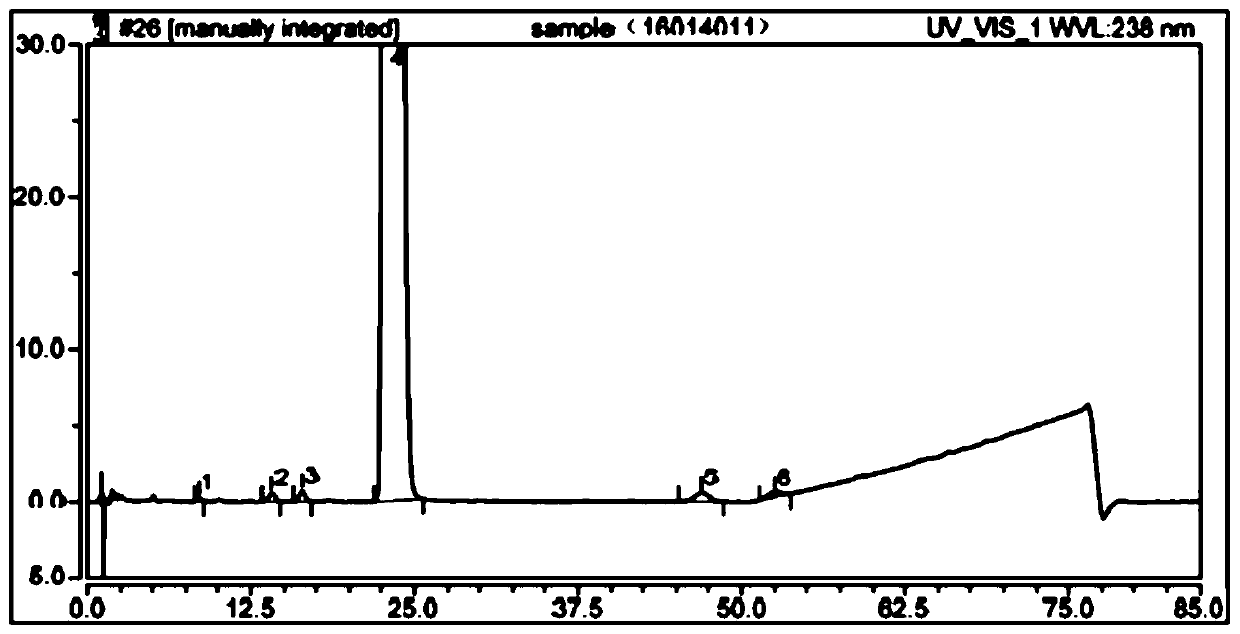
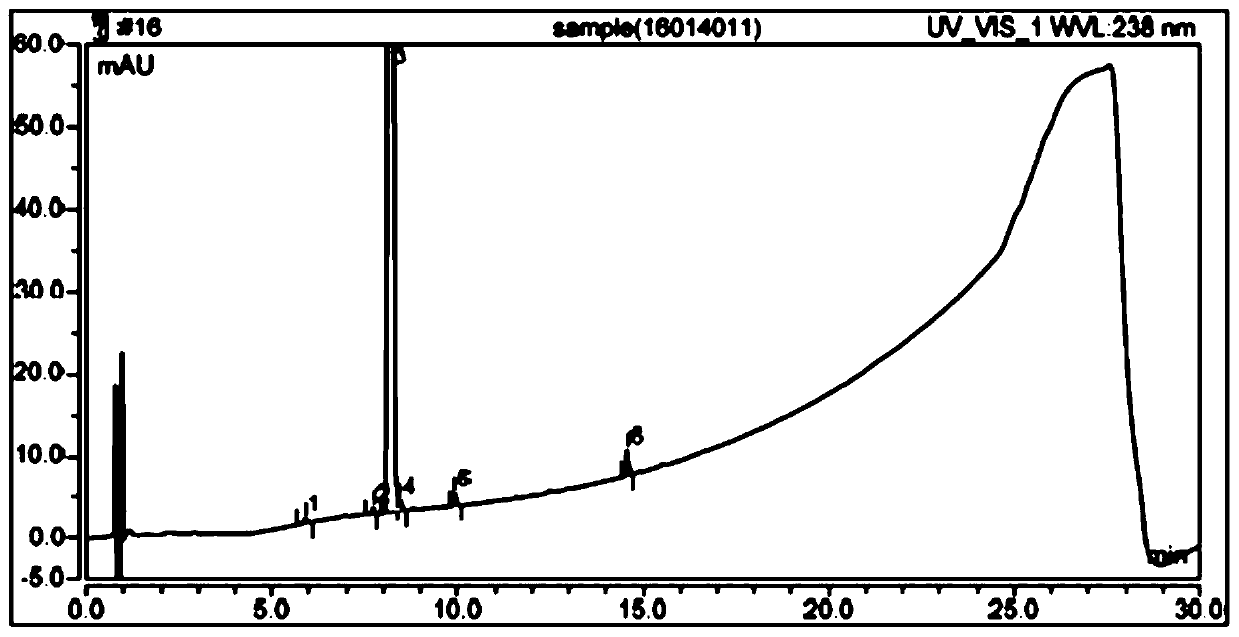
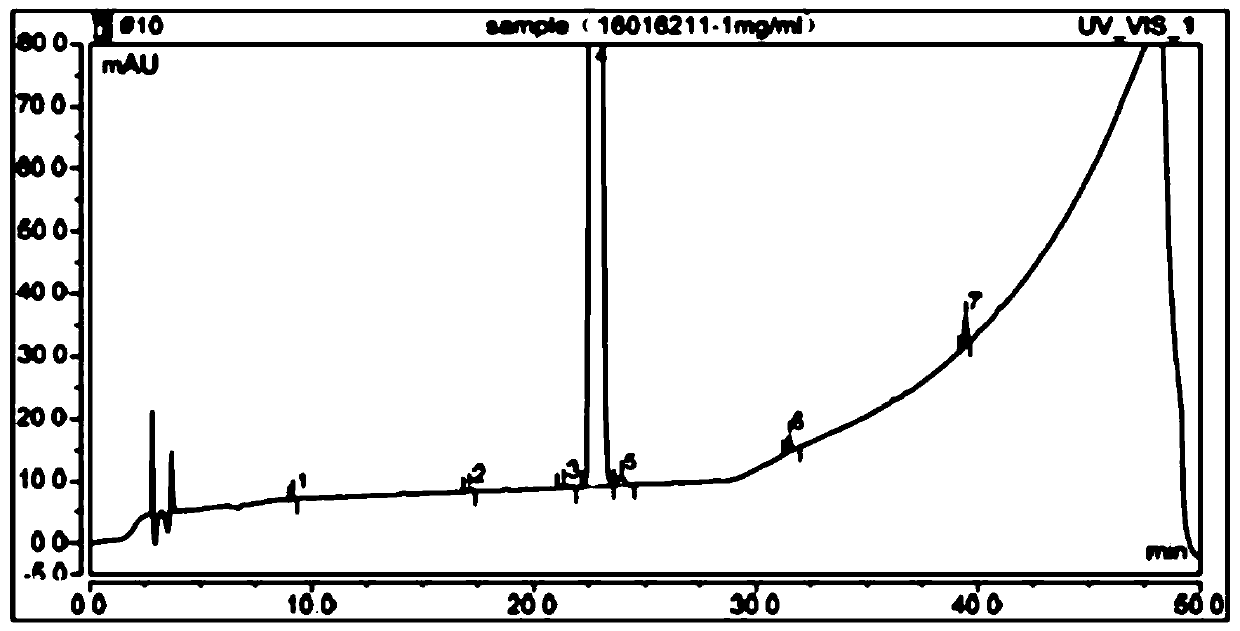
The detection method of the impurity of pravastatin sodium
A technology for the detection of pravastatin sodium, which is applied in the field of detection of impurities, can solve problems such as poor method reproducibility, short and fat peaks, irregular drift of retention time, etc., and achieve the effect of good reproducibility and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] A detection method of impurities of pravastatin sodium, comprising:
[0049] The concentration of pravastatin sodium in the need testing solution (self-developed preparation) of pravastatin sodium is 1mg / ml, and the concentration of pravastatin sodium in the need testing solution (reference preparation) of pravastatin sodium is 1mg / ml. ml.
[0050] Chromatographic conditions: the chromatographic column is Ultimate XB-C18 chromatographic column 4.6×250mm, 5μm. By volume, water: pH7.0 phosphate buffer: acetonitrile = 50:30:20 is mobile phase A, by volume, water: pH7.0 phosphate buffer: acetonitrile = 10:30:60 For mobile phase B, carry out gradient elution to the reference substance solution of pravastatin sodium and the test solution of pravastatin sodium respectively for 50min, the flow rate is 1ml / min, the column temperature is 25°C, the detection wavelength is 238nm, and the injection volume is 10μl , were eluted according to the following gradient elution procedure: ...
Embodiment 2
[0065] The difference between the detection method of the impurity of this pravastatin sodium and embodiment 1 is only:
[0066] Use water: pH7.0 phosphate buffer: acetonitrile = 52:30:18 as the mobile phase A, follow the gradient elution procedure as follows:
[0067] When t=0min, mobile phase A is 100%, and mobile phase B is 0%;
[0068] When t=0min~10min, the mobile phase A is 100%→100%, and the mobile phase B is 0%→0%;
[0069] When t=10min~55min, the mobile phase A is 100%→0%, and the mobile phase B is 0%→100%;
[0070] When t=55min~55.1min, the mobile phase A is 0%→100%, and the mobile phase B is 100%→0%;
[0071] When t=55.1min~60min, the mobile phase A is 100%, and the mobile phase B is 0%;
[0072] When t=50min, the mobile phase A is 100%, and the mobile phase B is 0%. The result is as Figure 5 As shown, the separation between the main component peak and the impurities before and after the main component peak is obviously increased, but the baseline is ugly.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com