Arsenic trioxide sustained-release pellet and preparation method thereof
A technology of arsenic trioxide and sustained-release pellets, which is applied in the fields of medical technology and pharmaceutical preparations, can solve the problems of low bioavailability, low patient compliance, and long administration time, and achieves easy industrial production, simple preparation process, and reduced administration. effect of times
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
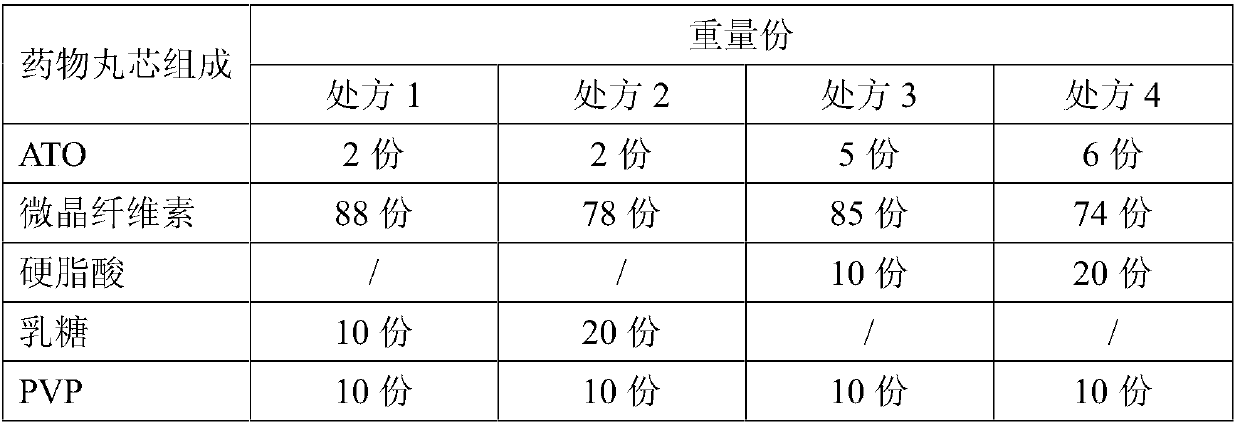
[0032] In the arsenic trioxide sustained-release pellets provided in this embodiment, the prescription composition and preparation method of the drug pellet core are as follows:
[0033] Table 1 Drug bolus core prescription composition
[0034]
[0035] Ball core preparation method: adopt extruding spheronization method to prepare the medicine ball core of above each prescription, concrete operation is as follows: after main drug and adjuvant are mixed by prescription ratio, add binding agent (with deionized water PVP is mixed with concentration 10% solution as adhesive). After the soft material is made, it is extruded in the extruder through the screen of the extruder, and the obtained strips are rounded in the spheronizer, then taken out and dried, and sieved to obtain small pellets of 18-35 mesh for later use. The pellet core yields prepared by each prescription are not less than 90%.
Embodiment 2
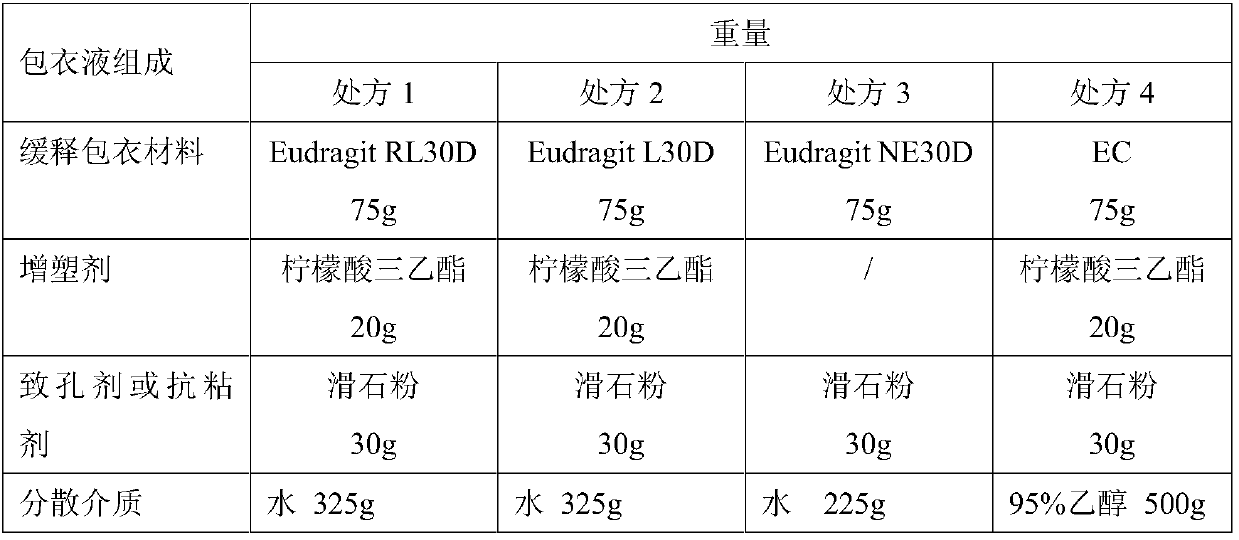
