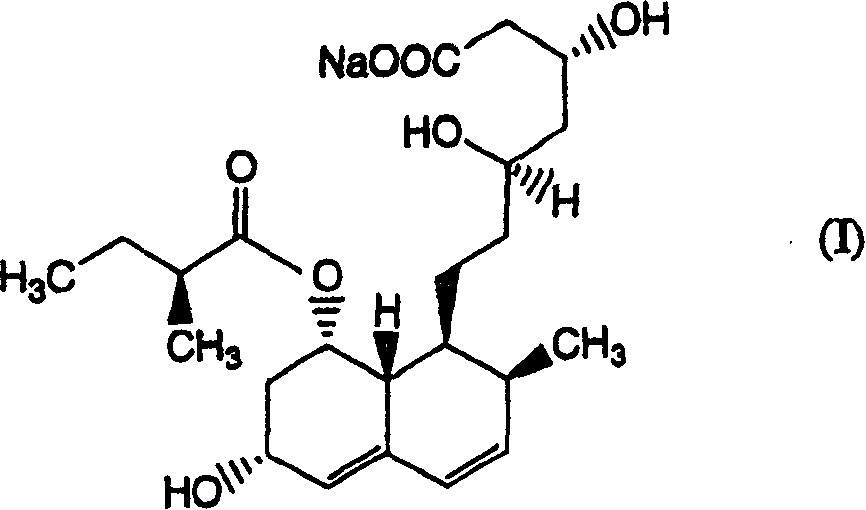
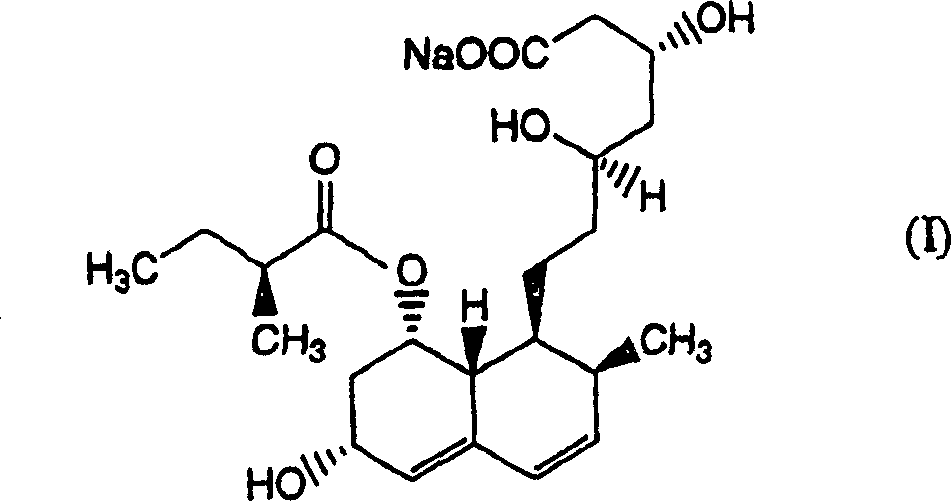
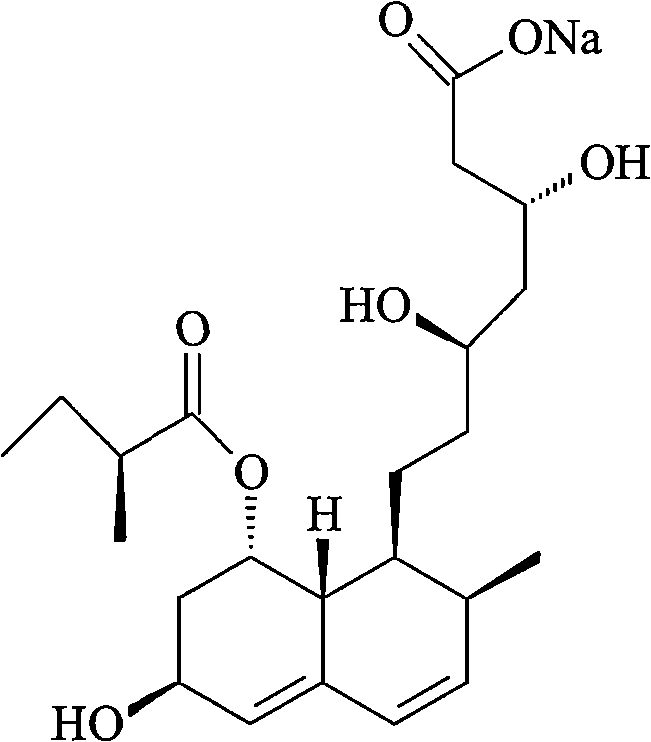
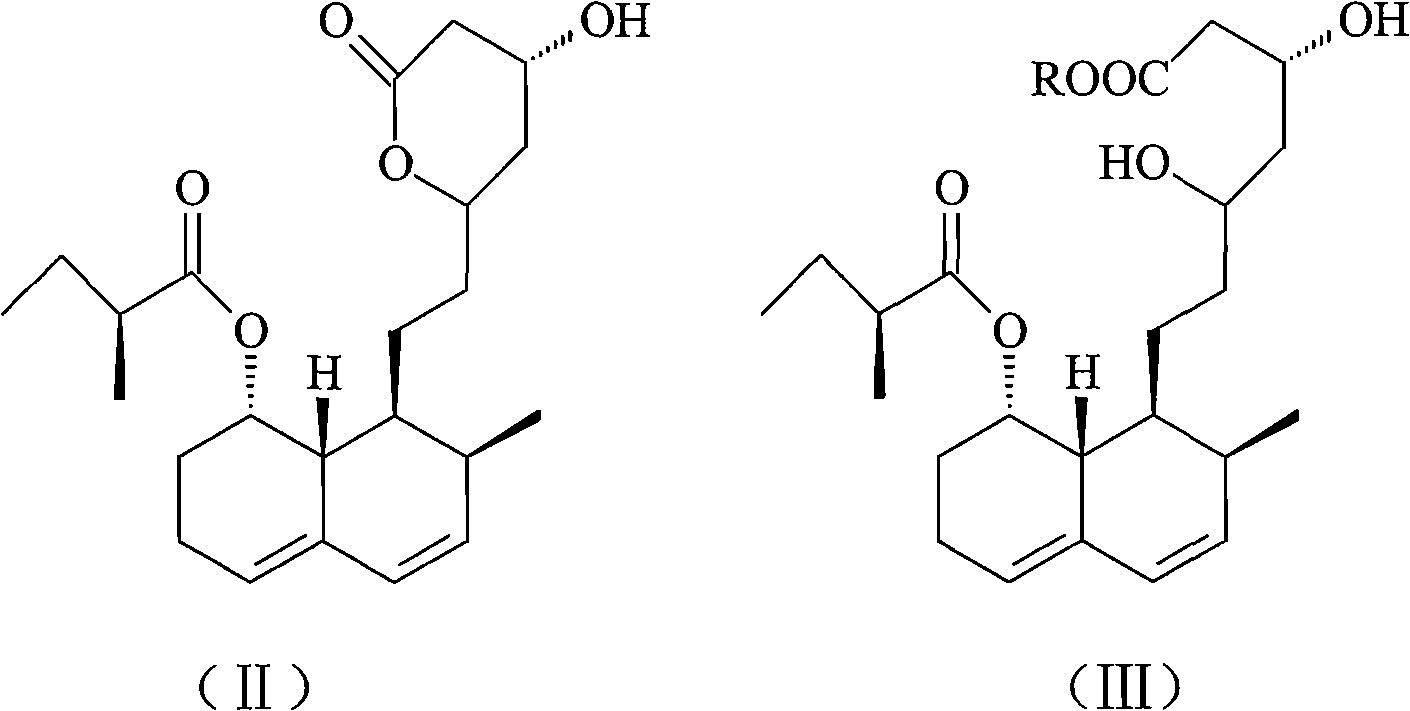
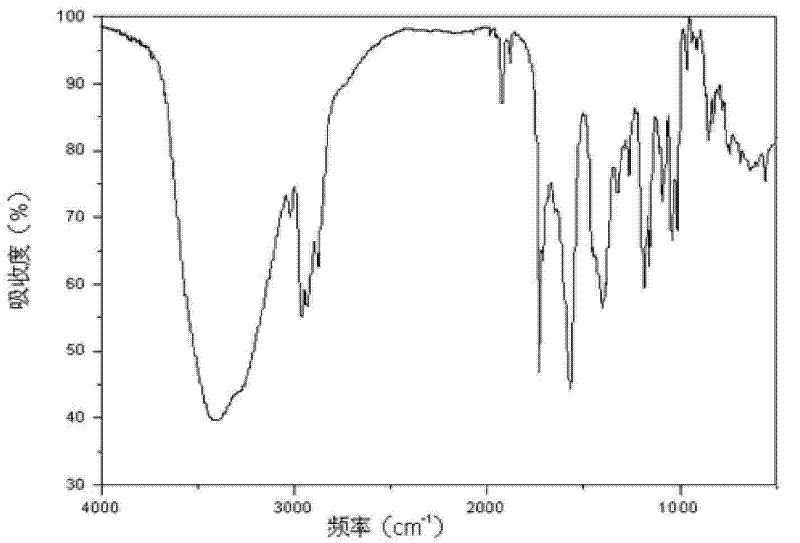
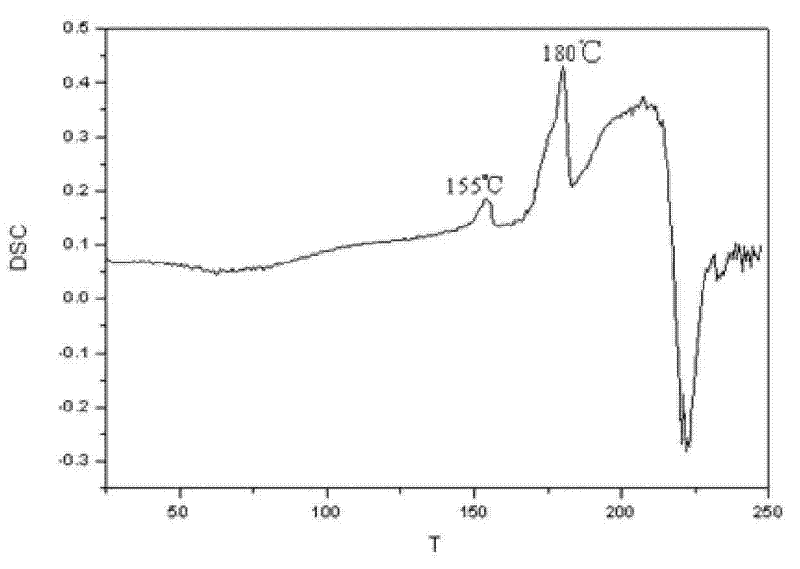
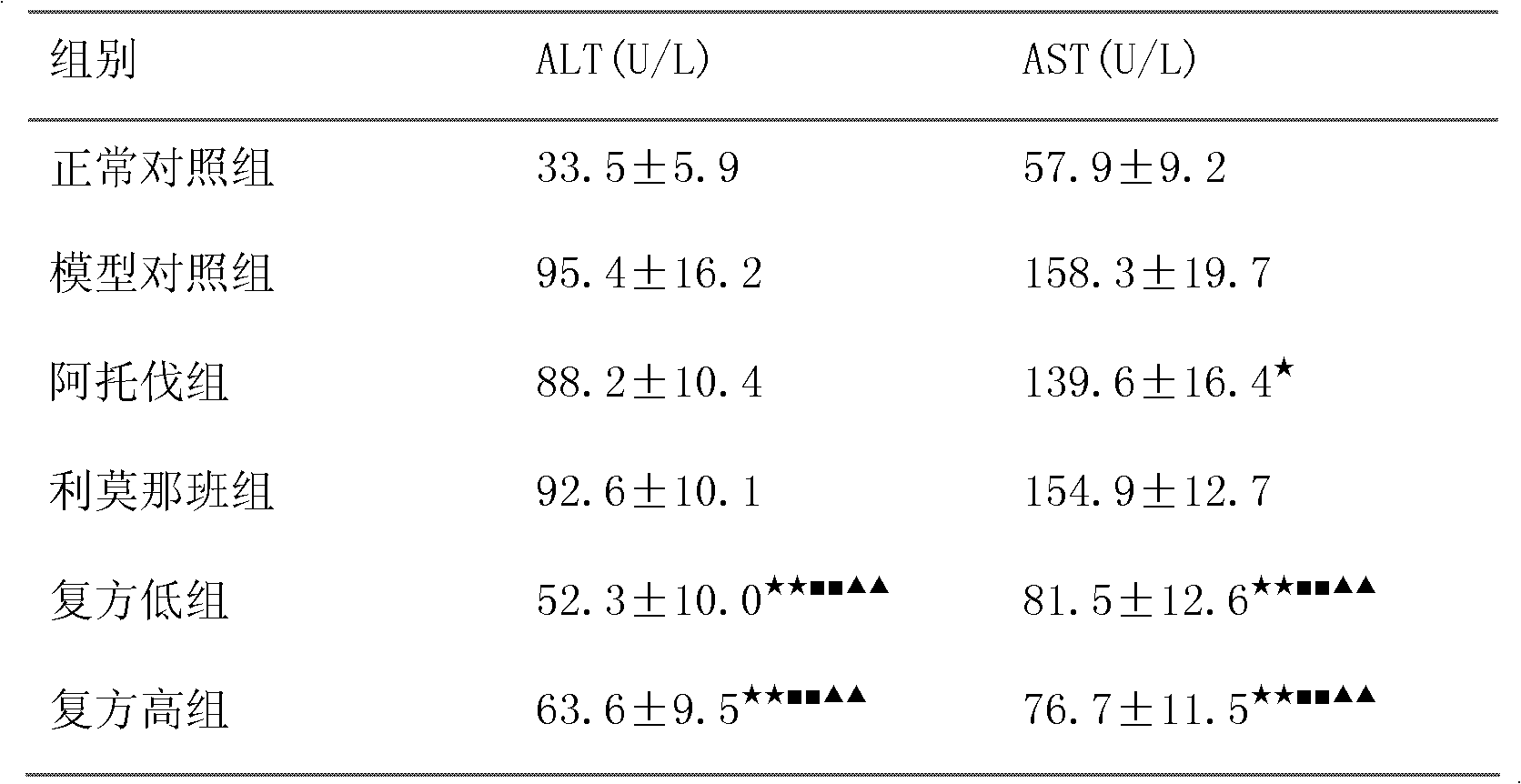
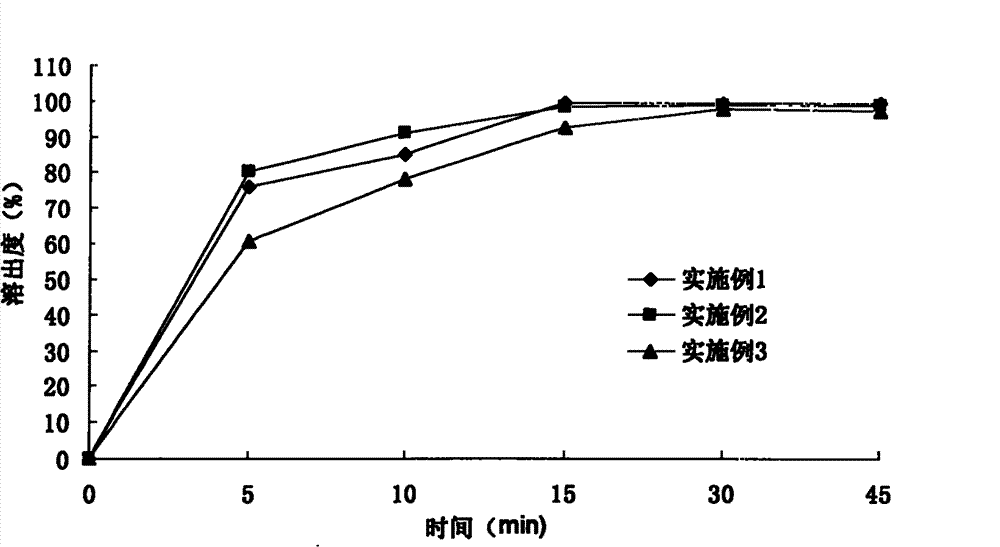
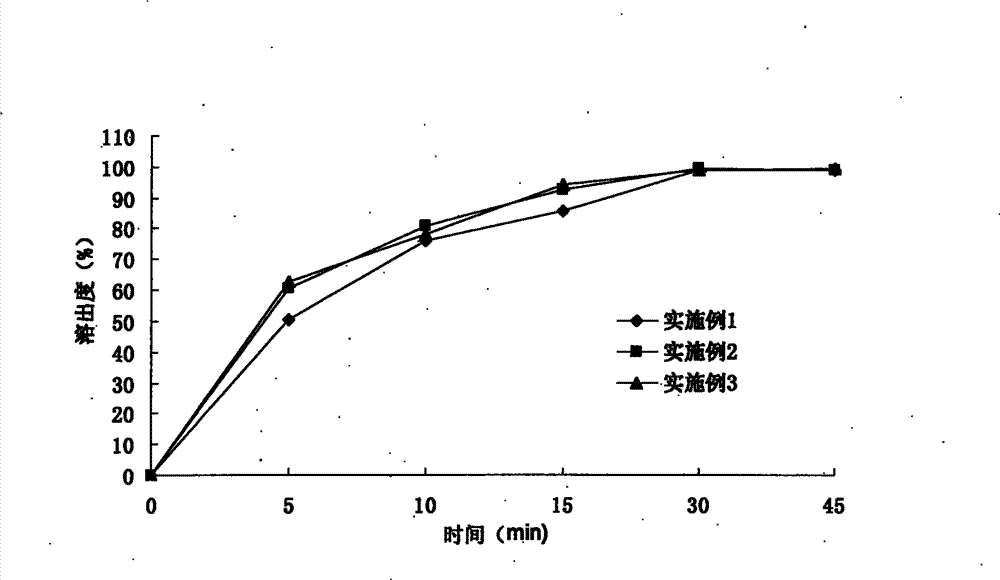
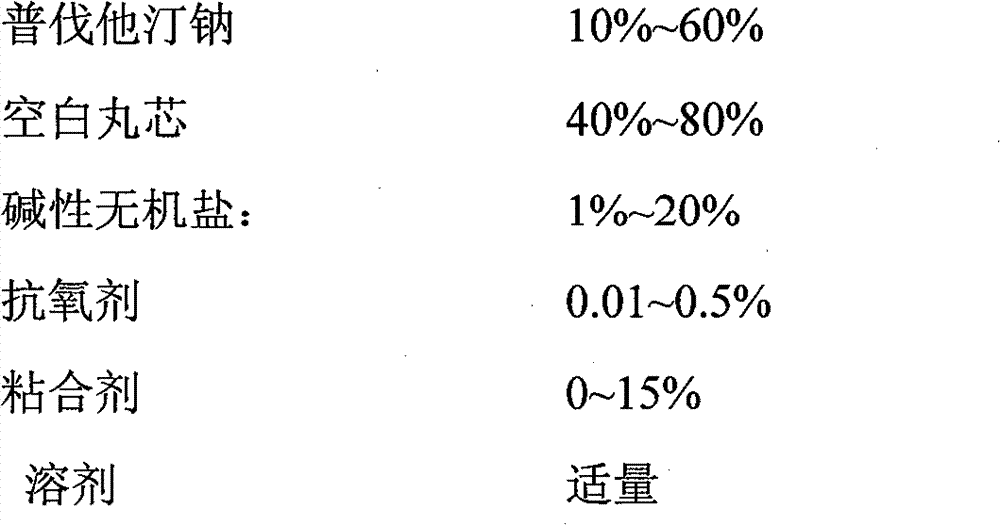
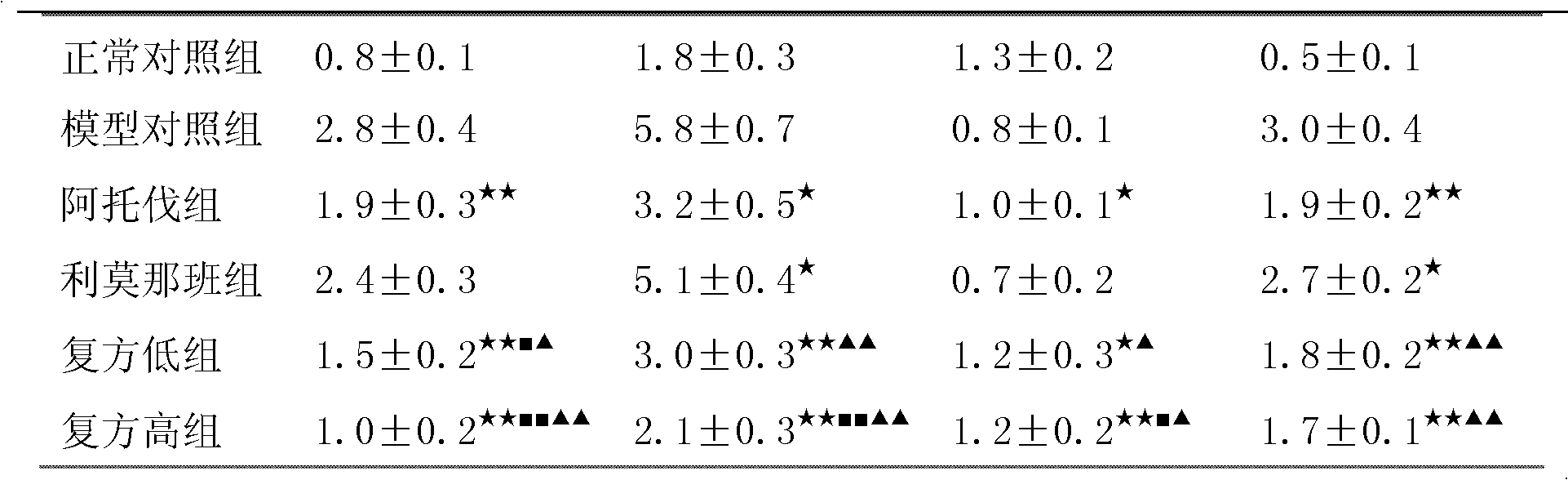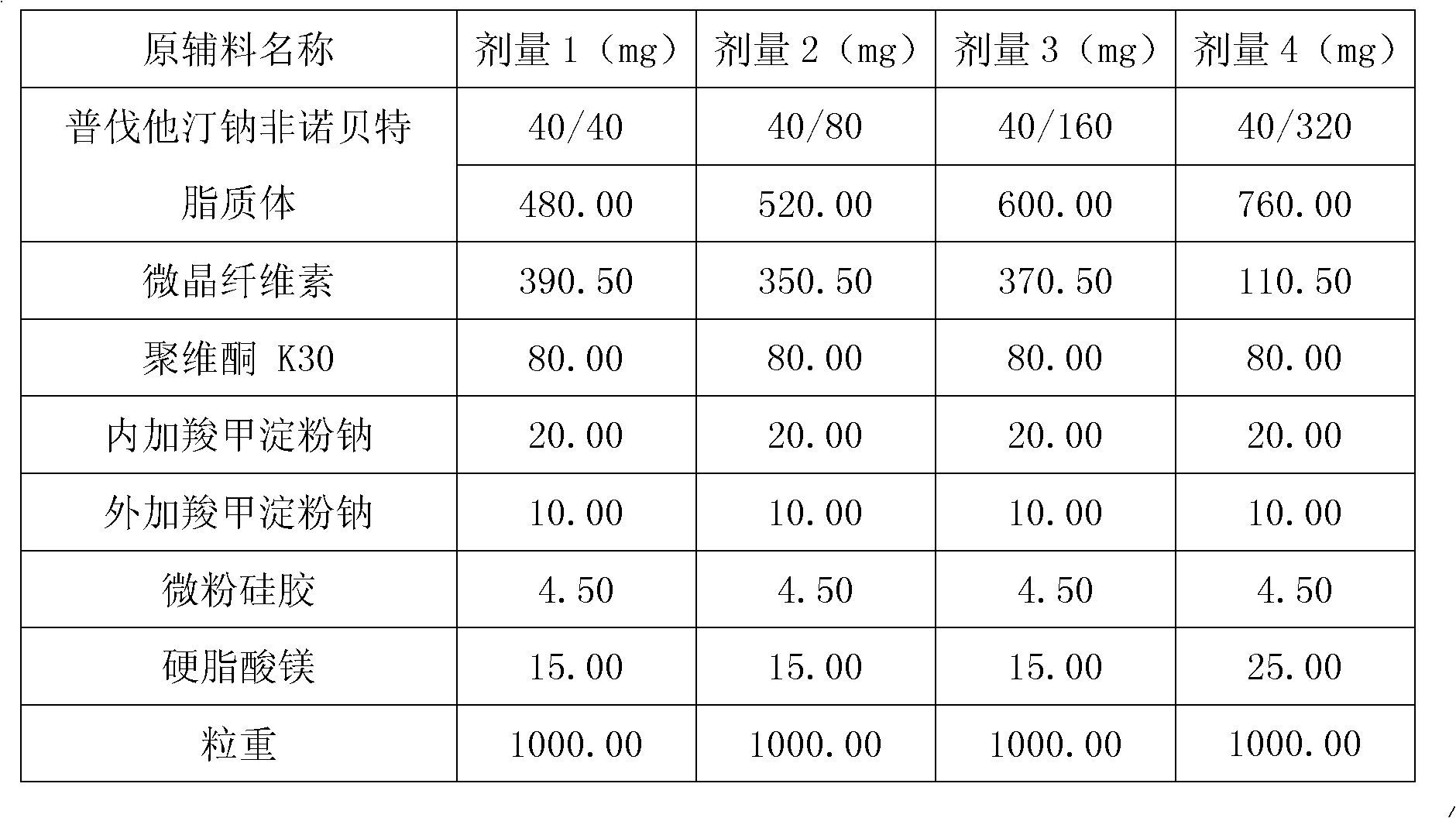Patents
Literature
53 results about "Pravastatin Sodium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
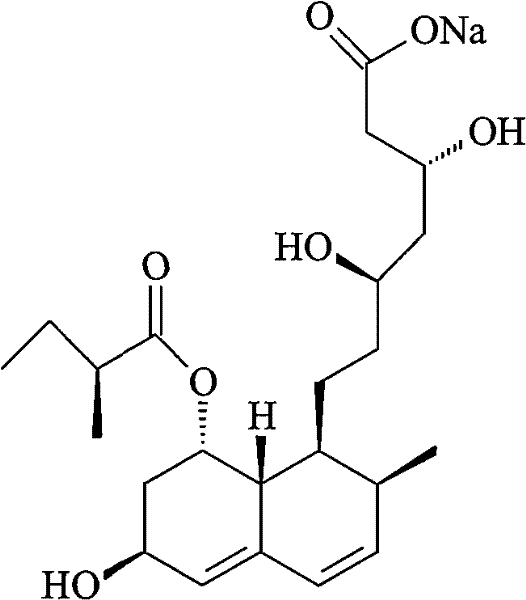
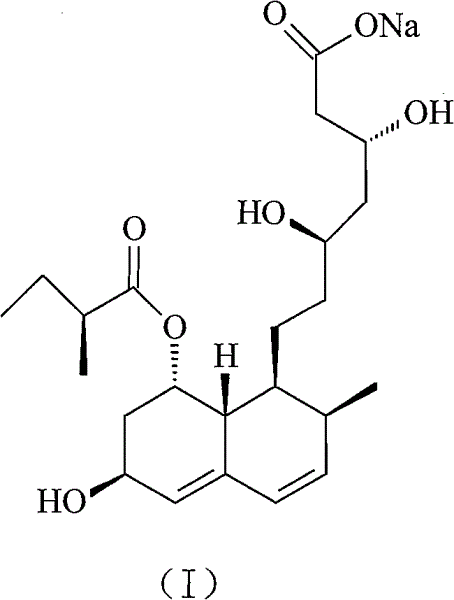
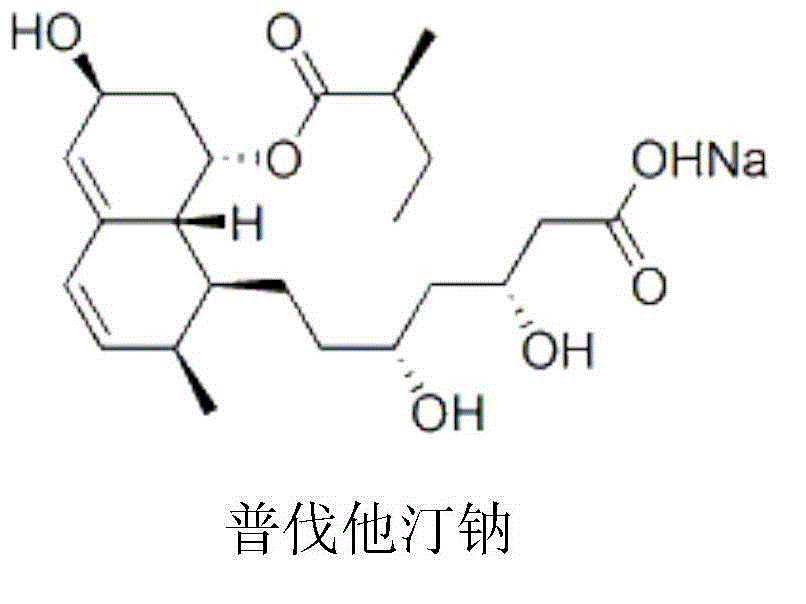
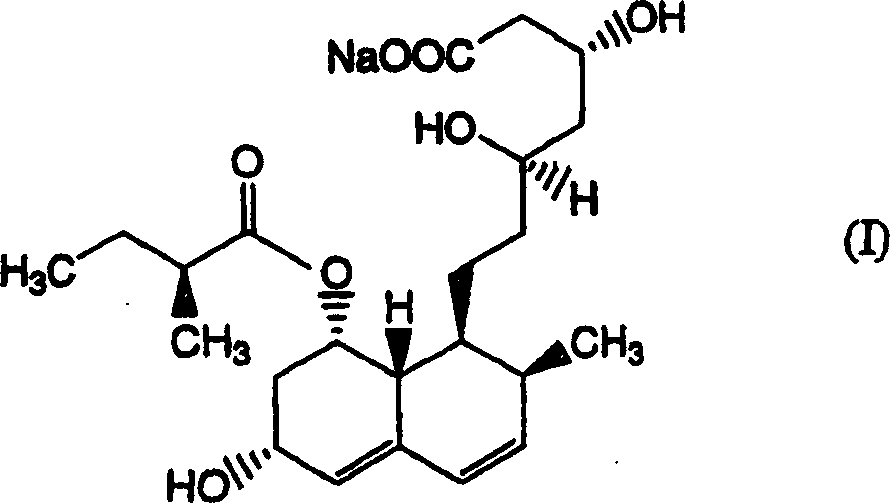
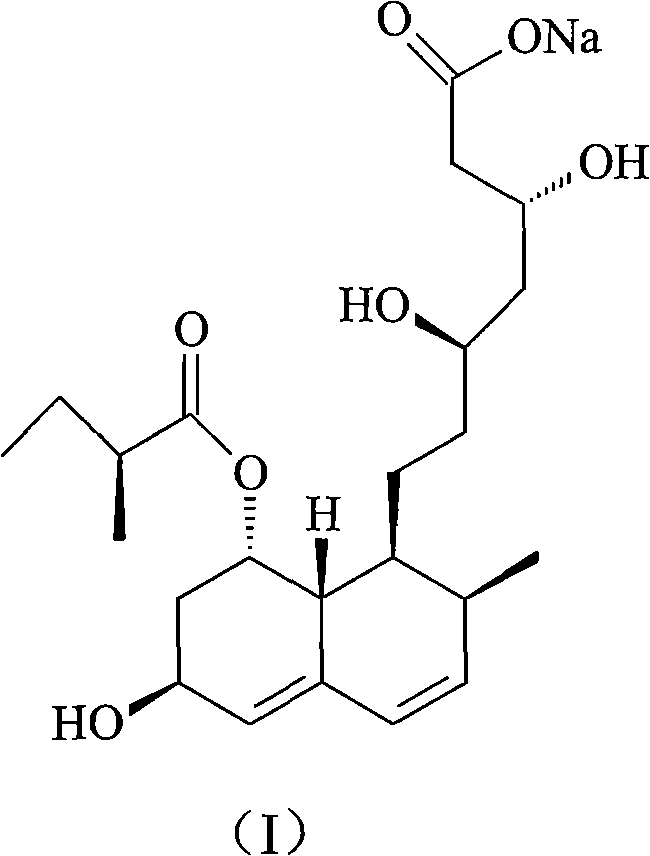
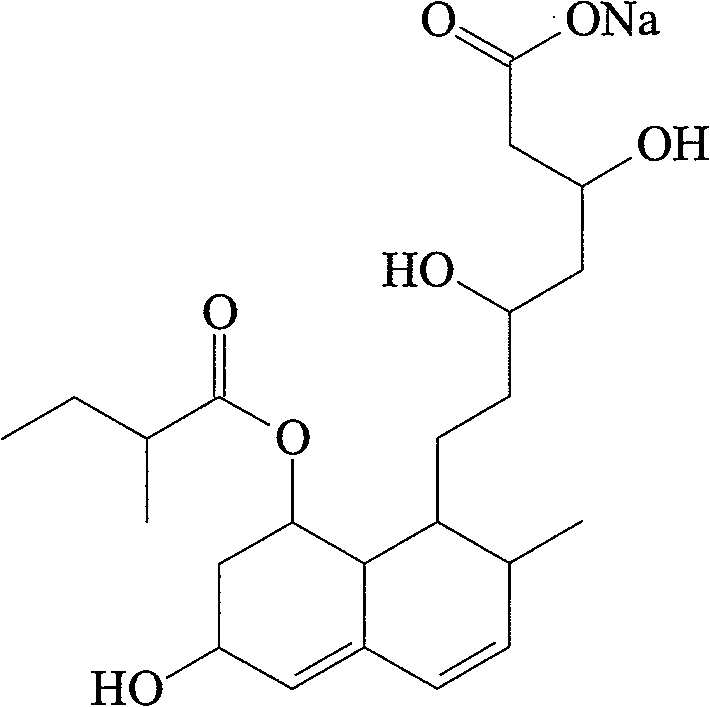
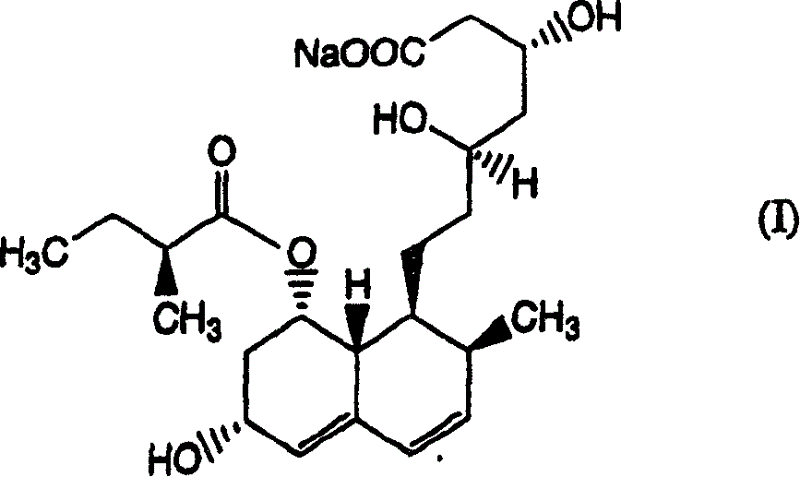
The sodium salt of pravastatin with cholesterol-lowering and potential antineoplastic activities. Pravastatin competitively inhibits hepatic hydroxymethyl-glutaryl coenzyme A (HMG-CoA) reductase, the enzyme which catalyzes the conversion of HMG-CoA to mevalonate, a key step in cholesterol synthesis. This agent lowers plasma cholesterol and lipoprotein levels, and modulates immune responses by suppressing MHC II (major histocompatibility complex II) on interferon gamma-stimulated, antigen-presenting cells such as human vascular endothelial cells. In addition, pravastatin, like other statins, exhibits pro-apoptotic, growth inhibitory, and pro-differentiation activities in a variety of tumor cells; these antineoplastic activities may be due, in part, to inhibition of the isoprenylation of Ras and Rho GTPases and related signaling cascades.
Solid preparation of sodium aspirin and sodium pravastatin medicinal composition
InactiveCN102204920AGood effectOvercome deficienciesPill deliveryEster active ingredientsAspirinLiposome
Owner:HAINAN YONGTIAN PHARMA INST
