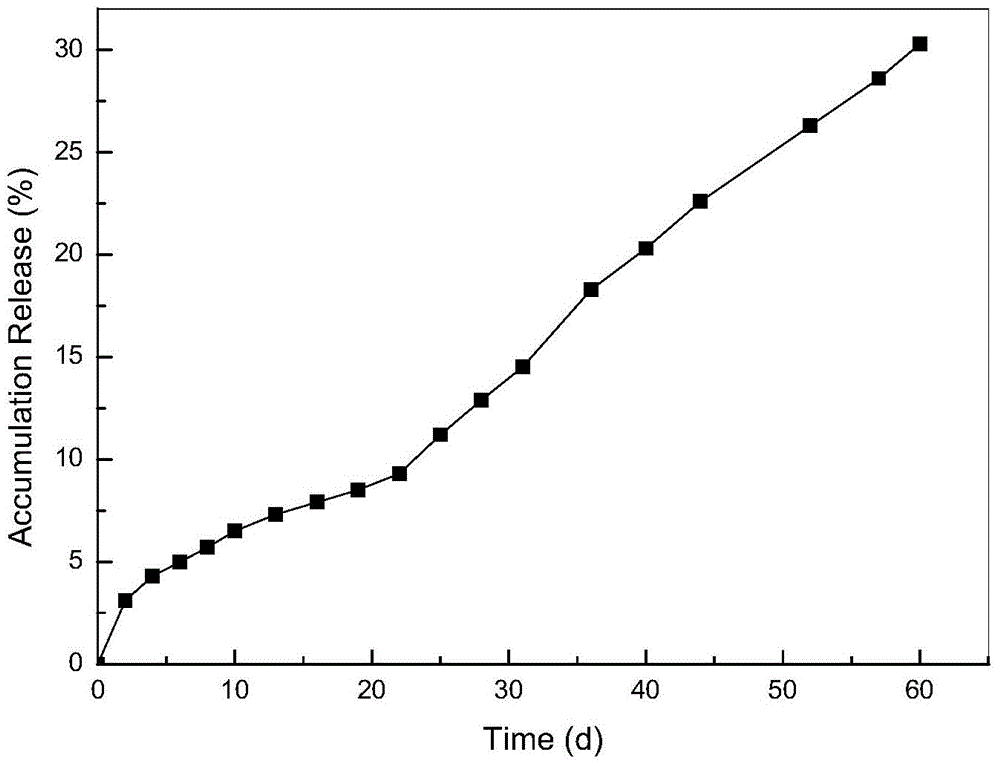
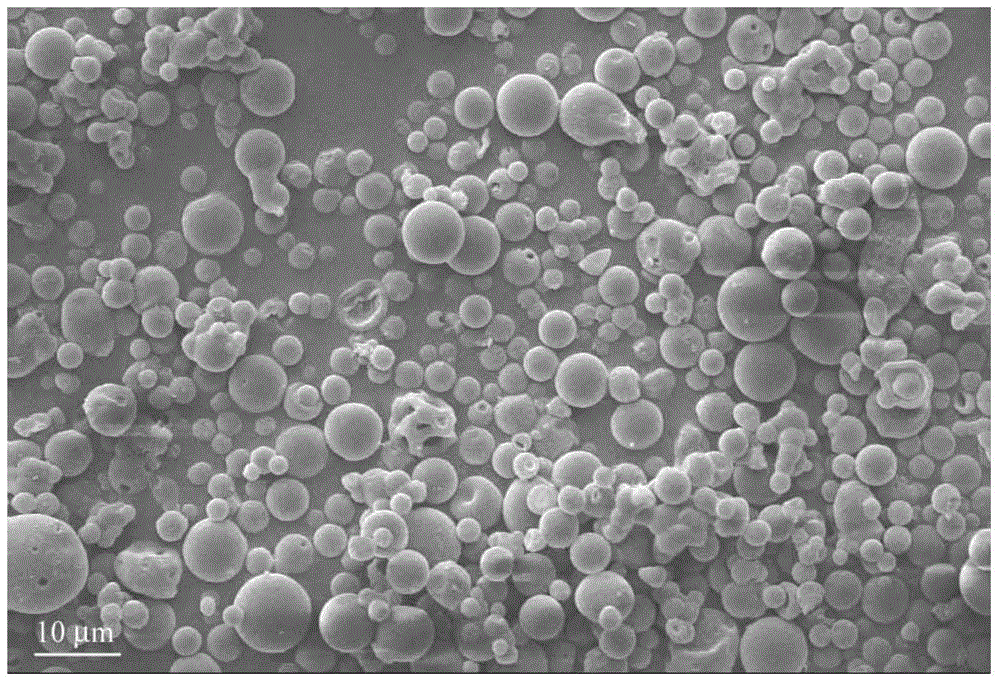
Method for preparing pravastatin sodium long-acting sustained release microsphere
A technology of pravastatin sodium and slow-release microspheres, applied in the field of preparation of pharmaceutical preparations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] 1. Preparation of long-acting sustained-release microspheres of pravastatin sodium
[0020] (1) Mix Tween-80 and liquid paraffin according to the volume ratio of Tween-80: liquid paraffin = 2: 100, and mix evenly under electric stirring at a speed of 500 r / min to obtain an oil phase;
[0021] (2) join the chitosan that viscosity is lower than 200mPa.s in the acetic acid solution that volume concentration is 1%, magnetic force stirs 1h, is made into the chitosan solution that mass concentration is 1.5%; Pravastatin sodium is dissolved in In the water, make the concentration of pravastatin sodium in water be 66.6g / L, obtain the pravastatin sodium aqueous solution; Then, mix the chitosan solution prepared with the pravastatin sodium aqueous solution, stir, and obtain the mass ratio Be the aqueous phase of pravastatin sodium: chitosan=1:1;
[0022] (3) dissolving genipin in 30% aqueous ethanol, so that the concentration of genipin in ethanol with a volume concentration of ...
Embodiment 2
[0032] 1. Preparation of long-acting sustained-release microspheres of pravastatin sodium
[0033] (1) Mix span-80 and ethyl benzoate according to the volume ratio of span-80: ethyl benzoate = 8: 100, and mix evenly under electric stirring at a speed of 1000r / min to obtain an oil phase;
