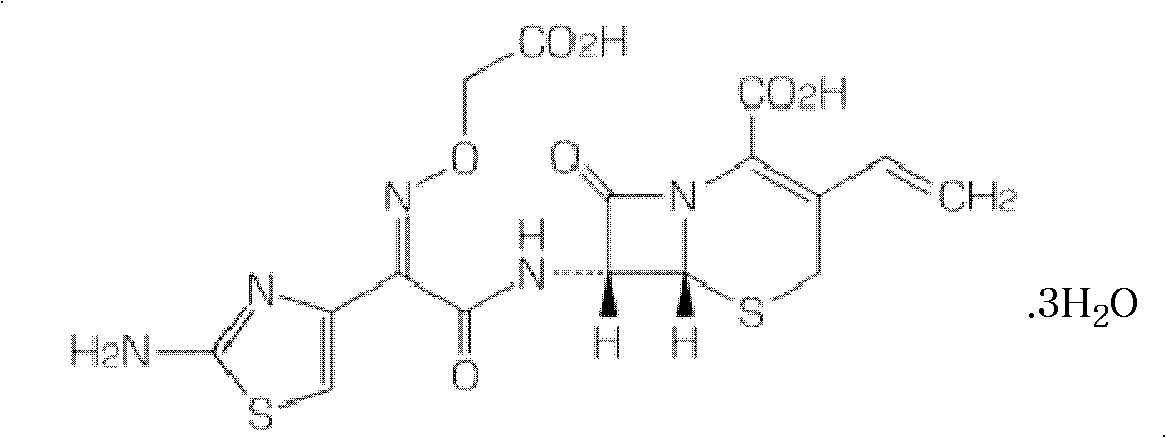
Dispersible tablet containing cefixime liposome and preparation method thereof
A cefixime and liposome technology, applied in the field of medicine, can solve the problems of poor solubility, stability, and bioavailability, and achieve the effects of improving bioavailability, improving bioavailability, and rapidly exerting drug effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
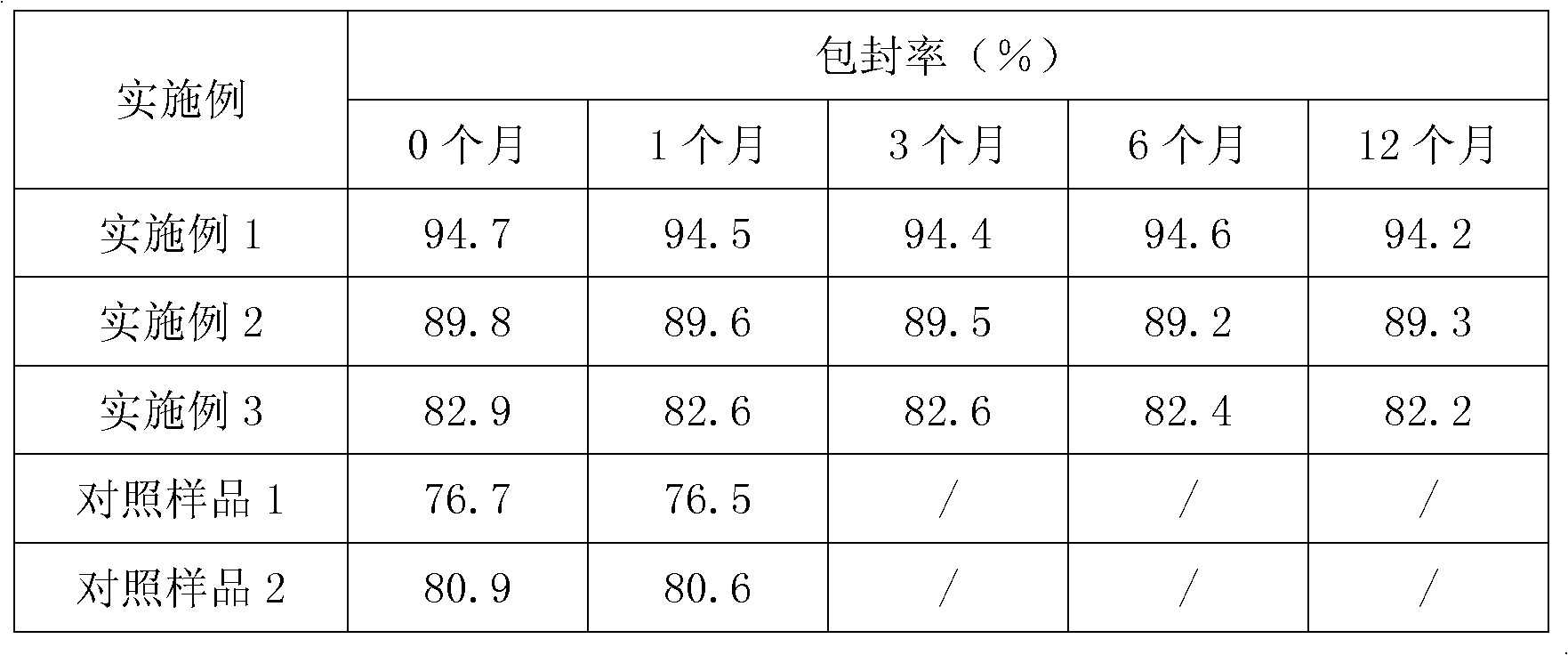
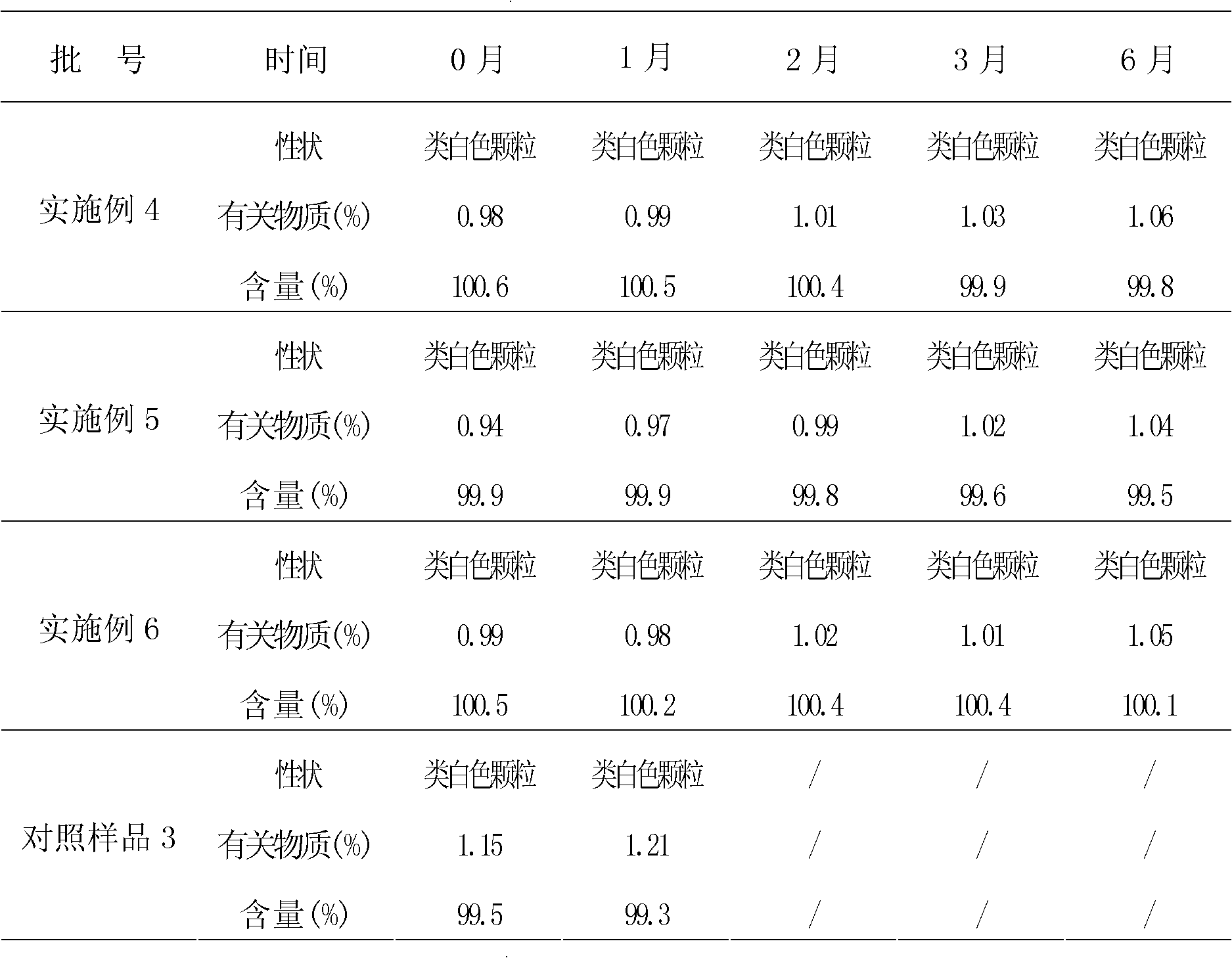
Embodiment 1
[0073] Embodiment 1: the preparation of cefixime liposome
[0074] Name of raw material
Dose 1 (mg)
Dose 2 (mg)
Dose 3 (mg)
50.00
100.00
200.00
Hydrogenated Soy Lecithin
200.00
400.00
800.00
Hydrogenated egg yolk lecithin
200.00
400.00
800.00
400.00
800.00
1600.00
Polysorbate 80
74.00
148.00
296.00
total
924.00
1848.00
3696.00
[0075] Preparation:
[0076] (A) Dissolve hydrogenated soybean lecithin, hydrogenated egg yolk lecithin, cholesterol and polysorbate 80 in an appropriate amount of dehydrated alcohol according to the prescription amount, and mix uniformly to obtain solution I; 30 times;