Pravastatin preparation formula
InactiveCN1507859AImprove stabilityDoes not affect body functionOrganic active ingredientsCardiovascular disorderEmulsionActive component
The present relates to a pravastatin preparation formula for curing angiocardiopathy. It is characterized by that said formula contains medicinal active component pravastatin sodium, at the same time contains one or several kinds of amino acids or amino acid salts as stabilizing agent, and the pH value of pravastatin preparation aqueous emulsion is 6.0-8.9.
Owner:SHANGHAI TECHWELL BIOPHARMACEUTICALS CO LTD
Purification method of pravastiatin sodium
InactiveCN1517331AHigh purityOrganic compound preparationCarboxylic acid esters preparationPurification methodsAlcohol
A process for purifying pravastiation sodium includes such steps as making the fermented liquid containing pravastatin sodium in contact with adsorptive resin, separating them from each other, washing the adsorptive resin with water, C1-4 alcohol, C1-4 ketone, or their mixture, making the eluted liquid in contact with the washed adsorptive resin, and collecting the eluted liquid containing the purified pravastation sodium. Its advantages are high purity of product and less environmental pollution.
Owner:SHANGHAI TECHWELL BIOPHARMACEUTICALS CO LTD
Novel Pseudonocardia sp. RMRC PAH4 and a process for bioconverting compactin into pravastatin using the same
The invention provides a novel microorganism Pseudonocardia sp. RMRC PAH4 characterized in that it is able to degrade high concentration of quinoline by enrichment culture, shows a high tolerance to compactin-sodium and possesses a high hydroxylation activity of converting compactin-sodium to pravastatin-sodium. The invention relates also a process for converting compactin-sodium into pravastatin-sodium by fermenting said novel microorganism Pseudonocardia sp. RMRC PAH4. Pravastain-sodium is a potent cholesterol-lowering agent used in treatment for hypercholesterolemia.
