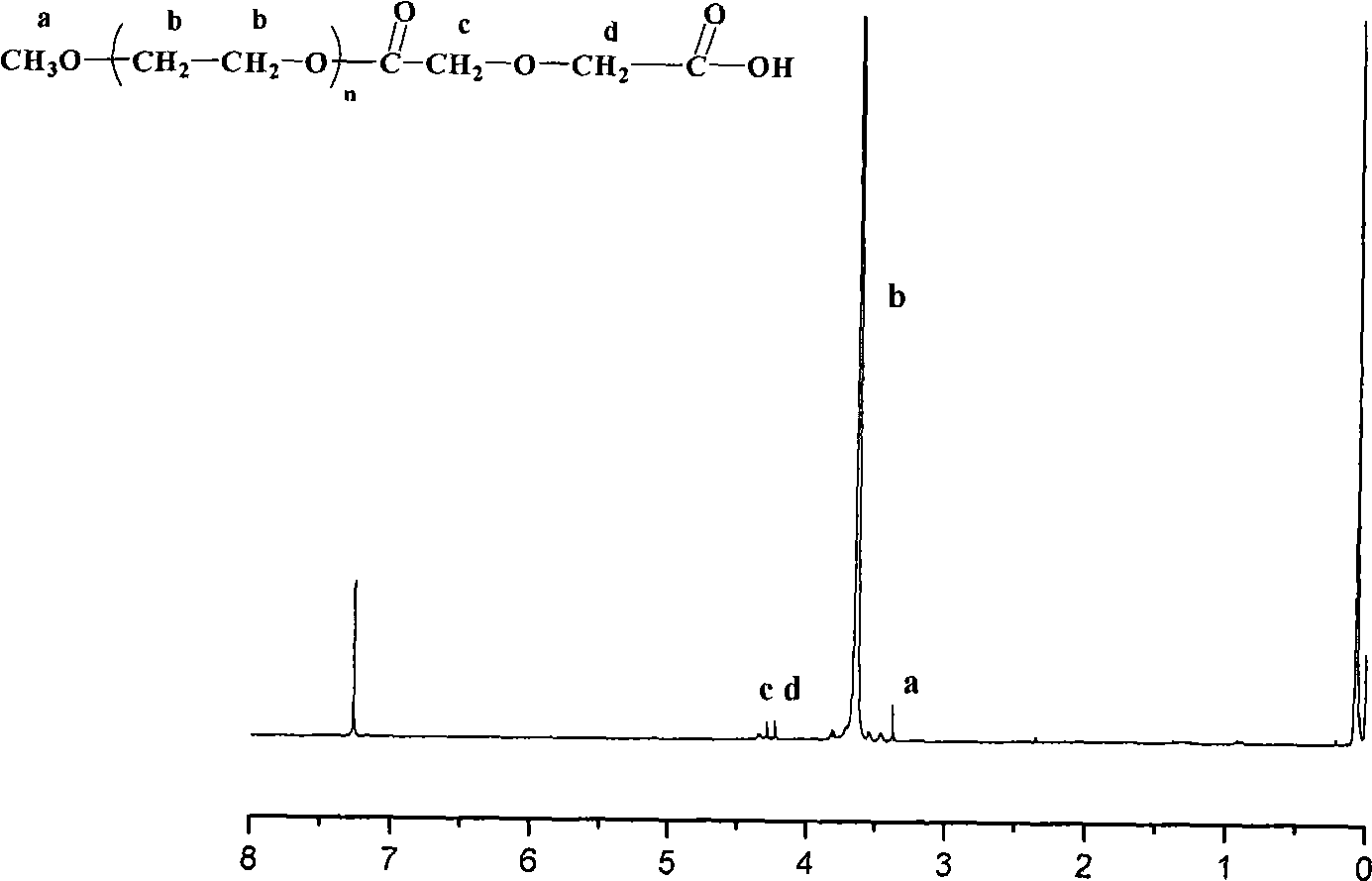
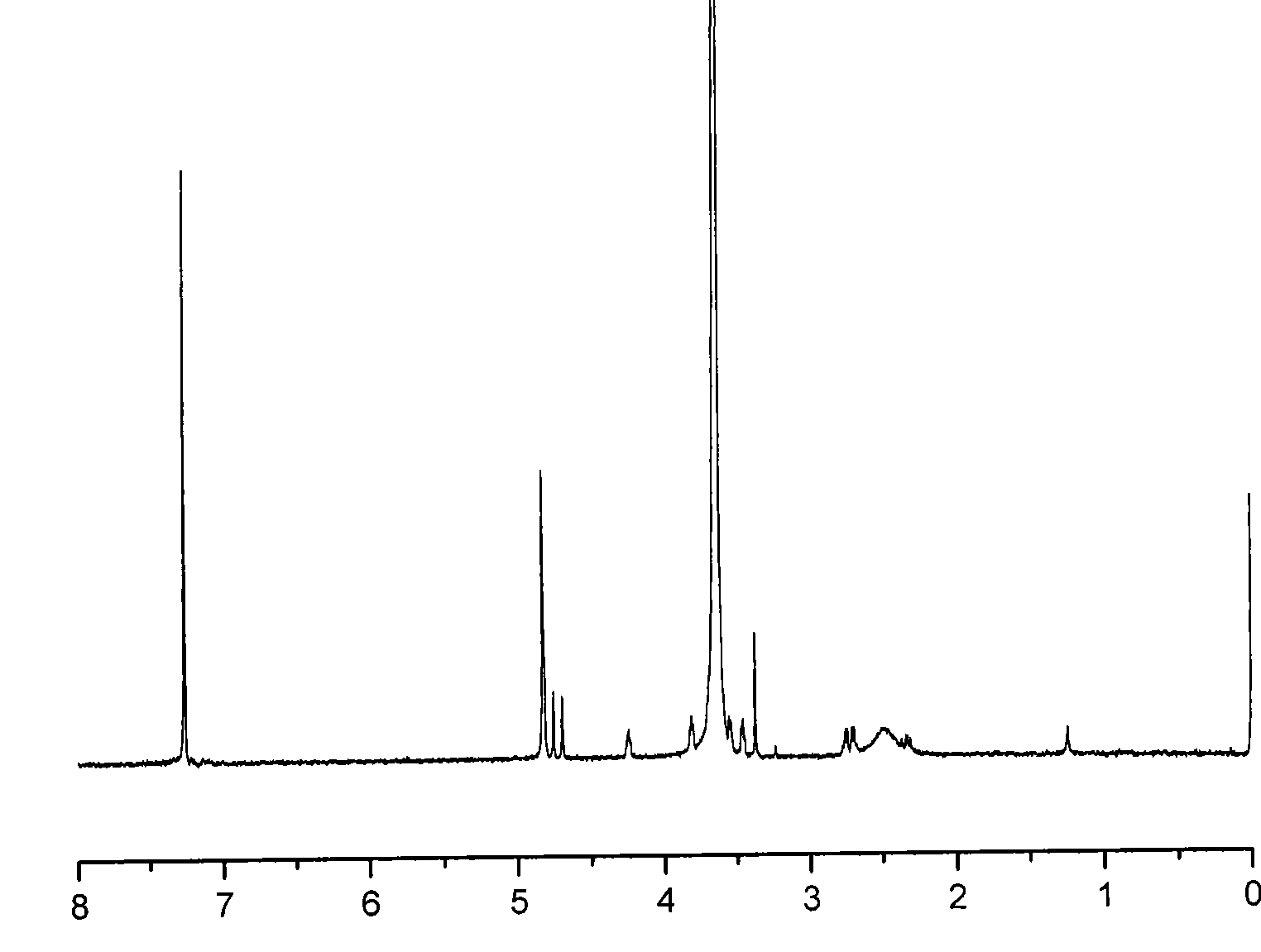
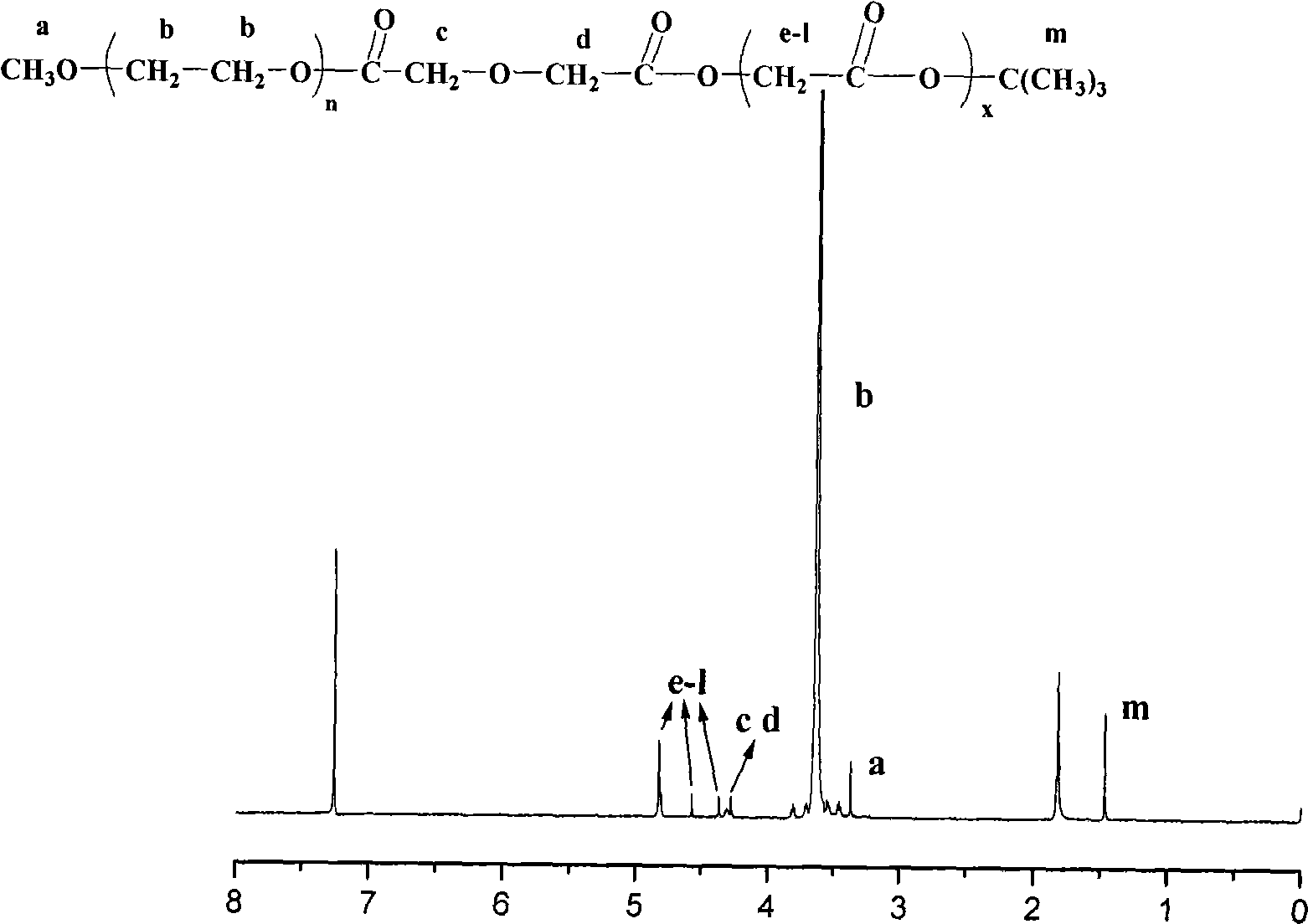Anti-tumor prodrug having accurate structure and taking novel amphipathic polymer as carrier and synthetic method thereof
An amphiphilic polymer, anti-tumor drug technology, applied in anti-tumor drugs, pharmaceutical formulations, organic active ingredients, etc., can solve problems such as different lengths of chain segments
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: Synthesis of polyethylene glycol 2000-succinic acid-glycolic acid octamer-paclitaxel
[0035] 1. Functionalization of Methoxylated Polyethylene Glycol
[0036]In a 100ml three-necked flask, equipped with a reflux condensing device and a magnetic stirrer, the baking reaction device was ventilated three times (high-purity argon), and under gas protection, 5g of terminal methoxypolyethylene glycol and pre-weighed D 1 g of dianhydride, and an equimolar amount of dimethylaminopyridine (DMAP), were added to 60 ml of anhydrous and oxygen-free tetrahydrofuran, and refluxed for 72 hours. After the reaction was completed, the solvent was concentrated, and then 10ml of 0.1M aqueous