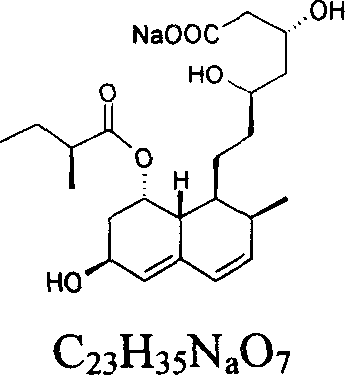
Pravastatin preparation formula
A technology of pravastatin and pravastatin sodium, which is applied in the formulation field of pravastatin preparations, can solve problems such as unfavorable absorption, damage to natural acidic environment, and influence on human body functions, and achieve good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Prepare the pravastatin preparation according to the following formula:
[0023] Element weight percentage (%)
[0024] Pravastatin sodium 5
[0025] Monosodium Glutamate 1
[0026] Lactose 74
[0027] Microcrystalline Cellulose 15
[0028] Croscarmellose Sodium 2.5
[0029] Polyvinylpyrrolidone 1.5
[0031] Weigh 2 grams of sodium glutamate and dissolve in an appropriate amount of water, adjust the pH value to 8.8, add 10 grams of pravastatin sodium and stir to dissolve, add 3 grams of polyvinylpyrrolidone and stir to dissolve, add 148 grams of lactose, 30 grams of microcrystalline cellulose and stir Mix evenly to form wet granules. The wet granules are passed through a 24-mesh sieve, and then dried in an airflow oven at 50°C for 3 hours. After drying, the granules are passed through a 30-mesh sieve. Magnesium fatty acid, stirred and mixed, and then punched into tablets.
[0032] After the above-mentioned tablet was stored for 2...
Embodiment 2
[0039] Prepare the pravastatin preparation according to the following formula:
[0040] Element weight percentage (%)
[0041] Pravastatin sodium 5
[0042] Lysine hydrochloride 5
[0043] Lactose 70
[0044] Microcrystalline Cellulose 15
[0045] Croscarmellose Sodium 2
[0046] Polyvinylpyrrolidone 1.5
[0048]Weigh 10 grams of lysine hydrochloride and dissolve in an appropriate amount of water, adjust the pH value to 8.8, add 10 grams of pravastatin sodium and stir to dissolve, add 3 grams of polyvinylpyrrolidone and stir to dissolve, add 140 grams of lactose, 30 grams of microcrystalline fiber Stir and mix evenly to make into wet granules. The wet granules are passed through a 24-mesh sieve, and then dried in an airflow oven at 50° C. for 3 hours. After drying, the granules are passed through a 30-mesh sieve. gram of magnesium stearate, stirred and mixed, and then punched into tablets.
[0049] After the above-mentioned tablet was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com