Owner:CHINESE GASOLINEEUM
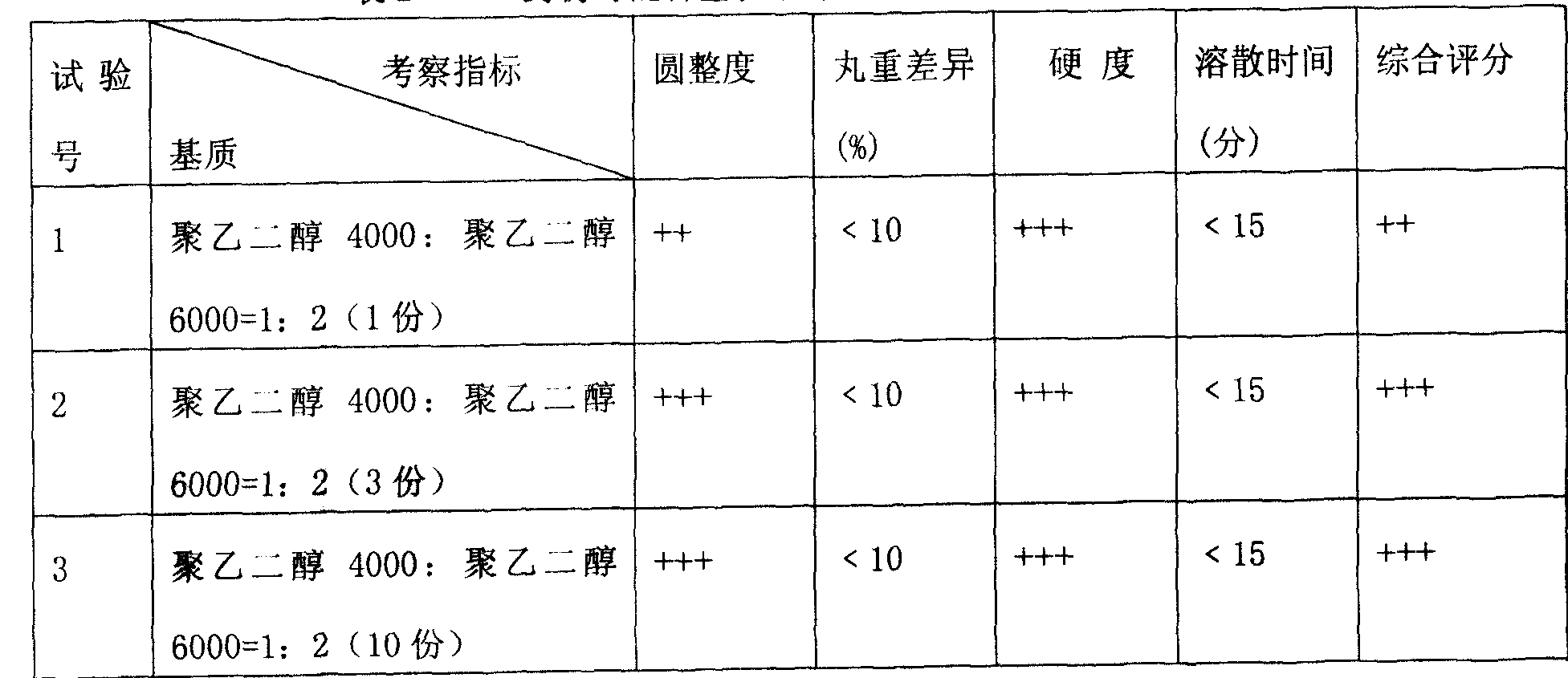
Pravastatin sodium pharmaceutical co-crystal and preparation method and application thereof
ActiveCN105175263AIncrease spawn rateEasy to moveUrea derivatives preparationAmino compound purification/separationMoisture absorptionLysine
The invention belongs to the technical field of organic pharmaceutical co-crystals, and particularly relates to a pravastatin sodium pharmaceutical co-crystal and a preparation method and application thereof. The pravastatin sodium pharmaceutical co-crystal comprises co-crystal formations and active components pravastatin sodium. The co-crystal formations are amino acid, urea, adamantine derivatives or other small molecule compounds. The result shows that before and after the co-crystal is formed, the moisture absorption performance of medicine is remarkably changed, for example, the co-crystal hygroscopicity of lysine and urea is increased, and a co-crystal formed by amantadine hydrochloride basically does not absorb moisture at the humidity of 70% or lower.
Owner:NAT INST FOR FOOD & DRUG CONTROL
Method for preparing vascular tissue engineering stent material carried with pravastatin sodium
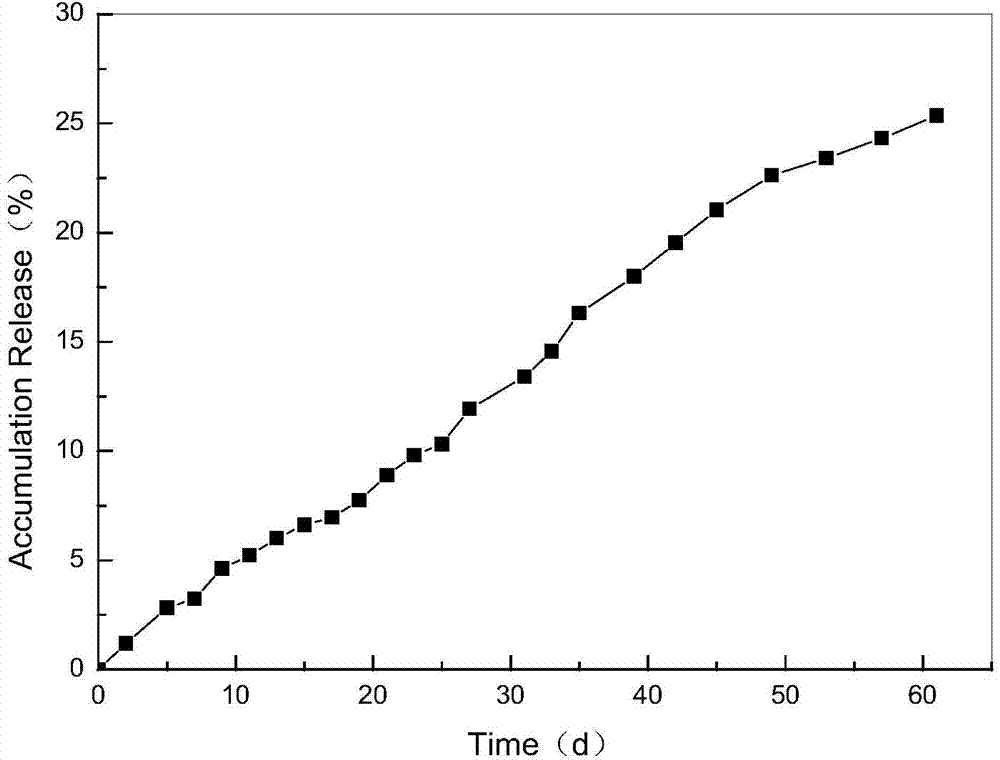
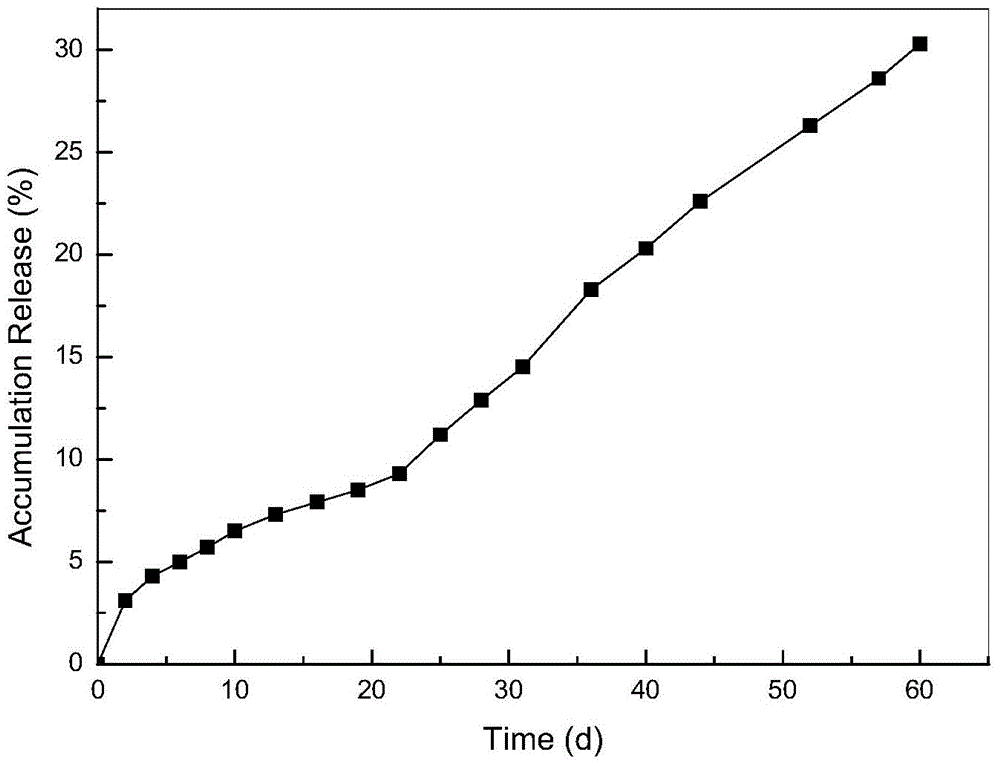
The invention relates to a method for preparing a vascular tissue engineering stent material carried with pravastatin sodium. The method consists of the following steps: (1) preparing chitosan solution and gelatin salutation; (2) preparing cross-linking agent solution; (3) mixing the chitosan solution, the gelatin salutation and the cross-linking agent solution, then adding chitosan microspheres entrapping pravastatin sodium, performing uniform mixing, pouring the mixture into a vascular stent mold, performing freeze-drying and then performing demolding; (4) repetitively washing the vascular stent after demolding and then performing freeze-drying. The vascular tissue engineering stent material carried with pravastatin sodium prepared by adopting the method has a pore diameter of 10-600mu m, the chitosan microspheres entrapping pravastatin sodium are well dispersed in the stent and the release time reaches more than 60 days.
Owner:SOUTHERN MEDICAL UNIVERSITY
Pravastatin sodium compound and novel preparation method thereof
InactiveCN102070447BHigh yieldHigh purityOrganic compound preparationCarboxylic acid esters preparationSodium bicarbonateSide effect
The invention provides a pravastatin sodium compound and a novel preparation method thereof. The method comprises the following steps of: (1) performing absorption and ion exchange on pravastatin sodium by using strongly acidic ion exchange resin; (2) eluting by using sodium carbonate or sodium bicarbonate aqueous solution at the concentration of 0.1 to 2 percent; and (3) separating and purifyingthe elution mother liquor by using a chromatographic column, collecting the eluted solution, washing by using water, drying by using a solid drying agent, concentrating under reduced pressure, evaporating to remove the eluted solution and obtaining a pravastatin sodium pure product. According to the high-purity pravastatin sodium compound prepared by the refining method, the purity and the content of the pravastatin sodium are increased greatly, the product quality of the preparation is improved, the toxic and side effects are reduced and the clinic medication safety is ensured; the method issimple in technology, low in cost and high in yield rate and is suitable for industrial production.
Owner:HAINAN MEIDA PHARMA
Freeze-dried powder of pravastatin sodium composition for injection
InactiveCN103599077AImprove solubilityShorten the dissolution timePowder deliveryMetabolism disorderSolubilityChitosan nanoparticles
The invention provides a freeze-dried powder of a pravastatin sodium composition for injection, and relates to the technical field of medicines and medicine production. The freeze-dried powder comprises the following raw materials by weight: 1 part of pravastatin sodium, 0.2-1.4 parts of chitosan nanoparticles and 100-200 parts of water for injection. The freeze-dried powder has the advantages that (1) chitosan, as a cosolvent, can increase solubility of pravastatin sodium in water and shorten dissolution time, and is helpful for clinical application; (2) the composition can significantly enhance a lipid-decreasing of pravastatin sodium, reduce usage amount of pravastatin sodium clinically and alleviate adverse effects of pravastatin sodium; in-vitro experiments demonstrate that the lipid-decreasing capacity of 10 mg of the freeze-dried powder of pravastatin sodium composition comprising the chitosan nanoparticles is equivalent to that of 20 mg of pravastatin sodium with no chitosan nanoparticles; and (3) chitosan nanoparticles can replace mannitol to be used as a freeze-drying skeleton agent, so that active effects of mannitol to a human body can be eliminated.
Owner:HAINAN WEI KANG PHARMA QIANSHAN
Method of purifying plavastatin
InactiveCN1690037AHigh purityMetabolism disorderOrganic compound preparationOrganic solventPurification methods
A process for the isolation or purification of pravastatin or a pharmacologically acceptable salt thereof comprising decomposing impurities using an inorganic acid, optionally in combination with an inorganic base.
Owner:SANKYO CO LTD
Pravastatin sodium compound and novel preparation method thereof
InactiveCN102070447AHigh yieldHigh purityOrganic compound preparationCarboxylic acid esters preparationSodium bicarbonateSide effect
The invention provides a pravastatin sodium compound and a novel preparation method thereof. The method comprises the following steps of: (1) performing absorption and ion exchange on pravastatin sodium by using strongly acidic ion exchange resin; (2) eluting by using sodium carbonate or sodium bicarbonate aqueous solution at the concentration of 0.1 to 2 percent; and (3) separating and purifying the elution mother liquor by using a chromatographic column, collecting the eluted solution, washing by using water, drying by using a solid drying agent, concentrating under reduced pressure, evaporating to remove the eluted solution and obtaining a pravastatin sodium pure product. According to the high-purity pravastatin sodium compound prepared by the refining method, the purity and the content of the pravastatin sodium are increased greatly, the product quality of the preparation is improved, the toxic and side effects are reduced and the clinic medication safety is ensured; the method is simple in technology, low in cost and high in yield rate and is suitable for industrial production.
