Method of purifying plavastatin
A purification method and pravastatin technology, applied in the field of salt separation or purification, can solve the problems of purity retention, reduction of pravastatin yield, unrecorded purity of pravastatin, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0160] Example 1 Extraction from culture concentrate with n-butyl acetate
[0161] (1a) Concentration of culture solution
[0162] After adjusting 10 L of the pravastatin culture solution after transformation culture to pH 12 with sodium hydroxide, it was heated to 50° C. and stirred for 30 minutes. After the culture solution was cooled to room temperature, 500 g of Celite 545 (trademark) (manufactured by CELITE CORPORATION) was added as a filter aid and filtered. 3 L of water was added to the separated bacterial cells, suspended again, and then filtered. The obtained two concentrates were combined to obtain 10 L of culture concentrate.
[0163] (1b) Extraction with n-butyl acetate
[0164] After adjusting the resulting culture concentrate to pH 5.7 with 25% sulfuric acid, 5 L of n-butyl acetate was added and stirred to extract pravastatin. The separated aqueous layer was adjusted to pH 5.7 with 75% sulfuric acid, then 5 L of n-butyl acetate was added, and stirred for ext...
Embodiment 2
[0165] Example 2 Extraction from culture concentrate with n-propyl acetate
[0166] N-propyl acetate was used to replace n-butyl acetate, and the same treatment as in Example 1 was carried out to obtain an aqueous sodium salt solution of pravastatin. As determined by HPLC (condition A), the purity of pravastatin sodium was 85% or above. As can be seen from the results of this example, high-purity pravastatin sodium can be obtained by using n-propyl acetate.
Embodiment 3
[0167] Example 3 Decomposition of impurities with phosphoric acid
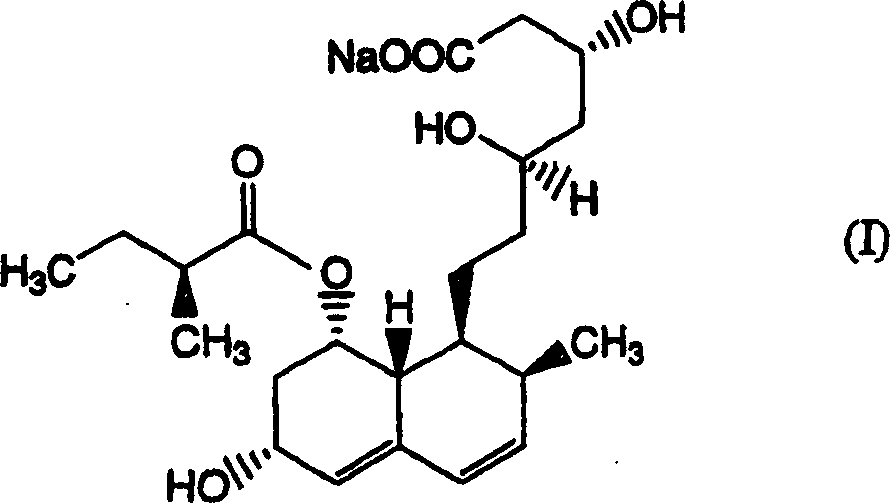
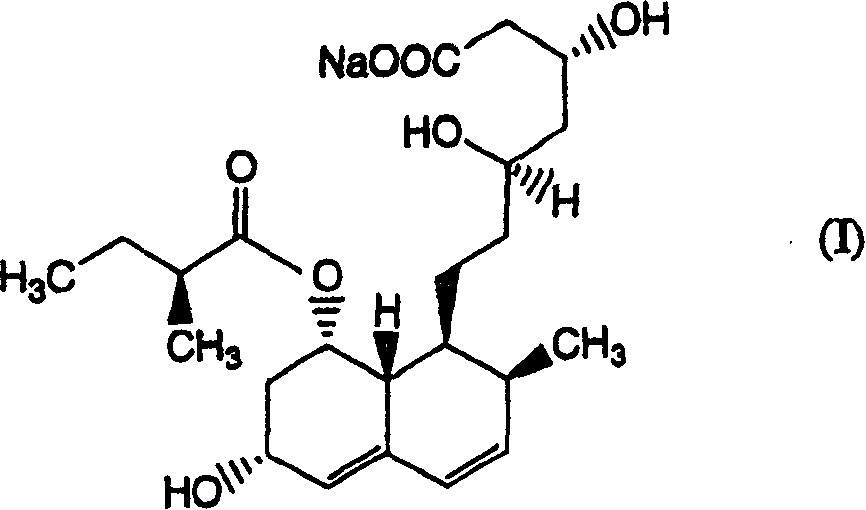
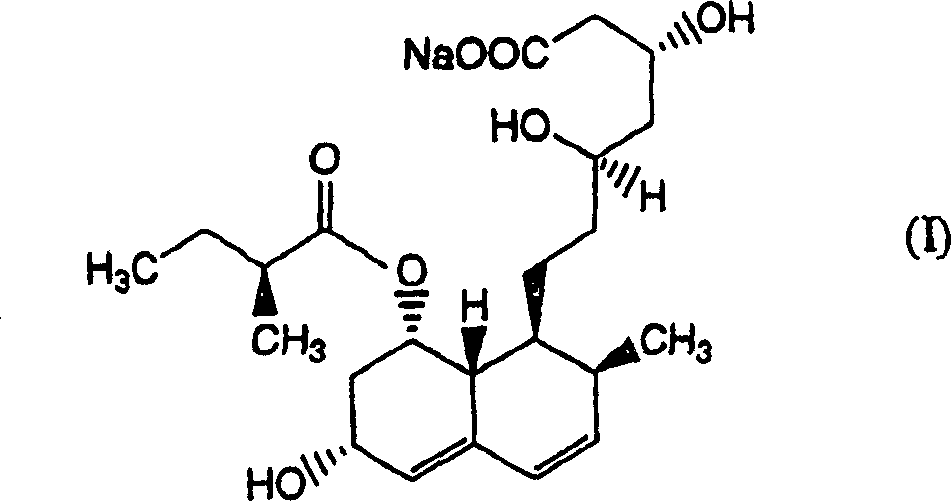
[0168] Add 350 ml of ethanol to the aqueous solution obtained in Example 1 [compound (I) / pravastatin sodium measured by HPLC (condition A) is 9.3%], adjust it to pH 3.0 with phosphoric acid, then stir at 50° C. for 10 minutes . Compound (I) / pravastatin sodium determined by HPLC (condition A) was 0.9%. From the results of this example, it was found that compound (I) can be significantly removed by using phosphoric acid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com