sodium bicarbonate solution was added to the residue, the filtrate was acidified with 10ml of 0.1M hydrochloric acid, the pH value of the solution was adjusted to 3-4 with hydrochloric acid, and then 100ml of ethyl acetate was used to Wash the water layer three times with ester, combine the ...
Embodiment 2
[0060] Example 2: Synthesis of polyethylene glycol 5000-succinic acid-glycolic acid octamer-paclitaxel
[0061] The reaction process is the same as in Example 1, but in the first step, in the there-necked flask of 100ml, a reflux condensing device and a magnetic stirrer are equipped, and the baking reaction device is ventilated three times (high-purity argon). Oxypolyethylene glycol, 1 g of succinic anhydride, and equimolar amounts of dimethylaminopyridine (DMAP) were added to 60 ml of anhydrous and oxygen-free tetrahydrofuran, and the mixture was refluxed for 72 hours. After the reaction was completed, the solvent was concentrated, and then 10ml of 0.1M aqueous sodium bicarbonate solution was added to the residue, the filtrate was acidified with 10ml of 0.1M hydrochloric acid, the pH value of the solution was adjusted to 3-4 with hydrochloric acid, and then 100ml of ethyl acetate was used to Wash the water layer three times with ester, combine the ethyl acetate solution and w...
Embodiment 3
[0062] Embodiment 3: Synthesis of polyethylene glycol 2000-diglycolic acid-glycolic acid octamer-paclitaxel
[0063] The reaction process is the same as in Example 1, but in the first step, in the there-necked flask of 100ml, a reflux condensing device and a magnetic stirrer are equipped, and the baking reaction device is ventilated three times (high-purity argon), and under gas protection, 5g polymer and 1 g of dihydroxyacetic anhydride, and an equimolar amount of dimethylaminopyridine (DMAP), added 60 ml of anhydrous and oxygen-free tetrahydrofuran, and refluxed for 72 hours. After the reaction was completed, the solvent was concentrated, and then 10ml of 0.1M aqueous sodium bicarbonate solution was added to the residue, the filtrate was acidified with 10ml of 0.1M hydrochloric acid, the pH value of the solution was adjusted to 3-4 with hydrochloric acid, and then 100ml of ethyl acetate was used to Wash the water layer three times with ester, combine the ethyl acetate soluti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com