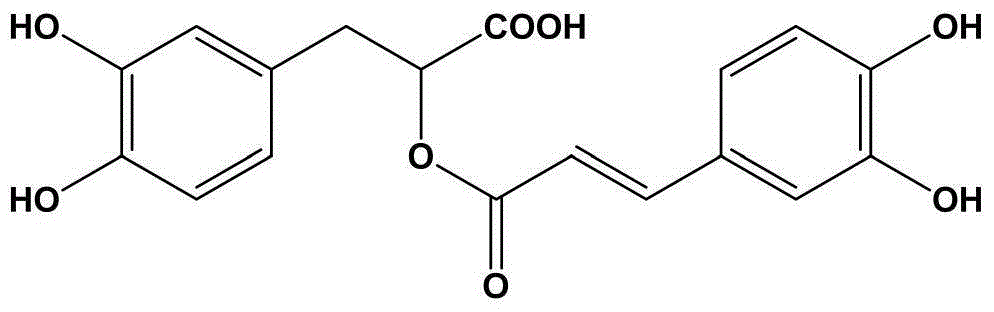
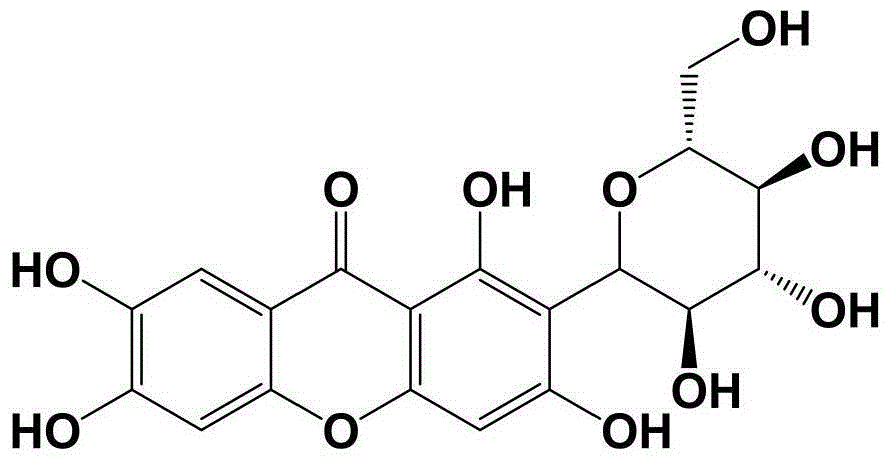
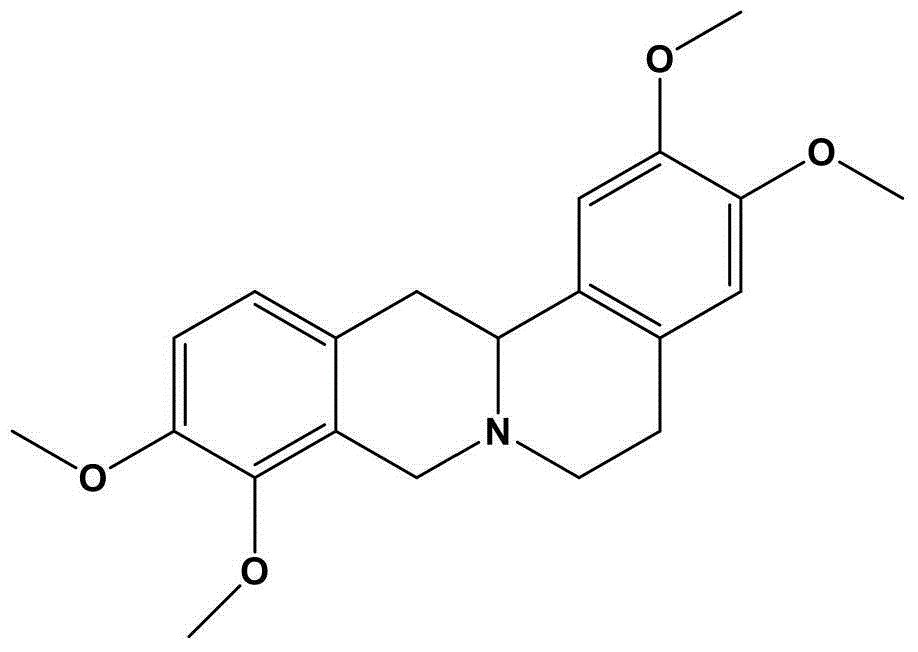
Oral preparation for analgesia, and preparation method of oral preparation
A technology for oral preparations and compositions, applied in the field of oral preparations and their preparation, can solve the problems of seriousness, addiction, gastrointestinal reactions, etc., and achieve the effects of reducing dosage, reducing toxic side effects, and improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1 Effect of pharmaceutical composition on mouse writhing times caused by acetic acid
[0028] Male ICR mice (20±2) g were randomly divided into groups according to body weight, 10 mice in each group, and each group was given therapeutic drugs by intragastric administration. The model group was intragastrically given an equal volume of normal saline. Intraperitoneal injection of acetic acid in mice caused large-scale and long-lasting pain stimulation in the deep abdominal cavity, resulting in writhing reactions in mice. After each dose group was administered for 1 hour, 0.1ml / 10g of 0.7% acetic acid saline solution was injected intraperitoneally, and the number of writhing reactions in each mouse within 20 minutes after the injection of acetic acid induced pain was recorded, and the writhing times of each administration group were calculated. Inhibition rate.
[0029] Writhing inhibition rate=[(the number of times of writhing in the control group-the number of ...
Embodiment 2
[0035] Example 2 Effect of pharmaceutical composition on hot plate-induced pain response in mice
[0036] Put female ICR mice (20±2) g on an intelligent hot plate instrument at 55±0.5°C, record the latency (s) from when the sole of the mouse touches the hot plate to the reaction of licking the rear paw as the pain threshold index, and remove the reaction Mice with latency 30s or jumping. Select 140 qualified mice with response latency within 10-30s, randomly group them into groups according to predrug pain threshold and body weight, and administer them by intragastric administration;
[0037] Model group: equal volume of saline,
[0038] Composition group: 50mg / kg, ig
[0039] After continuous intragastric administration for 5 days, the pain threshold of the mice in each administration group was measured once 30, 60, 90, and 120 minutes after the last administration, and the pain threshold exceeding 60s was calculated as 60s.
[0040] data to Said, using SPSS15.0 software...
Embodiment 3
[0044] Example 3 Tablet
[0045] prescription
[0046]
[0047] Preparation process: 1) Grinding the pharmaceutical composition and auxiliary materials through an 80-mesh sieve, fully mixing the pharmaceutical composition, methylcellulose and mannitol, 2) 50% ethanol soft material, granulating through a 18-mesh sieve, 60°C Dry it under a 16-mesh sieve, granulate it, add micropowder silica gel, mix evenly, and press it into tablets to get final product. The weight of the tablet is 400 mg, and the proportion of the pharmaceutical composition is that of the administration group 2 in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com