Blood vessel medicine support
A vascular stent and drug technology, applied in the field of medical devices, can solve the problem of the incidence of vascular restenosis not being effectively controlled, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
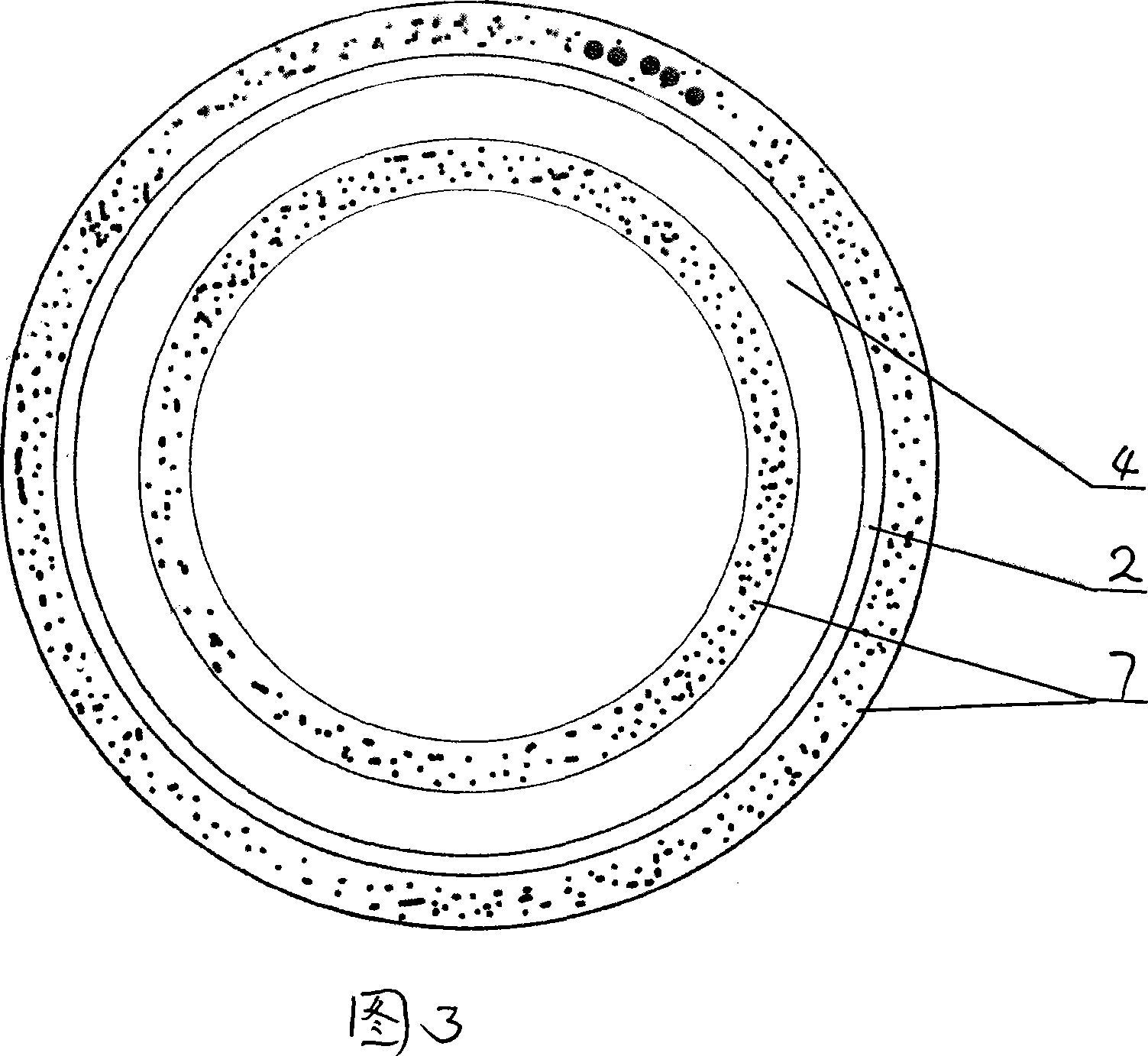
[0055] Embodiment 1: As shown in Figure 2, a porous adsorbed drug-coated stent, the surface of the stent 4 is coated with a polymer coating 6 with micropores 5, and the drug is absorbed on the polymer coating 6 by dip coating Microwell 5. Specifically: add 10g of tetraethyl orthosilicate (TEOS) to 10g of water, then add 0.1g of HCL, mix and disperse evenly at 40°C, form a sol in 2 hours, then spray on the surface of the stent, and then place the stent in an oven at 80°C, After curing for 24 hours, it was dried at 250-800° C. to densify it to form a porous silica gel ceramic-coated scaffold. Then soak the stent in a mixed solution of SIROLIMUS (sirolimus) and TACROLIMUS (tacrolimus) with a concentration of 10%-80% for 30 minutes. After taking out the stent, dry it under vacuum at 40°C to remove the paint, and then prepare SIROLIMUS (sirolimus) and TACROLIMUS (tacrolimus) drug-coated stents adsorbed by porous silica gel ceramics.
