Pharmaceutical composition of morphin-6-glucuronid for transdermal administration and preparation method and application of pharmaceutical composition
A glucuronide and transdermal drug delivery technology, which is applied to the transdermal drug composition of morphine-6-glucuronide and its preparation field, and can solve the problems of injection safety hazards, many adverse reactions, convulsions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
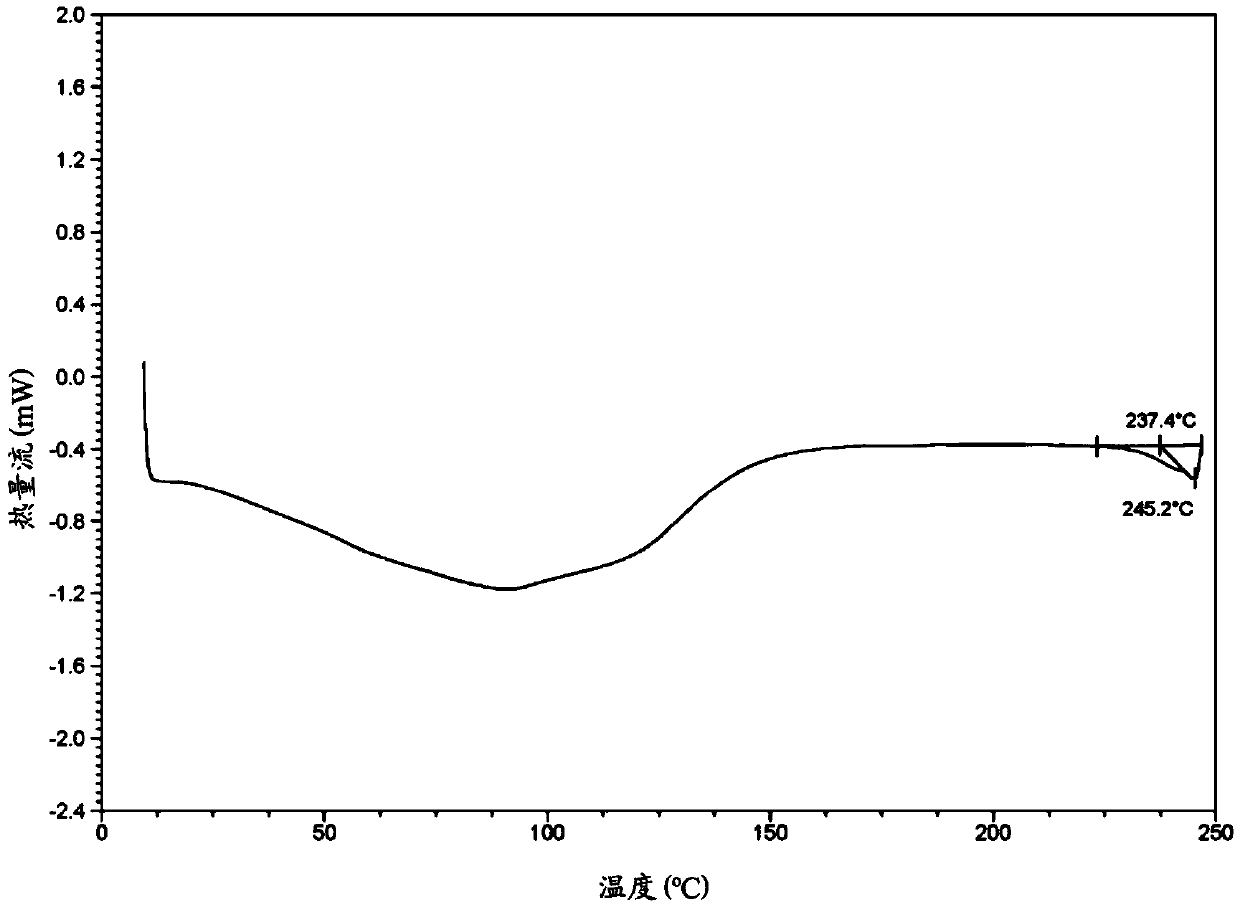
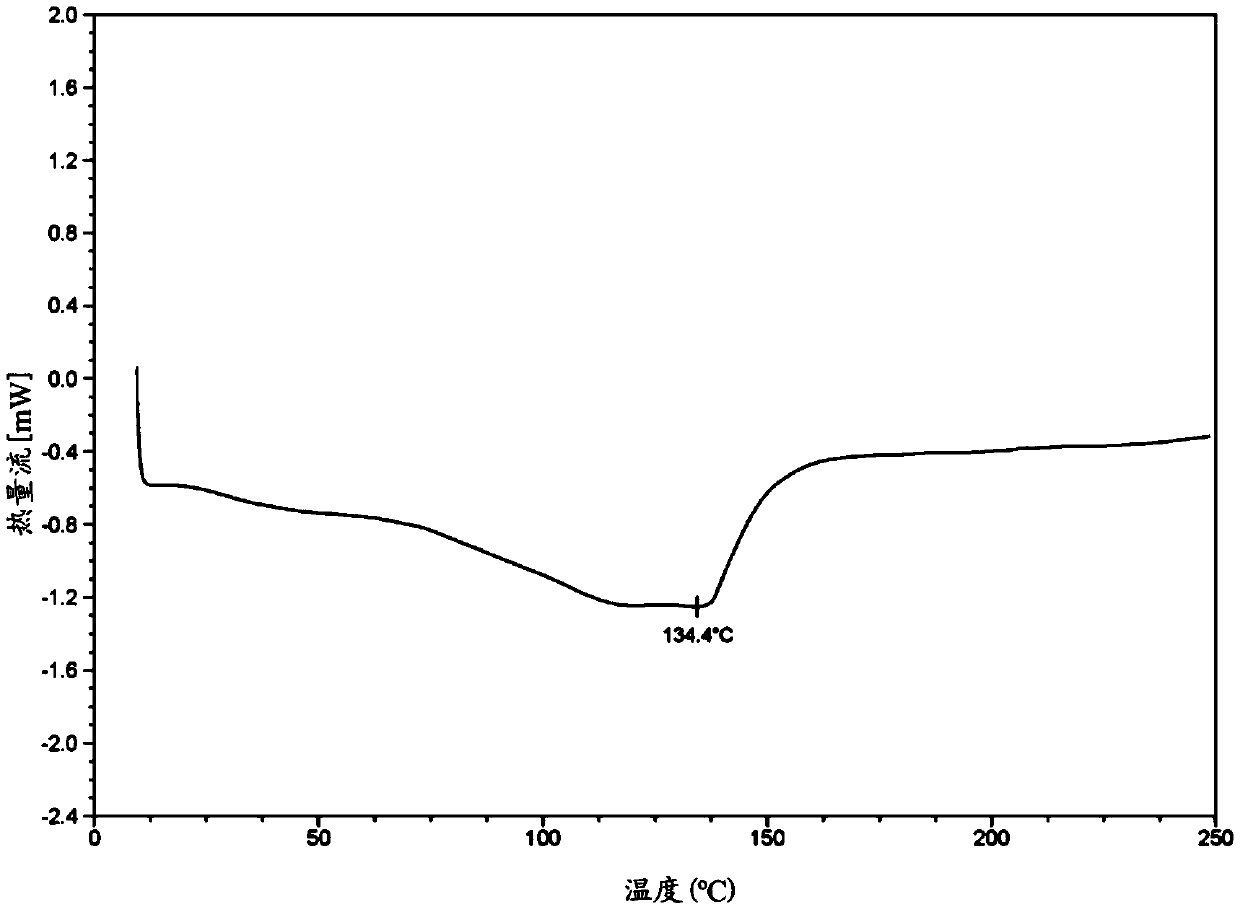
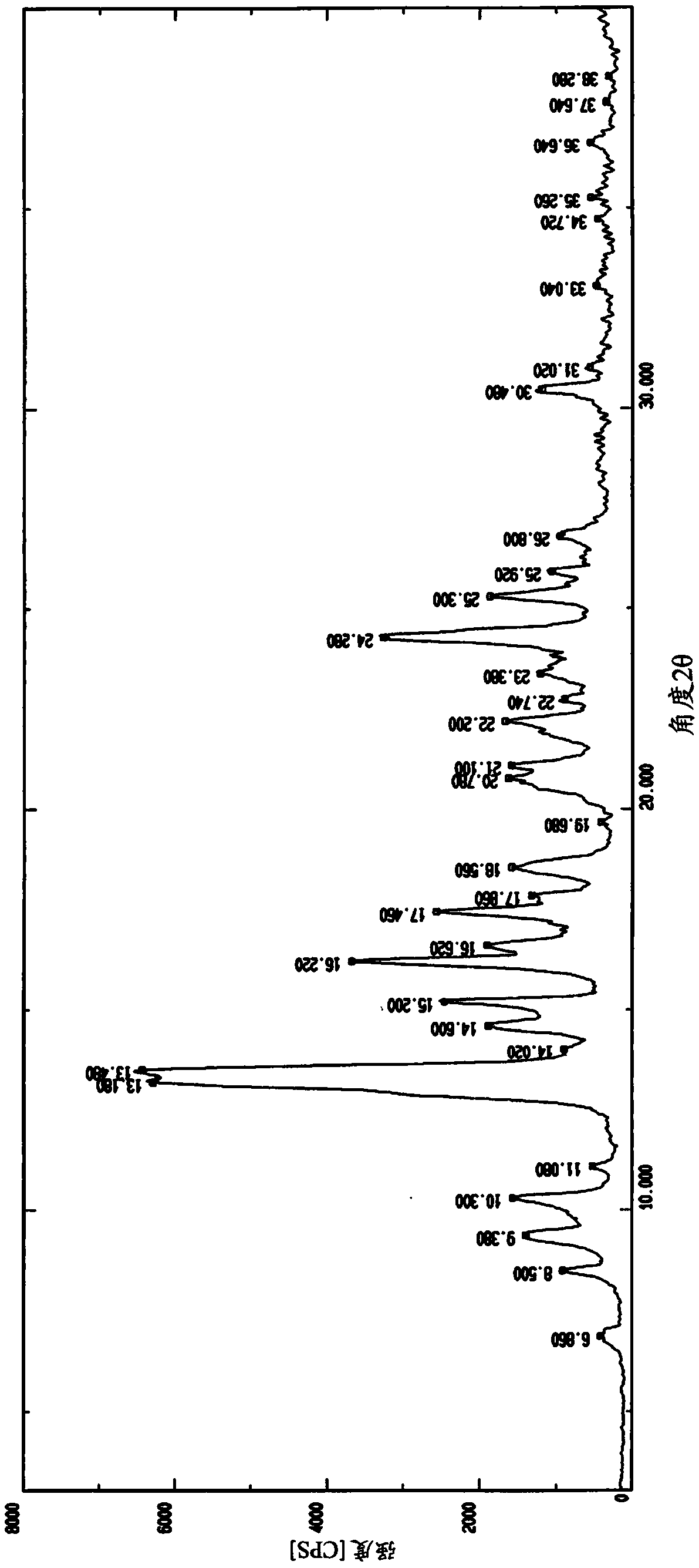
Image
Examples
Embodiment 1
[0066] Embodiment 1 Transdermal patch preparation of morphine-6-glucuronide hydrobromide
[0067] (1) Micronize 5 g of morphine-6-glucuronide hydrobromide, 2 g of povidone (PVP-S630) and 0.1 g of magnesium stearate, mix well to obtain a physical mixture, and add polyethylene glycol (Molecular weight 2000) 2.9g;
[0068] (2) Set the extrusion temperature of the twin-screw extruder to 120°C, start the screw after rising to the set temperature, add the physical mixture in step (1) to the extruder, heat-melt and extrude, It is extruded in the form of spherical particles to obtain amorphous particles, which are then micronized, and the particle size is controlled at about 100-150nm.