Owner:HAINAN MEIDA PHARMA
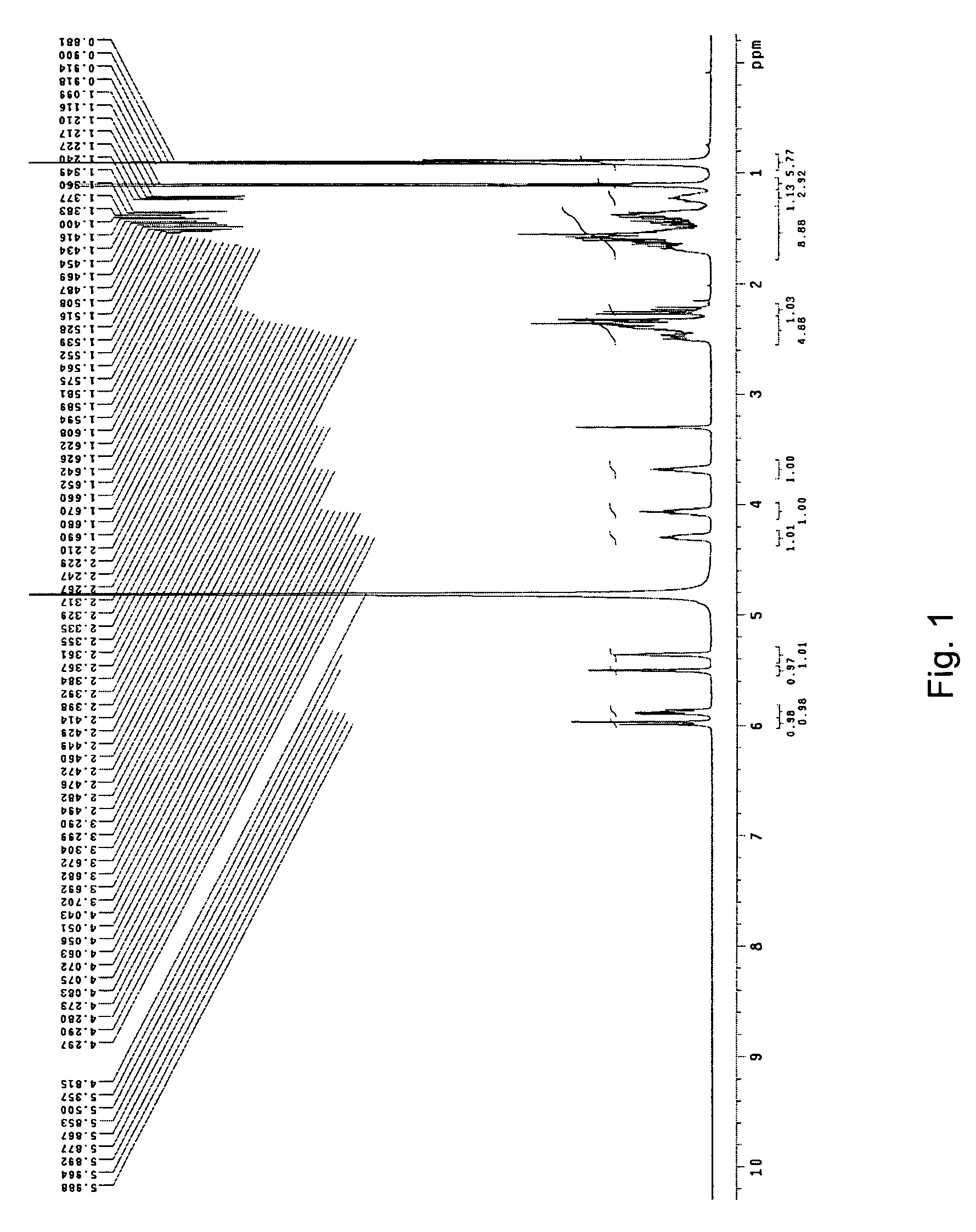
Methods of making pravastatin sodium
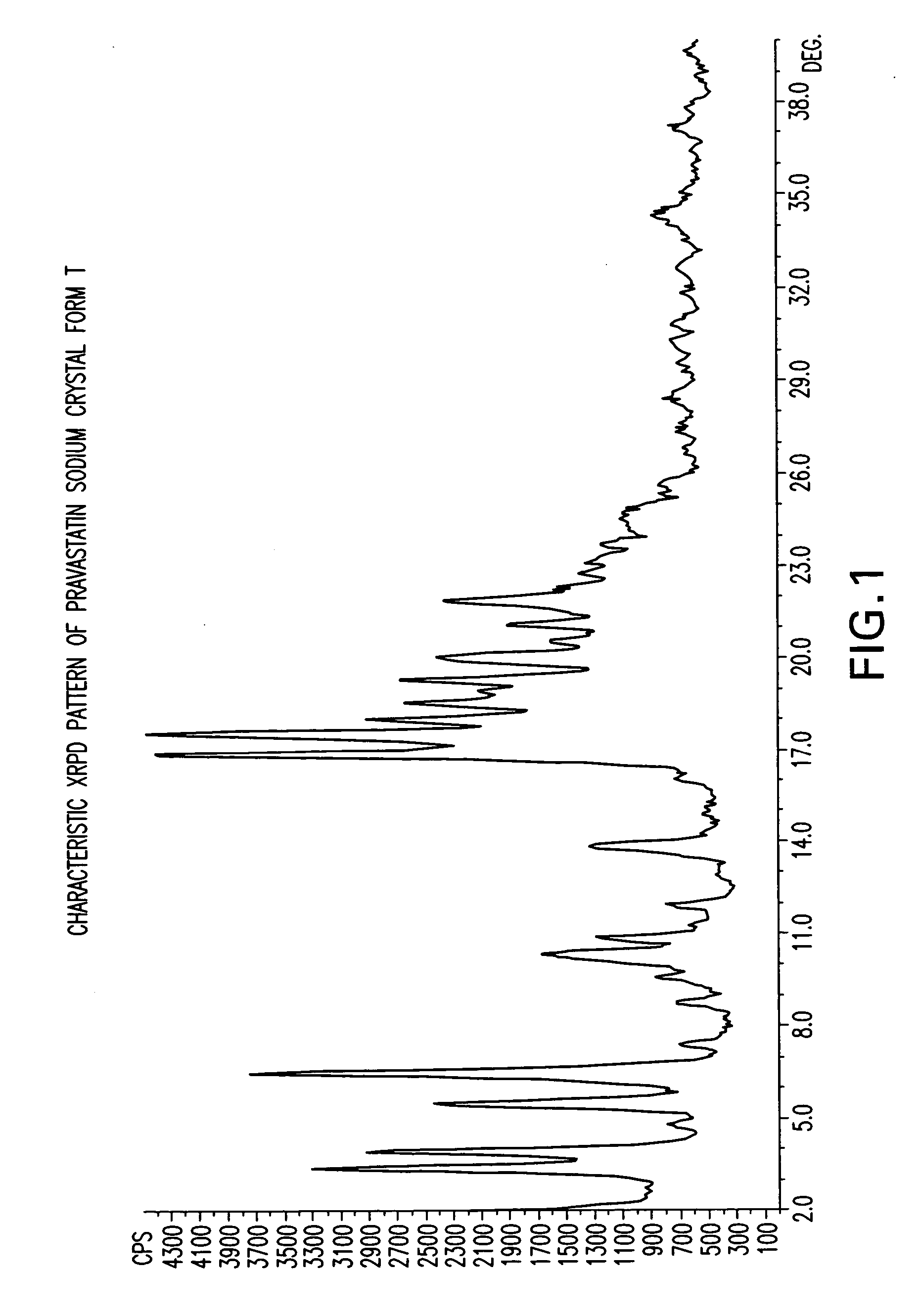
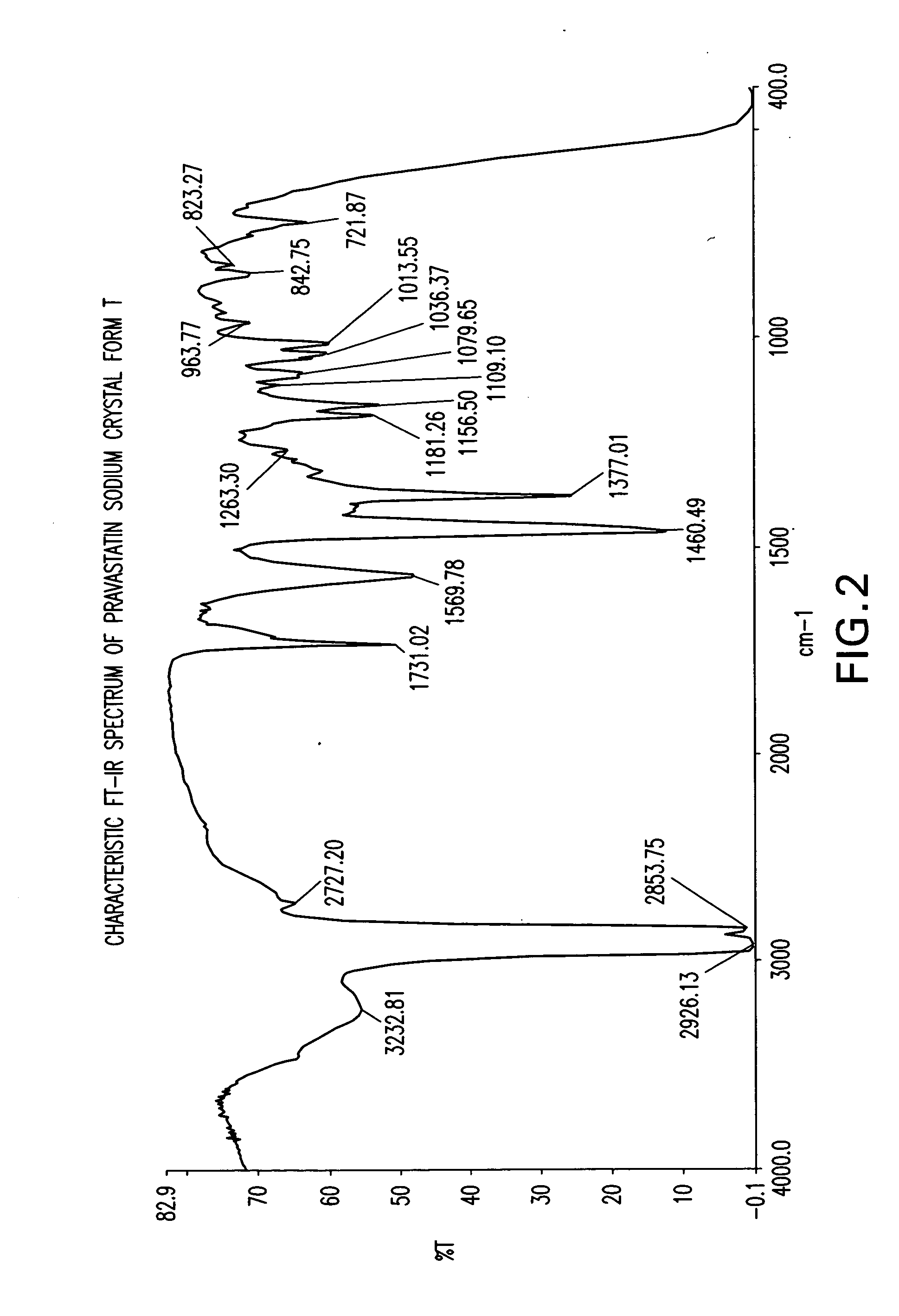
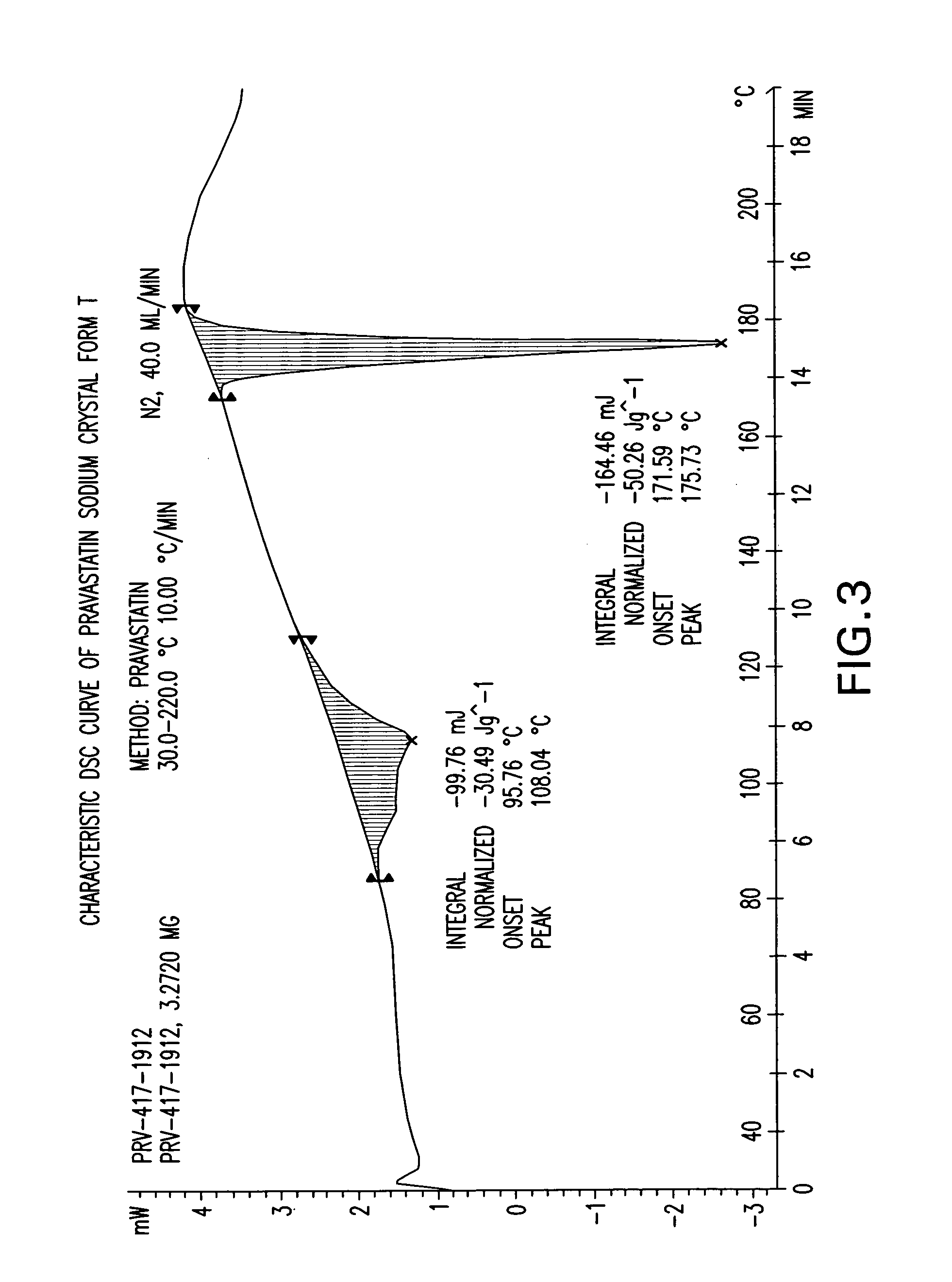
The invention encompasses a new crystalline form of pravastatin sodium characterized by X-ray powder diffraction peaks at 3.3, 3.9, 5.4, 6.4, 16.8, and 17.5 degrees two-theta, ±0.1 degrees two-theta and to methods of forming the crystalline form of the present invention and methods of making pravastatin Form B and Form D.
Owner:TEVE PHARMA USA INC
Method for extracting and purifying pravastatin sodium from fermentation liquor
ActiveCN109796333AReduce typesAvoid the risk of cross-contaminationCarboxylic acid esters separation/purificationFermentationImpurity
The invention provides a method for extracting and purifying pravastatin sodium from a fermentation liquor, and belongs to the field of biological medicine preparation. According to the method for extracting and purifying pravastatin sodium from the fermentation liquor, a large amount of impurities and pigments are removed by alkalizing and acidifying the fermentation liquor, and the effective components in an acidizing liquid are enriched by an adsorbent, so that the purity of an extraction substrate is improved, the quantity of the extraction substrate is greatly reduced, and the quality ofan extraction liquid is further improved; in the whole production process, a single solvent system is adopted, the process is not complicated, the generated three wastes are low in amount, and the method is suitable for large-scale production. The content of