[0034] (2) Adding chitosan with a viscosity of 200 to 400mPa.s into an acetic acid solution with a volume concentration of 1%, magnetic stirring for 5 hours, and preparing a chitosan solution with a mass concentration of 3%; dissolving pravastatin sodium In water, make the concentration of pravastatin sodium in water be 16.6g / L, obtain pravastatin sodium aqueous solution; Then, mix the prepared chitosan solution and pravastatin sodium aqueous solution, stir well, make the Ratio is the aqueous phase of pravastatin sodium: chitosan=1:4;
[0035](3) Genipin is dissolved in 40% aqueous ethanol, so that the concentration of genipin in 40% ethanol by volume concentration is 7.8g / L to obtain a cros...
Embodiment 3
[0042] 1. Preparation of long-acting sustained-release microspheres of pravastatin sodium
[0043] (1) Mix span-80 and liquid paraffin according to the volume ratio of span-80: liquid paraffin = 4: 100, and mix evenly under electric stirring at a speed of 850 r / min to obtain an oil phase;
[0044] (2) Add chitosan with a viscosity of 200 to 400mPa.s into an acetic acid solution with a volume concentration of 1%, magnetically stir for 1h, and prepare a chitosan solution with a mass concentration of 1.5%; dissolve pravastatin sodium In water, make the concentration of pravastatin sodium in water be 33.3g / L, obtain pravastatin sodium aqueous solution; Ratio is the aqueous phase of pravastatin sodium: chitosan=1:2;
[0045] (3) dissolving genipin in 90% aqueous ethanol, so that the concentration of genipin in ethanol with a volume concentration of 90% is 13g / L to obtain a crosslinking agent solution;
[0046] (4) Add the water phase prepared in step (2) dropwise to the oil phase...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| encapsulation rate | aaaaa | aaaaa |
| encapsulation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com