[0037] In the arsenic trioxide sustained-release pellets provided in this example, the coating solution formulation and preparation method of the sustained-release layer are as follows:
[0038] Table 2 Coating Solution Prescription Composition
[0039]
[0040] Coating process: the coating solution of above different prescriptions is wrapped outside the drug ball core, and concrete operation is as follows: open the fluidized bed, will prepare the ATO ball core (18 mesh to 35 Mesh) 500g into the fluidized bed, spray coating solution, after coating, respectively at 50°C (coating solution prescription 1), 40°C (coating solution prescription 2), 25°C (coating solution prescription 3) and 40°C (coating solution prescription 4) oven for aging for 24h.
Embodiment 3
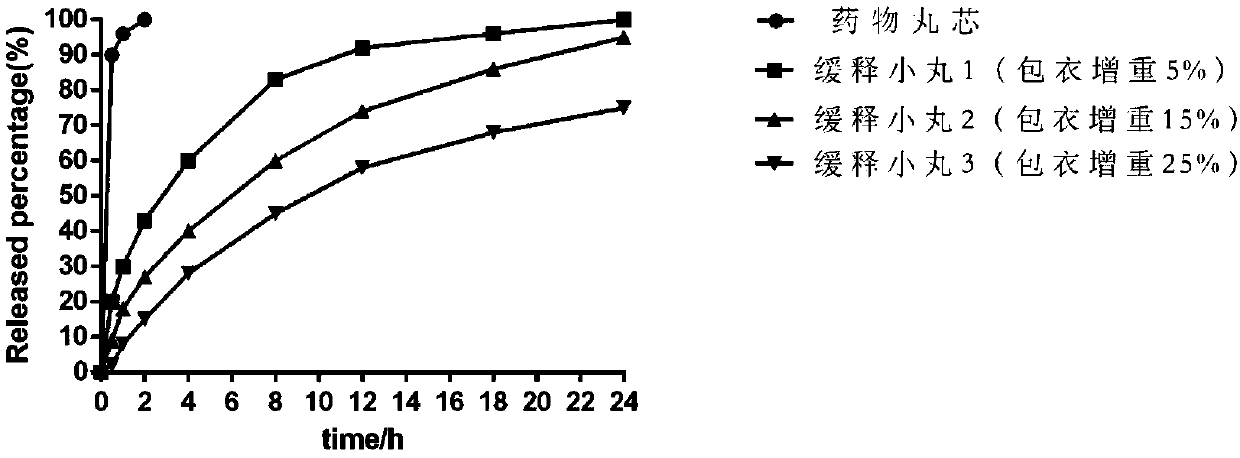
[0042] In this embodiment, an appropriate amount of slow-release pellets with different polymer wrapping amounts is taken, packed into capsules, and the release degree is measured. The specific results are as follows: figure 1 shown;
[0043] The release rate refers to the general rules of Part 4 of the 2015 edition of the "Chinese Pharmacopoeia", using the device of the first method of the dissolution and release rate determination method, using 1000ml of phosphate buffer solution with pH 7 as the solvent, and the speed is 100 rpm. Operate according to the law.
PUM
| Property | Measurement | Unit |
|---|---|---|
| aspect ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com