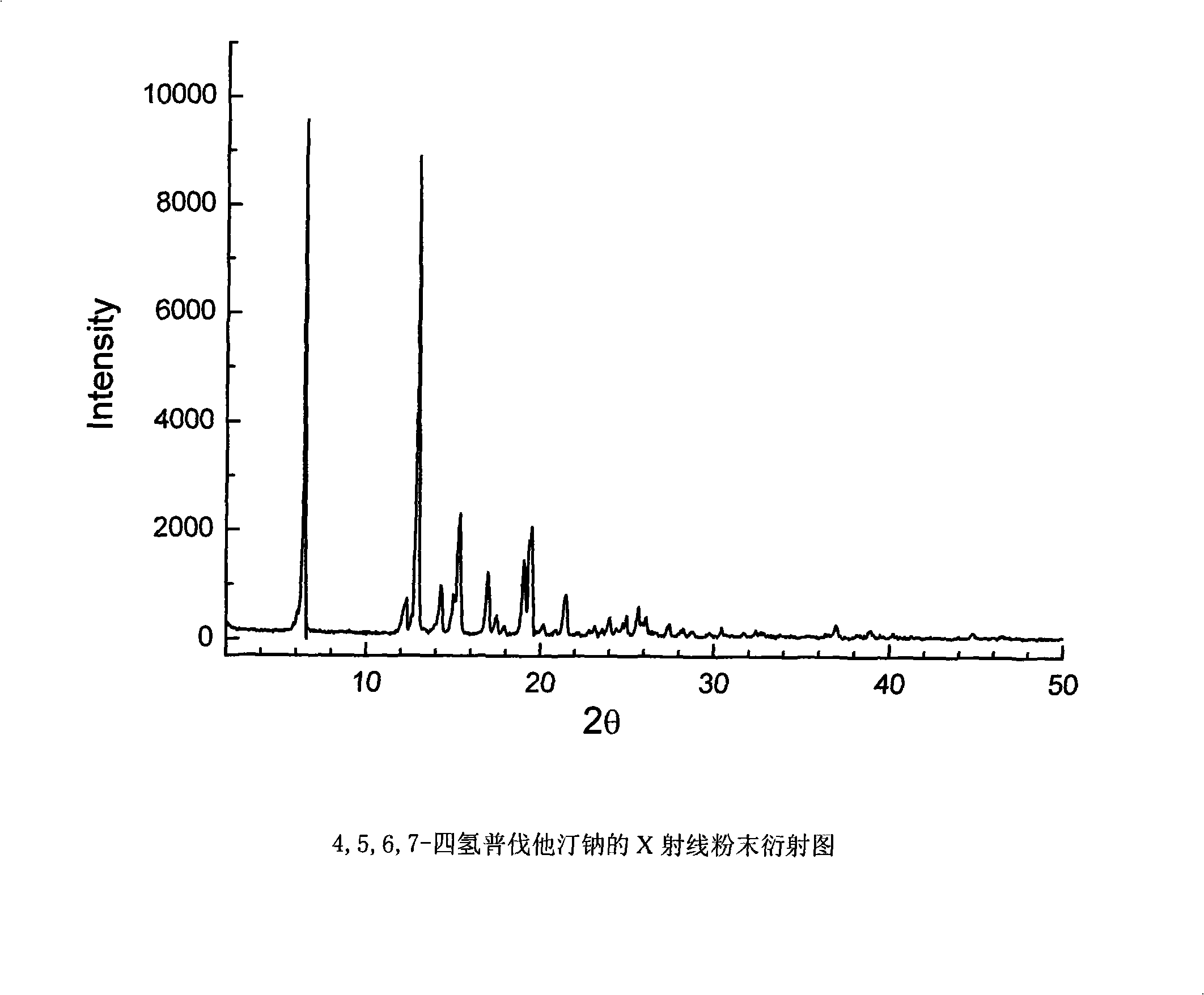
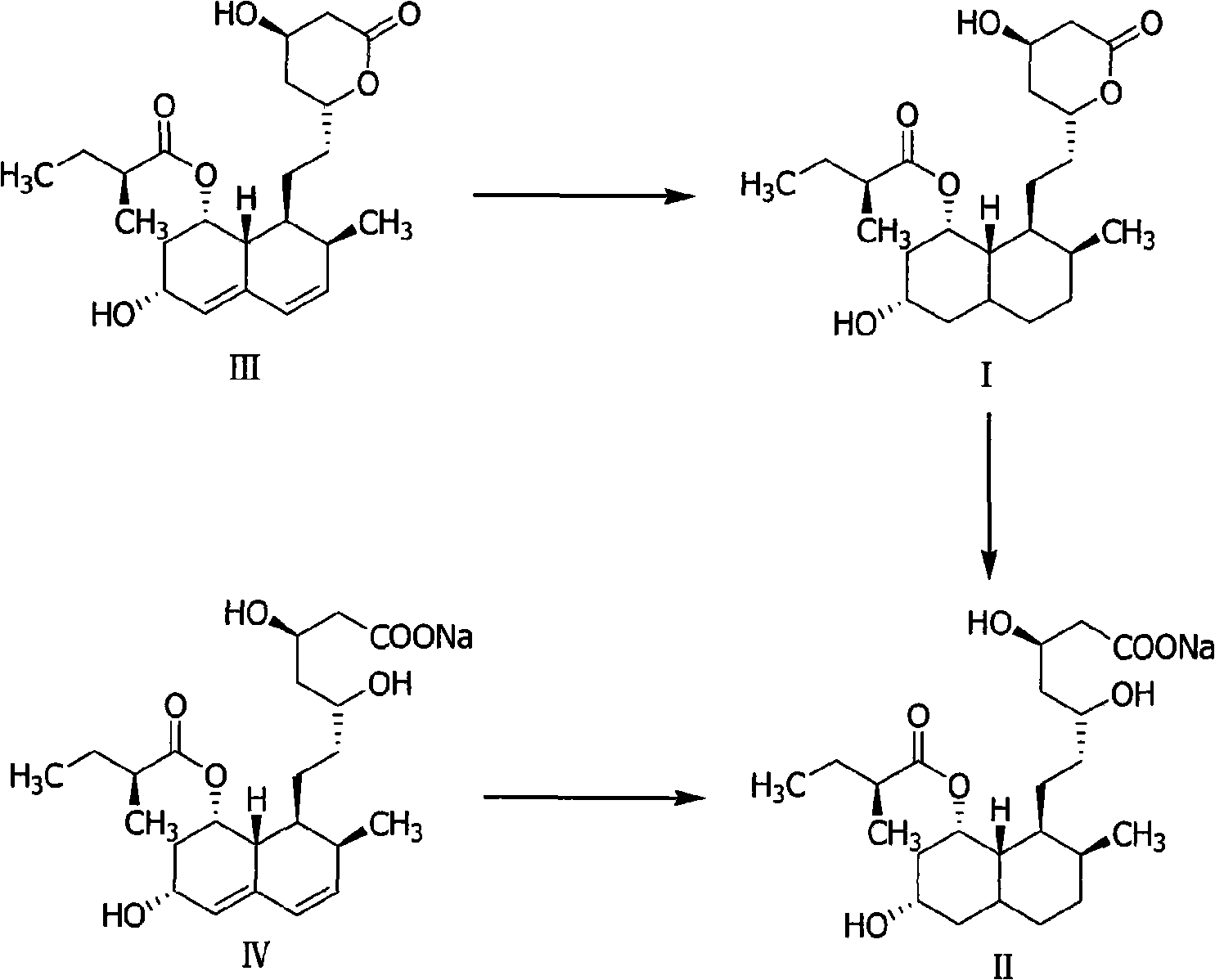
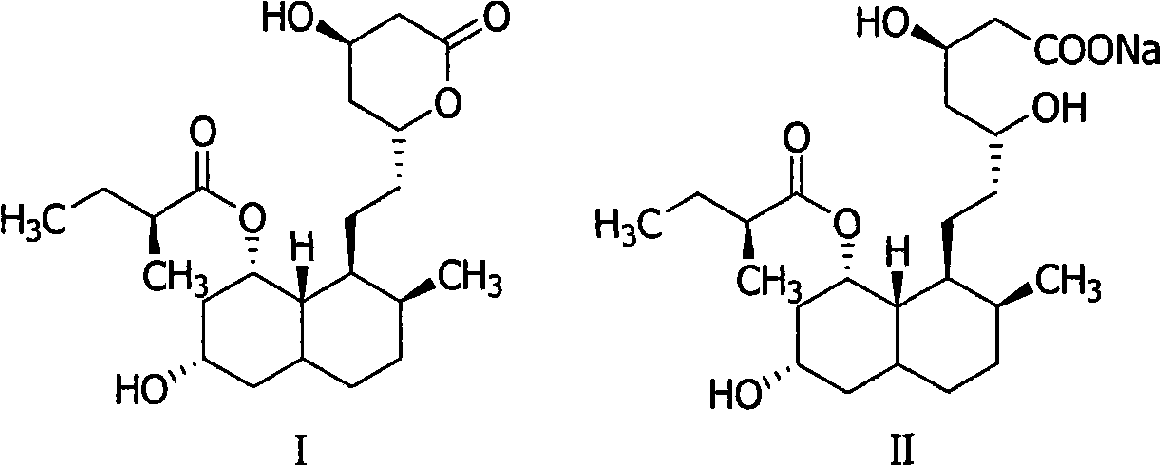
Method for preparing 4,5,6,7-tetrahydromevastatin and sodium salt thereof, and solid crystallization way
A technology of pravastatin sodium and pravastatin, which is applied in the field of preparation 4, can solve the problems of long hydrogenation reaction time, long synthesis steps, and low overall yield, and achieve simplified synthesis technology, simple preparation and operation, and improved yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The preparation of 8% Pd-carbon nanotubes refers to the methods in the literature (Feng Peng, Lei Zhang, Hongjuan Wang, Ping Lv, Hao Yu, Carbon, 2005, 43, 2405-2408). Add 1.3g of multi-walled carbon nanotubes into 70ml of concentrated sulfuric acid, 2 Reflux at 250°C for 20 hours under airflow, then filter and wash until no