Embodiment 2
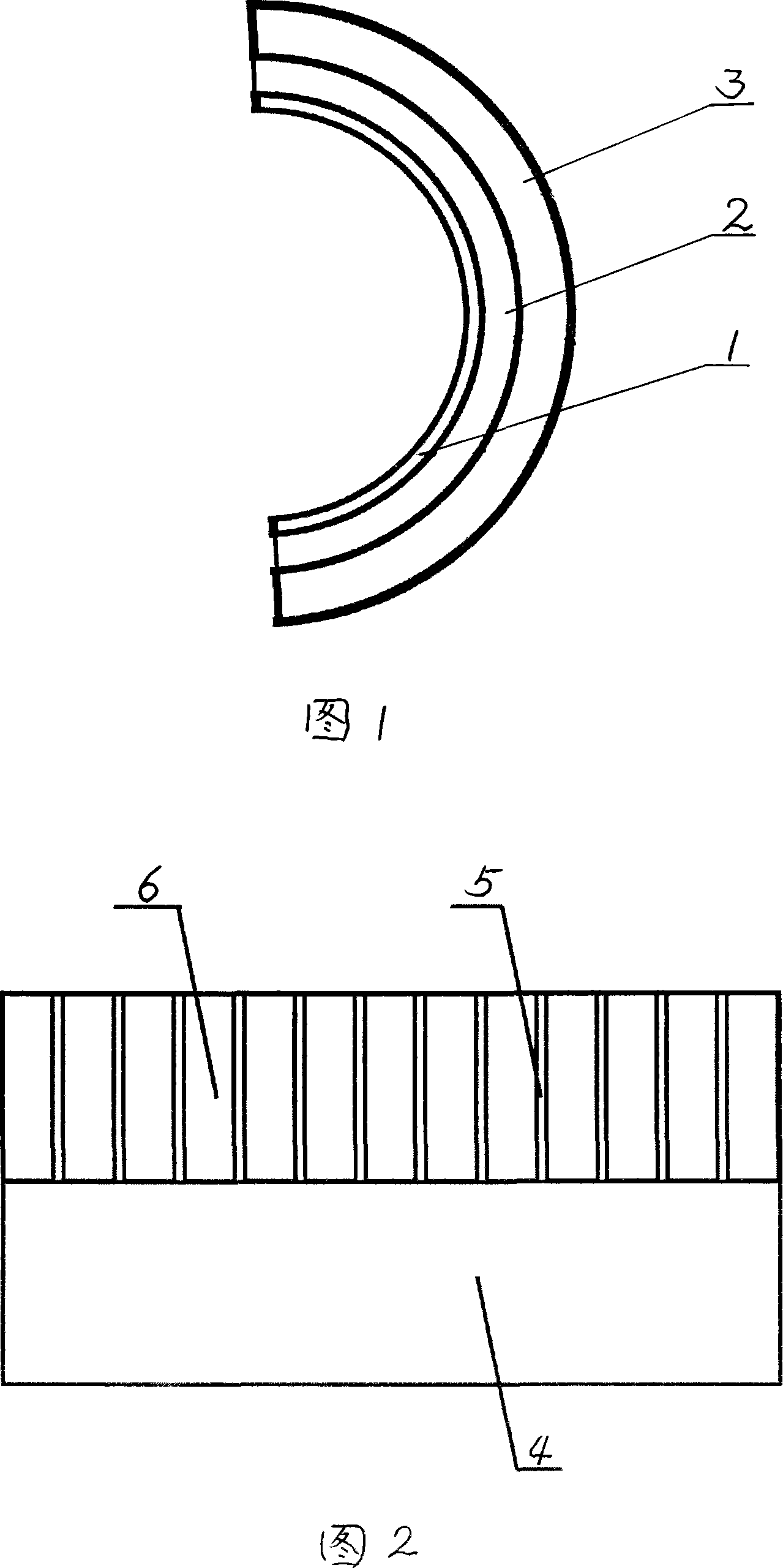
[0056] Embodiment 2: As shown in accompanying drawing 1, a sandwich-type embedded drug-coated stent, the surface of the stent is coated with a layer of pure polymer coating 1, and the polymer coating 1 is coated with a layer of drug coating 2. This layer is coated with a layer of biodegradable polymer or microporous polymer coating 3. Specifically: take 10 g of newly distilled methyl methacrylate monomer and put it into a clean dry conical flask, add initiator benzoyl peroxide (0.1% of monomer weight). In order to prevent water vapor from entering the Erlenmeyer flask during pre-polymerization, wrap a layer of cellophane on the mouth of the flask, and then tie it tightly with a rubber band. Heat the Erlenmeyer flask with a water bath at 80-90°C until the viscosity of the prepolymer in the bottle is close to that of glycerin, stop heating immediately and cool the prepolymer to room temperature with cold water. Spray this solution onto the stent. Put the film-coated stent abov...
Embodiment 3
[0057] Embodiment 3: As shown in accompanying drawing 1, a sandwich-type embedded drug-coated stent, the surface of the stent is coated with a layer of pure polymer coating 1, and the polymer coating 1 is coated with a layer of drug coating 2. A layer of biodegradable polymer microporous polymer coating 3 is applied outside the layer. Specifically: take 10 g of newly distilled methyl methacrylate monomer and put it into a clean dry conical flask, add initiator benzoyl peroxide (0.1% of monomer weight). In order to prevent water vapor from entering the Erlenmeyer flask during pre-polymerization, wrap a layer of cellophane on the mouth of the flask, and then tie it tightly with a rubber band. Heat the Erlenmeyer flask with a water bath at 80-90°C until the viscosity of the prepolymer in the bottle is close to that of glycerin, then stop heating immediately and cool the prepolymer to the room temperature with cold water. Spray this solution onto the stent. Put the film-coated s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com