Pravastatin sodium dispersible tablets and preparation method thereof
A technology of pravastatin sodium and dispersible tablets, which is applied in the field of pravastatin sodium dispersible tablets and its preparation, can solve the problems of reduced permeability of pravastatin sodium, low permeability and complexity of pravastatin sodium, and achieve Rapid disintegration, improved dissolution rate, and reduced inter-individual variation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Pravastatin Sodium 10g
[0039] Hydroxypropyl-β-cyclodextrin 45g
[0040] Mannitol 150g
[0041] Compressible starch 75g
[0042] Microcrystalline Cellulose 75g
[0043] Low-substituted hydroxypropyl cellulose 45g
[0044] 2% polyvinylpyrrolidone aqueous solution 650g
[0046] Made in 1000 pieces.
[0047] Preparation Process:
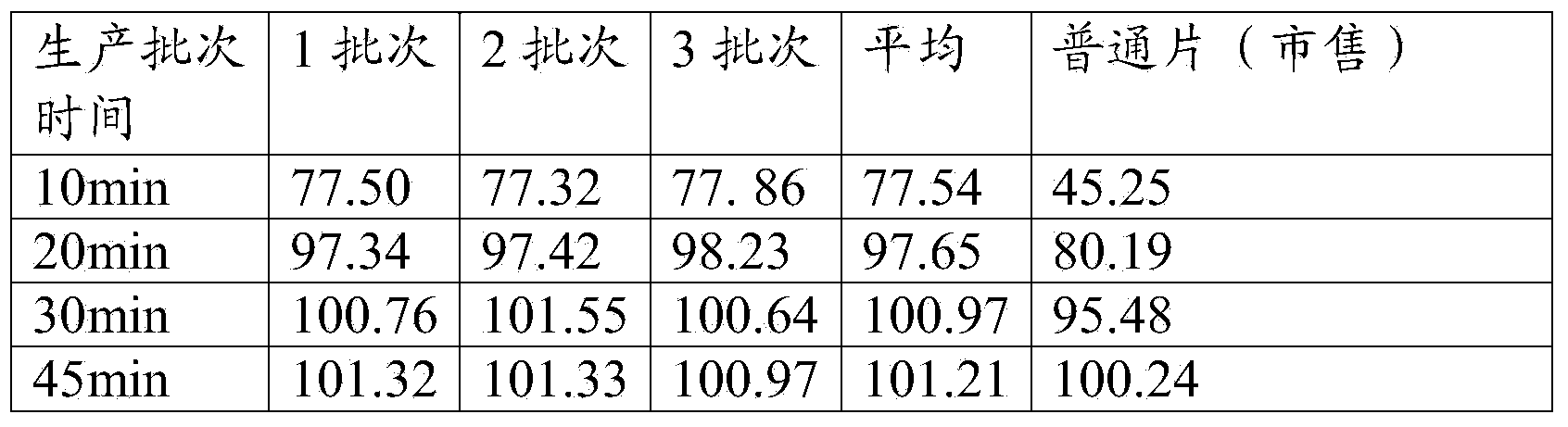
[0048] Take the prescription amount of hydroxypropyl-β-cyclodextrin and dissolve it in an appropriate amount of water to make a saturated aqueous solution. Pass pravastatin sodium through a 120-mesh sieve and dissolve it in an appropriate amount of ethanol. Under magnetic stirring, the ethanol solution of pravastatin sodium Slowly add the saturated aqueous solution of hydroxypropyl cyclodextrin, continue to stir at room temperature for 3 hours after all the addition is complete, and remove most of the ethanol by rotary evaporation at 45°C. Put it into a shallow tray and put it in the freezer of the refrigerator. The...
Embodiment 2
[0052] Pravastatin Sodium 10g
[0053] Hydroxypropyl-β-cyclodextrin 30g
[0054] Mannitol 150g
[0055] Compressible starch 75g
[0056] Microcrystalline Cellulose 75g
[0057] Low-substituted hydroxypropyl cellulose 45g
[0058] 2% polyvinylpyrrolidone aqueous solution 650g
[0060] Magnesium Lauryl Sulfate 1g
[0061] Made in 1000 pieces.
[0062] Preparation Process:
[0063] Take the prescription amount of hydroxypropyl-β-cyclodextrin and dissolve it in an appropriate amount of water to make a saturated aqueous solution. Pass pravastatin sodium through a 120-mesh sieve and dissolve it in an appropriate amount of ethanol. Under magnetic stirring, the ethanol solution of pravastatin sodium Slowly add the saturated aqueous solution of hydroxypropyl-β-cyclodextrin, continue stirring at room temperature for 4 hours after all the addition is complete, and remove most of the ethanol by rotary evaporation at 45°C. Put it into a shallow tray ...
Embodiment 3
[0067] Pravastatin Sodium 10g
[0068] Hydroxypropyl-β-cyclodextrin 45g
[0069] Mannitol 150g
[0070] Compressible starch 75g
[0071] Microcrystalline Cellulose 75g
[0072] Croscarmellose Sodium 45g
[0073] 2% polyvinylpyrrolidone aqueous solution 650g
[0075] Made in 1000 pieces.
[0076] Preparation Process:
[0077] Take the prescription amount of hydroxypropyl-β-cyclodextrin and dissolve it in an appropriate amount of water to make a saturated aqueous solution. Pass pravastatin sodium through a 120-mesh sieve and dissolve it in an appropriate amount of ethanol. Under magnetic stirring, the ethanol solution of pravastatin sodium Slowly add the saturated aqueous solution of hydroxypropyl cyclodextrin, continue to stir at room temperature for 2 hours after all the addition is complete, and remove most of the ethanol by rotary evaporation at 40°C. Put it into a shallow tray and put it in the freezer of the refrigerator. The pre-free...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com