Crystal form of Pravastatin Na, and preparation method and application thereof
A technology of pravastatin sodium and crystal, which is applied in the crystal form of pravastatin sodium and its preparation field, can solve the problems of harsh raw material requirements, poor repeatability, and small crystal particle size, and achieve large crystal particle size and simple operating conditions Easy to control, high crystallinity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Add 3.50 g of pravastatin sodium to 14 g of N,N-dimethylformamide solvent, heat up to 30° C. to dissolve it, and obtain a transparent light yellow solution. 42 g of ethyl acetate was added under stirring to crystallize. Cool to 0°C at 6min / °C, then continue stirring for 1h. The obtained light yellow suspension was vacuum-filtered, and the filtration speed was fast, and the filter cake was vacuum-dried at 40° C. to obtain 3.25 g of light yellow needle-shaped U-shaped crystals of pravastatin sodium. The product has high crystallinity, large crystal size and smooth surface.
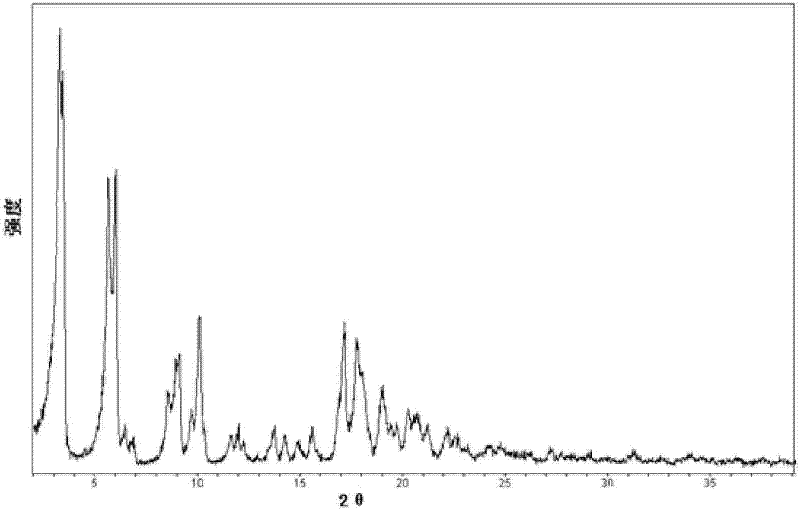
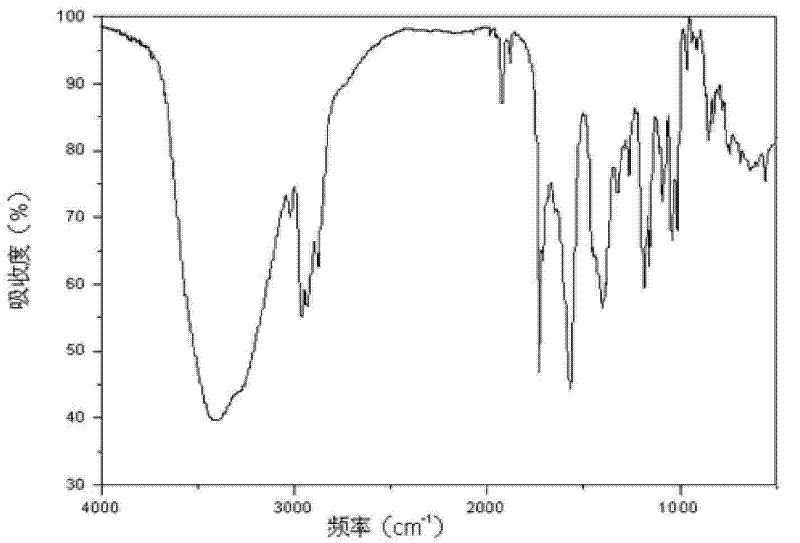
[0040] The XRD pattern of the product is as follows figure 1 As shown, at diffraction angles 2θ°=3.3, 5.6, 6.0, 6.4, 6.8, 8.5, 9.1, 10.1, 11.6, 12.0, 13.7, 14.3, 14.9, 15.5, 17.2, 17.8, 19.0, 20.2, 20.7, 21.3, and There is a characteristic peak at 22.2. Infrared absorption spectrum such as figure 2 Shown at 637, 687, 741, 781, 824, 843, 854, 913, 938, 965, 1015, 1039, 1091, 1109, 1158, 1185, 126...
Embodiment 2
[0042] Put 2.50g of pravastatin sodium into 12g of acetamide solvent, raise the temperature to 40°C, and stir to make it completely dissolved. 100 g of ethyl acetate was added under continuous stirring, and then the resulting suspension was stirred for 1 h. The suspension was filtered, and the filter cake was vacuum-dried at 50° C. to obtain 2.20 g of pale yellow needle-shaped U-shaped crystals of pravastatin sodium.
Embodiment 3
[0044] Put 5.00 g of pravastatin sodium into 15 g of N,N-dimethylformamide solvent, raise the temperature to 90° C., and stir to make it completely dissolved. 120 g of ethyl acetate was added under continuous stirring. The temperature was lowered to 30° C. at 10 min / ° C., and then the resulting suspension was stirred for 0.5 h. The resulting suspension was filtered and dried under vacuum at 70° C. to obtain 4.71 g of pale yellow U-shaped crystals of pravastatin sodium.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com