pravastatin sodium produced by the method is more than 98.8 percent, the content of main impurity 6-epipravastatin is less than 0.15%, the content of total related substances is less than 0.4%, the product quality accords with the EP9.6 edition quality standard (the content of the pravastatin sodium is between 97.0% and 102.0%; a main impurity A, namely6-epipravastatin, is less than or equal to 0.3%; the content of the total related substances is less than or equal to 0.6%) of qualified pravastatin sodium, the total yield can reach more than 75%, and the large-scale production prospect is good.
Owner:NEW FOUNDER HLDG DEV LLC +2
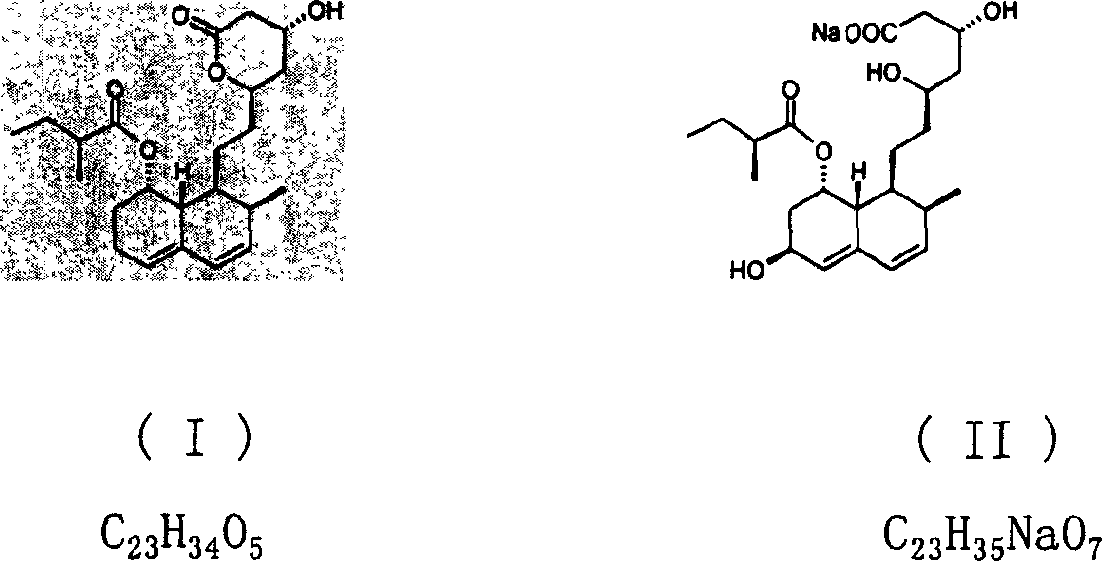
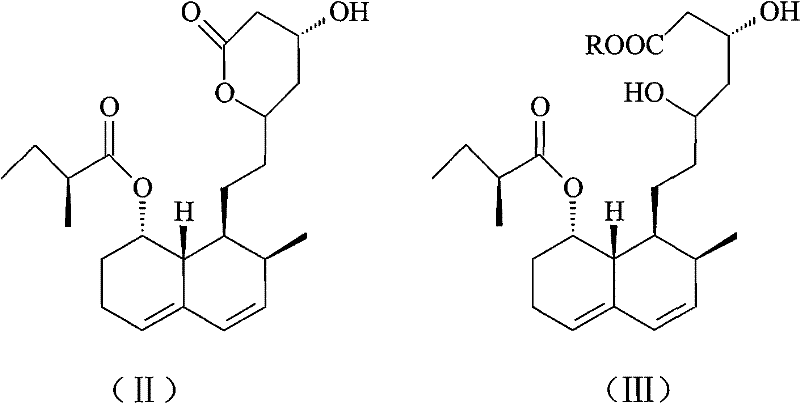
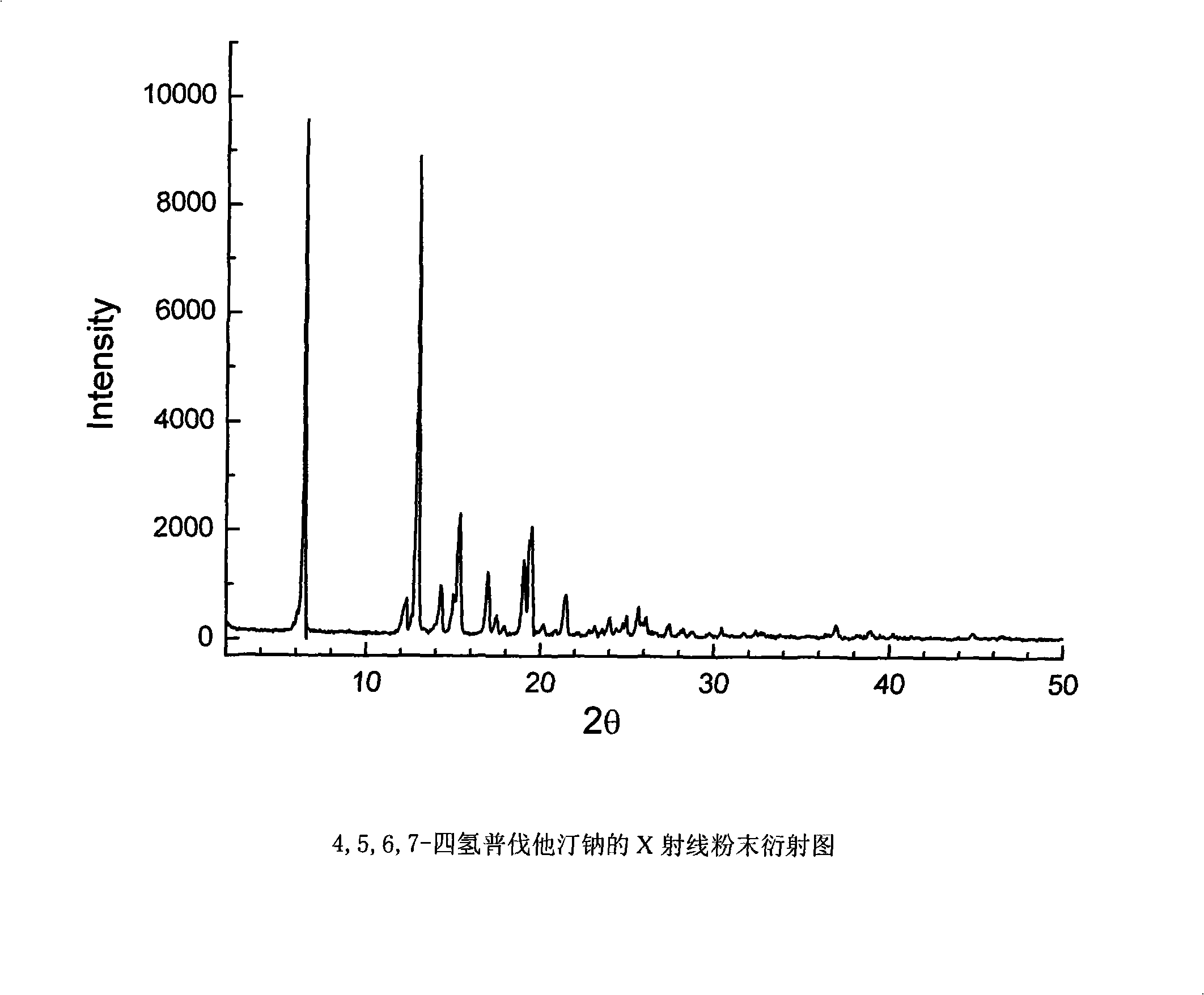
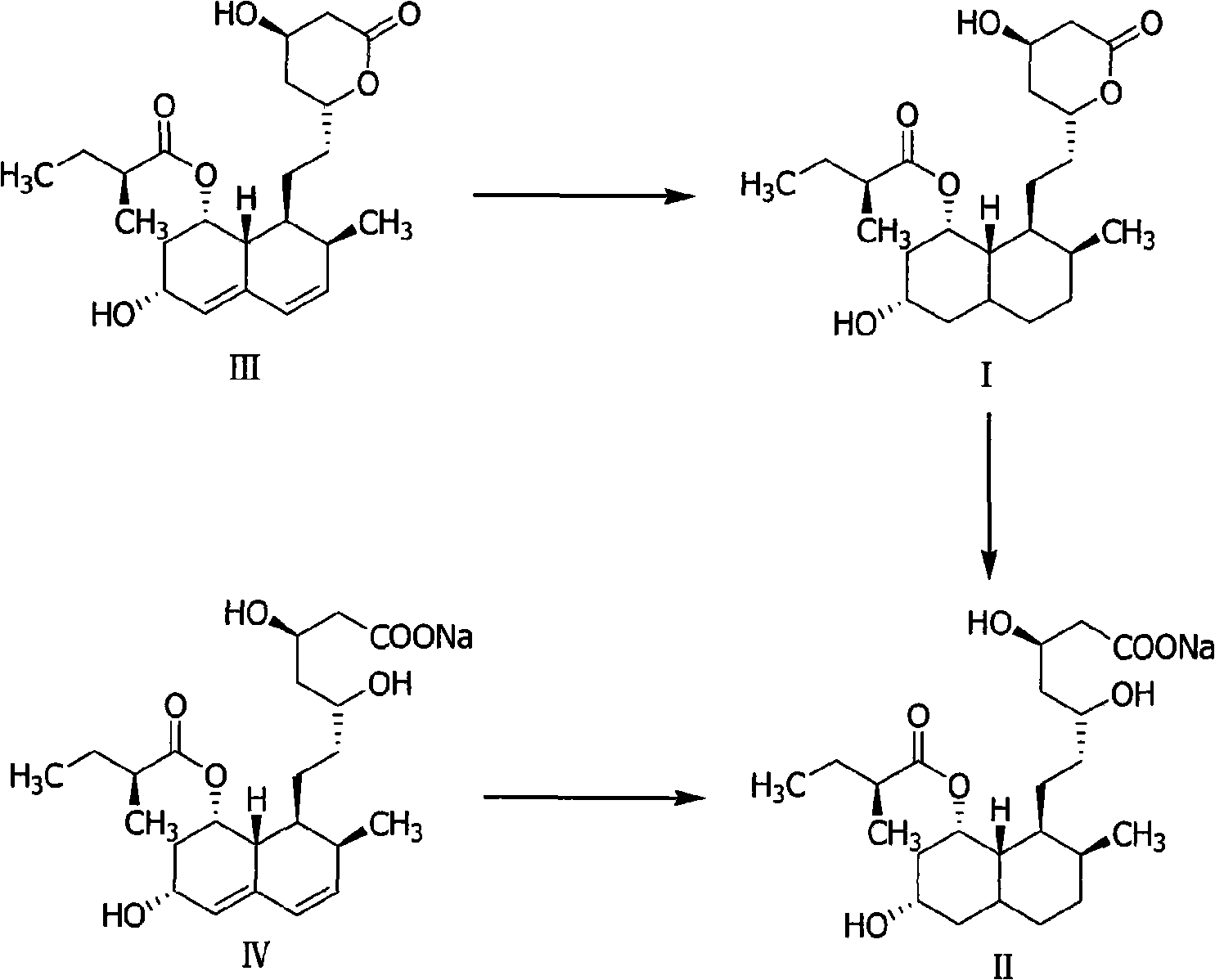
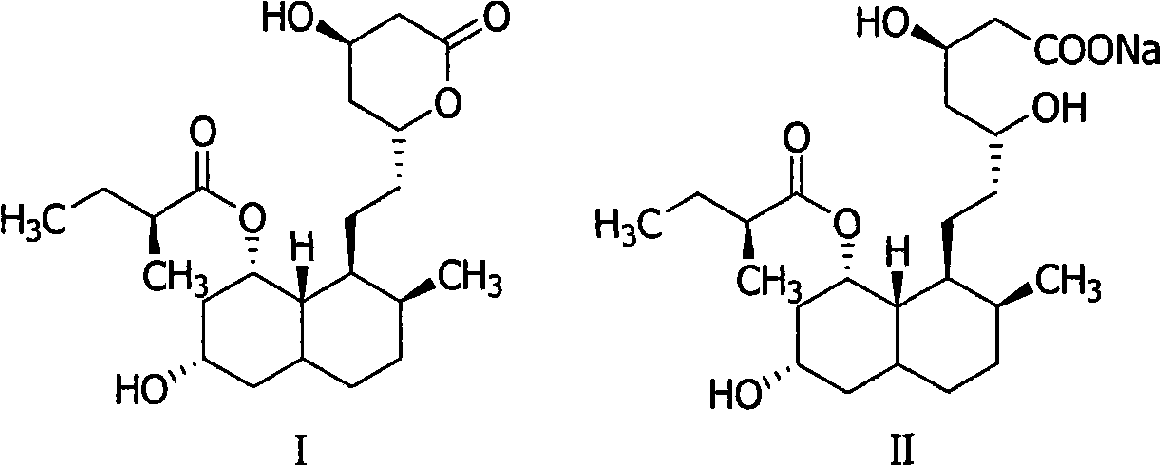
Method for preparing 4,5,6,7-tetrahydromevastatin and sodium salt thereof, and solid crystallization way
ActiveCN101348476AImprove responseShort reaction timeOrganic compound preparationCarboxylic acid esters preparationState of artCarbon nanotube
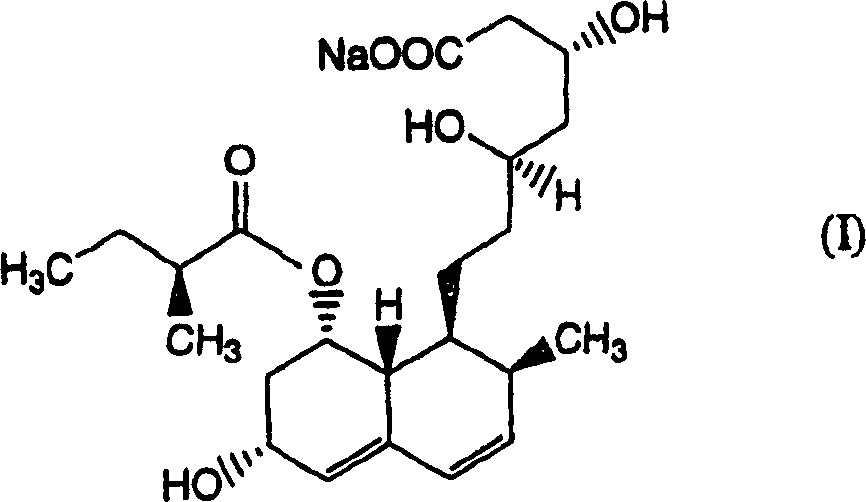
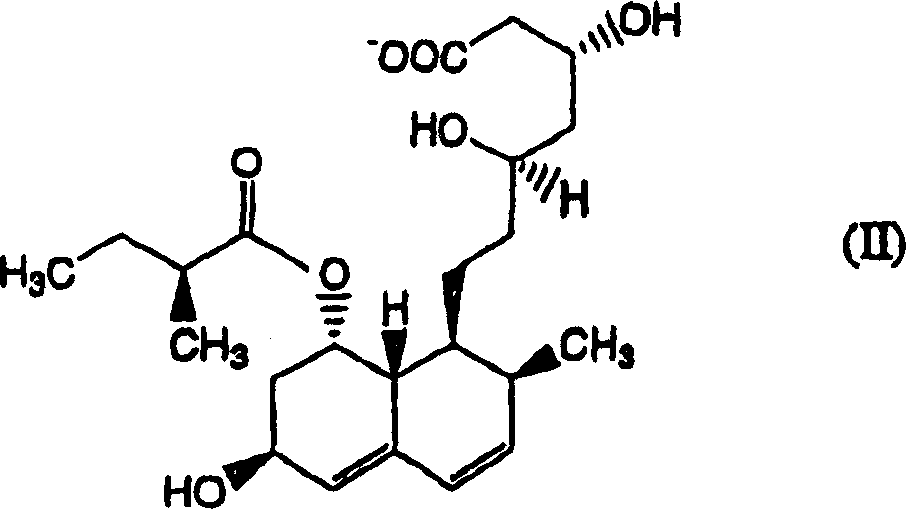
The invention relates to a novel method with easy operation for making 4,5,6,7-tetrahydro pravastatin (I) and the sodium salt (II) thereof. The method comprises a one-step deoxidization with pravastatin and sodium pravastatin as starting raw materials respectively to produce 4,5,6,7-tetrahydro pravastatin (I) and 4,5,6,7-tetrahydro pravastatin sodium, and particularly adopts a novel homemade Pd-nanometer tube, thereby overcoming the disadvantages in the prior art including long