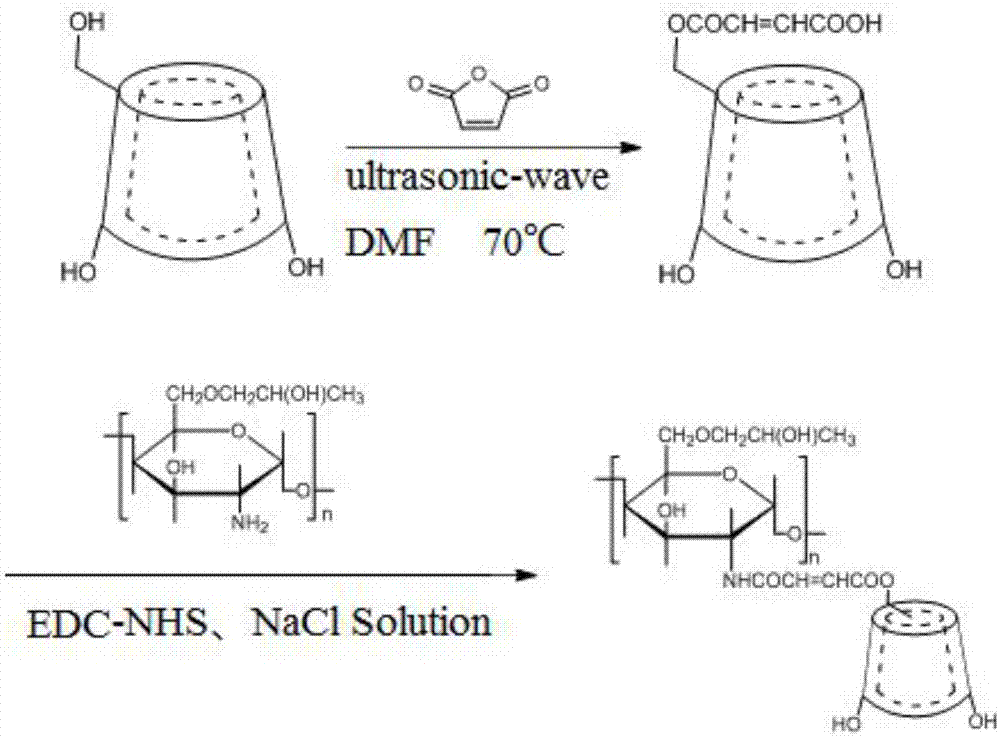
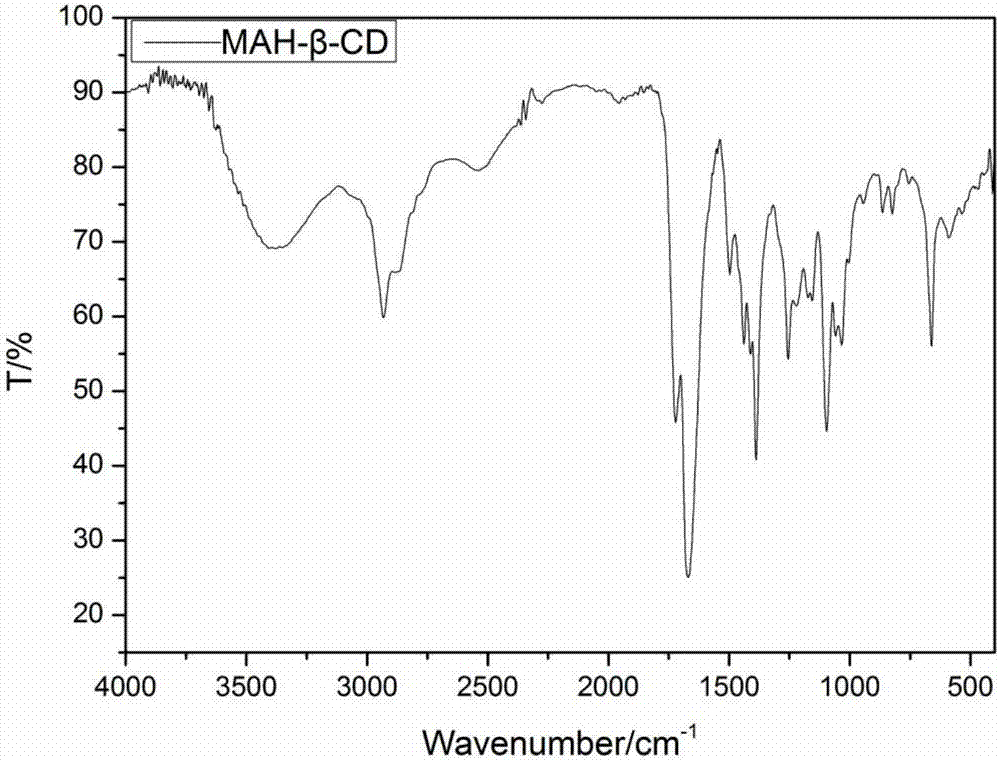
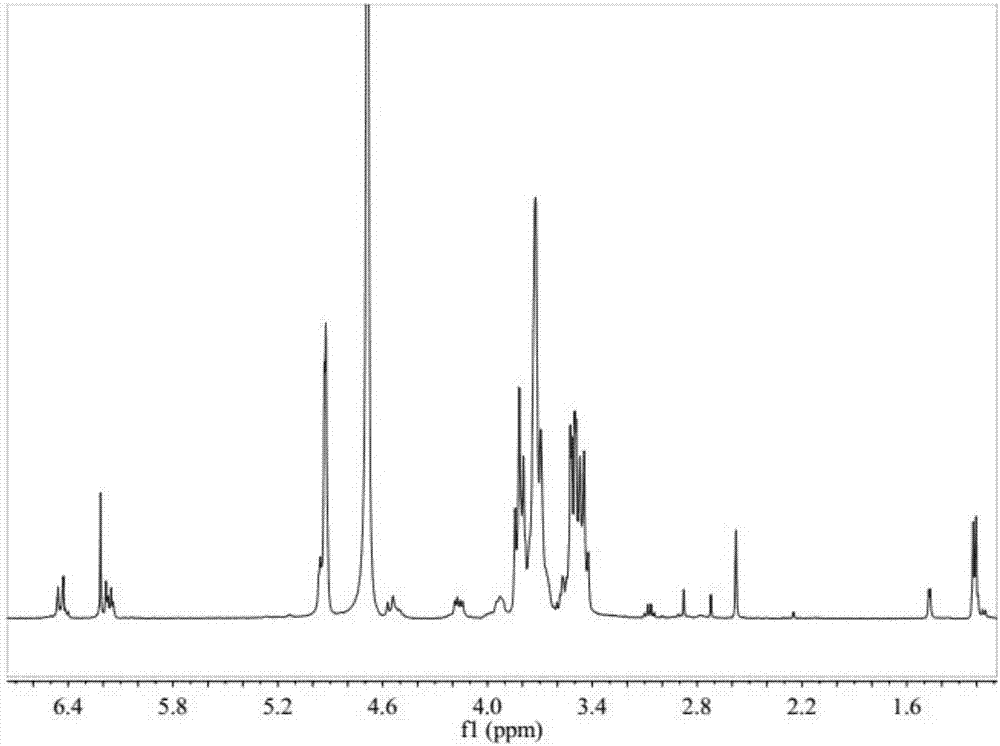
Beta-cyclodextrin derivative grafted hydroxypropyl chitosan hydrogel and preparation method thereof
A technology for grafting hydroxypropyl chitosan water and derivatives is applied in the field of β-cyclodextrin derivative grafting hydroxypropyl chitosan hydrogel and its preparation, which can solve the problem that the controlled release of hydrophobic drugs is difficult to play. function and other issues, to achieve good biocompatibility and expand the scope of application.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] (1) Weigh β-CD and prepare its saturated solution with distilled water at 80-90°C, then cool to room temperature, filter to obtain β-CD crystals, repeat the above operation once for the obtained β-CD crystals, and finally Vacuum drying at -90°C to obtain β-CD crystals with a purity of more than 99.9%;
[0050] (2) Weigh 17.04g (15mmol) of β-CD crystals obtained by recrystallization and 14.70g (150mmol) of maleic anhydride, add them to a 250ml three-necked flask equipped with a thermometer, and add 137ml N,N-dimethyl The three-necked flask was placed in a multi-functional ultrasonic extractor, the set temperature was 70°C, the ultrasonic time was 6h, and it was divided into three stages, the first stage, the ultrasonic frequency was 25KHz, the ultrasonic time was 2h, and the second stage , ultrasonic frequency 35KHz, ultrasonic time 2.5h, the third stage, ultrasonic frequency 45KHz, ultrasonic time 1.5h; take three clean reactors, respectively No. 1, No. 2, No. 3, No. 1 ...
Embodiment 2
