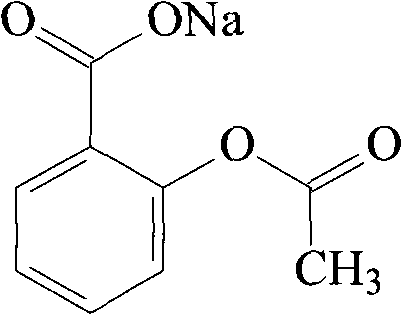
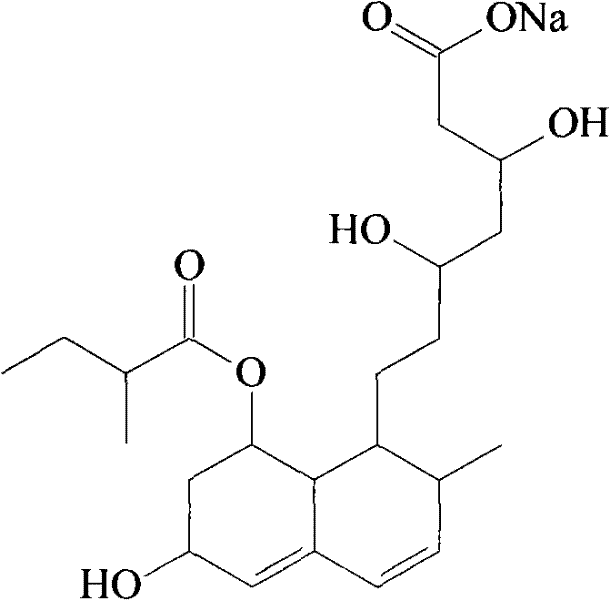
Solid preparation of sodium aspirin and sodium pravastatin medicinal composition
A technology of pravastatin sodium and aspirin sodium, which is applied in the field of aspirin sodium pravastatin sodium pharmaceutical composition solid preparation and aspirin sodium pravastatin sodium compound preparation, can solve the problem of poor liposome stability and encapsulation rate, etc. problems, to achieve the effect of improving bioavailability, high encapsulation efficiency, and prolonging cycle time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1 Preparation of Aspirin Sodium Pravastatin Sodium Liposome Tablets
[0052] Prescription (1000 tablets)
[0053]
[0054]
[0055] Preparation Process
[0056] (1) 300g of hydrogenated soybean lecithin, 80g of cholesterol and 150g of poloxamer 188 are dissolved in 10000ml of a volume ratio of 1:3 in the mixed solvent of chloroform and n-butanol to obtain lipid solution;
[0057] (2) Place the above-mentioned lipid solution in a pear-shaped bottle, and remove the mixed solvent by rotary evaporation in a constant temperature water bath at 55° C. to form a uniform lipid film;
[0058] (3) Disperse 20 g of aspirin sodium and 81 g of pravastatin sodium in 900 ml of water, add it to a pear-shaped bottle and shake gently, so that the lipid film is eluted and dispersed in a hydration medium for dissolution to obtain a liposome suspension;
[0059] (4) Place the above-mentioned suspension in an ultrasonic instrument and sonicate to a translucent colloidal soluti...
Embodiment 2
[0064] Example 2 Preparation of Aspirin Sodium Pravastatin Sodium Liposome Tablets
[0065] Prescription (1000 tablets)
[0066]
[0067]
[0068] Preparation Process
[0069] (1) 600g of hydrogenated soybean lecithin, 200g of cholesterol and 300g of poloxamer 188 are dissolved in 20000ml of a volume ratio of 1:3 in a mixed solvent of chloroform and n-butanol to obtain a lipid solution;
[0070] (2) Place the above-mentioned lipid solution in a pear-shaped bottle, and remove the mixed solvent by rotary evaporation in a constant temperature water bath at 45° C. to form a uniform lipid film;
[0071] (3) Disperse 20 g of aspirin sodium and 81 g of pravastatin sodium in 900 ml of water, add it to a pear-shaped bottle and shake gently, so that the lipid film is eluted and dispersed in a hydration medium for dissolution to obtain a liposome suspension;
[0072] (4) Place the above-mentioned suspension in an ultrasonic instrument and sonicate to a translucent colloidal solut...
Embodiment 3
[0077] Example 3 Preparation of Aspirin Sodium Pravastatin Sodium Liposome Tablets
[0078] Prescription (1000 tablets)
[0079]
[0080]
[0081] Preparation Process
[0082] (1) 450g hydrogenated soybean lecithin, 140g cholesterol and 220g poloxamer 188 are dissolved in 15000ml volume ratio is in the mixed solvent of chloroform and n-butanol of 1: 3, obtain lipid solution;
[0083] (2) Place the above-mentioned lipid solution in a pear-shaped bottle, and remove the mixed solvent by rotary evaporation in a constant temperature water bath at 50° C. to form a uniform lipid film;
[0084] (3) Disperse 40 g of aspirin sodium and 81 g of pravastatin sodium in 1300 ml of water, add to a pear-shaped bottle and shake gently, so that the lipid film is eluted and dispersed in a hydration medium for dissolving to obtain a liposome suspension;
[0085] (4) Place the above-mentioned suspension in an ultrasonic instrument and sonicate to a translucent colloidal solution;
[0086] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com