synthesis procedures, complicated reaction, low total yield and long hydrogenation period, dramatically lowering the production cost, and being greatly suitable for industrialized production. The invention also relates to the method for preparing the solid crystal of 4,5,6,7-tetrahydro pravastatin (I) and the sodium salt (II) thereof.
Owner:LIVZON NEW NORTH RIVER PHARMA +1
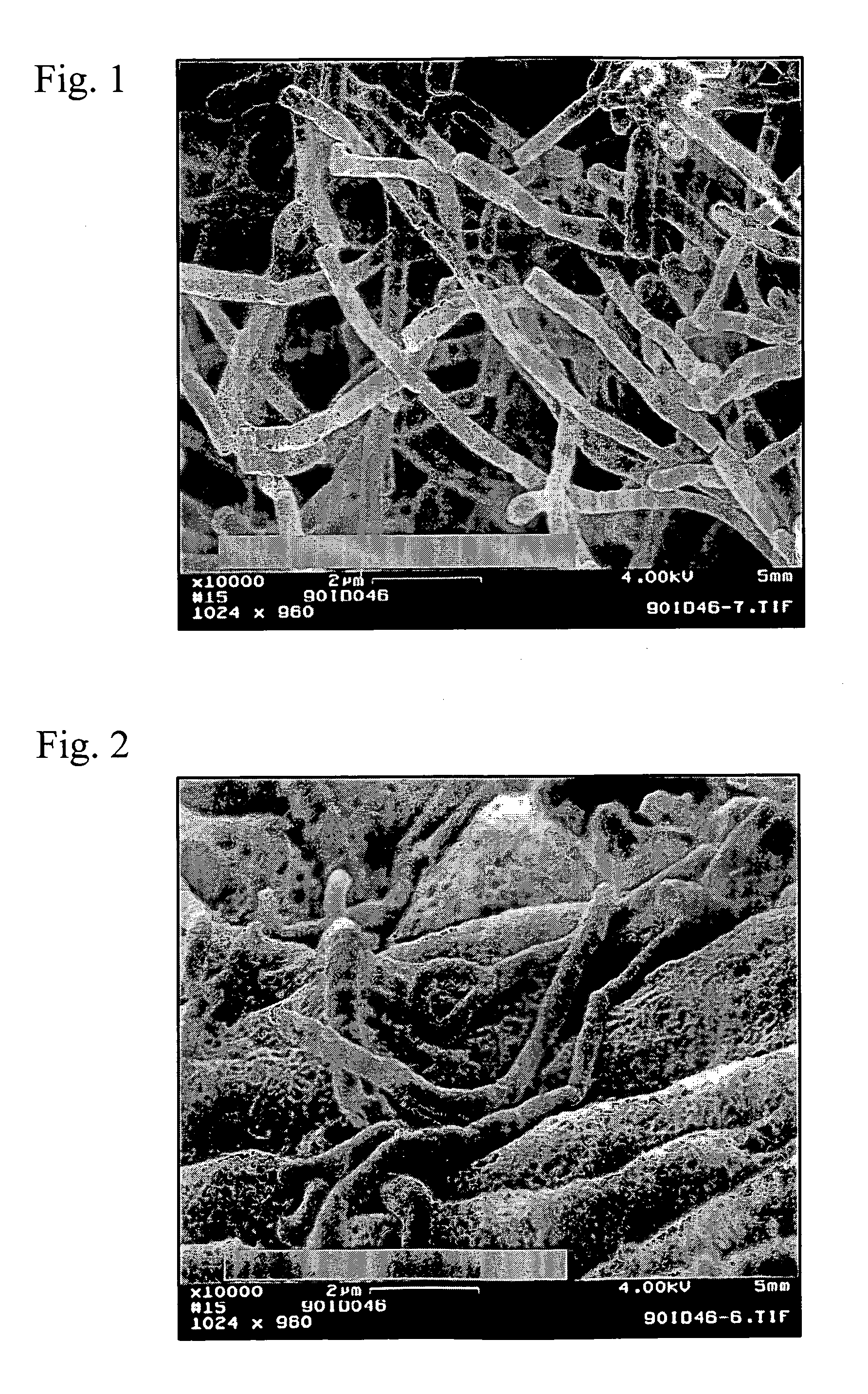
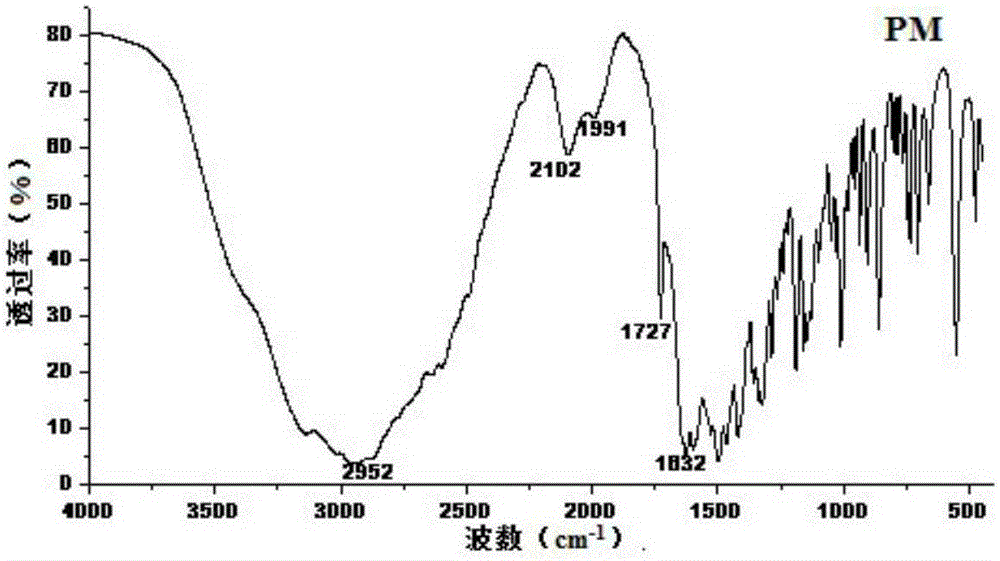
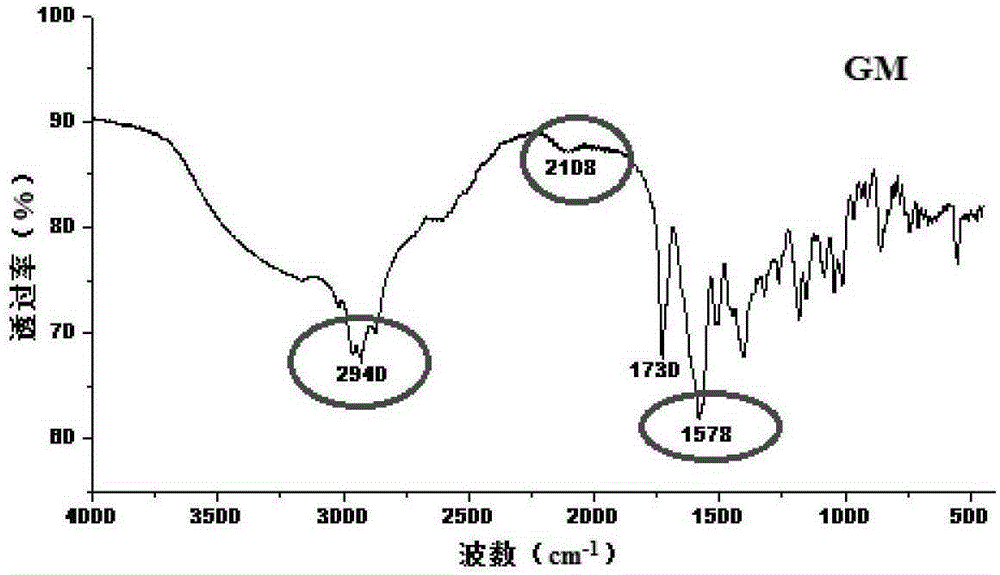
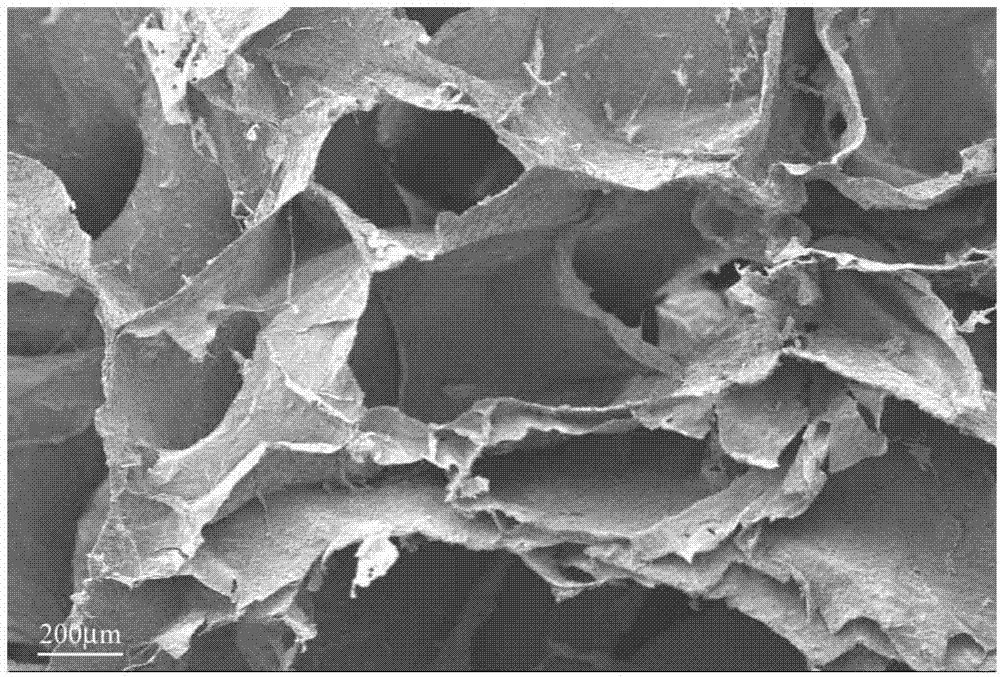
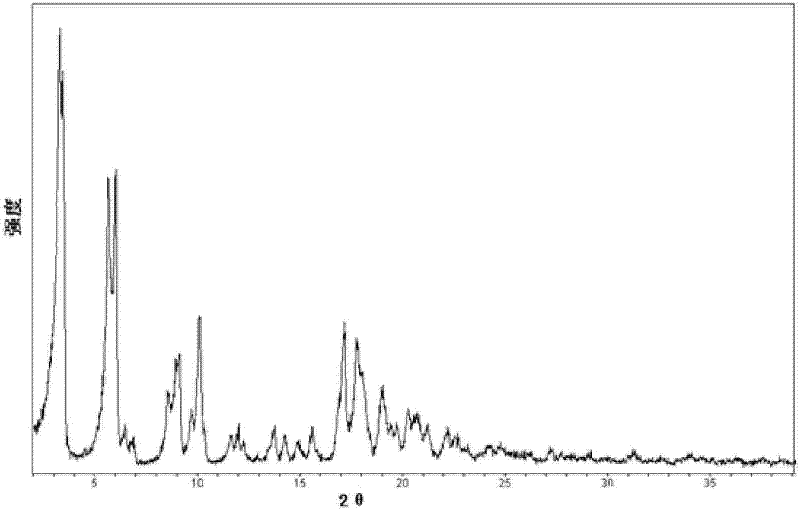
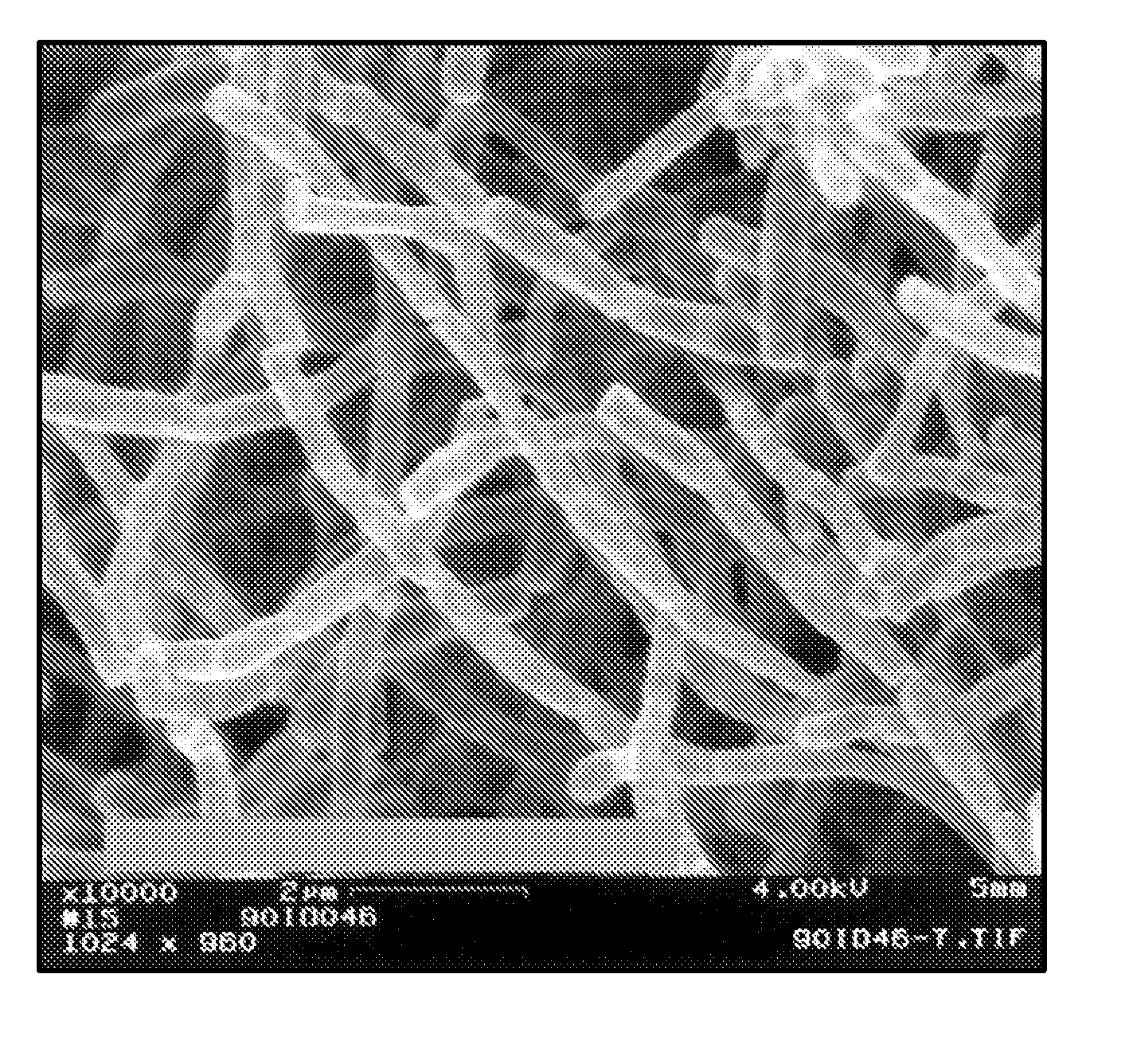
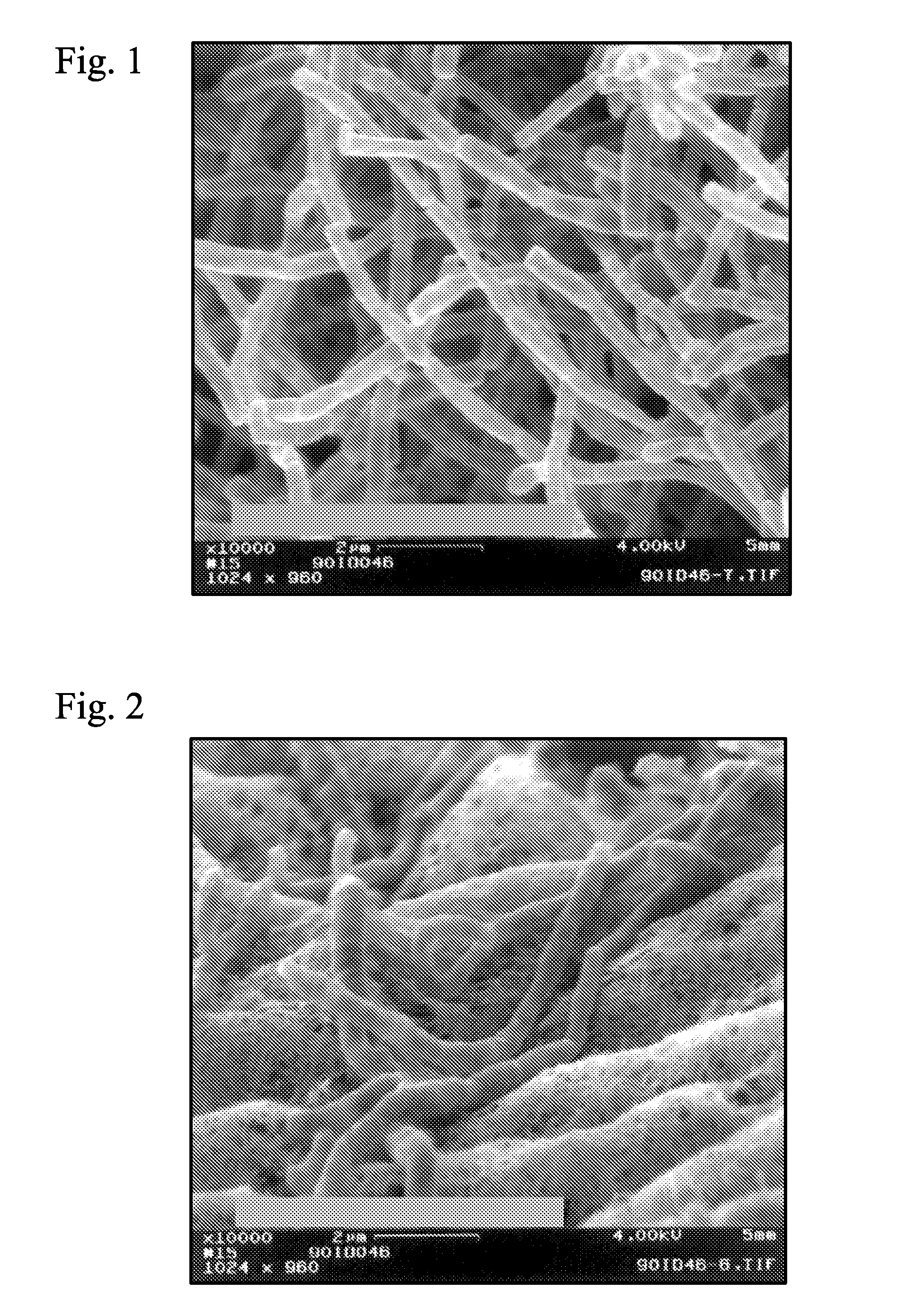
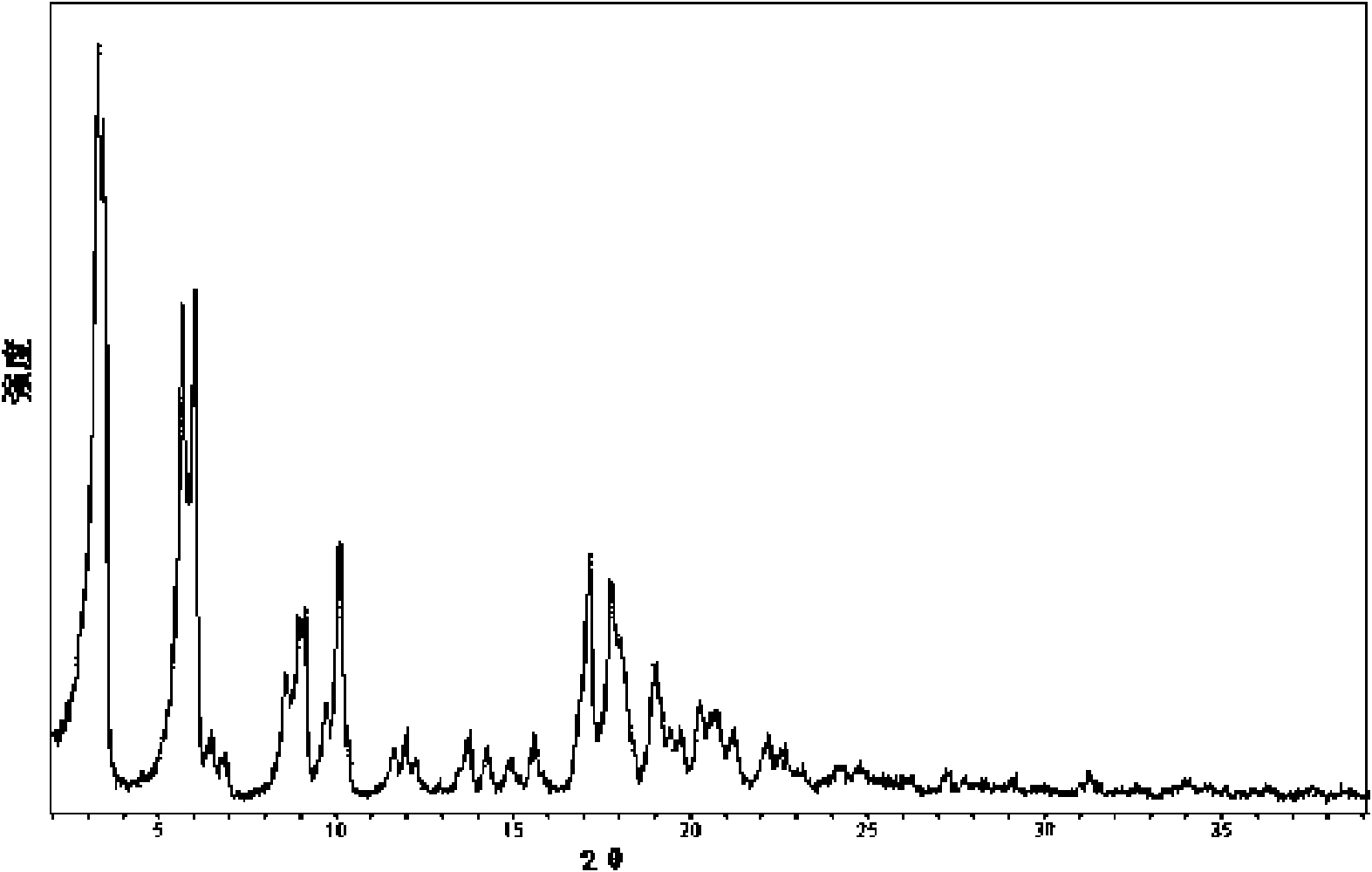
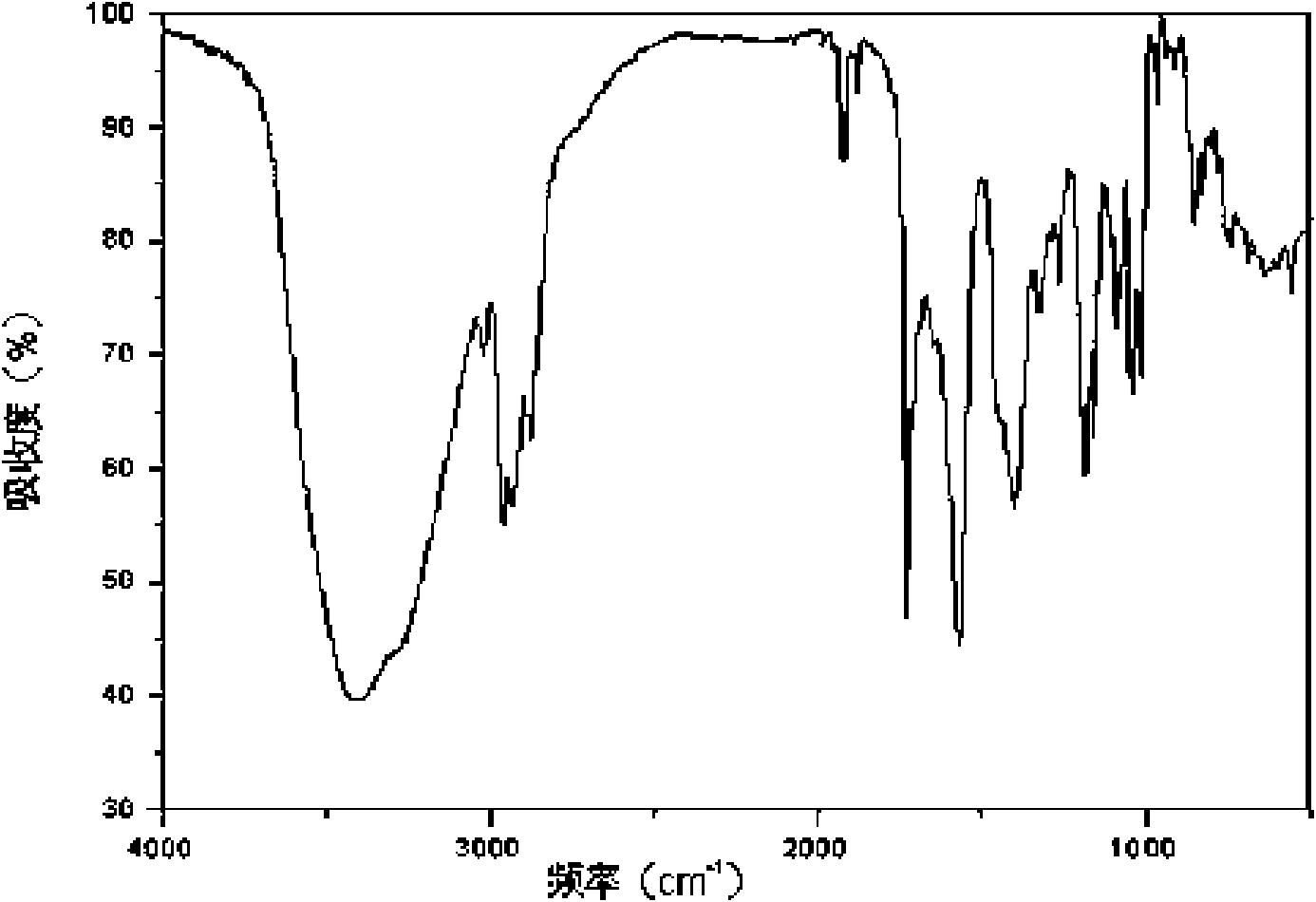
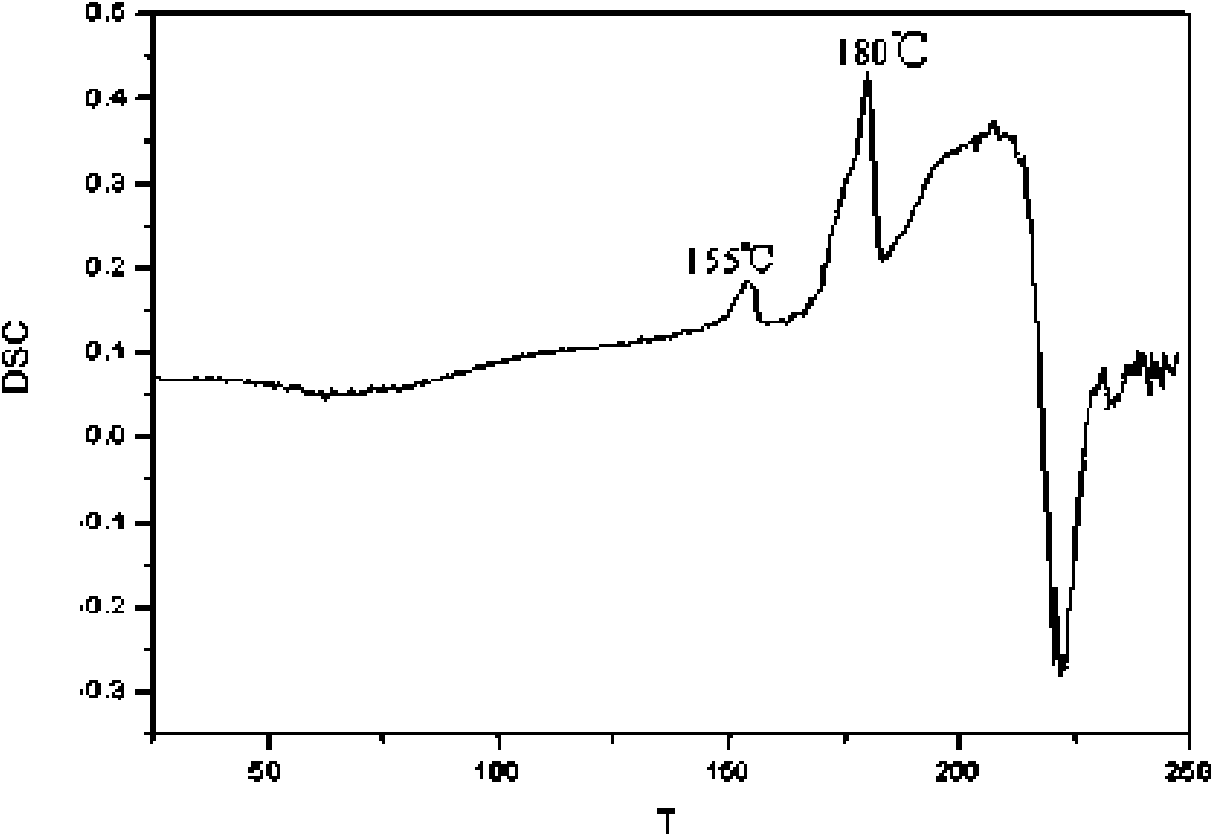
Crystal form of Pravastatin Na, and preparation method and application thereof
InactiveCN101648867BLarge granularityReduce filter timeMetabolism disorderCarboxylic acid esters separation/purificationScanning electron microscopeCrystallinity
Owner:TIANJIN UNIV +1
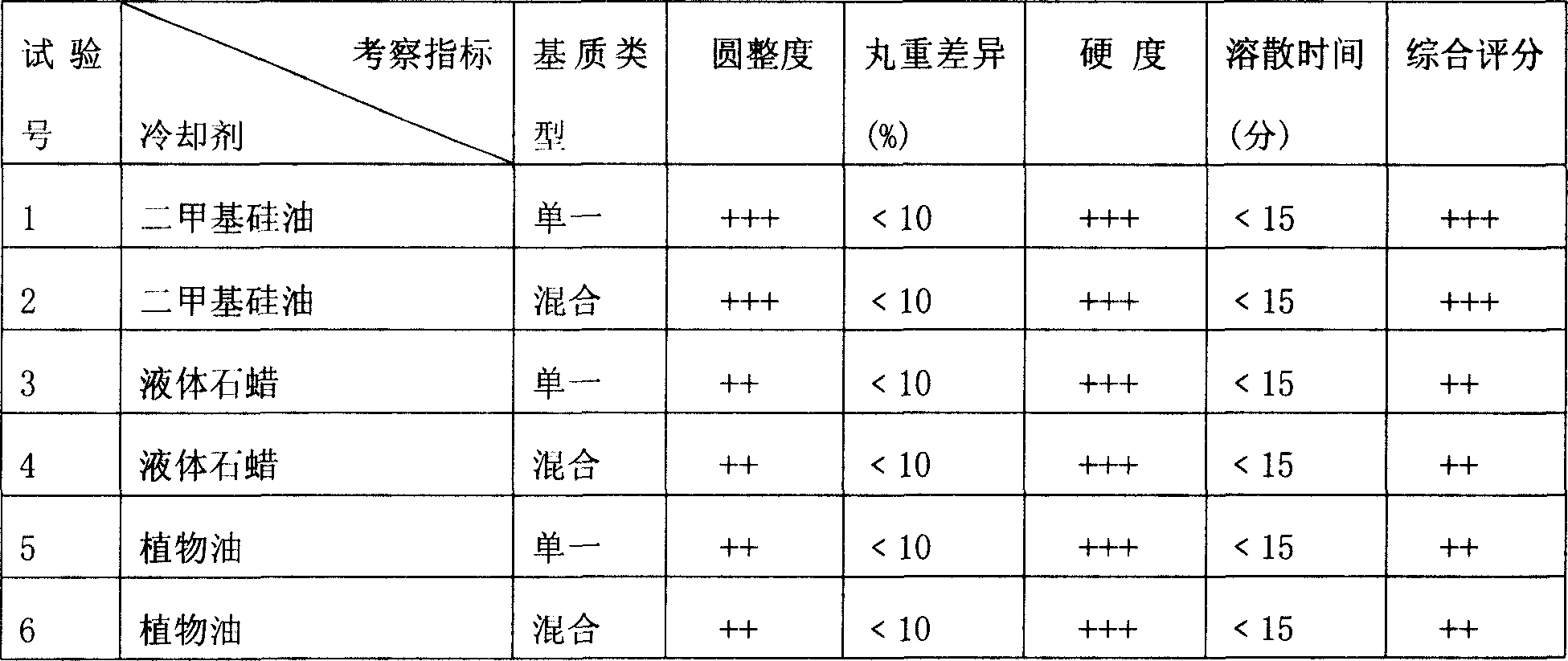
Composition containing statins and application thereof
ActiveCN102416015AHighlight substantiveSignificant progressDigestive systemEster active ingredientsLovastatinAdditive ingredient
The invention relates to a composition containing statins and application thereof. The active ingredient of the composition is composed of statins and rimonabant, wherein the statins comprise atorvastatin calcium, simvastatin, pravastatin sodium and rosuvastatin calcium. The pharmaceutical composition provided by the invention can effectively prevent or treat fatty liver.