[0077] (B) take by weighing the cefixime of recipe quantity, add the phosphate buffer saline solution that an appropriate pH value is 7.9 and make it dissolve completely, obtain cefixime solution;
[0078...
Embodiment 2
[0084] Embodiment 2: the preparation of cefixime liposome
[0085] Name of raw material
Dose 1 (mg)
Dose 2 (mg)
Dose 3 (mg)
50.00
50.00
100.00
Hydrogenated Soy Lecithin
162.50
325.00
650.00
Hydrogenated egg yolk lecithin
162.50
325.00
650.00
cholesterol
325.00
650.00
1300.00
Polysorbate 80
60.00
120.00
240.00
total
760.00
1520.00
3040.00
[0086] Preparation:
[0087] (A) Dissolve hydrogenated soybean lecithin, hydrogenated egg yolk lecithin, cholesterol and polysorbate 80 in an appropriate amount of dehydrated alcohol according to the prescription amount, and mix uniformly to obtain solution I; 25 times;
[0088] (B) take by weighing the cefixime of recipe quantity, add the phosphate buffer saline solution that proper pH value is 7.6 and make it dissolve completely, obtain cefixime solution;
[0089] (C) take ...
Embodiment 3
[0095] Embodiment 3: the preparation of cefixime liposome
[0096] Name of raw material
Dose 1 (mg)
Dose 2 (mg)
Dose 3 (mg)
50.00
100.00
200.00
Hydrogenated Soy Lecithin
140.00
280.00
560.00
Hydrogenated egg yolk lecithin
140.00
280.00
560.00
cholesterol
280.00
560.00
1120.00
Polysorbate 80
50.00
100.00
200.00
total
660.00
1320.00
2640.00
[0097] Preparation:
[0098] (A) Dissolve hydrogenated soybean lecithin, hydrogenated egg yolk lecithin, cholesterol and polysorbate 80 in an appropriate amount of dehydrated alcohol according to the prescription amount, and mix uniformly to obtain solution I; 35 times;
[0099] (B) take by weighing the cefixime of recipe quantity, add the phosphate buffer saline solution that an appropriate pH value is 7.0 and make it dissolve completely, obtain cefixime solution;
[0100...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com