Drug sustained release composition sparingly soluble or slightly soluble in water, and preparation method thereof
A slow-release composition and water-insoluble technology, which can be used in drug delivery, pharmaceutical formulations, non-active ingredients of polymer compounds, etc., can solve the problems of inconvenient clinical use and poor patient compliance, and achieve effective blood drug concentration, Good stability, avoiding the effect of delayed release platform
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
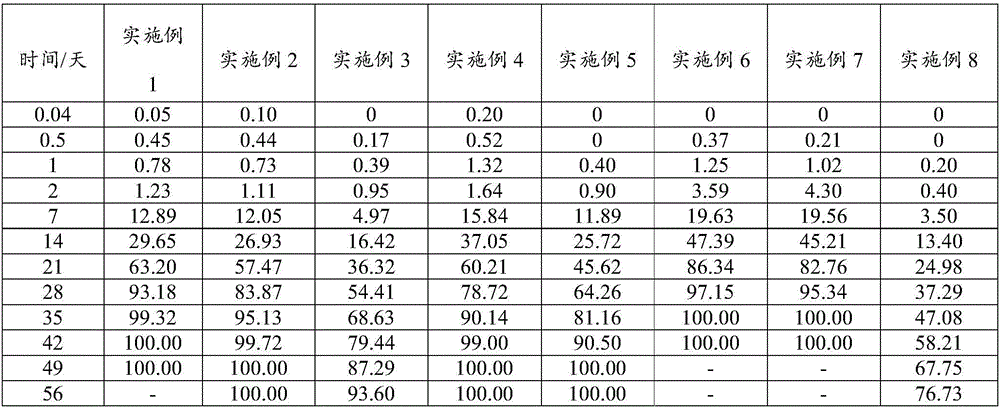
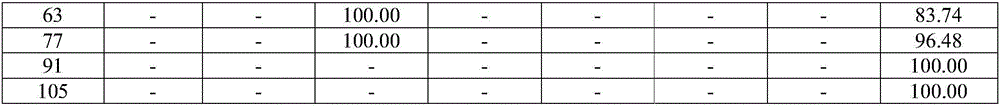
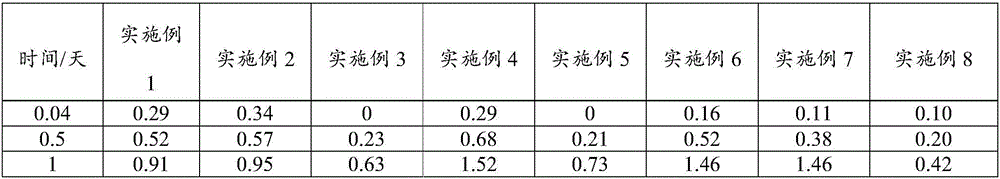
Examples
Embodiment 1
[0076]An embodiment of the poorly water-soluble / slightly soluble drug sustained-release composition of the present invention, the non-solvent preparation raw materials of the poorly water-soluble / slightly soluble drug sustained-release composition in this embodiment contain the following components in mass percentage : Poorly water-soluble / slightly soluble drug: Entecavir 25%, poorly water-soluble polymer: PLGA 74.9%, release modifier: mixture of stearic acid and PEG6000 0.1%.
[0077] Wherein, the molar ratio of lactide to glycolide in the PLGA is 90:10, the weight average molecular weight of the PLGA is 25kDa, the viscosity is 0.24dL / g, and the PLGA has a terminal ester group; the hard In the mixture of fatty acid and PEG6000, the mass percent content of PEG6000 in the release regulator is 70%.
[0078] The poorly water-soluble / slightly soluble drug sustained-release composition described in this example is prepared by the following method:
[0079] (1) The poorly water-sol...
Embodiment 2
[0084] An embodiment of the poorly water-soluble / slightly soluble drug sustained-release composition of the present invention, the non-solvent preparation raw materials of the poorly water-soluble / slightly soluble drug sustained-release composition in this embodiment contain the following components in mass percentage : Poorly water-soluble / slightly soluble drug: aripiprazole 27%, poorly water-soluble polymer: PLGA 72.5%, release modifier: mixture of behenic acid and PEG4000 0.5%.
[0085] Wherein, the molar ratio of lactide to glycolide in the PLGA is 95:5, the weight average molecular weight of the PLGA is 30kDa, the viscosity is 0.29dL / g, and the PLGA has a terminal ester group; In the mixture of sputic acid and PEG4000, the mass percent content of the PEG4000 in the release modifier is 60%.
[0086] The poorly water-soluble / slightly soluble drug sustained-release composition described in this example is prepared by the following method:
[0087] (1) Dissolve the poorly wa...
Embodiment 3
[0092] An embodiment of the poorly water-soluble / slightly soluble drug sustained-release composition of the present invention, the non-solvent preparation raw materials of the poorly water-soluble / slightly soluble drug sustained-release composition in this embodiment contain the following components in mass percentage : Poorly water-soluble / slightly soluble drug: risperidone 30%, poorly water-soluble polymer: PLA 69.2%, release regulator: behenic acid 0.8%.
[0093] Wherein, the weight average molecular weight of the PLA is 35Da, the viscosity is 0.31dL / g, and the PLGA has a terminal ester group.
[0094] The poorly water-soluble / slightly soluble drug sustained-release composition described in this example is prepared by the following method:
[0095] (1) Dissolve the poorly water-soluble polymer in the organic solvent toluene, then add the poorly water-soluble / slightly soluble drug and the release regulator, and obtain the internal oil phase after dissolving; wherein, the poo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com