[0069] (3) Weigh 5 g of micronized amorphous particles prepared in step (2), 0.2 g of soybean lecithin, 0.03 g of cholesterol, 3 g of absolute ethanol, and 1.77 g of water;
[0070] (4) Dissolving soybean lecithin, cholesterol, and morphine-6-glucuronide hydrobromide microparticles in absolute ethan...
Embodiment 2
[0074] Embodiment 2 Transdermal patch preparation of morphine-6-glucuronide hydrochloride
[0075] (1) Micronize morphine-6-glucuronide hydrochloride 6g, povidone (PVP-S630) 1g, and talcum powder 0.2g, mix well to obtain a physical mixture, add polyethylene glycol (molecular weight 3000 )2.8g;
[0076] (2) Set the extrusion temperature of the twin-screw extruder to 100°C, start the screw after rising to the set temperature, add the physical mixture in step (1) to the extruder, heat-melt and extrude, It is extruded in the form of spherical particles to obtain amorphous particles, which are then micronized, and the particle size is controlled at about 150-200nm.
[0077] (3) Weigh 5 g of micronized amorphous particles prepared in step (2), 0.2 g of phosphatidylcholine, 0.08 g of cholesterol, 3 g of propylene glycol, and 1.72 g of water;
[0078] (4) Dissolving phosphatidylcholine, cholesterol, and morphine-6-glucuronide hydrochloride microparticles in propylene glycol, and hea...
Embodiment 3
[0082] Embodiment 3 Transdermal patch preparation of morphine-6-glucuronide sulfate
[0083] (1) morphine-6-glucuronide sulfate 7g and povidone (PVP-VA64) 1.5g magnesium stearate 0.1g are micronized, mix homogeneously, make physical mixture, add polyethylene glycol (molecular weight 4000) 1.4g;
[0084] (2) Set the extrusion temperature of the twin-screw extruder to 140°C, start the screw after rising to the set temperature, add the physical mixture in step (1) to the extruder, heat-melt and extrude, It is extruded in the form of spherical particles to obtain amorphous particles, which are then micronized, and the particle size is controlled at about 250-300nm.
[0085] (3) Weighing 7 g of micronized amorphous particles prepared in step (2), 2 g of dipalmitoylphosphatidylcholine, 0.3 g of cholesterol, 30 g of absolute ethanol, and 60.7 g of water;
[0086] (4) Dissolving dipalmitoylphosphatidylcholine, cholesterol, and morphine-6-glucuronide sulfate particles in absolute eth...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com