Yiqing granule preparing method
A technology of granules and Coptis chinensis, applied in the field of preparation of Yiqing granules, can solve the problems that ordinary people dare not use Yiqing granules with confidence, the solubility of Yiqing granules is unqualified, and affects the solubility of granules, etc., and achieves qualified solubility and is easy to promote , easy-to-grasp effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
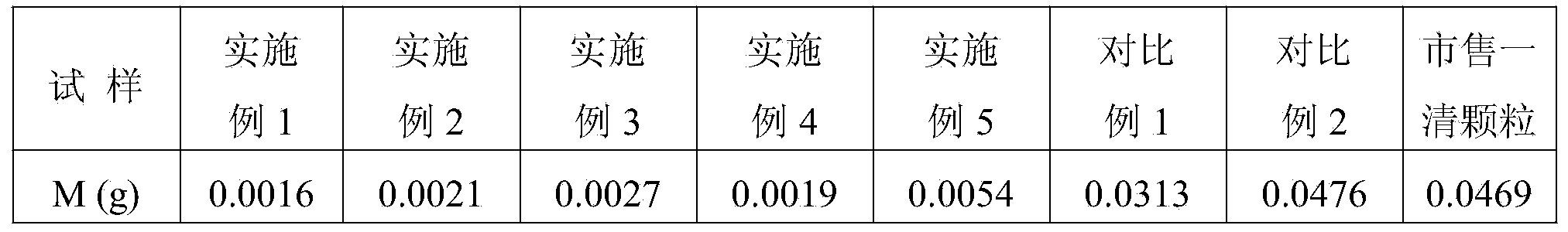
Examples
Embodiment 1
[0032](1) Take 264g of Coptidis rhizome, 800g of rhubarb, and 400g of Scutellaria baicalensis, add water to decoct 3 times respectively, the first time is 1.5h, the second and third times are 1h each, the decoctions are combined, filtered, and the filtrate is concentrated under reduced pressure to 70°C to measure the relative time The clear paste with a density of 1.15 was spray-dried to obtain Coptidis rhizome dry extract powder, Rhubarb dry extract powder and Scutellaria baicalensis dry extract powder;
[0033] (2) Take the Coptidis Rhizoma dry extract powder prepared in step (1), add 100 g of dextrin and 3 g of sodium cyclamate, mix well, add 20 ml of 40% ethanol aqueous solution, make granules, and dry to obtain Coptidis Rhizoma Granules;
[0034] (3) Take the rhubarb dry extract powder obtained in step (1), add 300 g of dextrin and 6 g of sodium cyclamate, mix well, add 50 ml of ethanol aqueous solution containing sodium hydroxide (hydrogen in ethanol aqueous solution cont...
Embodiment 2
[0038] (1) Take 320g of Coptidis rhizome, 700g of rhubarb and 450g of Scutellaria baicalensis, decoct with water for 3 times respectively, 1.5h for the first time, 1h for the second and 3 times respectively, combine the decoctions, filter, and concentrate the filtrate under reduced pressure to 70°C to measure the relative time The clear paste with a density of 1.10 was spray-dried to obtain Coptidis rhizome dry extract powder, Rhubarb dry extract powder and Scutellaria baicalensis dry extract powder;
[0039] (2) Take the Coptidis Rhizoma dry extract powder prepared in step (1), add 100 g of lactose and 5 g of sodium cyclamate, mix well, add 15 ml of 20% ethanol aqueous solution, make granules, and dry to obtain Coptidis Rhizoma Granules;
[0040] (3) Take the rhubarb dry extract powder obtained in step (1), add lactose 300g, sodium cyclamate 5g, mix well, add 45ml of ethanol aqueous solution containing sodium hydroxide (hydroxide in ethanol aqueous solution containing sodium h...
Embodiment 3
[0044] (1) Take 150g of Coptidis rhizome, 600g of rhubarb, and 200g of Scutellaria baicalensis, decoct with water for 3 times, 1.5 hours for the first time, 1 hour for the second and third times, combine the decoctions, filter, and concentrate the filtrate to 70°C under reduced pressure to measure the relative time The clear paste with a density of 1.08 was spray-dried to obtain Coptidis rhizome dry extract powder, Rhubarb dry extract powder and Scutellaria baicalensis dry extract powder;
[0045] (2) Take the Coptidis rhizome dry extract powder obtained in step (1), add 47 g of soluble starch and 3 g of sodium cyclamate, mix well, add 10 ml of 50% ethanol aqueous solution, make granules, and dry to obtain Coptidis rhizome granules;
[0046] (3) Get the rhubarb dry extract powder obtained in step (1), add 196g of soluble starch, 4g of sodium cyclamate, mix well, add 40ml of ethanol aqueous solution containing sodium hydroxide (hydrogen in ethanol aqueous solution containing sod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com