Owner:南通金羽禽业发展有限公司
Pravastatin sodium liposome solid preparation
InactiveCN102133187AAvoid influenceReduce stimulationMetabolism disorderPharmaceutical non-active ingredientsAcetic acidSide effect
The invention discloses a pravastatin sodium liposome preparation. The pravastatin sodium liposome is mainly prepared from 1 weight part of pravastatin sodium, 3 to 10 weight parts of glycerol monoacetate, 1.5 to 6 weight parts of poloxamer 188 and 0.05 to 5 weight parts of sodium deoxycholate. The invention further discloses a pravastatin sodium liposome solid preparation. The solid preparation is prepared from the pravastatin sodium liposome preparation and other pharmaceutically common excipients. The product quality of the preparation is improved and the toxic or side effects are reduced.
Owner:HAINAN MEIDA PHARMA
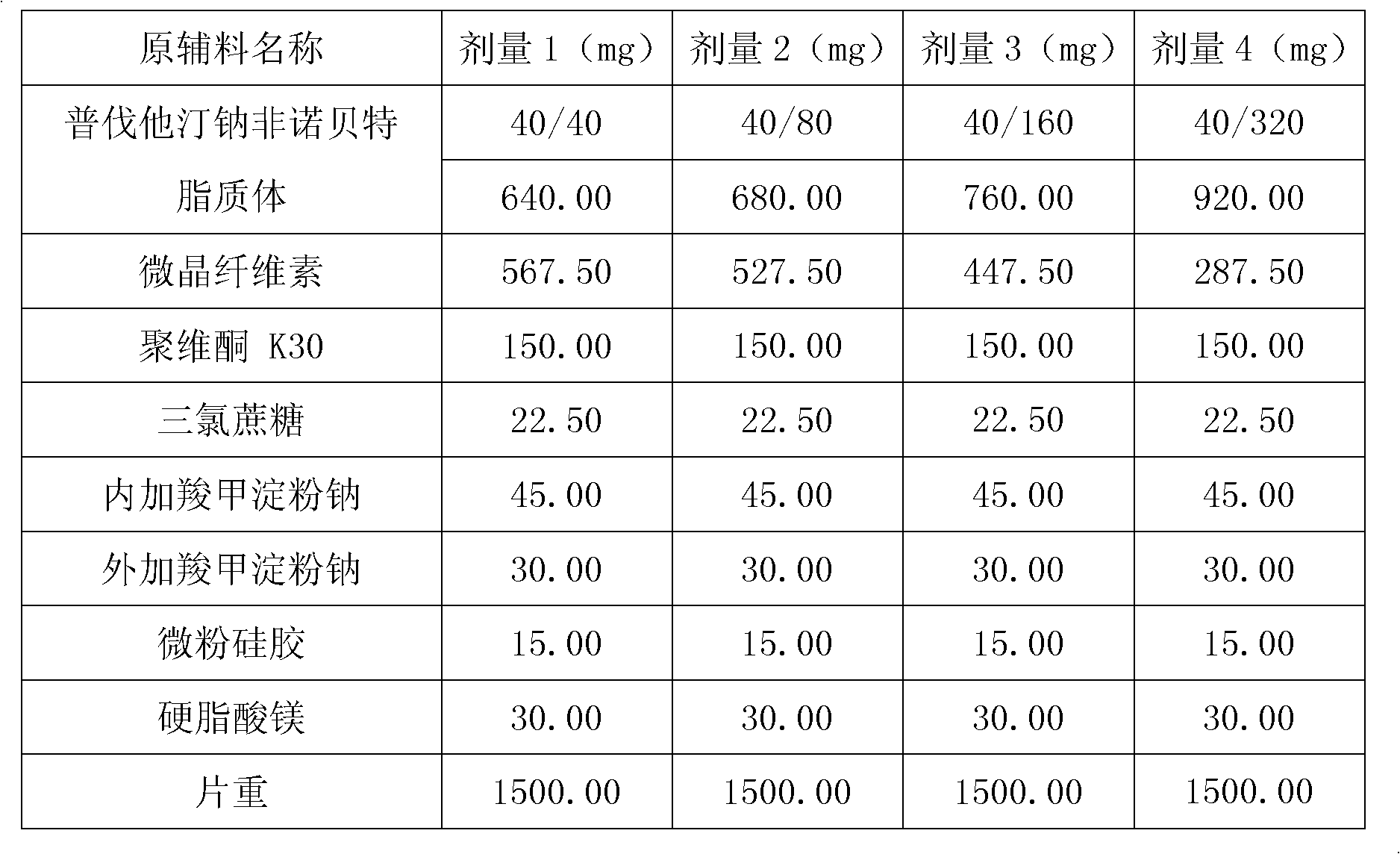
Medicine composition containing pravastatin sodium fenofibrate liposome and preparation method of medicine composition
InactiveCN102552143AHigh content of the main drugHigh dissolution rateMetabolism disorderPharmaceutical non-active ingredientsCholesterolDissolution
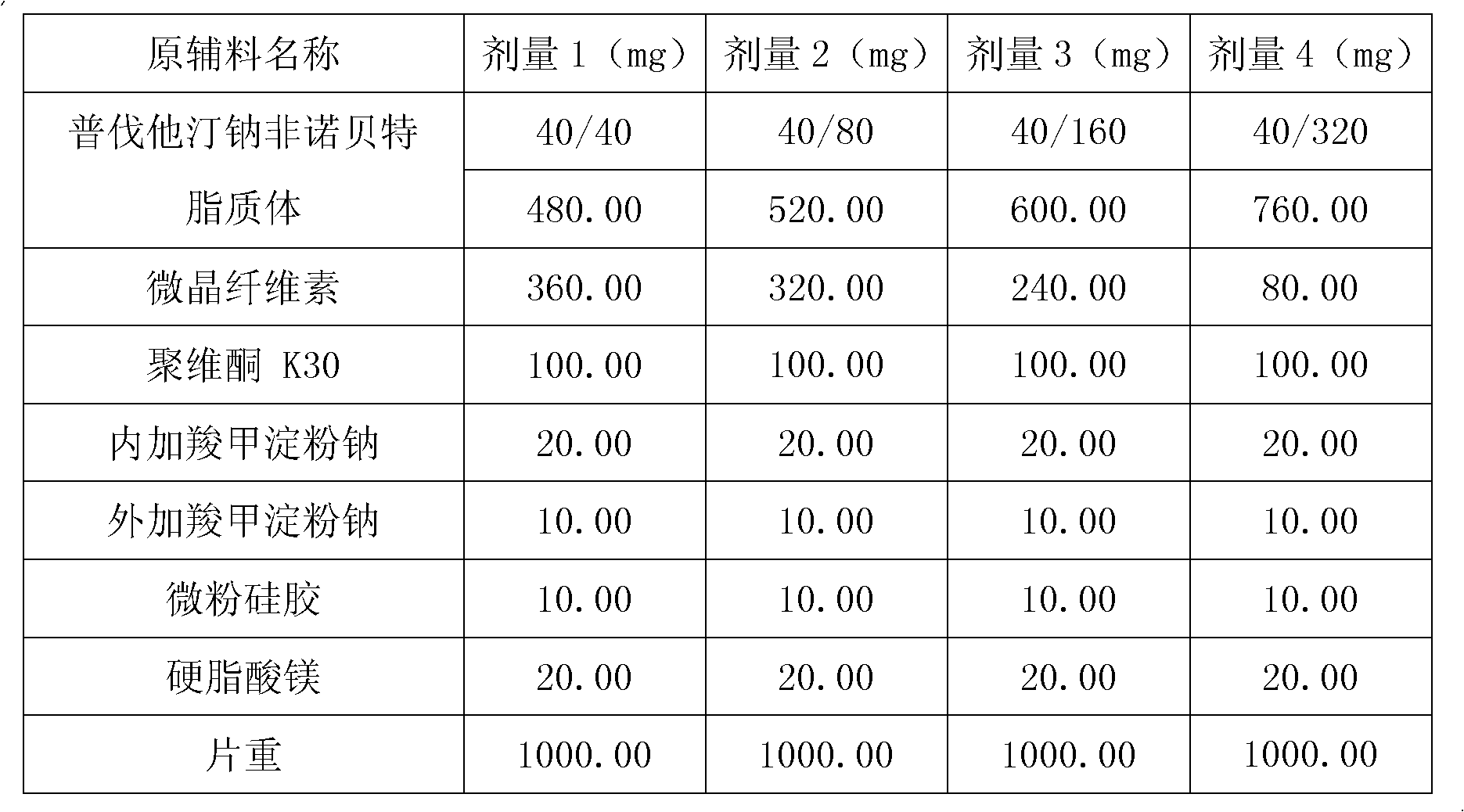
The invention relates to a medicine composition containing a pravastatin sodium fenofibrate liposome and a preparation method of the medicine composition. The medicine composition comprises a pravastatin sodium fenofibrate liposome and at least one pharmaceutically acceptable vector, wherein the pravastatin sodium fenofibrate liposome comprises pravastatin sodium, fenofibrate, phospholipid and cholesterol. The medicine composition prepared by the pravastatin sodium fenofibrate liposome not only satisfies the requirements of Chinese Pharmacopoeia and has the advantages of faster dissolution, faster medicine efficacy, high bioavailability, good stability as well as worth of being widely promoted and applied when being compared with the common pravastatin sodium fenofibrate medicine composition.
Owner:海南欣莱医药科技股份有限公司
Method for preparing pravastatin sodium long-acting sustained release microsphere
InactiveCN104906047AControl release speedMetabolism disorderPharmaceutical non-active ingredientsCross-linkMicrosphere
The invention relates to a method for preparing a pravastatin sodium long-acting sustained release microsphere. The method comprises the first step of oil phase preparing, the second step of water phase preparing, the third step of cross-linking agent solution preparing, the fourth step of mixing a water phase and an oil phase to obtain w / o emulsion and the fifth step of adding a cross-linking agent for cross-linking solidifying, centrifugal separating and vacuum drying to obtain the pravastatin sodium long-acting sustained release microsphere. The pravastatin sodium long-acting sustained release microsphere prepared through the method has the advantages of being long in releasing time and capable of being degraded.
Owner:SOUTHERN MEDICAL UNIVERSITY
Composition comprising pravastatin
InactiveCN1679535AHigh purityOrganic active ingredientsOrganic compound preparationOrganic solventPurification methods
A method of isolating or purifying pravastatin or its pharmaceutically acceptable salt characterized by involving, in the process of isolating or purifying pravastatin or its pharmacologically acceptable salt, the step of extracting pravastatin using an organic solvent represented by the formula CH3CO2R (wherein R represents an alkyl group having 3 or more carbon atoms) or the step of decomposing impurities using an inorganic acid or an inorganic base; and compositions containing pravastatin sodium thus obtained.
Owner:SANKYO CO LTD
A kind of pravastatin sodium dispersible tablet and preparation method thereof
InactiveCN103861117BImprove bioavailabilitySmall inter-individual differencesMetabolism disorderPharmaceutical non-active ingredientsAdhesiveIn vivo
The invention belongs to the field of pharmaceutical preparations and in particular relates to pravastatin sodium dispersible tablets and a preparation method thereof. Because the oral absorption bioavailability of pravastatin sodium is low, and the difference between medication individuals is large, the invention provides pravastatin sodium dispersible tablets. The pravastatin sodium dispersible tablets contain pravastatin sodium, hydroxypropyl-beta-cyclodextrin, filler, a disintegrating agent, an adhesive and a lubricating agent. The pravastatin sodium easily dissolves in water after being subjected to inclusion of hydroxypropyl-beta-cyclodextrin (HP-beta-CD), the stability of the medicine is improved, release of the medicine in vivo is promoted, the absorption is improved, the bioavailability is improved, and the difference between medication individuals is reduced.
Owner:兰丽伟
Pravastatin sodium tablet as well as application and preparation method thereof
ActiveCN101732267BImprove stabilityEasy to storeMetabolism disorderPill deliveryFiller ExcipientDrug product
The invention provides a pravastatin sodium tablet as well as application and a preparation method thereof. The pravastatin sodium tablet contains pravastatin sodium, mannitol as filler, a basifier, a caking agent and a lubricating agent. The pravastatin sodium tablet is prepared by using a direct tablet compressing method, has higher stability and is beneficial to the storage and the transportation of medicines.
Owner:LIVZON PHARM GRP INC +1
Pravastatin sodium dispersible tablets and preparation method thereof
InactiveCN103861117AImprove bioavailabilitySmall inter-individual differencesMetabolism disorderPharmaceutical non-active ingredientsAdhesiveIn vivo
The invention belongs to the field of pharmaceutical preparations and in particular relates to pravastatin sodium dispersible tablets and a preparation method thereof. Because the oral absorption bioavailability of pravastatin sodium is low, and the difference between medication individuals is large, the invention provides pravastatin sodium dispersible tablets. The pravastatin sodium dispersible tablets contain pravastatin sodium, hydroxypropyl-beta-cyclodextrin, filler, a disintegrating agent, an adhesive and a lubricating agent. The pravastatin sodium easily dissolves in water after being subjected to inclusion of hydroxypropyl-beta-cyclodextrin (HP-beta-CD), the stability of the medicine is improved, release of the medicine in vivo is promoted, the absorption is improved, the bioavailability is improved, and the difference between medication individuals is reduced.
Owner:兰丽伟
PravastatinNa pills, and preparation method
InactiveCN101049299ARapid dissolutionReduce dissolutionMetabolism disorderPill deliveryStereochemistryPravastatin Sodium
A dripping pill of pravastatin sodium for treating hyperlipemia is prepared from the pravastatin sodium and the matrix of dripping pill. Its preparing process is also disclosed.
Owner:陈茜
Oral administration solid preparation containing component for reducing blood fat
The invention relates to an oral drug preparation containing pravastatin, in particular to an oral solid formulation with reinforced effect of pravastatin, which comprises effective amount of pravastatin and pharmaceutical adjuvant, wherein the pharmaceutical adjuvant comprises an enwrap agent; the weight proportion of the pravastatin and the wrapping agent ranges from 1: 0.2 to 1: 10. The oral drug has the advantages of quick effects, reinforced active components and easy administration without uncomfortable feeling.
Owner:姚俊华
Novel pseudonocardia sp. rmrc pah4 and a process for bioconverting compactin into pravastatin using the same
The invention provides a novel microorganism Pseudonocardia sp. RMRC PAH4 characterized in that it is able to degrade high concentration of quinoline by enrichment culture, shows a high tolerance to compactin-sodium and possesses a high hydroxylation activity of converting compactin-sodium to pravastatin-sodium. The invention relates also a process for converting compactin-sodium into pravastatin-sodium by fermenting said novel microorganism Pseudonocardia sp. RMRC PAH4. Pravastain-sodium is a potent cholesterol-lowering agent used in treatment for hypercholesterolemia.