sulfate ions are detected, neutralize with NaOH solution and wash until no sodium ions are detected, then add to the solution containing PdCl 2 in the aqueous solution, stirred overnight, filtered, washed with deionized water, dried at 120°C for 12 hours, and passed H2 at 80°C for 12 hours to obtain 1.18g of 8% Pd-carbon nanotubes.
Embodiment 2
[0035]4,5,6, the preparation of 7-tetrahydropravastatin (I):
[0036] Add 2.0g of pravastatin (4.93mmol) and 200ml of ethanol to a 500ml three-necked flask, stir to dissolve, add 0.1g of 10% Pd-C, pass in hydrogen at 120°C and 4mpa until no hydrogen is absorbed, react for about 100min, filter , concentrated to obtain 4,5,6,7-tetrahydropravastatin (I) crude product as a colorless oil 1.84g, yield 91%.
[0037] MS (ESI) m / z: 433.3 (M+Na) + ; 1 H-NMR (CD 3 COCD 3 , 400MHz) δ: 5.20(s, 1H, 13-H), 4.51(s, 1H, 1-H), 4.24(s, 1H, 16-H), 3.76(s, 1H, 3-H), 2.60 -2.54(q, 3H, 15-2H, 20-H), 1.91(m, 3H, 17-2H, 10-H), 1.15(m, 5H, 23-CH 3 , 11-2H), 0.86(t, 3H, J=7.6Hz, 18-CH 3 ), 0.76(t, 3H, J=6.8Hz, 22-CH 3 ); 13 C-NMR (CDCl 3 , 100MHz) δ: 176.1, 170.8, 76.4, 70.0, 65.9, 62.2, 43.0, 42.8, 41.6, 39.7, 39.5, 38.4, 35.9, 35.4, 32.8, 32.5, 28.7, 27.8, 26.5, 24.5, 16.7, 11.6, 11.5 .
Embodiment 3
[0039] 4,5,6, the preparation of 7-tetrahydropravastatin (I):
[0040] Add 2.0g of pravastatin (4.93mmol) and 200ml of ethanol to a 500ml three-necked flask, stir to dissolve, add 0.1g of 8% Pd-carbon nanotubes, feed hydrogen at 120°C and 4mpa until no more hydrogen is absorbed, and react for about 110min , filtered, and concentrated to obtain 1.92 g of crude 4,5,6,7-tetrahydropravastatin (I) as a colorless oil, with a yield of 95%. The spectrum shown in the sample is consistent with the spectrum obtained in Example 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com