[0056] (1) Weigh 17.04g (15mmol) of β-CD crystals obtained by recrystallization and 14.70g (150mmol) of maleic anhydride, add them to a 250ml three-necked flask equipped with a thermometer, and add 170ml N,N-dimethyl The three-necked flask was placed in a multi-functional ultrasonic extractor, the set temperature was 70°C, and the ultrasonic time was 5 hours. It was divided into three stages. In the first stage, the ultrasonic frequency was 25KHz, and the ultrasonic time was 2.5 hours. In the first stage, the ultrasonic frequency is 35KHz, and the ultrasonic time is 1.5h. In the third stage, the ultrasonic frequency is 45KHz, and the ultrasonic time is 1h; three clean reactors are taken, namely No. 1, No. 2, and No. 3, and 50ml Dichloromethane, the three-necked flask is connected to the No. 1 reactor with a two-way peristaltic pump and peristaltic pump tube to form a circulation system. After the first stage of the ultrasonic process, the liquid in the three-necked flask is pum...
Embodiment 3
[0059] (1) Weigh 8.52g (7.5mmol) of β-CD crystals obtained by recrystallization and 7.35g (75mmol) of maleic anhydride, add them to a 250ml three-necked flask equipped with a thermometer, and add 43ml of N,N-2 Methyl formamide, the three-necked flask was placed in a multi-functional ultrasonic extractor, the set temperature was 60°C, the ultrasonic time was 6h, divided into three stages, the first stage, ultrasonic frequency 25KHz, ultrasonic time 2h, the second stage, ultrasonic frequency 35KHz, ultrasonic time 3h, third stage, ultrasonic frequency 45KHz, ultrasonic time 1h; take three clean reactors, namely No. 1, No. 2, No. 3, No. 1 reactor is equipped with 17ml three Chloromethane, the three-necked flask is connected to the No. 1 reactor with a two-way peristaltic pump and peristaltic pump tube to form a circulation system. After the first stage of the ultrasonic process is completed, the liquid in the three-necked flask is pumped into the No. 1 reactor, and the No. 1 react...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com