Owner:CHINESE GASOLINEEUM
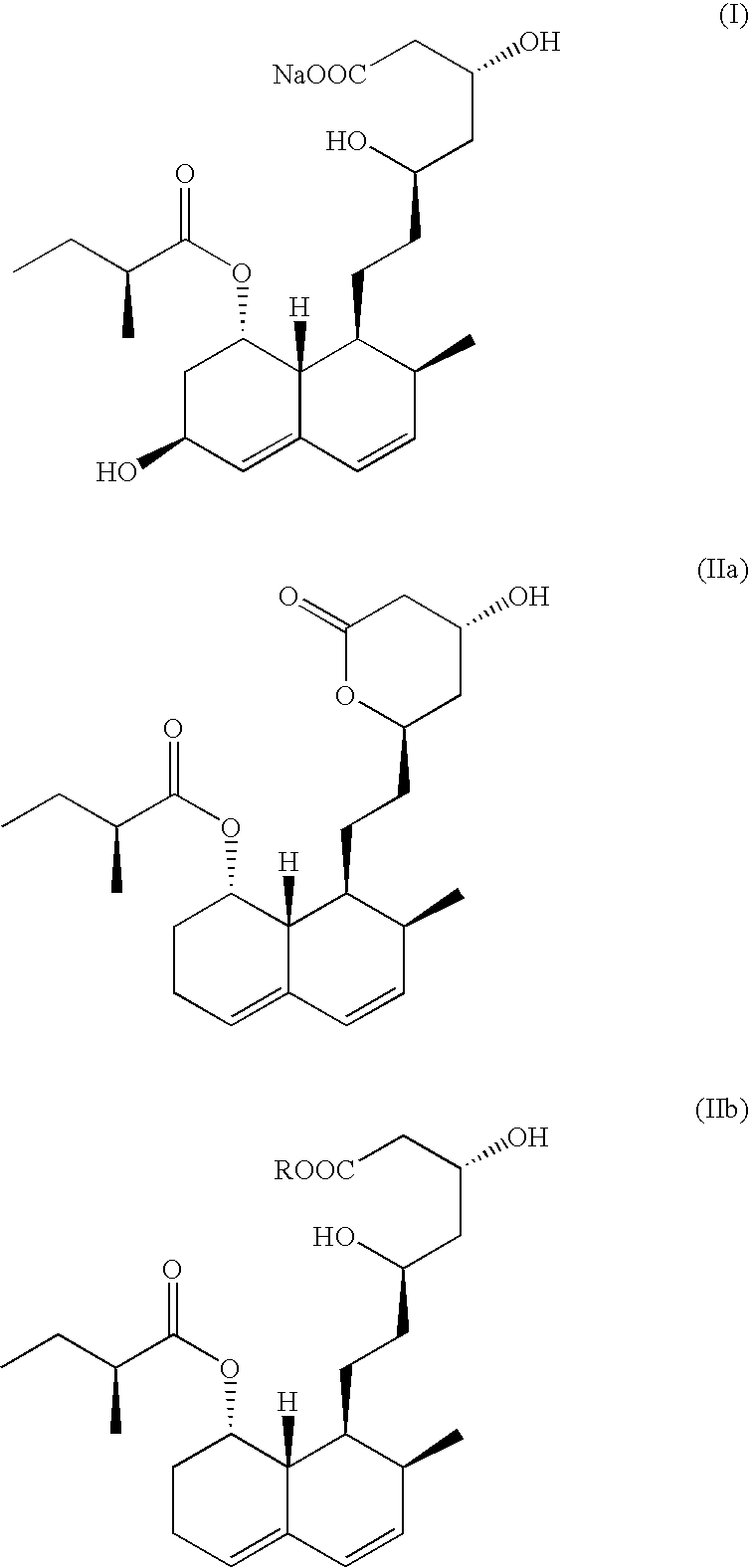
Pravastatin sodium substantially free of pravastatin lactone and EPI-pravastatin, and compositions containing same
The present invention provides pravastatin sodium substantially free of pravastatin lactone and epiprava, the C-6 epimer of pravastatin. The present invention further provides a novel process for recovering pravastatin sodium from a fermentation broth in such high purity. The process includes the stages of forming an solution of the compound by extraction, obtaining an ammonium salt of pravastatin from the solution, purifying the ammonium salt of the compound and transposing the salt of the compound to pravastatin sodium.
Owner:TEVA GYOGYSZERGYAR ZARTKORUEN MUKODO RESZVENYTARSASAG
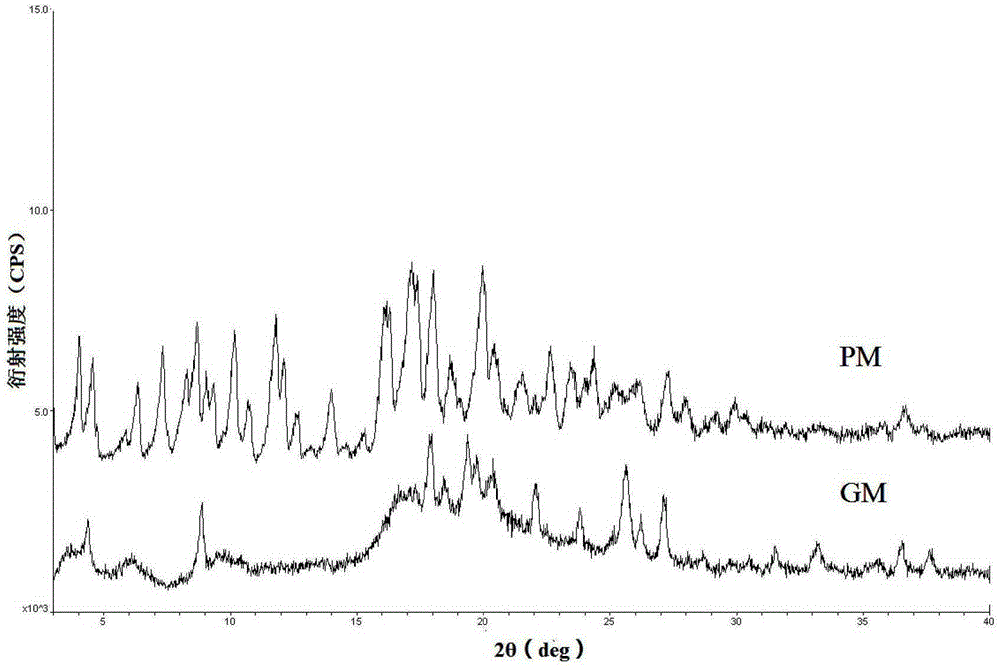
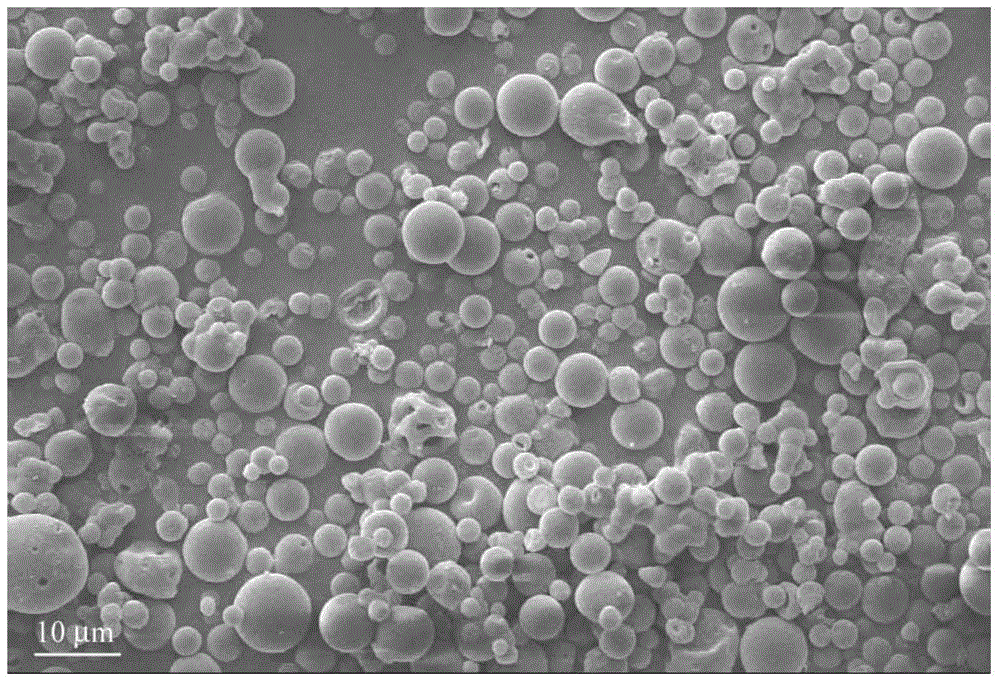
Crystal form of Pravastatin Na, and preparation method and application thereof
InactiveCN101648867ALarge granularityReduce filter timeMetabolism disorderCarboxylic acid esters separation/purificationMass ratioX-ray
The present invention relates to a crystal form of Pravastatin Na, and a preparation method and application thereof. The crystal form is named as a U crystal form. In an X-ray powder diffraction pattern, diffraction angle of 20 degrees, infrared spectrum and endothermic peak in the curve characteristic of a differential scanning calorimeter are defined. The Pravastatin Na in any forms is dissolvedin an acid amide solvent to form a solution, the temperature is preferably 30 to 90 DEG C, and the mass ratio of the acid amide solvent to the Pravastatin Na is preferably 3 to 7:1; a dissolving agent is added in the Pravastatin Na solution, the mass of the dissolving agent is 3 to 15 times of that of the solvent, and then a suspension is formed; the suspension is separated and dried, and the U crystal form of the Pravastatin Na is obtained. Through the XRD pattern and the photographs of a scanning electron microscope, it is confirmed that the product has the advantages of high crystallinity,large crystal size, smooth crystal surface and high filtering speed, and the filtering time of crystal mush with the same density and same volume can be saved by more than one half. The U-form Pravastatin Na crystal as a medicine composite has the purpose of treating atherosclerosis or hypercholesterolemia.
Owner:TIANJIN UNIV +1
Preparation method of fenofibrate and pravastatin sodium compound preparation
The invention provides a preparation method of a compound composition containing active ingredients fenofibrate and pravastatin sodium. The preparation method is characterized in that the pravastatin sodium exists in the composition in the form of quick-release pellets; the fenofibrate exists in the composition in the form of solid dispersoid or semi-solid particles. The preparation method of the composition aims to solve the problem about the bioavailability of the fenofibrate and the pravastatin sodium with completely different solubility and the problem about the stability when the fenofibrate and the pravastatin exist in the same component commonly.
Owner:肖广常
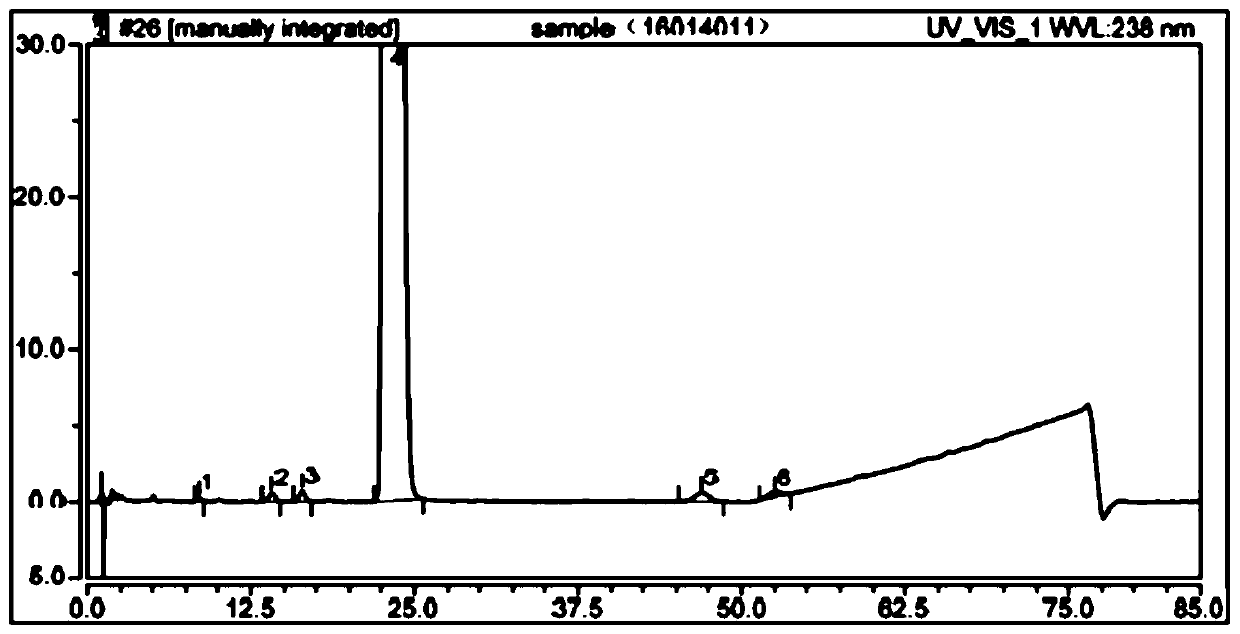
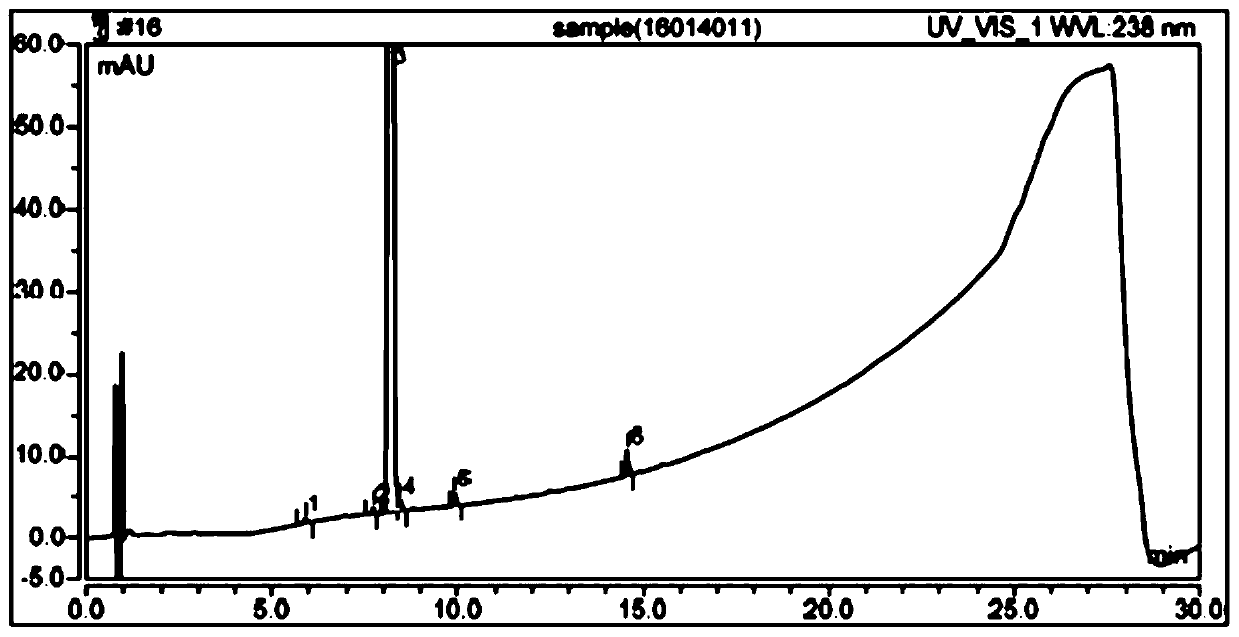
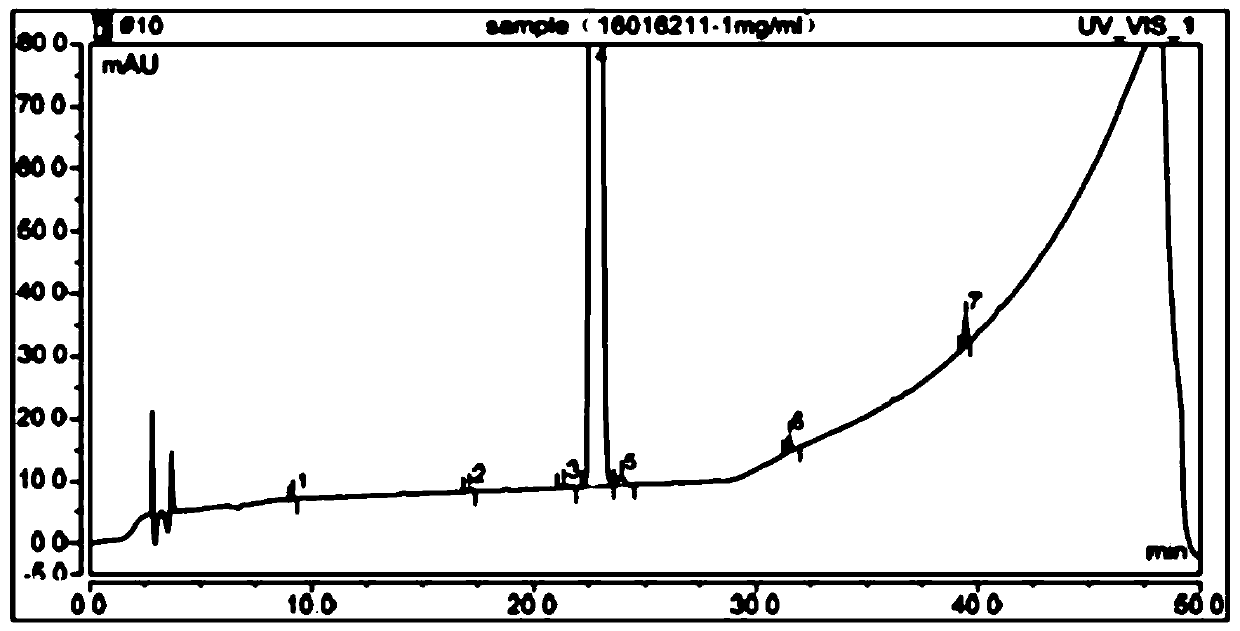
The detection method of the impurity of pravastatin sodium
ActiveCN109521116BQuality improvementGood separation detectionComponent separationPhosphatePhosphoric acid
The invention provides a detection method for impurities of pravastatin sodium, belonging to the technical field of drug detection. The chromatographic conditions of the detection method are listed asbelow: in an Ultimate XB-C18 chromatographic column, water-phosphate buffer with a pH value of 7.0-acetonitrile at a volume ratio of (48-52) to 30 to (18-22) is taken as a mobile phase A, the water-phosphate buffer with the pH value of 7.0-acetonitrile at a volume ratio of 10 to 30 to 60 is taken as a mobile phase B, enabling the reference solution and test solution of the pravastatin sodium to be subjected to gradient elution for 50-60 min, in the period, enabling the percentage of the volume of the mobile phase B accounting for the total volume dose of mobile phases to be increased from 0%to 100%, afterwards, decreasing to 0% and then eluting for 4-5 min. In the chromatograms of relevant substances obtained by the detection method, the resolutions of a main component peak and impurities before and after the main component peak are both greater than 2, baseline separation can be achieved, the reproducibility is good, the impurities and pravastatin sodium samples are effectively separated and detected, and the quality of the pravastatin sodium is controlled.
Owner:瀚晖制药有限公司
Microorganism and the process for preparation of pravastatin sodium
The invention provides a novel microorganism producing pravastatin sodium, as well as the method for producing pravastatin sodium by using this microorganism. Micropolyspora roseoalba CGMCC 0624 of the invention is highly tolerant to mevastatin sodium, and has a high transformation rate of mevastatin sodium, and can produce pravastatin sodium with a high efficiency and low cost.
Owner:SHANGHAI TECHWELL BIOPHARMACEUTICALS CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com